Asymmetric and Symmetric Protein Arginine Dimethylation: Concept and Postprandial Effects of High-Fat Protein Meals in Healthy Overweight Men
Abstract
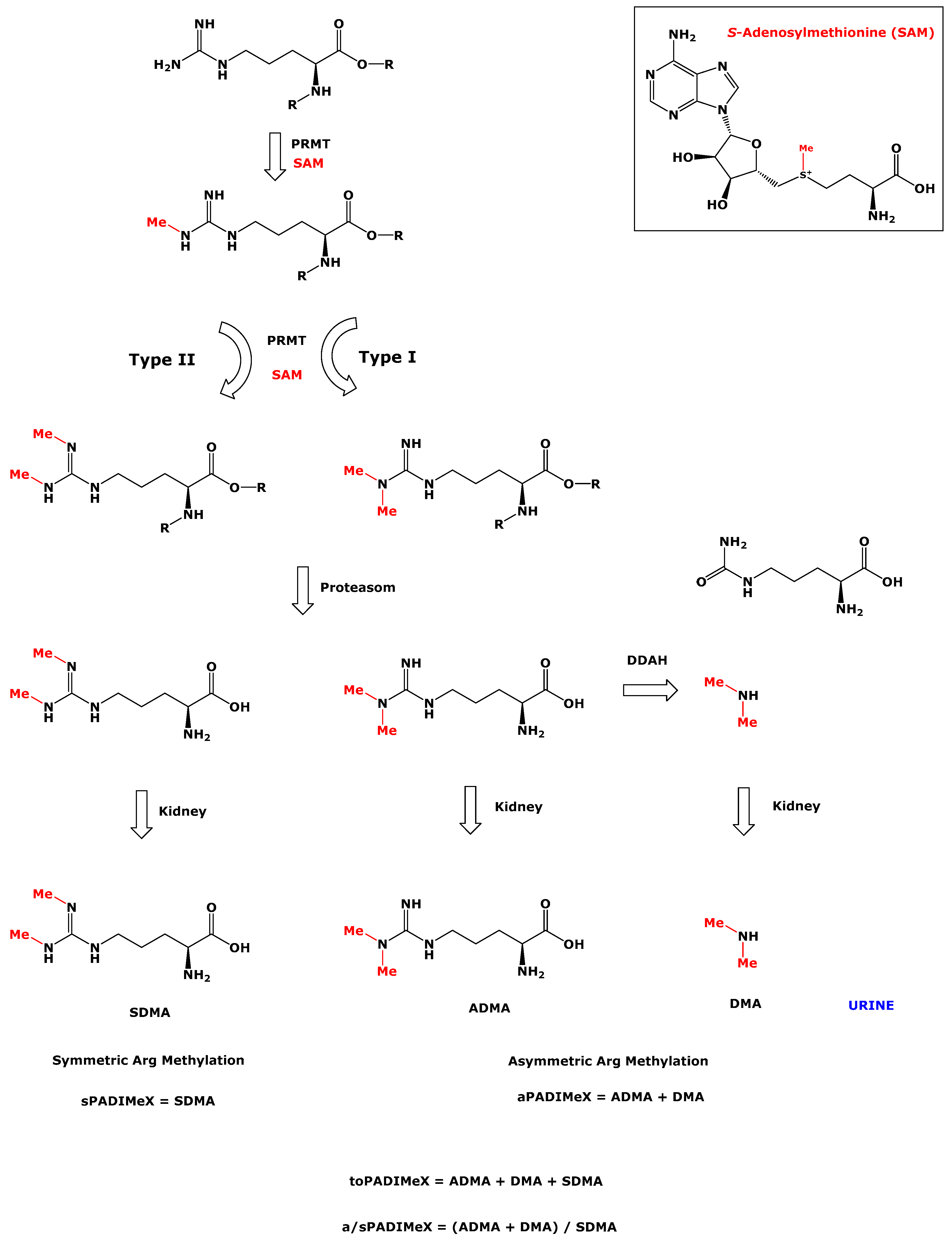
1. Introduction
2. Materials and Methods
2.1. Ingestion of High-Fat Protein Meals by Healthy Overweight Men
2.2. Measurement of Urinary ADMA, DMA, SDMA, Creatinine, and Quality Control
2.3. Measurement of Inflammation and Cardiovascular Biomarkers
2.4. Statistical Analyses
3. Results
4. Discussion
Author Contributions
Funding
Conflicts of Interest
References
- Wu, G.; Bazer, F.W.; Davis, T.A.; Kim, S.W.; Li, P.; Marc Rhoads, J.; Carey Satterfield, M.; Smith, S.B.; Spencer, T.E.; Yin, Y. Arginine metabolism and nutrition in growth, health and disease. Amino Acids 2009, 37, 153–168. [Google Scholar] [CrossRef] [PubMed]
- Wu, G. Functional amino acids in nutrition and health. Amino Acids 2013, 45, 407–411. [Google Scholar] [CrossRef] [PubMed]
- Moncada, S.; Higgs, A. The L-arginine-nitric oxide pathway. N. Engl. J. Med. 1993, 329, 2002–2012. [Google Scholar] [CrossRef] [PubMed]
- Leiper, J.; Vallance, P. Biological significance of endogenous methylarginines that inhibit nitric oxide synthases. Cardiovasc. Res. 1999, 43, 542–548. [Google Scholar] [CrossRef]
- Tsikas, D.; Böger, R.H.; Sandmann, J.; Bode-Böger, S.M.; Frölich, J.C. Endogenous nitric oxide synthase inhibitors are responsible for the L-arginine paradox. FEBS Lett. 2000, 478, 1–3. [Google Scholar] [CrossRef]
- Tsikas, D. Does the inhibitory action of asymmetric dimethylarginine (ADMA) on the endothelial nitric oxide synthase activity explain its importance in the cardiovascular system? The ADMA paradox. J. Controversies Biomed. Res. 2017, 3, 16–22. [Google Scholar] [CrossRef][Green Version]
- Tsikas, D.; Bollenbach, A.; Hanff, E.; Kayacelebi, A.A. Asymmetric dimethylarginine (ADMA), symmetric dimethylarginine (SDMA) and homoarginine (hArg): The ADMA, SDMA and hArg paradoxes. Cardiovasc. Diabetol. 2018, 17, 1. [Google Scholar] [CrossRef]
- Blanc, R.S.; Richard, S. Arginine Methylation: The Coming of Age. Mol. Cell 2017, 65, 8–24. [Google Scholar] [CrossRef]
- Blanc, R.S.; Richard, S. Regenerating muscle with arginine methylation. Transcription 2017, 8, 175–178. [Google Scholar] [CrossRef]
- Peng, C.; Wong, C.C. The story of protein arginine methylation: Characterization, regulation, and function. Expert Rev. Proteomics 2017, 14, 157–170. [Google Scholar] [CrossRef]
- Zoccali, C.; Bode-Böger, S.; Mallamaci, F.; Benedetto, F.; Tripepi, G.; Malatino, L.; Cataliotti, A.; Bellanuova, I.; Fermo, I.; Frölich, J.; et al. Plasma concentration of asymmetrical dimethylarginine and mortality in patients with end-stage renal disease: A prospective study. Lancet 2001, 358, 2113–2117. [Google Scholar] [CrossRef]
- Zoccali, C.; Benedetto, F.A.; Maas, R.; Mallamaci, F.; Tripepi, G.; Malatino, L.S.; Böger, R. Asymmetric dimethylarginine, C-reactive protein, and carotid intima-media thickness in end-stage renal disease. JASN 2002, 13, 490–496. [Google Scholar] [PubMed]
- Frenay, A.R.; van den Berg, E.; de Borst, M.H.; Beckmann, B.; Tsikas, D.; Feelisch, M.; Navis, G.; Bakker, S.J.; van Goor, H. Plasma ADMA associates with all-cause mortality in renal transplant recipients. Amino Acids 2015, 47, 1941–1949. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Schlesinger, S.; Sonntag, S.R.; Lieb, W.; Maas, R. Asymmetric and symmetric dimethylarginine as risk markers for total mortality and cardiovascular outcomes: A systematic review and meta-analysis of prospective studies. PLoS ONE 2016, 11, e0165811. [Google Scholar] [CrossRef] [PubMed]
- Emrich, I.E.; Zawada, A.M.; Martens-Lobenhoffer, J.; Fliser, D.; Wagenpfeil, S.; Heine, G.H.; Bode-Böger, S.M. Symmetric dimethylarginine (SDMA) outperforms asymmetric dimethylarginine (ADMA) and other methylarginines as predictor of renal and cardiovascular outcome in non-dialysis chronic kidney disease. Clin. Res. Cardiol. 2018, 107, 201–213. [Google Scholar] [CrossRef] [PubMed]
- Zobel, E.H.; von Scholten, B.J.; Reinhard, H.; Persson, F.; Teerlink, T.; Hansen, T.W.; Parving, H.H.; Jacobsen, P.K.; Rossing, P. Symmetric and asymmetric dimethylarginine as risk markers of cardiovascular disease, all-cause mortality and deterioration in kidney function in persons with type 2 diabetes and microalbuminuria. Cardiovasc. Diabetol. 2017, 16, 88. [Google Scholar] [CrossRef]
- Beltran-Alvarez, P.; Pagans, S.; Brugada, R. The cardiac sodium channel is post-translationally modified by arginine methylation. J. Proteom Res. 2011, 10, 3712–3719. [Google Scholar] [CrossRef]
- Beltran-Alvarez, P.; Tarradas, A.; Chiva, C.; Perez-Serra, A.; Batlle, M.; Perez-Villa, F.; Schulte, U.; Sabido, E.; Brugada, R.; Pagans, S. Identification of N-terminal protein acetylation and arginine methylation of the voltage-gated sodium channel in end-stage heart failure human heart. J. Mol. Cell. Cardiol. 2014, 76, 126–129. [Google Scholar] [CrossRef]
- Beltran-Alvarez, P.; Feixas, F.; Osuna, S.; Diaz-Hernandez, R.; Brugada, R.; Pagans, S. Interplay between R513 methylation and S516 phosphorylation of the cardiac voltage-gated sodium channel. Amino Acids 2015, 47, 429–434. [Google Scholar] [CrossRef]
- Achan, V.; Broadhead, M.; Malaki, M.; Whitley, G.; Leiper, J.; MacAllister, R.; Vallance, P. Asymmetric dimethylarginine causes hypertension and cardiac dysfunction in humans and is actively metabolized by dimethylarginine dimethylaminohydrolase. Arterioscl. Thromb. Vascular Biol. 2003, 23, 1455–1459. [Google Scholar] [CrossRef]
- Nijveldt, R.J.; Van Leeuwen, P.A.; Van Guldener, C.; Stehouwer, C.D.; Rauwerda, J.A.; Teerlink, T. Net renal extraction of asymmetrical (ADMA) and symmetrical (SDMA) dimethylarginine in fasting humans. Nephrology Dialysis Transplantation 2002, 17, 1999–2002. [Google Scholar] [CrossRef] [PubMed]
- Bollenbach, A.; Hanff, E.; Beckmann, B.; Kruger, R.; Tsikas, D. GC-MS quantification of urinary symmetric dimethylarginine (SDMA), a whole-body symmetric l-arginine methylation index. Anal. Biochem. 2018, 556, 40–44. [Google Scholar] [CrossRef] [PubMed]
- Kuo, H.C.; Hsu, C.N.; Huang, C.F.; Lo, M.H.; Chien, S.J.; Tain, Y.L. Urinary arginine methylation index associated with ambulatory blood pressure abnormalities in children with chronic kidney disease. JASH 2012, 6, 385–392. [Google Scholar] [CrossRef]
- Tsikas, D.; Thum, T.; Becker, T.; Pham, V.V.; Chobanyan, K.; Mitschke, A.; Beckmann, B.; Gutzki, F.M.; Bauersachs, J.; Stichtenoth, D.O. Accurate quantification of dimethylamine (DMA) in human urine by gas chromatography-mass spectrometry as pentafluorobenzamide derivative: Evaluation of the relationship between DMA and its precursor asymmetric dimethylarginine (ADMA) in health and disease. J. Chromatogr. B 2007, 851, 229–239. [Google Scholar] [CrossRef] [PubMed]
- Asatoor, A.M.; Simenhoff, M.L. The origin of urinary dimethylamine. Biochim. Biophys. Acta 1965, 111, 384–392. [Google Scholar] [CrossRef]
- Mitchell, S.C.; Zhang, A.Q.; Smith, R.L. Dimethylamine and diet. Food Chem. Toxicol. 2008, 46, 1734–1738. [Google Scholar] [CrossRef] [PubMed]
- Servillo, L.; Giovane, A.; Cautela, D.; Castaldo, D.; Balestrieri, M.L. The methylarginines NMMA, ADMA, and SDMA are ubiquitous constituents of the main vegetables of human nutrition. Nitric Oxide 2013, 30, 43–48. [Google Scholar] [CrossRef] [PubMed]
- Engeli, S.; Tsikas, D.; Lehmann, A.C.; Bohnke, J.; Haas, V.; Strauss, A.; Janke, J.; Gorzelniak, K.; Luft, F.C.; Jordan, J. Influence of dietary fat ingestion on asymmetrical dimethylarginine in lean and obese human subjects. Nutr. Metab. Cardiovasc. Dis. 2012, 22, 720–726. [Google Scholar] [CrossRef] [PubMed]
- Kayacelebi, A.A.; Langen, J.; Weigt-Usinger, K.; Chobanyan-Jurgens, K.; Mariotti, F.; Schneider, J.Y.; Rothmann, S.; Frolich, J.C.; Atzler, D.; Choe, C.U.; et al. Biosynthesis of homoarginine (hArg) and asymmetric dimethylarginine (ADMA) from acutely and chronically administered free L-arginine in humans. Amino Acids 2015, 47, 1893–1908. [Google Scholar] [CrossRef] [PubMed]
- Mariotti, F.; Valette, M.; Lopez, C.; Fouillet, H.; Famelart, M.H.; Mathe, V.; Airinei, G.; Benamouzig, R.; Gaudichon, C.; Tome, D.; et al. Casein compared with whey proteins affects the organization of dietary fat during digestion and attenuates the postprandial triglyceride response to a mixed high-fat meal in healthy, overweight men. J. Nutr. 2015, 145, 2657–2664. [Google Scholar] [CrossRef] [PubMed]
- Tsikas, D.; Schubert, B.; Gutzki, F.M.; Sandmann, J.; Frölich, J.C. Quantitative determination of circulating and urinary asymmetric dimethylarginine (ADMA) in humans by gas chromatography-tandem mass spectrometry as methyl ester tri(N-pentafluoropropionyl) derivative. J. Chromatogr. B 2003, 798, 87–99. [Google Scholar] [CrossRef]
- Tsikas, D.; Beckmann, B.; Gutzki, F.M.; Jordan, J. Simultaneous gas chromatography-tandem mass spectrometry quantification of symmetric and asymmetric dimethylarginine in human urine. Anal. Biochem. 2011, 413, 60–62. [Google Scholar] [CrossRef]
- Torremans, A.; Marescau, B.; Vanholder, R.; De Smet, R.; Billiouw, J.M.; De Deyn, P.P. The low nanomolar levels of N G-monomethylarginine in serum and urine of patients with chronic renal insufficiency are not significantly different from control levels. Amino Acids 2003, 24, 375–381. [Google Scholar] [CrossRef] [PubMed]
- Martens-Lobenhoffer, J.; Rodionov, R.N.; Drust, A.; Bode-Boger, S.M. Detection and quantification of alpha-keto-delta-(N(G),N(G)-dimethylguanidino)valeric acid: A metabolite of asymmetric dimethylarginine. Anal. Biochem. 2011, 419, 234–240. [Google Scholar] [CrossRef] [PubMed]
- Martens-Lobenhoffer, J.; Rodionov, R.N.; Bode-Böger, S.M. Determination of asymmetric Nalpha-acetyldimethylarginine in humans: A phase II metabolite of asymmetric dimethylarginine. Anal. Biochem. 2014, 452, 25–30. [Google Scholar] [CrossRef] [PubMed]
- Schneider, J.Y.; Rothmann, S.; Schröder, F.; Langen, J.; Lücke, T.; Mariotti, F.; Huneau, J.F.; Frölich, J.C.; Tsikas, D. Effects of chronic oral L-arginine administration on the L-arginine/NO pathway in patients with peripheral arterial occlusive disease or coronary artery disease: L-Arginine prevents renal loss of nitrite, the major NO reservoir. Amino Acids 2015, 47, 1961–1974. [Google Scholar] [CrossRef]
- Said, M.Y.; Bollenbach, A.; Minovic, I.; van Londen, M.; Frenay, A.R.; de Borst, M.H.; van den Berg, E.; Kayacelebi, A.A.; Tsikas, D.; van Goor, H.; et al. Plasma ADMA, urinary ADMA excretion, and late mortality in renal transplant recipients. Amino Acids 2019. [Google Scholar] [CrossRef] [PubMed]
| Table 1a | ADMA | SDMA | DMA |
| SDMA (µmol/L) | 0.939, p < 0.0001 | ||
| DMA (µmol/L) | 0.900, p < 0.0001 | 0.923, p < 0.0001 | |
| Creatinine (mmol/L) | 0.842, p < 0.0001 | 0.886, p < 0.0001 | 0.883, p < 0.0001 |
| Table 1b | ADMA | SDMA | DMA |
| SDMA (µmol/mmol) | 0.811, p < 0.0001 | ||
| DMA (µmol/mmol) | 0.652, p < 0.0001 | 0.765, p < 0.0001 | |
| toPADiMeX (µmol/mmol) | 0.755, p < 0.0001 | 0.842, p < 0.0001 | 0.981, p < 0.0001 |
| Glucose | TAG | apoB48 | NEFA | Insulin | TNF-α | IL-6 | MCP-1 | sICAM-1 | sVCAM-1 | MPO | E-Selectin | tPAI-1 | RI | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| TAG | 0.086 | |||||||||||||
| apoB48 | 0.034 | 0.785 c | ||||||||||||
| NEFA | −0.265 | 0.099 | 0.055 | |||||||||||
| Insulin | 0.249 | −0.225 | −0.062 | −0.312 | ||||||||||
| TNF-α | 0.395 a | −0.082 | −0.010 | −0.154 | 0.372 a | |||||||||
| IL-6 | 0.489 a | −0.026 | 0.023 | −0.158 | 0.446 a | 0.847 c | ||||||||
| MCP-1 | 0.109 | −0.116 | 0.077 | −0.301 | 0.557 b | 0.690 c | 0.754 c | |||||||
| sICAM-1 | −0.235 | 0.076 | 0.121 | −0.218 | 0.495 a | 0.198 | 0.164 | 0.283 | ||||||
| sVCAM-1 | −0.231 | −0.048 | 0.090 | −0.471 a | 0.303 | −0.012 | −0.059 | 0.131 | 0.726 c | |||||
| MPO | −0.187 | −0.050 | −0.108 | −0.164 | 0.073 | −0.212 | −0.108 | 0.118 | −0.010 | 0.063 | ||||
| E-Selectin | −0.242 | −0.281 | −0.224 | −0.403 a | 0.454 a | 0.216 | −0.028 | 0.225 | 0.630 c | 0.598 c | 0.203 | |||
| tPAI-1 | 0.126 | −0.171 | −0.153 | −0.061 | 0.089 | 0.004 | 0.168 | 0.016 | 0.059 | −0.073 | 0.039 | 0.168 | ||
| RI | −0.104 | −0.163 | −0.169 | −0.467 a | 0.197 | 0.055 | −0.074 | 0.033 | 0.348 | 0.485 a | −0.092 | 0.565 b | 0.111 | |
| SI | −0.087 | 0.436 a | 0.317 | 0.296 | −0.022 | 0.017 | 0.058 | 0.118 | −0.025 | −0.156 | 0.136 | −0.226 | −0.673 | −0.236 |
| ADMA | SDMA | DMA | toPADiMeX | a/sPADiMeX | BMI | |
|---|---|---|---|---|---|---|
| BMI | 0.072 | 0.168 | 0.057 | 0.064 | −0.279 | |
| Glucose | 0.249 | 0.328 (*) | 0.312 (*) | 0.332 (*) | −0.068 | −0.191 |
| TAG | −0.143 | −0.141 | −0.164 | −0.180 | −0.125 | −0.235 |
| apoB48 | −0.285 | −0.260 | −0.236 | −0.264 | −0.017 | −0.076 |
| NEFA | 0.171 | 0.167 | 0.156 | 0.150 | −0.107 | −0.117 |
| Insulin | 0.197 | 0.240 | 0.160 | 0.189 | −0.205 | 0.458 * |
| TNF-α | 0.360 * | 0.526 ** | 0.392 * | 0.422 * | −0.241 | 0.310 (*) |
| IL-6 | 0.349 (*) | 0.496 ** | 0.370 * | 0.411 | −0.298 | 0.200 |
| MCP-1 | 0.116 | 0.279 | 0.172 | 0.198 | −0.212 | 0.339 (*) |
| sICAM-1 | 0.134 | 0.126 | −0.021 | −0.013 | −0.376 * | 0.627 *** |
| sVCAM-1 | −0.207 | −0.208 | −0.291 | −0.302 | −0.208 | 0.415 * |
| MPO | −0.214 | −0.187 | −0.217 | −0.223 | −0.233 | 0.113 |
| E-Selectin | −0.103 | −0.034 | −0.150 | −0.147 | −0.257 | 0.774 *** |
| tPAI-1 | −0.001 | 0.004 | 0.080 | 0.071 | 0.094 | 0.295 |
| Basal_RI | 0.066 | −0.012 | 0.056 | 0.053 | 0.186 | 0.217 |
| Basal_SI | 0.005 | 0.008 | −0.126 | −0.103 | −0.317 (*) | −0.211 |
| Measure | T0 | T2 | T4 | T6 | ANOVA |
|---|---|---|---|---|---|
| Creatinine | 10.9 (5.5–17.0) | 12.0 (8.6–21.9) | 17.4 ± 9.5 T4 vs. T0: p = 0.0007 | 8.9 (5.2–15.4) T6 vs. T4: p = 0.0011 | p = 0.0021 |
| ADMA | 3.59 ± 1.38 | 3.62 ± 1.3 | 3.43 ± 1.23 | 3.17 ± 1.09 T6 vs. T2: p = 0.0006 | p = 0.0718 |
| DMA | 26.9 (19.9–35.1) | 27.5 (20.3–36.1) | 33.7 (22.2–37.3) T4 vs. T0: p = 0.0027 | 30.8 ± 10.1 | p = 0.0190 |
| SDMA | 4.45 (3.48–5.59) | 4.78 (3.1–6.3) | 4.52 (3.35–5.86) | 4.05 (3.22–5.64) T6 vs. T2: p = 0.0499 | p = 0.2687 |
| aPADiMeX | 29.9 (24.3–39) | 30.7 (22.2–40.4) | 37.4 (24.8–41.9) T4 vs. T0: p = 0.0290 | 33.9 ± 10.9 | p = 0.0677 |
| toPADiMeX | 33.5 (29.9–44.0) | 33.8 (28.2–45.4) | 37.6 (26.1–43.0) | 38.3 ± 11.4 | p = 0.2980 |
| a/sPADiMeX | 7.21 (5.25–8.63) | 7.57 ± 3.15 | 7.90 ± 2.81 | 8.46 ± 3.19 | p = 0.2213 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bollenbach, A.; Huneau, J.-F.; Mariotti, F.; Tsikas, D. Asymmetric and Symmetric Protein Arginine Dimethylation: Concept and Postprandial Effects of High-Fat Protein Meals in Healthy Overweight Men. Nutrients 2019, 11, 1463. https://doi.org/10.3390/nu11071463
Bollenbach A, Huneau J-F, Mariotti F, Tsikas D. Asymmetric and Symmetric Protein Arginine Dimethylation: Concept and Postprandial Effects of High-Fat Protein Meals in Healthy Overweight Men. Nutrients. 2019; 11(7):1463. https://doi.org/10.3390/nu11071463
Chicago/Turabian StyleBollenbach, Alexander, Jean-François Huneau, François Mariotti, and Dimitrios Tsikas. 2019. "Asymmetric and Symmetric Protein Arginine Dimethylation: Concept and Postprandial Effects of High-Fat Protein Meals in Healthy Overweight Men" Nutrients 11, no. 7: 1463. https://doi.org/10.3390/nu11071463
APA StyleBollenbach, A., Huneau, J.-F., Mariotti, F., & Tsikas, D. (2019). Asymmetric and Symmetric Protein Arginine Dimethylation: Concept and Postprandial Effects of High-Fat Protein Meals in Healthy Overweight Men. Nutrients, 11(7), 1463. https://doi.org/10.3390/nu11071463







