Effect of 1 Year Krill Oil Supplementation on Cognitive Achievement of Dutch Adolescents: A Double-Blind Randomized Controlled Trial
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.1.1. Recruitment Procedure
2.1.2. Participants
2.1.3. Randomization and Blinding
2.1.4. Intervention
2.2. Data Collection
2.2.1. Blood Analyses
2.2.2. Cognitive Measurements
2.2.3. Other Measurements
2.3. Statistical Analyses
2.3.1. Sample Size
2.3.2. Group Comparisons, Treatment Guess and Adherence
2.3.3. Imputing and Recoding Covariates
2.3.4. Cognitive Measurements
3. Results
3.1. Fatty Acids Concentrations
3.2. Treatment Guess
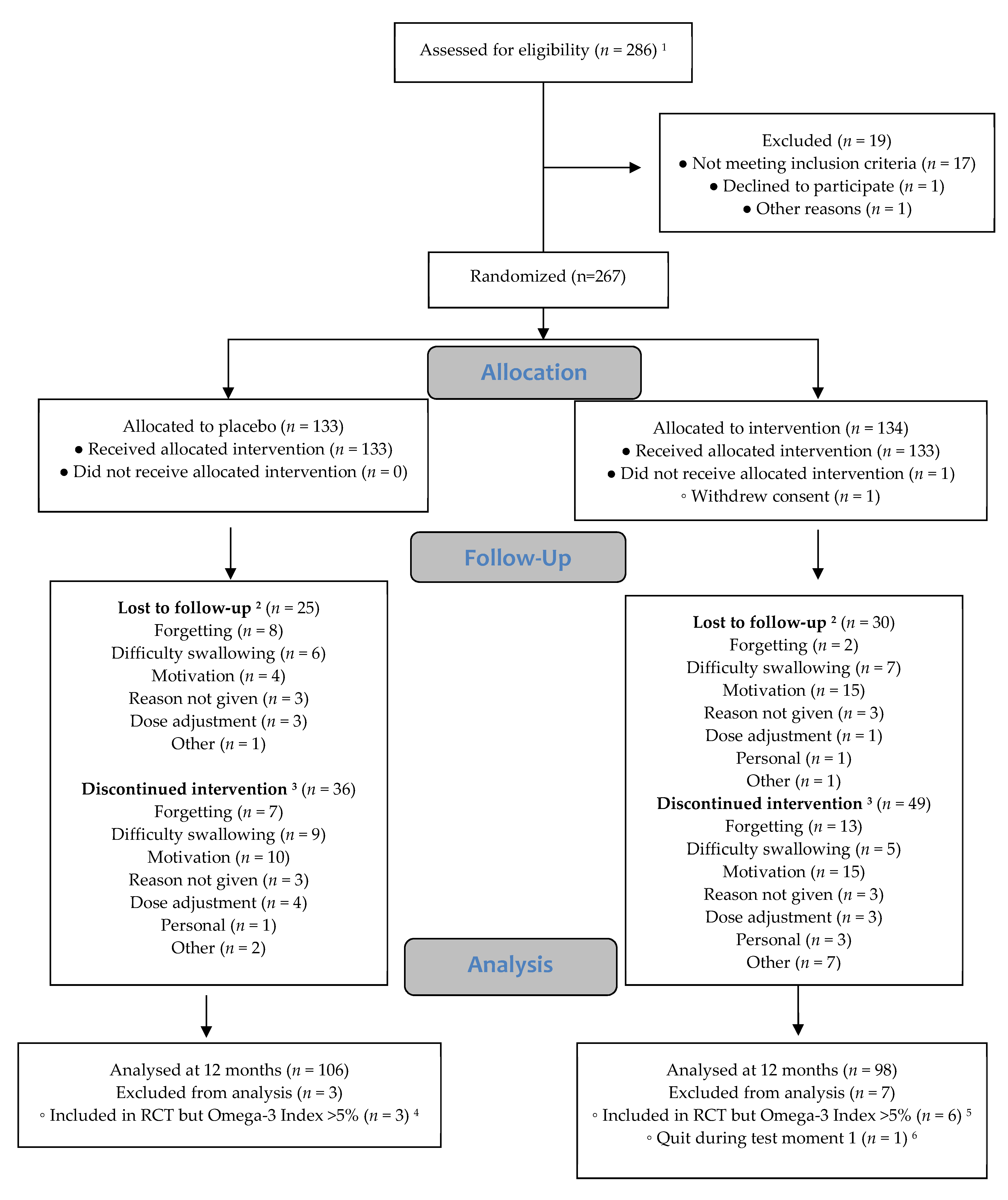
3.3. Drop-Out and Adherence
3.4. Neuropsychological Tests
3.5. Side Effects
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Aarsetoey, H.; Grundt, H.; Nygaard, O.; Nilsen, D.W. The role of long-chained marine n-3 polyunsaturated fatty acids in cardiovascular disease. Cardiol. Res. Pract. 2012, 2012, 303456. [Google Scholar] [CrossRef] [PubMed]
- Abbatecola, A.M.; Evans, W.; Paolisso, G. Pufa supplements and type 2 diabetes in the elderly. Curr. Pharm. Des. 2009, 15, 4126–4134. [Google Scholar] [CrossRef]
- Fotuhi, M.; Mohassel, P.; Yaffe, K. Fish consumption, long-chain omega-3 fatty acids and risk of cognitive decline or alzheimer disease: A complex association. Nat. Clin. Pract. Neurol. 2009, 5, 140–152. [Google Scholar] [CrossRef]
- Schuchardt, J.P.; Huss, M.; Stauss-Grabo, M.; Hahn, A. Significance of long-chain polyunsaturated fatty acids (pufas) for the development and behaviour of children. Eur. J. Pediatr. 2010, 169, 149–164. [Google Scholar] [CrossRef]
- Assisi, A.; Banzi, R.; Buonocore, C.; Capasso, F.; Di Muzio, V.; Michelacci, F.; Renzo, D.; Tafuri, G.; Trotta, F.; Vitocolonna, M.; et al. Fish oil and mental health: The role of n-3 long-chain polyunsaturated fatty acids in cognitive development and neurological disorders. Int. Clin. Psychopharmacol. 2006, 21, 319–336. [Google Scholar] [CrossRef]
- Parletta, N.; Milte, C.M.; Meyer, B.J. Nutritional modulation of cognitive function and mental health. J. Nutr. Biochem. 2013, 24, 725–743. [Google Scholar] [CrossRef] [Green Version]
- Karr, J.E.; Alexander, J.E.; Winningham, R.G. Omega-3 polyunsaturated fatty acids and cognition throughout the lifespan: A review. Nutr. Neurosci. 2011, 14, 216–225. [Google Scholar] [CrossRef] [PubMed]
- Yurko-Mauro, K.; Alexander, D.D.; Van Elswyk, M.E. Docosahexaenoic acid and adult memory: A systematic review and meta-analysis. PLoS ONE 2015, 10, e0120391. [Google Scholar] [CrossRef] [PubMed]
- Eilander, A.; Hundscheid, D.C.; Osendarp, S.J.; Transler, C.; Zock, P.L. Effects of n-3 long chain polyunsaturated fatty acid supplementation on visual and cognitive development throughout childhood: A review of human studies. Prostaglandins Leukot. Essent. Fat. Acids 2007, 76, 189–203. [Google Scholar] [CrossRef]
- Ryan, A.S.; Astwood, J.D.; Gautier, S.; Kuratko, C.N.; Nelson, E.B.; Salem, N., Jr. Effects of long-chain polyunsaturated fatty acid supplementation on neurodevelopment in childhood: A review of human studies. Prostaglandins Leukot. Essent. Fat. Acids 2010, 82, 305–314. [Google Scholar] [CrossRef] [PubMed]
- Gogtay, N.; Giedd, J.N.; Lusk, L.; Hayashi, K.M.; Greenstein, D.; Vaituzis, A.C.; Nugent, T.F.; Herman, D.H.; Clasen, L.S.; Toga, A.W.; et al. Dynamic mapping of human cortical development during childhood through early adulthood. Proc. Natl. Acad. Sci. USA 2004, 101, 8174–8179. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paus, T. Mapping brain maturation and cognitive development during adolescence. Trends Cogn. Sci. 2005, 9, 60–68. [Google Scholar] [CrossRef]
- Bull, R.; Espy, K.A.; Wiebe, S.A. Short-term memory, working memory, and executive functioning in preschoolers: Longitudinal predictors of mathematical achievement at age 7 years. Dev. Neuropsychol. 2008, 33, 205–228. [Google Scholar] [CrossRef] [PubMed]
- McNamara, R.K.; Carlson, S.E. Role of omega-3 fatty acids in brain development and function: Potential implications for the pathogenesis and prevention of psychopathology. Prostaglandins Leukot. Essent. Fat. Acids 2006, 75, 329–349. [Google Scholar] [CrossRef] [PubMed]
- McNamara, R.K.; Able, J.; Jandacek, R.; Rider, T.; Tso, P.; Eliassen, J.C.; Alfieri, D.; Weber, W.; Jarvis, K.; DelBello, M.P.; et al. Docosahexaenoic acid supplementation increases prefrontal cortex activation during sustained attention in healthy boys: A placebo-controlled, dose-ranging, functional magnetic resonance imaging study. Am. J. Clin. Nutr. 2010, 91, 1060–1067. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.L.; Winkvist, A.; Aberg, M.A.; Aberg, N.; Sundberg, R.; Toren, K.; Brisman, J. Fish consumption and school grades in swedish adolescents: A study of the large general population. Acta Paediatr. 2010, 99, 72–77. [Google Scholar] [CrossRef]
- Äberg, M.A.L.; Äberg, N.; Brisman, J.; Sundberg, R.; Winkvist, A.; Torén, K. Fish intake of swedish male adolescents is a predictor of cognitive performance. Acta Paediatr. 2009, 98, 555–560. [Google Scholar] [CrossRef] [PubMed]
- De Groot, R.H.M.; Ouwehand, C.; Jolles, J. Eating the right amount of fish: Inverted u-shape association between fish consumption and cognitive performance and academic achievement in dutch adolescents. Prostaglandins Leukot. Essent. Fat. Acids 2012, 86, 113–117. [Google Scholar] [CrossRef]
- Harris, W.S.; Von Schacky, C. The omega-3 index: A new risk factor for death from coronary heart disease? Prev. Med. 2004, 39, 212–220. [Google Scholar] [CrossRef]
- Van der Wurff, I.S.; von Schacky, C.; Berge, K.; Kirschner, P.A.; de Groot, R.H. A protocol for a randomised controlled trial investigating the effect of increasing omega-3 index with krill oil supplementation on learning, cognition, behaviour and visual processing in typically developing adolescents. BMJ Open 2016, 6, e011790. [Google Scholar] [CrossRef]
- Van Rossum, C.T.M.; Fransen, H.P.; Verkaik-Kloosterman, J.; Buurma-Rethans, E.J.M.; Ocké, M.C.; Rossum, C.T.M.V.; Fransen, H.P.; Verkaik-Kloosterman, J.; Buurma-Rethans, E.J.M.; Ocké, M.C. Dutch National Food Consumption Survey 2007–2010—Diet of Children and Adults Aged 7 to 69 Years; Rijksinstituut voor Volksgezondheid en Milieu: Bilthoven, The Netherlands, 2011; p. 59. [Google Scholar]
- Health Council of the Netherlands. Guidelines for a Healthy Diet 2006; Health Council of the Netherlands: The Hague, The Netherlands, 2006.
- Harris, W.S.; von Schacky, C.; Park, Y. Standardizing Methods for Assessing Omega-3 Fatty Acid Biostatus; McNamara, R.K., Ed.; Nova Science Publishers, Inc.: Hauppauge, NY, USA, 2013; pp. 385–398. [Google Scholar]
- Van der Elst, W.; van Boxtel, M.P.; van Breukelen, G.J.; Jolles, J. The letter digit substitution test: Normative data for 1858 healthy participants aged 24–81 from the maastricht aging study (maas): Influence of age, education, and sex. J. Clin. Exp. Neuropsychol. 2006, 28, 998–1009. [Google Scholar] [CrossRef]
- Van der Elst, W.; Dekker, S.; Hurks, P.; Jolles, J. The letter digit substitution test: Demographic influences and regression-based normative data for school-aged children. Arch. Clin. Neuropsychol. 2012, 27, 433–439. [Google Scholar] [CrossRef] [PubMed]
- Brickenkam, R.; Zillmerr, E. The d2 Test of Attention; Hogrefe & Huber Publishers: Seattle, WA, USA, 1998. [Google Scholar]
- Van der Elst, W.; van Boxtel, M.P.J.; Van Breukelen, G.J.P.; Jolles, J. The concept shifting test: Adult normative data. Psychol. Assess. 2006, 18, 424–432. [Google Scholar] [CrossRef]
- Petersen, A.C.; Crockett, L.; Richards, M.; Boxer, A. A self-report measure of pubertal status: Reliability, validity, and initial norms. J. Youth Adolesc. 1988, 17, 117–133. [Google Scholar] [CrossRef]
- Pinheiro, J.; Bates, D.; DebRoy, S.; Sarkar, D.; Team, R.C. Nlme: Linear and nonlinear mixed effects models. R Package Version 2013, 3, 111. [Google Scholar]
- Bates, D.; Mächler, M.; Bolker, B.; Walker, S. Fitting linear mixed-effects models using lme4. J. Stat. Softw. 2015, 67. [Google Scholar] [CrossRef]
- Christensen, R.H.B. Ordinal—Regression Models for Ordinal Data, R package version 2015.6-28; R Foundation for Statistical Computing: Vienna, Austria, 2015. [Google Scholar]
- Richardson, A.J.; Puri, B.K. A randomized double-blind, placebo-controlled study of the effects of supplementation with highly unsaturated fatty acids on adhd-related symptoms in children with specific learning difficulties. Prog. Neuropsychopharmacol. Biol. Psychiatry 2002, 26, 233–239. [Google Scholar] [CrossRef]
- Richardson, A.J.; Montgomery, P. The oxford-durham study: A randomized, controlled trial of dietary supplementation with fatty acids in children with developmental coordination disorder. Pediatrics 2005, 115, 1360–1366. [Google Scholar] [CrossRef]
- Dalton, A.; Wolmarans, P.; Witthuhn, R.C.; van Stuijvenberg, M.E.; Swanevelder, S.A.; Smuts, C.M. A randomised control trial in schoolchildren showed improvement in cognitive function after consuming a bread spread, containing fish flour from a marine source. Prostaglandins Leukot. Essent. Fat. Acids 2009, 80, 143–149. [Google Scholar] [CrossRef] [PubMed]
- Parletta, N.; Cooper, P.; Gent, D.N.; Petkov, J.; O’Dea, K. Effects of fish oil supplementation on learning and behaviour of children from australian indigenous remote community schools: A randomised controlled trial. Prostaglandins Leukot. Essent. Fat. Acids 2013, 89, 71–79. [Google Scholar] [CrossRef] [PubMed]
- Portillo-Reyes, V.; Pérez-García, M.; Loya-Méndez, Y.; Puente, A.E. Clinical significance of neuropsychological improvement after supplementation with omega-3 in 8–12 years old malnourished mexican children: A randomized, double-blind, placebo and treatment clinical trial. Res. Dev. Disabil. 2014, 35, 861–870. [Google Scholar] [CrossRef]
- Kirby, A.; Woodward, A.; Jackson, S.; Wang, Y.; Crawford, M.A. A double-blind, placebo-controlled study investigating the effects of omega-3 supplementation in children aged 8–10 years from a mainstream school population. Res. Dev. Disabil. 2010, 31, 718–730. [Google Scholar] [CrossRef]
- Osendarp, S.J.; Baghurst, K.I.; Bryan, J.; Calvaresi, E.; Hughes, D.; Hussaini, M.; Karyadi, S.J.; van Klinken, B.J.; van der Knaap, H.C.; Lukito, W.; et al. Effect of a 12-mo micronutrient intervention on learning and memory in well-nourished and marginally nourished school-aged children: 2 parallel, randomized, placebo-controlled studies in australia and indonesia. Am. J. Clin. Nutr. 2007, 86, 1082–1093. [Google Scholar]
- Baumgartner, J.; Smuts, C.M.; Malan, L.; Kvalsvig, J.; van Stuijvenberg, M.E.; Hurrell, R.F.; Zimmermann, M.B. Effect of iron and n-3 fatty acid supplementation, alone and in combination, on cognition in school children: A randomized, double-blind, placebo-controlled intervention in south africa. Am. J. Clin. Nutr. 2012, 96, 1327–1338. [Google Scholar] [CrossRef]
- Sinn, N.; Bryan, J.; Wilson, C. Cognitive effects of polyunsaturated fatty acids in children with attention deficit hyperactivity disorder symptoms: A randomised controlled trial. Prostaglandins Leukot. Essent. Fat. Acids 2008, 78, 311–326. [Google Scholar] [CrossRef]
- Ryan, A.S.; Nelson, E.B. Assessing the effect of docosahexaenoic acid on cognitive functions in healthy, preschool children: A randomized, placebo-controlled, double-blind study. Clin. Pediatr. 2008, 47, 355–362. [Google Scholar] [CrossRef]
- Kennedy, D.; Jackson, P.A.; Elliott, J.M.; Scholey, A.B.; Robertson, B.C.; Greer, J.; Tiplady, B.; Buchanan, T.; Haskell, C.F. Cognitive and mood effects of 8 weeks’ supplementation with 400 mg or 1000 mg of the omega-3 essential fatty acid docosahexaenoic acid (dha) in healthy children aged 10–12 years. Nutr. Neurosci. 2009, 12, 48–56. [Google Scholar] [CrossRef]
- Muthayya, S.; Eilander, A.; Transler, C.; Thomas, T.; van der Knaap, H.C.; Srinivasan, K.; van Klinken, B.J.; Osendarp, S.J.; Kurpad, A.V. Effect of fortification with multiple micronutrients and n-3 fatty acids on growth and cognitive performance in indian schoolchildren: The champion (children’s health and mental performance influenced by optimal nutrition) study. Am. J. Clin. Nutr. 2009, 89, 1766–1775. [Google Scholar] [CrossRef]
- Bauer, I.; Hughes, M.; Rowsell, R.; Cockerell, R.; Pipingas, A.; Crewther, S.; Crewther, D. Omega-3 supplementation improves cognition and modifies brain activation in young adults. Hum. Psychopharmacol. 2014, 29, 133–144. [Google Scholar] [CrossRef]
- Van Der Wurff, I.S.M.; Von Schacky, C.; Berge, K.; Zeegers, M.P.; Kirschner, P.A.; de Groot, R.H.M. Association between blood omega-3 index and cognition in typically developing dutch adolescents. Nutrients 2016, 8, 13. [Google Scholar] [CrossRef]
- Van der Wurff, I.S.M.; Meyer, B.J.; de Groot, R.H.M. A review of recruitment, adherence and drop-out rates in omega-3 polyunsaturated fatty acid supplementation trials in children and adolescents. Nutrients 2017, 9, 474. [Google Scholar] [CrossRef]
- Tammam, J.D.; Steinsaltz, D.; Bester, D.W.; Semb-Andenaes, T.; Stein, J.F. A randomised double-blind placebo-controlled trial investigating the behavioural effects of vitamin, mineral and n-3 fatty acid supplementation in typically developing adolescent schoolchildren. Br. J. Nutr. 2016, 115, 361–373. [Google Scholar] [CrossRef]

| Placebo (M ± SD or N (%)) | N | Krill (M ± SD or N (%)) | N | p4 | |
|---|---|---|---|---|---|
| Age (years) | 14.07 ± 0.48 | 130 | 14.15 ± 0.51 | 126 | 0.185 |
| Male/female N (%) | 51/79 (39/61) | 130 | 72/54 (57/43) | 126 | 0.004 |
| Smoking 1 (no/yes) | 117/12 (91/9) | 129 | 113/12 (90/10) | 125 | 0.935 |
| BMI 2 | 20.13 ± 3.05 | 123 | 19.84 ± 2.96 | 116 | 0.455 |
| Alcohol units per week 3 | 0.34 ± 1.16 | 129 | 0.61 ± 2.29 | 126 | 0.234 |
| Level of parental education (low/high) | 54/69 | 123 | 49/66 | 115 | 0.840 |
| Baseline | 3 Months | 6 Months 1 | 12 Months 2 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Fatty acid (%wt/wt of total FA) | placebo (n 130) Mean ± SD | krill (n 126) Mean ± SD | p | placebo (n 124) Mean ± SD | Krill 3 (n 118) Mean ± SD | p | placebo (n 116) Mean ± SD | krill (n 108) Mean ± SD | p | placebo (n 104) Mean ± SD | krill (n 95) Mean ± SD | p |
| AA 20:4n-6 | 11.19 ± 1.36 | 11.12 ± 1.18 | 0.657 | 10.97 ± 1.18 | 10.26 ± 1.13 | <0.001 | 11.02 ± 1.49 | 10.28 ± 1.41 | <0.001 | 11.15 ± 1.30 | 10.72 ± 1.49 | 0.029 |
| EPA 20:5n-3 | 0.38 ± 0.14 | 0.38 ± 0.15 | 0.860 | 0.43 ± 0.15 | 0.93 ± 0.58 | <0.001 | 0.41 ± 0.14 | 0.95 ± 0.69 | <0.001 | 0.40 ± 0.12 | 0.75 ± 0.58 | <0.001 |
| ObA 22:5n-6 | 0.43 ± 0.11 | 0.45 ± 0.10 | 0.154 | 0.41 ± 0.17 | 0.32 ± 0.12 | <0.001 | 0.42 ± 0.09 | 0.32 ± 0.11 | <0.001 | 0.38 ± 0.12 | 0.34 ± 0.13 | 0.038 |
| DPA 22:5n-3 | 1.22 ± 0.20 | 1.22 ± 0.17 | 0.858 | 1.29 ± 0.23 | 1.58 ± 0.34 | <0.001 | 1.30 ± 0.20 | 1.54 ± 0.35 | <0.001 | 1.30 ± 0.19 | 1.47 ± 0.31 | <0.001 |
| DHA22:6n-3 | 2.60 ± 0.44 | 2.49 ± 0.46 | 0.055 | 2.61 ± 0.52 | 3.25 ± 0.73 | <0.001 | 2.69 ± 0.53 | 3.40 ± 0.90 | <0.001 | 2.72 ± 0.54 | 3.20 ± 0.84 | <0.001 |
| O3I | 3.83 ± 0.54 | 3.71 ± 0.55 | 0.106 | 3.88 ± 0.64 | 5.10 ± 1.29 | <0.001 | 3.95 ± 0.64 | 5.29 ± 1.61 | <0.001 | 3.98 ± 0.63 | 4.86 ± 1.43 | <0.001 |
| Baseline | 6 Months | 12 Months | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Placebo Mean ± SD Range | Krill Mean ± SD Range | p | Placebo Mean ± SD Range | Krill Mean ± SD Range | p | Placebo Mean ± SD Range | Krill Mean ± SD Range | p | |
| LDST (number) | 35.02 ± 5.73 21–53 | 33.98 ± 5.22 15–49 | 0.131 | 37.56 ± 6.13 24–52 | 36.44 ± 6.46 12–50 | 0.184 | 39.79 ± 5.78 26–59 | 38.39 ± 5.81 25–53 | 0.085 |
| D2 total (number) | 421.42 ± 57.69 310–562 | 416.37 ± 55.92 294–287 | 0.478 | 468.88 ± 61.71 314–636 | 461.41 ± 57.68 308–607 | 0.350 | 506.08 ± 65.88 370–653 | 494.22 ± 57.00 352–619 | 0.175 |
| D2 correct (number) | 164.82 ± 24.34 103–232 | 162.71 ± 22.29 111–223 | 0.472 | 187.91 ± 28.47 134–279 | 184.87 ± 25.59 128–261 | 0.403 | 206.88 ± 34.09 151–291 | 201.29 ± 28.17 140–271 | 0.208 |
| D2 error of commission (number) | 11.97 ± 9.55 0–58 | 11.67 ± 12.11 0–109 | 0.824 | 10.0 ± 9.62 0–55 | 9.44 ± 7.81 0–40 | 0.631 | 9.14 ± 10.59 0–56 | 8.22 ± 6.90 0–37 | 0.469 |
| D2 error of omission (number) | 1.38 ± 1.72 0–9 | 1.22 ± 1.49 0–6 | 0.442 | 1.12 ± 1.74 0–10 | 1.06 ± 1.35 0–7 | 0.793 | 0.85 ± 1.64 0–10 | 0.68 ± 1.10 0–6 | 0.388 |
| Shifting score (s) | 11.69 ± 6.51 –1.27–37.81 | 11.79 ± 7.21 1.39–38.33 | 0.912 | 10.30 ± 5.59 –3.83–36.58 | 9.99 ± 7.06 –2.35–36.41 | 0.715 | 10.26 ± 5.69 0.99–27.18 | 9.98 ± 7.20 –8.21–40.01 | 0.760 |
| Interferences score (s) | 31.64 ± 8.74 16.69–67.90 | 30.51 ± 7.79 16.34–52.32 | 0.274 | 28.01 ± 6.60 14.56–68.40 | 27.83 ± 6.14 16.31–42.29 | 0.834 | 26.77 ± 6.76 14.19–59.31 | 25.92 ± 5.31 16.75–40.67 | 0.321 |
| Digit Span Forward (digits) | 5.55 ± 0.83 3–8 | 5.61 ± 0.89 3–8 | 0.547 | 5.37 ± 0.80 4–8 | 5.53 ± 1.05 3–8 | 0.211 | 5.68 ± 0.98 3–8 | 5.64 ± 0.90 3–8 | 0.783 |
| Digit Span Backward (digits) | 4.58 ± 0.98 2–7 | 4.52 ± 1.00 2–7 | 0.668 | 4.71 ± 0.81 2–7 | 4.69 ± 0.91 2–7 | 0.816 | 4.83 ± 0.96 3–7 | 4.65 ± 1.11 3–7 | 0.224 |
| Outcome Variable | Predictor | Estimate (SE) | 95%CI | Estimate (SE) | 95%CI | ||
|---|---|---|---|---|---|---|---|
| D2-Total | Test moment | T2 | 48.40 (3.62) | (41.39; 55.41) | T2 | 47.90 (3.01) | (42.06; 53.74) |
| T3 | 89.00 (3.83) | (81.59; 96.41) | T3 | 84.88 (3.07) | (78.93; 90.84) | ||
| Condition | Krill | −7.99 (8.06) | (−23.62; 7.65) | O3I | −0.20 (1.63) | (−3.36; 2.95) | |
| Interaction | T2 × krill | −0.99 (5.28) | (−11.21; 9.23) | ||||
| T3 × krill | −9.17 (5.47) | (−19.77; 1.43) | |||||
| D2-Correct | Test moment | T2 | 23.01 (1.55) | (20.00; 26.02) | T2 | 23.01 (1.30) | (20.50; 25.53) |
| T3 | 43.53 (1.64) | (40.35; 46.71) | T3 | 41.63 (1.32) | (39.07; 44.19) | ||
| Condition | Krill | −1.53 (3.70) | (−8.72; 5.66) | O3I | −0.49 (0.71) | (−1.85; 0.88) | |
| Interaction | T2 × krill | −0.63 (2.26) | (−5.01; 3.76) | ||||
| T3 × krill | −4.71 (2.35) | (−9.26; −0.17) | |||||
| D2-F1 | Test moment | T2 | −0.13 (0.04) | (−0.21; −0.04) | T2 | −0.14 (0.04) | (−0.21; −0.06) |
| T3 | −0.24 (0.05) | (−0.34; −0.14) | T3 | −0.27 (0.04) | (−0.34; −0.19) | ||
| Condition | Krill | −0.10 (0.10) | (−0.29; 0.11) | O3I | 0.01 (0.02) | (−0.03; 0.05) | |
| Interaction | T2 × krill | −0.001 (0.06) | (−0.13; 0.11) | ||||
| T3 × krill | −0.05 (0.07) | (−0.18; 0.10) | |||||
| D2-F2 | Test moment | T2 | −0.24 (0.13) | (−0.48; 0.01) | T2 | −0.13 (0.10) | (−0.30; 0.06) |
| T3 | −0.53 (0.14) | (−0.82; −0.28) | T3 | −0.56 (0.12) | (−0.78; −0.34) | ||
| Condition | Krill | −0.23 (0.16) | (−0.55; 0.08) | O3I | −0.03 (0.05) | (−0.14; 0.06) | |
| Interaction | T2 × krill | 0.17 (0.19) | (−0.18; 0.52) | ||||
| T3 × krill | −0.04 (0.23) | (−0.45; 0.38) | |||||
| Test moment | T2 | 2.48 (0.48) | (1.55; 3.41) | T2 | 2.42 (0.39) | (1.66; 3.18) | |
| LDST | T3 | 4.74 (0.50) | (3.76; 5.72) | T3 | 4.56 (0.40) | (3.78; 5.33) | |
| Condition | Krill | −0.18 (0.81) | (−1.75; 1.39) | O3I | −0.07 (0.20) | (−0.46; 0.32) | |
| Interaction | T2 × krill | −0.23 (0.70) | (−1.59; 1.12) | ||||
| T3 × krill | −0.32 (0.72) | (−1.71; 1.08) | |||||
| Shifting | Test moment | T2 | −1.59 (0.81) | (−3.16; −0.02) | T2 | −1.80 (0.64) | (−3.04; −0.56) |
| T3 | −1.23 (0.85) | (−2.87; 0.41) | T3 | −1.87 (0.66) | (−3.15; −0.60) | ||
| Condition | Krill | −0.004 (0.90) | (−1.75; 1.74) | O3I | 0.06 (0.27) | (−0.47; 0.58) | |
| Interaction | T2 × krill | −0.31 (1.18) | (−2.61; 1.98) | ||||
| T3 × krill | −1.48 (1.22) | (−3.85; 0.88) | |||||
| Interference | Test moment | T2 | −4.01 (0.72) | (−5.40; −2.62) | T2 | −3.45 (0.58) | (−4.57; −2.32) |
| T3 | −5.41 (0.75) | (−6.87; −3.96) | T3 | −5.38 (0.59) | (−6.53; −4.24) | ||
| Condition | Krill | −1.10 (0.97) | (−2.98; 0.77) | O3I | −0.01 (0.28) | (−0.55; 0.53) | |
| Interaction | T2 × krill | 1.14 (1.05) | (−0.89; 3.16) | ||||
| T3 × krill | −0.12 (1.08) | (−2.21; 1.97) |
| Outcome Variable | Predictor | Odds Ratio (SE) | 95%CI | Odds Ratio (SE) | 95%CI | |
|---|---|---|---|---|---|---|
| Digit Span forward | ||||||
| Test moment | T2 | 0.44 (1.32) | (0.26; 0.76) | T2 | 0.54 (1.25) | (0.35; 0.84) |
| T3 | 1.15 (1.33) | (0.66; 2.02) | T3 | 1.16 (1.25) | (0.75; 1.80) | |
| Condition 1 | Krill | 0.85 (1.41) | (0.43; 1.68) | O3I | 1.08 (1.11) | (0.88; 1.32) |
| Interaction 2 | T2 × krill | 1.88 (1.50) | (0.85; 4.14) | |||
| T3 × krill | 1.22 (1.51) | (0.54; 2.73) | ||||
| Digit Span backward | ||||||
| Test moment | T2 | 1.38 (1.30) | (0.83; 2.30) | T2 | 1.49 (1.23) | (1.00; 2.23) |
| T3 | 1.61 (1.32) | (0.93; 2.78) | T3 | 1.61 (1.24) | (1.06; 2.46) | |
| Condition 1 | Krill | 0.85 (1.37) | (0.46; 1.58) | O3I | 1.00 (1.10) | (0.83; 1.21) |
| Interaction 2 | T2 × krill | 1.15 (1.46) | (0.55; 2.42) | |||
| T3 × krill | 0.98 (1.49) | (0.45; 2.13) |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
van der Wurff, I.S.M.; von Schacky, C.; Bergeland, T.; Leontjevas, R.; Zeegers, M.P.; Jolles, J.; Kirschner, P.A.; de Groot, R.H.M. Effect of 1 Year Krill Oil Supplementation on Cognitive Achievement of Dutch Adolescents: A Double-Blind Randomized Controlled Trial. Nutrients 2019, 11, 1230. https://doi.org/10.3390/nu11061230
van der Wurff ISM, von Schacky C, Bergeland T, Leontjevas R, Zeegers MP, Jolles J, Kirschner PA, de Groot RHM. Effect of 1 Year Krill Oil Supplementation on Cognitive Achievement of Dutch Adolescents: A Double-Blind Randomized Controlled Trial. Nutrients. 2019; 11(6):1230. https://doi.org/10.3390/nu11061230
Chicago/Turabian Stylevan der Wurff, Inge S.M., Clemens von Schacky, Trygve Bergeland, Roeslan Leontjevas, Maurice P. Zeegers, Jelle Jolles, Paul A. Kirschner, and Renate H.M. de Groot. 2019. "Effect of 1 Year Krill Oil Supplementation on Cognitive Achievement of Dutch Adolescents: A Double-Blind Randomized Controlled Trial" Nutrients 11, no. 6: 1230. https://doi.org/10.3390/nu11061230
APA Stylevan der Wurff, I. S. M., von Schacky, C., Bergeland, T., Leontjevas, R., Zeegers, M. P., Jolles, J., Kirschner, P. A., & de Groot, R. H. M. (2019). Effect of 1 Year Krill Oil Supplementation on Cognitive Achievement of Dutch Adolescents: A Double-Blind Randomized Controlled Trial. Nutrients, 11(6), 1230. https://doi.org/10.3390/nu11061230







