Circulating Salicylic Acid and Metabolic Profile after 1-Year Nutritional–Behavioral Intervention in Children with Obesity
Abstract
1. Introduction
2. Experimental Section
2.1. Anthropometry and Blood Pressure
2.2. Biochemistry
2.3. Serum Salicylic Acid Determination
2.4. Dietary Intake
2.5. Intervention
2.6. Statistical Analysis
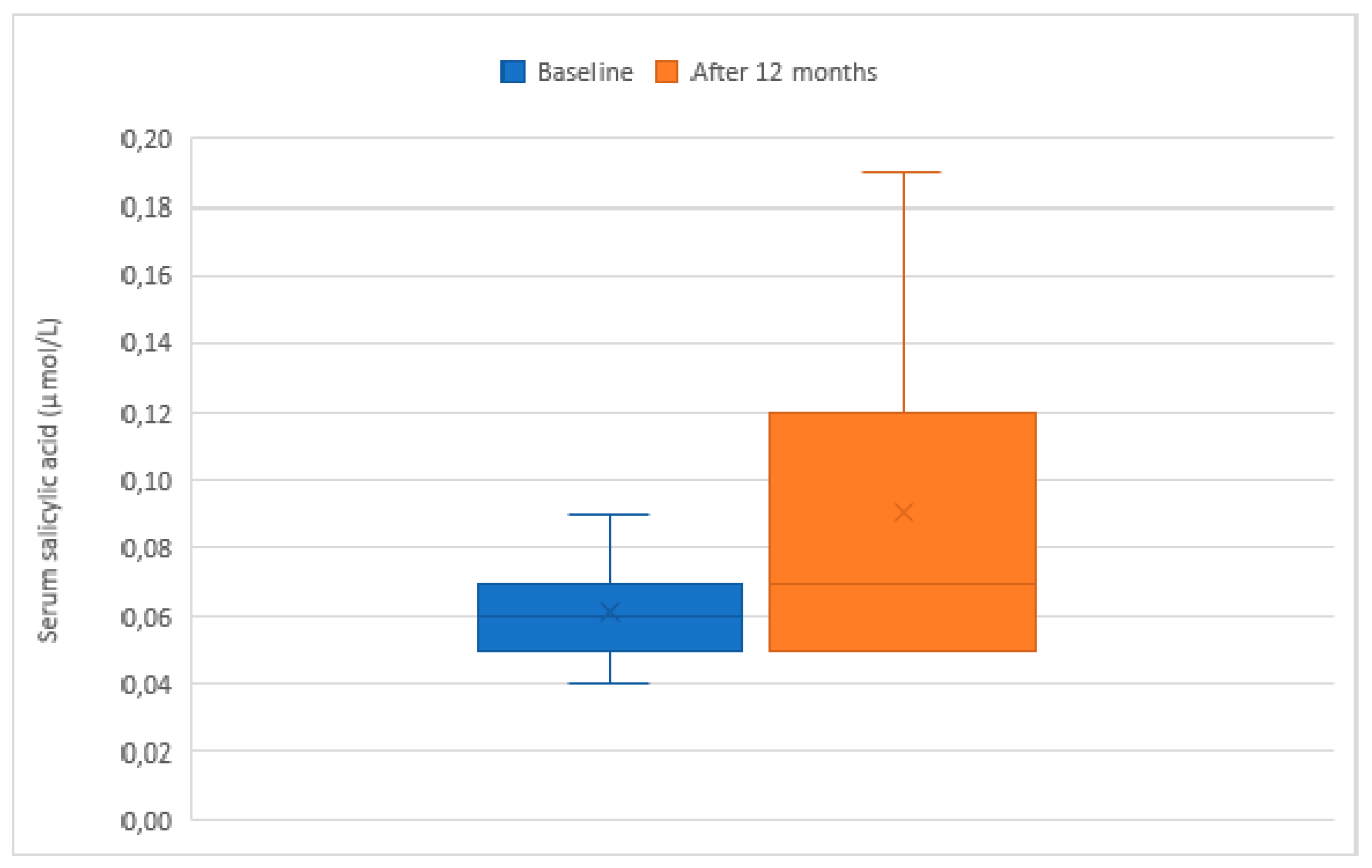
3. Results
4. Discussion
Author Contributions
Funding
Conflicts of Interest
References
- Paterson, J.R.; Lawrence, J.R. Salicylic acid: A link between aspirin, diet and the prevention of colorectal cancer. QJM 2001, 94, 445–448. [Google Scholar] [CrossRef] [PubMed]
- Paterson, J.; Baxter, G.; Lawrence, J.; Duthie, G. Is there a role for dietary salicylates in health? Proc. Nutr. Soc. 2006, 65, 93–96. [Google Scholar] [CrossRef] [PubMed]
- Wood, A.; Baxter, G.; Thies, F.; Kyle, J.; Duthie, G. A systematic review of salicylates in foods: Estimated daily intake of a Scottish population. Mol. Nutr. Food Res. 2011, 55, S7–S14. [Google Scholar] [CrossRef]
- Zheng, S.L.; Roddick, A.J. Association of aspirin use for primary prevention with cardiovascular events and bleeding events: A systematic review and meta-analysis. JAMA 2019, 321, 277–287. [Google Scholar] [CrossRef]
- Simon, A.; Hawley, M.D.; Fullerton, F.A.; Ross, J.D.; Schertzer, C.; Chevtzoff, K.J.; Walker, M.W.; Peggie, D.; Zibrova, K.; Green, A.; et al. The ancient drug salicylate directly activates amp-activated protein kinase. Science 2012, 336, 918–922. [Google Scholar]
- Blacklock, C.J.; Lawrence, J.R.; Wiles, D.; Malcolm, E.A.; Gibson, I.H.; Kelly, C.J.; Paterson, J.R. Salicylic acid in the serum of subjects not taking aspirin. Comparison of salicylic acid concentrations in the serum of vegetarians, non-vegetarians, and patients taking low dose aspirin. J. Clin. Pathol. 2001, 54, 553–555. [Google Scholar] [CrossRef]
- Lassandro, C.; Banderali, G.; Mariani, B.; Battezzati, A.; Diaferio, L.; Miniello, V.L.; Radaelli, G.; Verduci, E. Serum salicylic acid and fruit and vegetable consumption in obese and normal-weight children: A pilot-study. Int. J. Food Sci. Nutr. 2016. [Google Scholar] [CrossRef] [PubMed]
- Makki, K.; Froguel, P.; Wolowczuk, I. Adipose tissue in obesity-related inflammation and insulin resistance: Cells, cytokines, and chemokines. ISRN Inflamm. 2013. [Google Scholar] [CrossRef]
- Lakshman, R.; Elks, C.E.; Ong, K.K. Childhood obesity. Circulation 2012, 126, 1770–1779. [Google Scholar] [CrossRef]
- Cole, T.J.; Bellizzi, M.C.; Flegal, K.M.; Dietz, W.H. Establishing a standard definition for child overweight and obesity worldwide: International survey. BMJ 2000, 320, 1240–1243. [Google Scholar] [CrossRef]
- Cole, T.J. The LMS method for constructing normalized growth standards. Eur. J. Clin. Nutr. 1990, 44, 45–60. [Google Scholar] [PubMed]
- Cacciari, E.; Milani, S.; Balsamo, A.; Dammacco, F.; de Luca, F.; Chiarelli, F.; Pasquino, A.M.; Tonini, G.; Vanelli, M. Italian cross-sectional growth charts for height, weight and BMI (6–20 years). Eur. J. Clin. Nutr. 2002, 56, 171–180. [Google Scholar] [CrossRef]
- Moreno, L.A.; Rodríguez, G.; Guillén, J.; Rabanaque, M.J.; León, J.F.; Ariño, A. Anthropometric measurements in both sides of the body in the assessment of nutritional status in prepubertal children. Eur. J. Clin. Nutr. 2002, 56, 1208–1215. [Google Scholar] [CrossRef]
- Eszter, V.; Frances, A.; Tylavsky, A.; Lyytikäinen, H.S.; Markku, A.; Sulin, C. Assessing Body Composition With DXA and Bioimpedance: Effects of Obesity, Physical Activity, and Age. Obesity 2008, 16, 700–705. [Google Scholar]
- Joseph, T.; Flynn, D.C.; Kaelber, C.M.; Baker-Smith, D.B.; Aaron, E.C.; Stephen, R.D.; Sarah, D.; de Ferranti, J.M.; Dionne, B.F.; Susan, K.; et al. Clinical Practice Guideline for Screening and Management of High Blood Pressure in Children and Adolescents. Pediatrics 2017, 140, 3. [Google Scholar]
- Van Gammeren, A.J.; van Gool, N.; de Groot, M.J.; Cobbaert, C.M. Analytical performance evaluation of the Cobas 6000 analyzer—Special emphasis on trueness verification. Clin. Chem. Lab. Med. 2008, 46, 863–871. [Google Scholar] [CrossRef] [PubMed]
- Matthews, D.R.; Hosker, J.P.; Rudenski, A.S.; Naylor, B.A.; Treacher, D.F.; Turner, R.C. Homeostasis model assessment: Insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985, 28, 412–419. [Google Scholar] [CrossRef] [PubMed]
- Katz, A.; Nambi, S.S.; Mather, K.; Baron, A.D.; Follmann, D.A.; Sullivan, G.; Quon, M.J. Quantitative insulin sensitivity check index: A simple, accurate method for assessing insulin sensitivity in humans. J. Clin. Endocrinol. Metab. 2000, 85, 2402–2410. [Google Scholar] [CrossRef]
- Simental-Mendıa, L.E.; Rodrıguez-Moraan, M.; Guerrero-Romero, F. The product of fasting glucose and triglycerides as surrogate for identifying insulin resistance in apparently healthy subjects. Metab. Syndr. Relat. Disord. 2008, 6, 299–304. [Google Scholar] [CrossRef]
- Mohd Nor, N.S.; Lee, S.; Bacha, F.; Tfayli, H.; Arslanian, S. Triglyceride glucose index as a surrogate measure of insulin sensitivity in obese adolescents with normoglycemia, prediabetes, and type 2 diabetes mellitus: Comparison with the hyperinsulinemic-euglycemic clamp. Pediatr. Diabetes 2015. [Google Scholar] [CrossRef]
- Dobiasova, M. Atherogenic Index of plasma [log(triglycerides/hdl-cholesterol)]: Theoretical and practical implications. Clin Chem. 2004, 50, 1113–1115. [Google Scholar] [CrossRef]
- Dobiásová, M.; Frohlich, J. The plasma parameter log (TG/HDL-C) as an atherogenic index: Correlation with lipoprotein particle size and esterification rate in apoB-lipoprotein-depleted plasma (FER(HDL)). Clin. Biochem. 2001, 34, 583–588. [Google Scholar] [CrossRef]
- Spadafranca, A.; Bertoli, S.; Fiorillo, G.; Testolin, G.; Battezzati, A. Circulating salicylic acid is related to fruit and vegetable consumption in healthy subjects. Br. J. Nutr. 2007, 98, 802–806. [Google Scholar] [CrossRef] [PubMed]
- Sirok, D.; Pátfalusi, M.; Szeleczky, G.; Somorjai, G.; Greskovits, D.; Monostory, K. Robust and sensitive LC / MS-MS method for simultaneous detection of acetylsalicylic acid and salicylic acid in human plasma. Microchem. J. 2018, 136, 200–208. [Google Scholar]
- Swain, A.R.; Dutton, S.P.; Truswell, A.S. Salicylates in foods. J. Am. Diet. Assoc. 1985, 85, 950–960. [Google Scholar] [PubMed]
- Società Italiana di Pediatria (SIP) (Italian Society of Pediatrics). Childhood obesity: Consensus on Prevention, Diagnosis and Therapy. Available online: http://www.ecog-obesity.eu/papers/Consensus_Italia.pdf (accessed on 16 October 2015).
- Società Italiana di Nutrizione Umana (SINU) (Italian Society of Human Nutrition). Nutrients and Energy Reference Intake Levels, IV Revision; Società Italiana di Nutrizione Umana: Milan, Italy, 2014. [Google Scholar]
- Michie, S.; Ashford, S.; Sniehotta, F.F.; Dombrowski, S.U.; Bishop, A.; French, D.P. A refined taxonomy of behaviour change techniques to help people change their physical activity and healthy eating behaviours: The CALO-RE taxonomy. Psychol. Health 2011, 26, 1479–1498. [Google Scholar] [CrossRef]
- Bach-Faig, A.; Berry, E.M.; Lairon, D.; Reguant, J.; Trichopoulou, A.; Dernini, S.; Medina, F.X.; Battino, M.; Belahsen, R.; Miranda, G. Mediterranean diet pyramid today. Science and cultural updates. Public Health Nutr. 2011, 14, 2274–2284. [Google Scholar] [CrossRef]
- Kolsgaard, M.L.; Joner, G.; Brunborg, C.; Anderssen, S.A.; Tonstad, S.; Andersen, L.F. Reduction in BMI z-score and improvement in cardiometabolic risk factors in obese children and adolescents. The oslo adiposity intervention study—A hospital/public health nurse combined treatment. BMC Pediatr. 2011, 11, 47. [Google Scholar] [CrossRef]
- Blüher, S.; Petroff, D.; Wagner, A.; Warich, K.; Gausche, R.; Klemm, T.; Wagner, M.; Keller, A. The one year exercise and lifestyle intervention program KLAKS: Effects on anthropometric parameters, cardiometabolic risk factors and glycemic control in childhood obesity. Metabolism 2014, 63, 422–430. [Google Scholar] [CrossRef]
- Anton, G.; Maffeis, C. L’obesità nel bambino e rischio metabolico a lungo termine: Obiettivo di intervento. Giornale Italiano dell’Arteriosclerosi 2013, 4, 46–63. [Google Scholar]
- Verduci, E.; Lassandro, C.; Giacchero, R.; Miniello, V.L.; Banderali, G.; Radaelli, G. Change in metabolic profile after 1-year nutritional-behavioral intervention in obese children. Nutrients 2015, 7, 10089–10099. [Google Scholar] [CrossRef]
- Uysal, Y.; Wolters, B.; Knop, C.; Reinehr, T. Components of the metabolic syndrome are negative predictors of weight loss in obese children with lifestyle intervention. Clin. Nutr. 2014, 33, 620–625. [Google Scholar] [CrossRef]
- Pedrosa, C.; Oliveira, B.M.; Albuquerque, I.; Simões-Pereira, C.; Vaz-de-Almeida, M.D.; Correia, F. Markers of metabolic syndrome in obese children before and after 1-year lifestyle intervention program. Eur. J. Nutr. 2011, 50, 391–400. [Google Scholar] [CrossRef] [PubMed]
- Santiprabhob, J.; Leewanun, C.; Limprayoon, K.; Kiattisakthavee, P.; Wongarn, R.; Aanpreung, P.; Likitmaskul, S. Outcomes of group-based treatment program with parental involvement for the management of childhood and adolescent obesity. Patient Educ. Couns. 2014, 97, 67–74. [Google Scholar] [CrossRef]
- Ho, M.; Garnett, S.P.; Baur, L.; Burrows, T.; Stewart, L.; Neve, M.; Collins, C. Effectiveness of lifestyle interventions in child obesity: Systematic review with meta-analysis. Pediatrics 2012, 130, e1647–e1671. [Google Scholar] [CrossRef] [PubMed]
- Rumore, M.M.; Kim, K.S. Potential role of salicylates in type 2 diabetes. Ann. Pharmacother. 2010, 44, 1207–1221. [Google Scholar] [CrossRef] [PubMed]
- Lara-Castro, C.; Fu, Y.; Chung, B.H.; Garvey, W.T. Adiponectin and the metabolic syndrome: Mechanisms mediating risk for metabolic and cardiovascular disease. Curr. Opin. Lipidol. 2007, 18, 263–270. [Google Scholar] [CrossRef]
- Marely, G.; Figueroa-Pérez, M.A.; Gallegos-Corona, M.R.-G.; Rosalía, R.-C. Salicylic acid elicitation during cultivation of the peppermint plant improves anti-diabetic effects of its infusions. Food Funct. 2015, 6, 1865. [Google Scholar]
- Valerio, G.; Maffeis, C.; Saggese, G.; Ambruzzi, M.A.; Balsamo, A.; Bellone, S.; Bergamini, M.; Bernasconi, S.; Bona, G.; Calcaterra, V.; et al. Diagnosis, treatment and prevention of pediatric obesity: Consensus position statement of the Italian Society for Pediatric Endocrinology and Diabetology and the Italian Society of Pediatrics. Ital. J. Pediatr. 2018, 44, 88. [Google Scholar] [CrossRef] [PubMed]
| Variable | Baseline (n = 49) | End of Intervention (n = 49) | p * | Reference Values a |
|---|---|---|---|---|
| Average of the 7-day record Energy | ||||
| kcal | 2324.08 (534.50) | 1743.08 (439.47) | <0.001 * | 1380–3170 kcal/day depending on age and sex |
| kJ | 9728.61 (2902.43) | 7296.53 (2386.26) | ||
| Protein | ||||
| g | 98.28 (35.96) | 70.60 (43.85) | <0.001 * | |
| % Energy | 17 (3) | 16 (3) | 0.102 | <15% Energy |
| Carbohydrates | ||||
| g | 321.20 (96.58) | 263.87 (62.42) | <0.001 * | |
| % Energy | 54 (6) | 56 (5) | 0.076 | 45–60% Energy |
| Fat | ||||
| g | 81.84 (29.88) | 55.44 (26.12) | <0.001 * | |
| % Energy | 32 (5) | 28 (5) | <0.001 * | 20–35% Energy |
| Fiber | ||||
| g | 17.34 (5.95) | 18.87 (6.74) | 0.236 | |
| g/1000 kcal | 7.97 (1.26) | 10.59 (1.67) | <0.001 * | 8.4 g/1000 kcal |
| Fruit and vegetables | ||||
| Average of the 7-day record | ||||
| Amount (g) | 203.50 (91.17) | 252.27 (93.74) | <0.001 * | ≥ 400 g/day |
| Last day record | ||||
| Amount (g) | 235.68 (96.72) | 313.43 (98.03) | <0.001 * | ≥ 400 g/day |
| Baseline (n = 49) | End of Intervention (n = 49) | ||
|---|---|---|---|
| Variable | Mean (SD); Median (25th–75th centile) | Mean (SD); Median (25th–75th centile) | p * |
| Glucose metabolism Glucose (mg/dL) | |||
| 82.64 (7.92); 82.50 (78.00–88.50) | 81.98 (8.53); 82.00 (75.00–86.50) | 0.978 | |
| Insulin (nU/L) | 19.93 (13.98); 17.30 (10.70–22.68) | 19.69 (11.05); 15.20 (12.05–28.05) | 0.525 |
| HOMA-IR | 4.20 (3.29); 3.42 (2.06–5.19) | 4.03 (2.28); 3.23 (2.37–6.02) | 0.460 |
| HOMA-β% | 391.55 (232.52); 288.00 (256.80–529.07) | 462.75 (441.41); 346.63 (198.81–477.00) | 0.069 |
| QUICK index | 0.32 (0.03); 0.32 (0.30–0.34) | 0.32 (0.03); 0.32 (0.29–0.33) | 0.959 |
| TyG index | 4.52 (0.20); 4.55 (4.40–4.59) | 4.48 (0.23); 4.49 (4.35–4.62) | <0.001 * |
| Lipid profile Total cholesterol (mg/dL) | |||
| 159.23 (19.17); 161.00 (144.00–176.50) | 160.89 (24.03); 157.00 (142.00–181.50) | 0.201 | |
| LDL cholesterol (mg/dL) | 92.19 (18.15); 92.50 (81.00–106.00) | 90.94 (30.80); 91.00 (66.00–113.00) | 0.930 |
| HDL cholesterol (mg/dL) | 45.92 (9.43); 43.00 (38.00–55.00) | 51.53 (10.83); 48.00 (43.00–58.00) | <0.001 * |
| Triglycerides (mg/dL) | 111.58 (46.66); 99.00 (82.00–119.50) | 104.55 (48.25); 95.00 (71.50–137.00) | <0.001 * |
| Triglycerides/HDL cholesterol | 2.57 (1.28); 2.18 (1.69–3.00) | 2.18 (1.22); 2.20 (1.21–2.84) | <0.001 * |
| LDL/HDL cholesterol | 2.13 (0.77); 1.97 (1.49–2.48) | 1.89 (0.82); 1.69 (1.15–2.69) | <0.001 * |
| AIP | 0.36 (0.21); 0.34 (0.23–0.48) | 0.27 (0.25); 0.34 (0.08–0.45) | <0.001 * |
| Baseline (n = 49) | End of Intervention (n = 49) | ||
|---|---|---|---|
| Variable | Mean (SD); Median (25th–75th centile) | Mean (SD); Median (25th–75th centile) | p * |
| Anthropometric parameters | |||
| BMI z-score | 3.14 (0.79); 2.92 (2.72–3.70) | 3.02 (0.82); 2.77 (2.40–3.73) | <0.001 * |
| Tricipital skinfold | 30.79 (5.26); 29.40 (27.00–35.00) | 31.84 (5.59); 34.00 (27.00–37.00) | 0.293 |
| WHtR | 0.62 (0.06); 0.62 (0.57–0.64) | 0.61 (0.07); 0.60 (0.56–0.66) | <0.001 * |
| FM (gr) | 23581.82 (11582.97); 20650.00 (16450.00–25600.00) | 25546.941 (12670.54); 24050.00 (18250.00–32700.00) | 0.253 |
| FFM (gr) | 34722.73 (8102.45); 34900.00 (28100.00–43300.00) | 39797.73 (7941.49); 42100.00 (32700.00–47225.00) | <0.001 * |
| FM (%) | 38.80 (7.30); 38.00 (33.85–39.80) | 38.65 (7.18); 38.10 (33.40–42.05) | 0.126 |
| FFM (%) | 61.48 (7.49); 63.40 (60.25–66.20) | 61.39 (7.17); 61.90 (57.95–66.60) | <0.001 * |
| PAS (mmHg) | 116.85 (11.22); 117.00 (109.75–127.00) | 118.25 (10.40); 120.00 (111.00–126.00) | 0.939 |
| PAD (mmHg) | 61.24 (10.31); 58.00 (56.00–62.00) | 64.98 (8.71); 62.50 (58.00–70.00) | 0.146 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vizzari, G.; Sommariva, M.C.; Dei Cas, M.; Bertoli, S.; Vizzuso, S.; Radaelli, G.; Battezzati, A.; Paroni, R.; Verduci, E. Circulating Salicylic Acid and Metabolic Profile after 1-Year Nutritional–Behavioral Intervention in Children with Obesity. Nutrients 2019, 11, 1091. https://doi.org/10.3390/nu11051091
Vizzari G, Sommariva MC, Dei Cas M, Bertoli S, Vizzuso S, Radaelli G, Battezzati A, Paroni R, Verduci E. Circulating Salicylic Acid and Metabolic Profile after 1-Year Nutritional–Behavioral Intervention in Children with Obesity. Nutrients. 2019; 11(5):1091. https://doi.org/10.3390/nu11051091
Chicago/Turabian StyleVizzari, Giulia, Maria Chiara Sommariva, Michele Dei Cas, Simona Bertoli, Sara Vizzuso, Giovanni Radaelli, Alberto Battezzati, Rita Paroni, and Elvira Verduci. 2019. "Circulating Salicylic Acid and Metabolic Profile after 1-Year Nutritional–Behavioral Intervention in Children with Obesity" Nutrients 11, no. 5: 1091. https://doi.org/10.3390/nu11051091
APA StyleVizzari, G., Sommariva, M. C., Dei Cas, M., Bertoli, S., Vizzuso, S., Radaelli, G., Battezzati, A., Paroni, R., & Verduci, E. (2019). Circulating Salicylic Acid and Metabolic Profile after 1-Year Nutritional–Behavioral Intervention in Children with Obesity. Nutrients, 11(5), 1091. https://doi.org/10.3390/nu11051091








