Polyphenol-Enriched Plum Extract Enhances Myotubule Formation and Anabolism while Attenuating Colon Cancer-induced Cellular Damage in C2C12 Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Composition of the PE60-Plum Extract
2.3. Characterization of Anti-Oxidation Capacity of the Plum Extract
2.4. Cell Culture
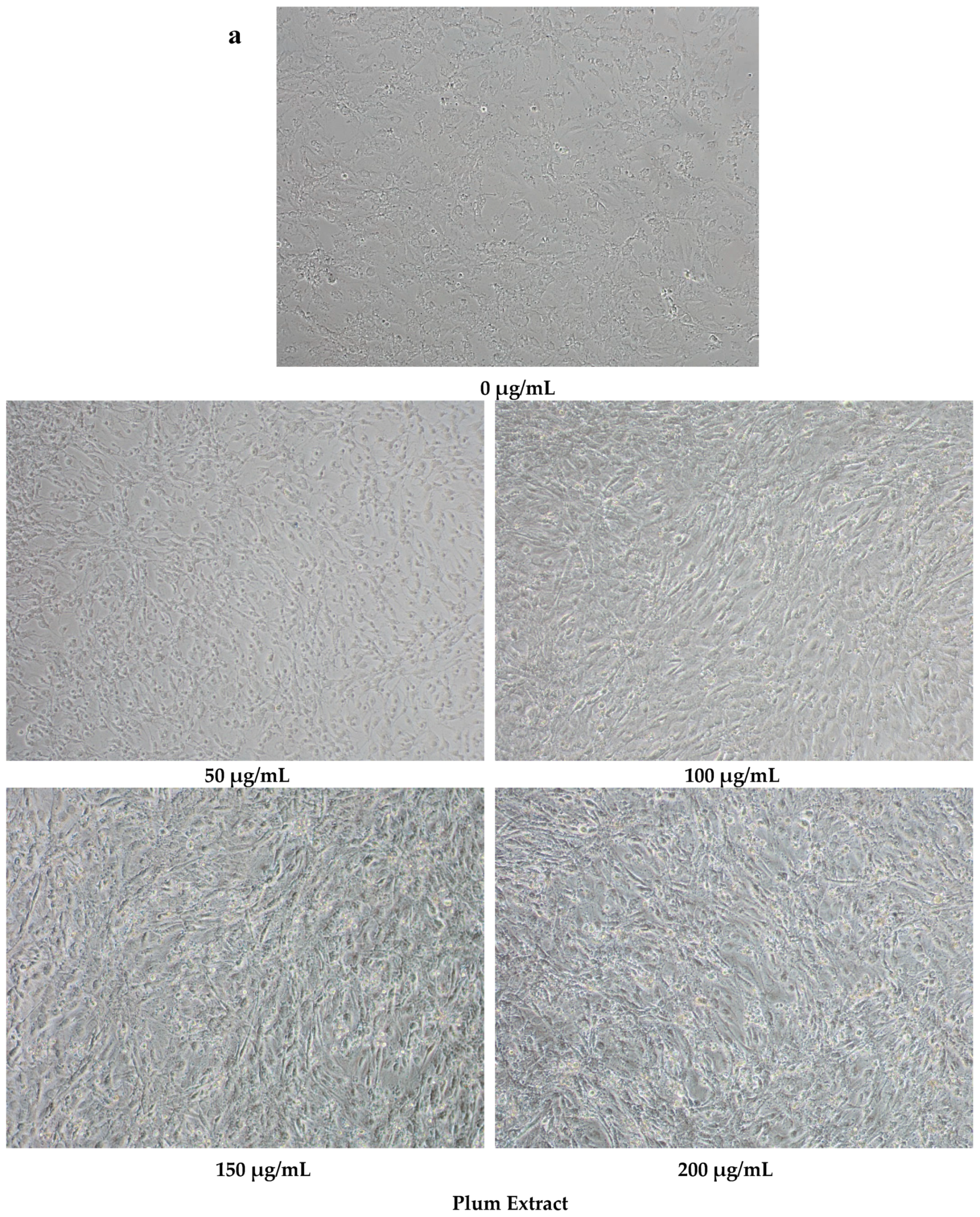
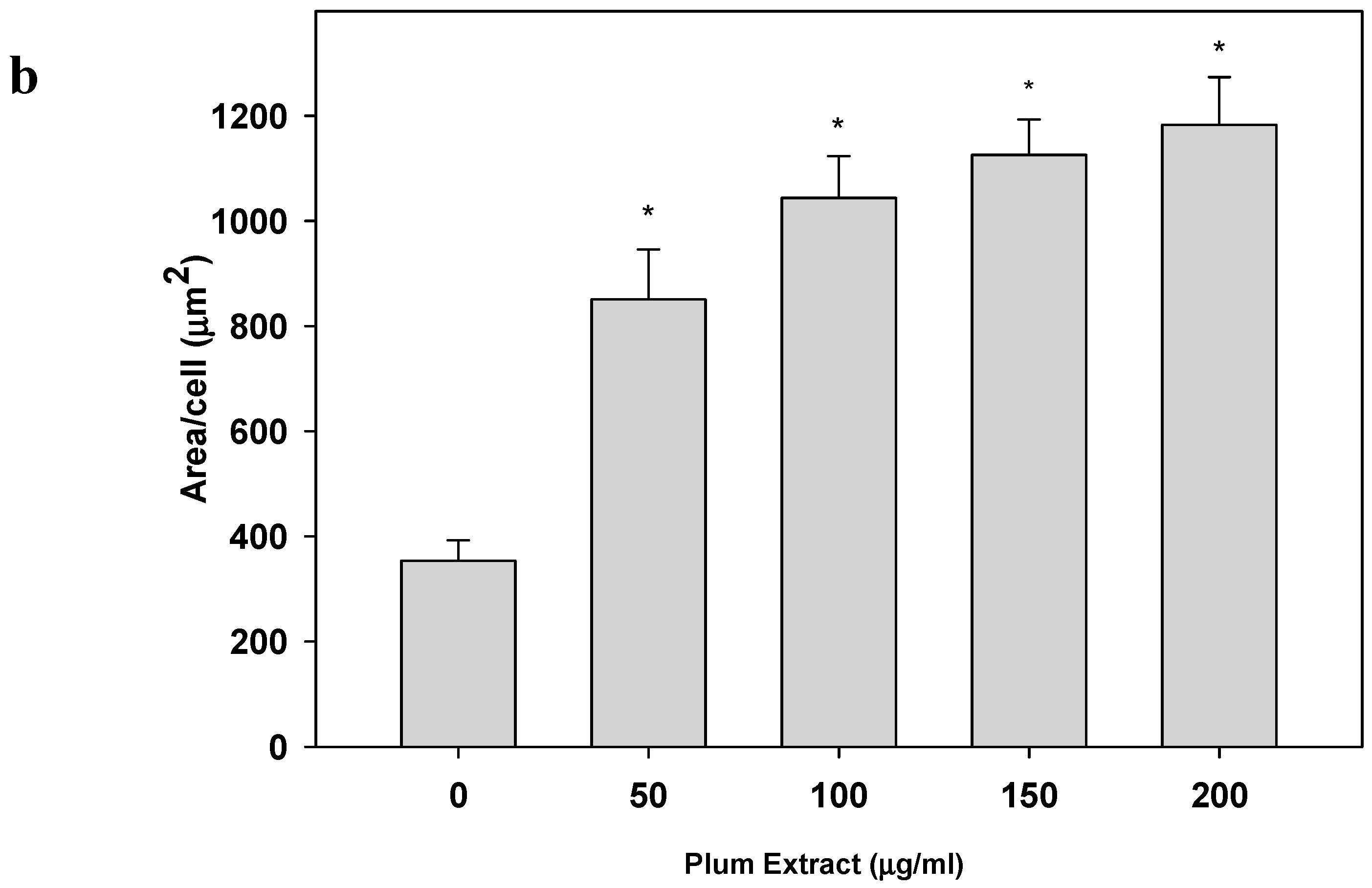
2.5. Determination of C2C12 Myoblast Cell Size
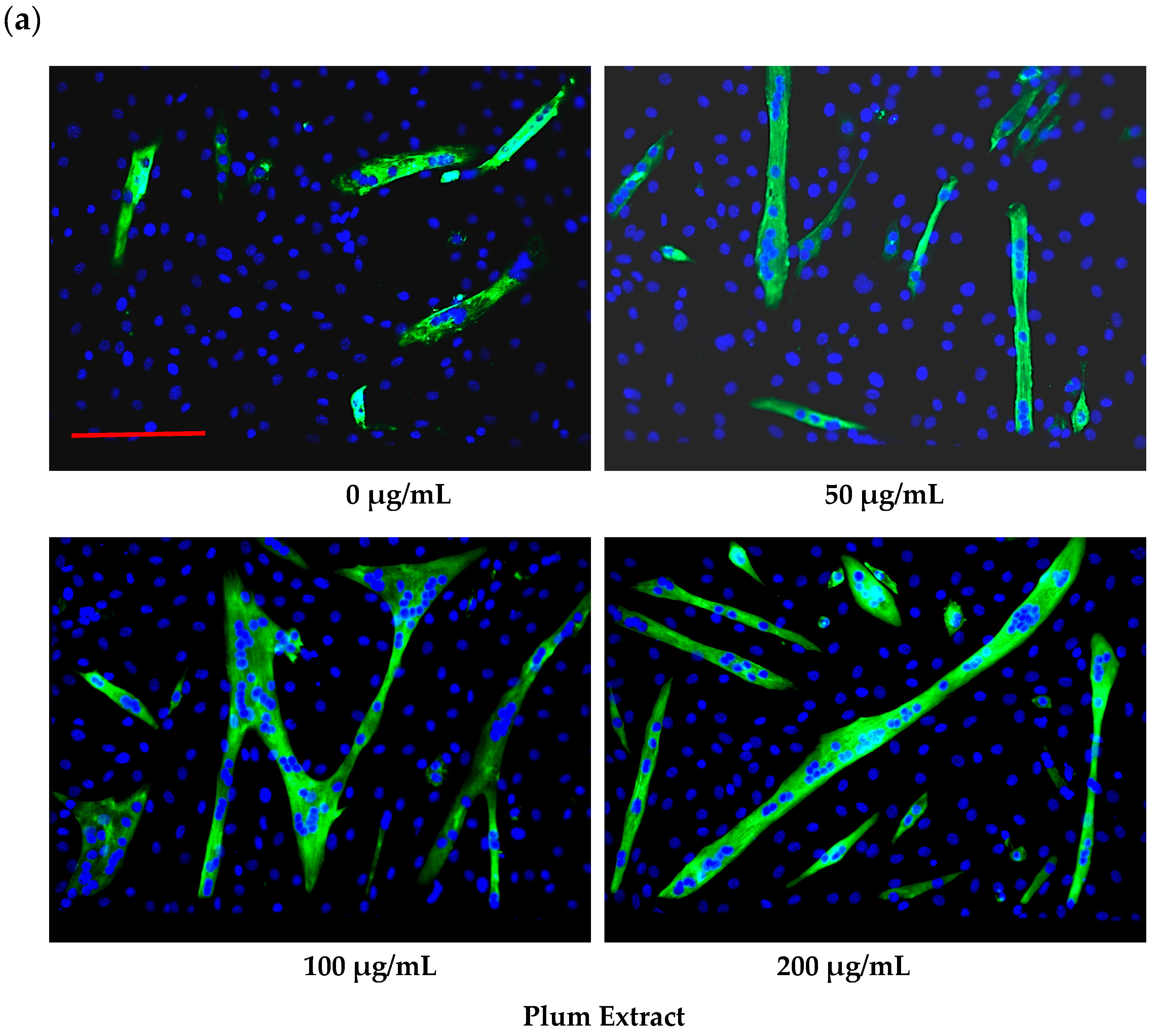
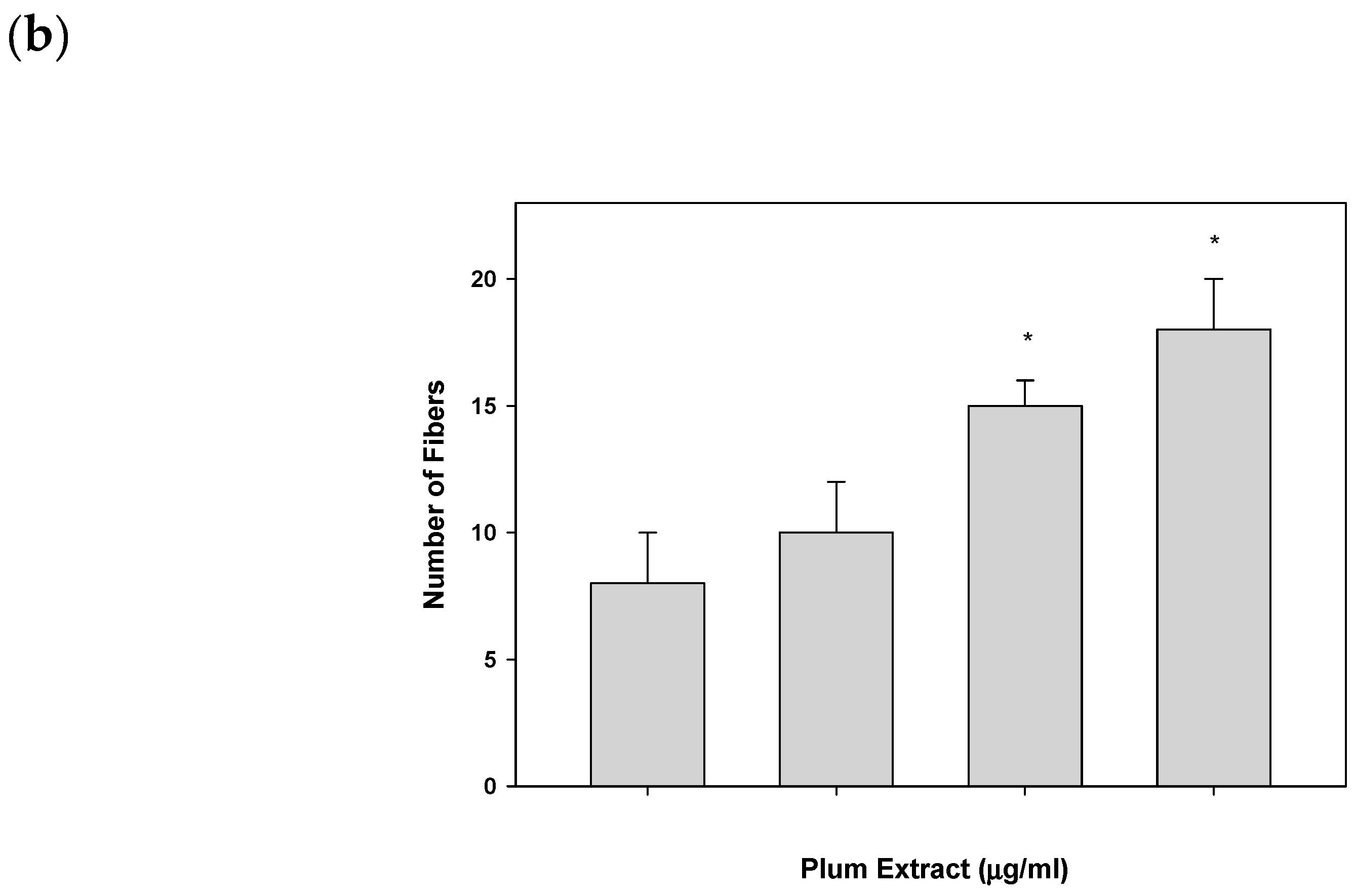
2.6. Assaying C2C12 Myoblast Differentiation
2.7. Protein Synthesis in Cultured C2C12 Myotubules
2.8. Protein Degradation in C1C12 Myotubules
2.9. Determination of Insulin-Like Growth Factor-1 (IGF-1) Expression
2.10. Determination of NFkB Activation
2.11. Effect of Plum Extract on Colon-26 Proliferation and its’ Soluble Factor Induced Cytotoxicity on C2C12 Myotubules
2.12. Data Analysis
3. Results
3.1. Characterization of PE60 Plum Extract Composition and Anti-Oxidation Properties
3.2. Effect of PE60 Plum Extract on C2C12 Myoblast Size and Differentiation
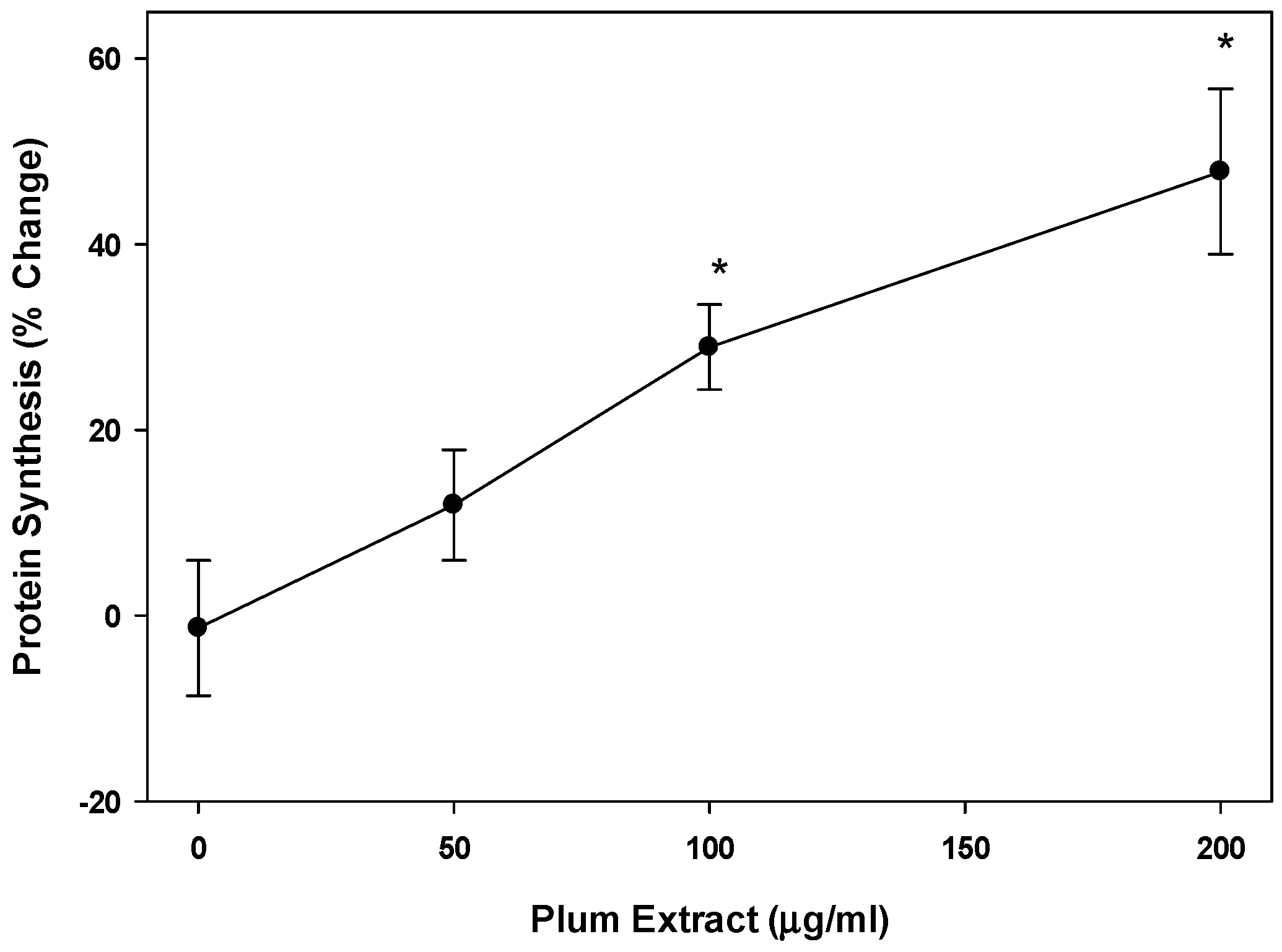
3.3. Effect of PE60 Plum Extract on Myotubule Protein Synthesis
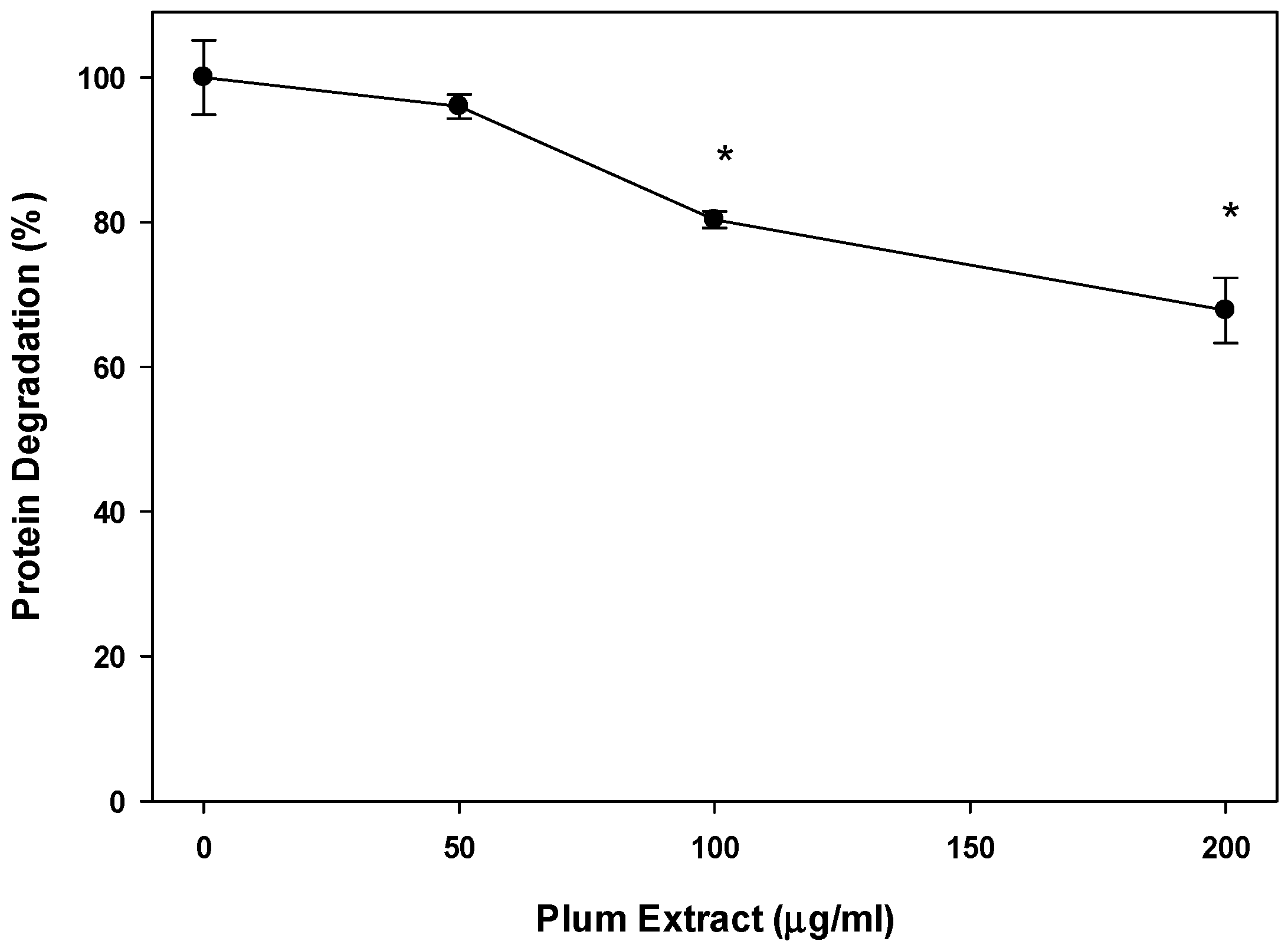
3.4. Effect of PE60 Plum Extract on Myotubules Protein Degradation
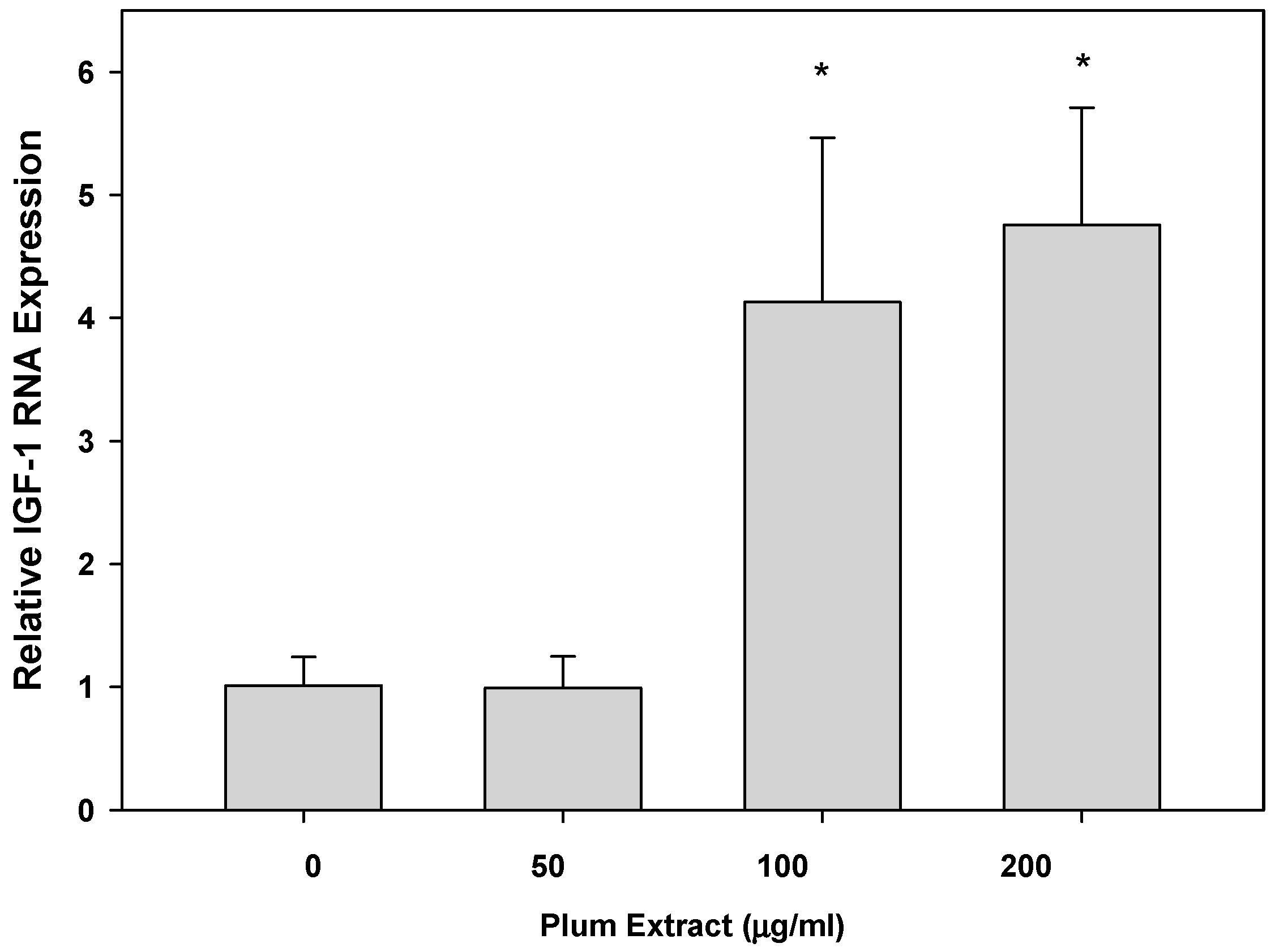
3.5. Effect of PE60 Plum Extract on IGF-1 Expression in Myotubules
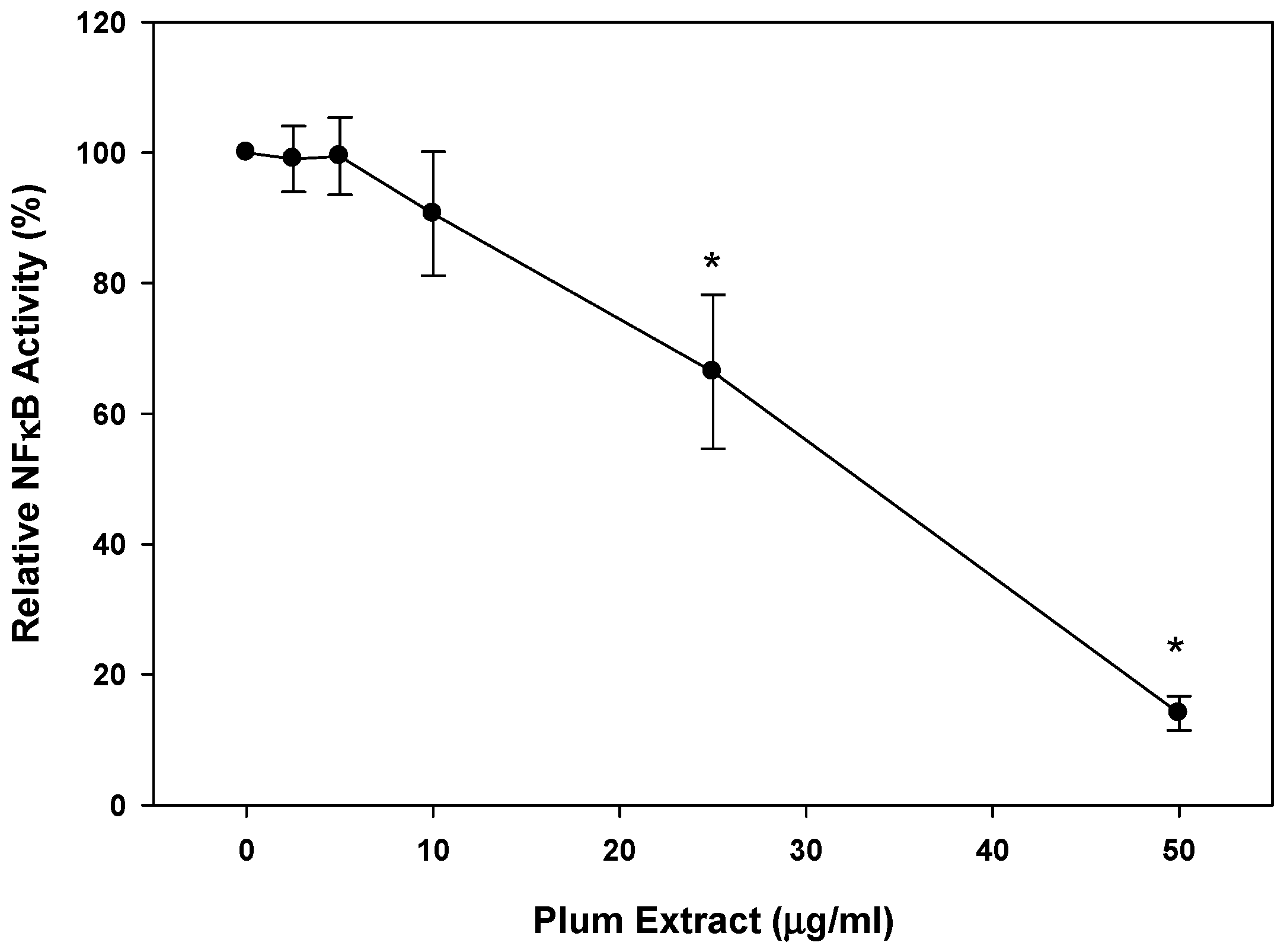
3.6. Anti-Inflammatory Effect of PE60 plum Extract in Vitro
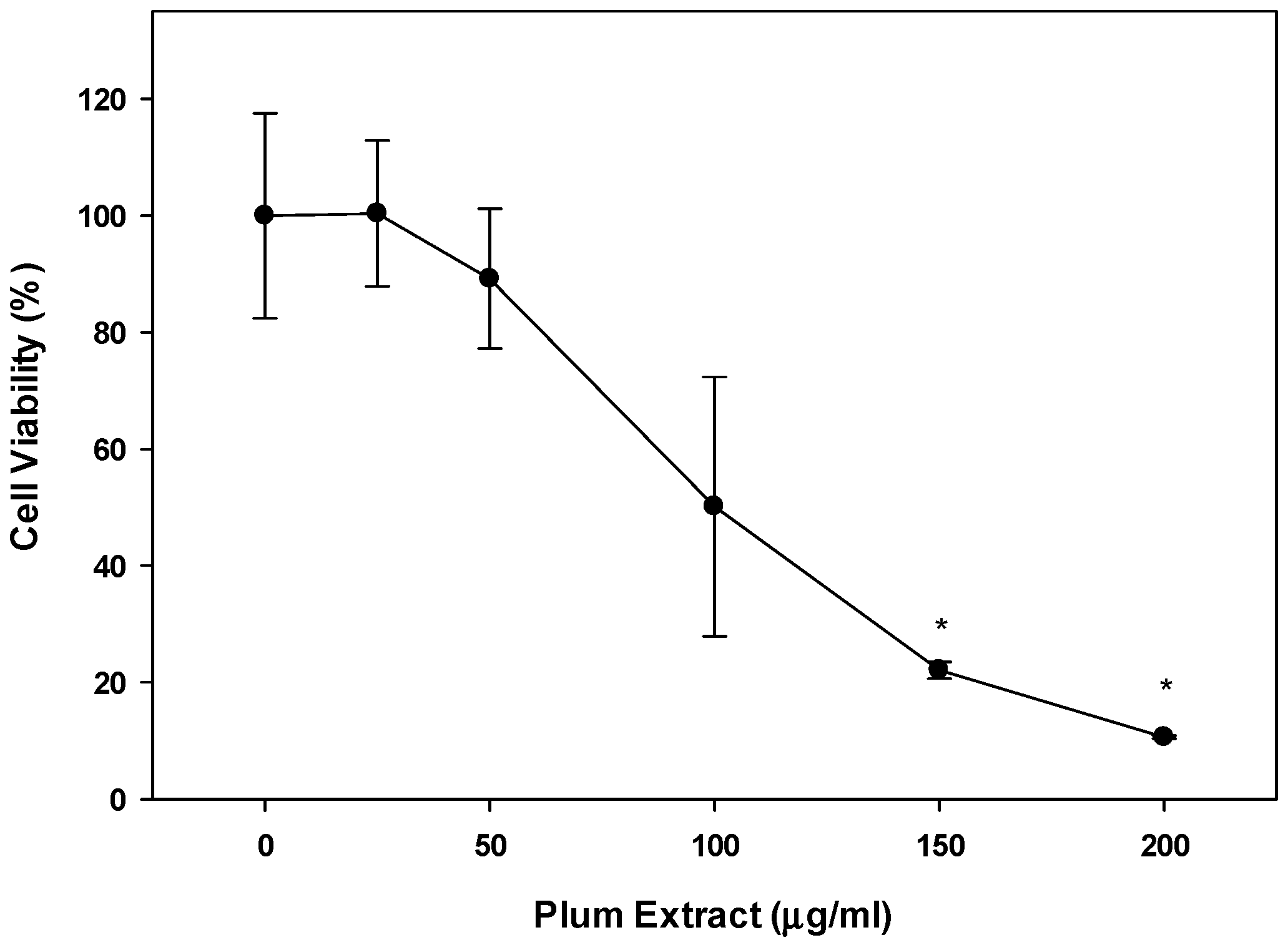
3.7. Effect of PE60 Plum Extract on Colon-26 Mouse Adenocarcinoma Cell Line
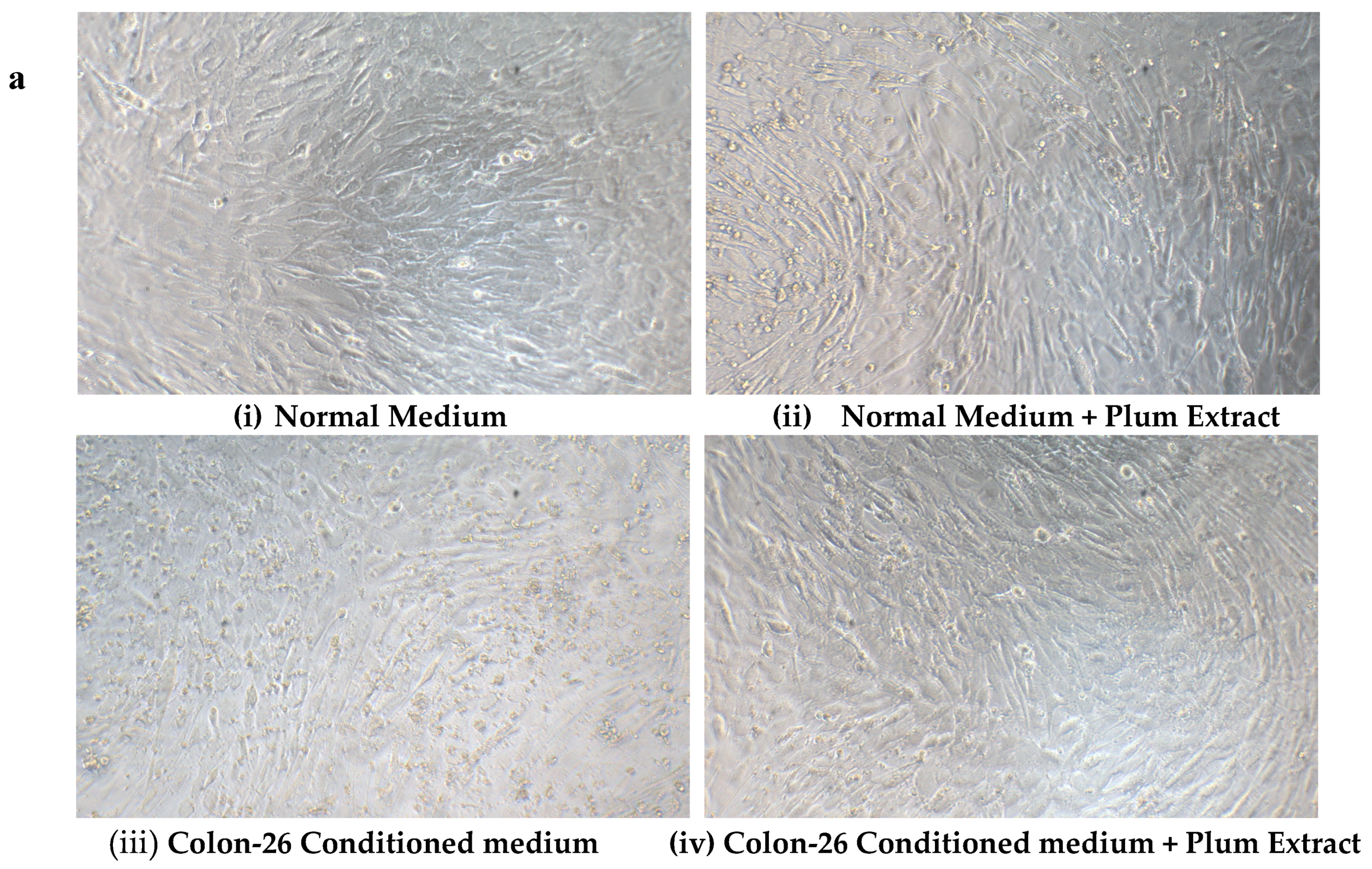
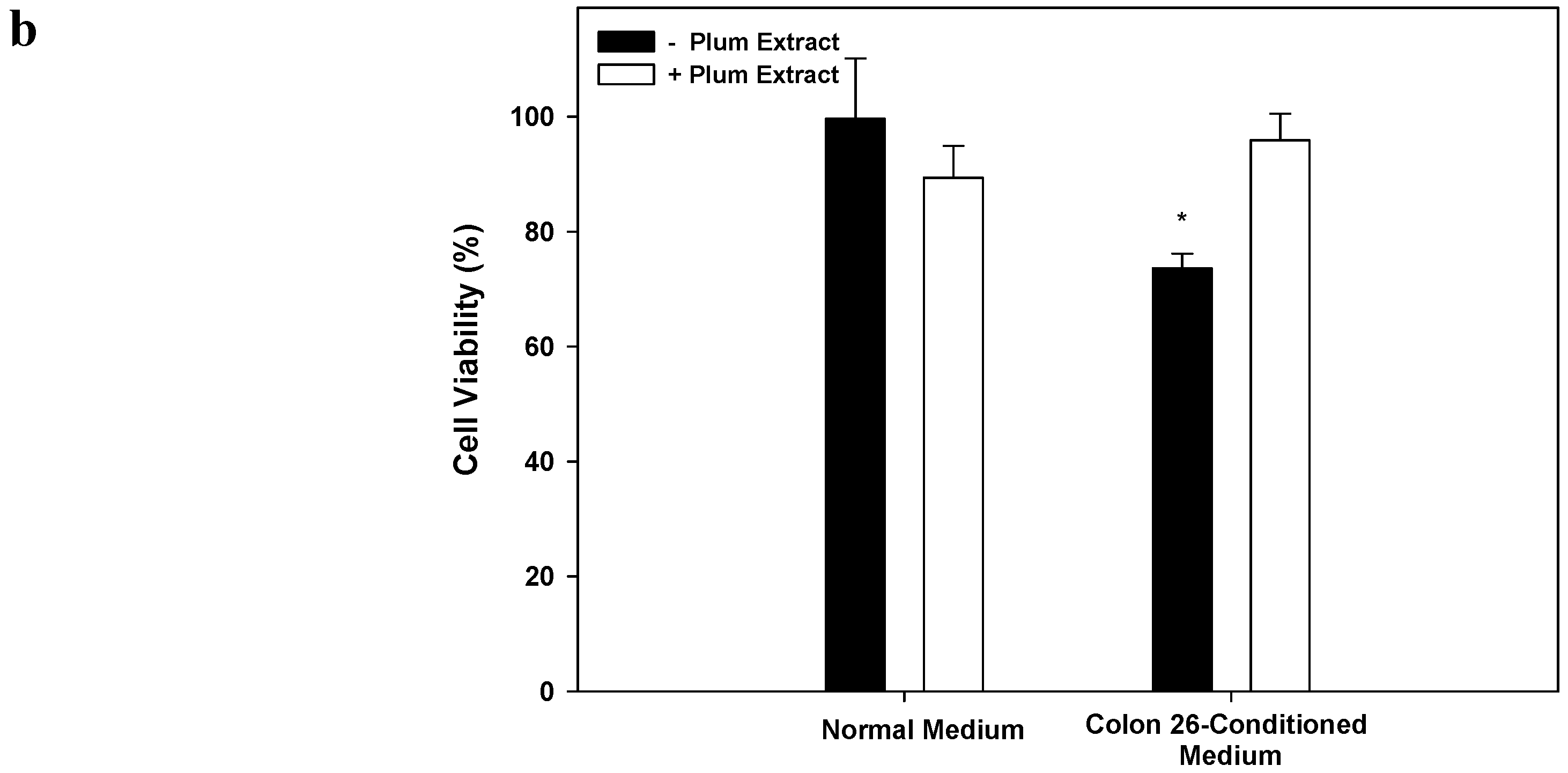
3.8. Effect of PE60 Plum Extract on C2C12 Cell Viability in Response to Colon-26 Cells Cytotoxicity-Inducing Factors
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Nixon, D.W.; Heymsfield, S.B.; Cohen, A.E.; Lutne, M.H.; Ansley, J.; Lawson, D.H.; Rudman, D. Protein calorie under-nutrition in hospitalized cancer patients. Am. J. Med. 1980, 68, 683–690. [Google Scholar] [CrossRef]
- Warren, S. The immediate causes of death in cancer. Am. J. Med. Sci. 1932, 184, 610–616. [Google Scholar] [CrossRef]
- Evans, W.J.; Morley, J.E.; Argiles, J.; Bales, C.; Baracos, V.; Guttridge, D.; Jatoi, A.; Kalantar-Zadeh, K.; Lochs, H.; Mantovani, G.; et al. Cachexia: A new definition. Clin. Nutr. 2008, 27, 793–799. [Google Scholar] [CrossRef]
- Fearon, K.C.; Glass, D.J.; Guttridge, D.C. Cancer cachexia: Mediators, signaling, and metabolic pathways. Cell Metab. 2012, 16, 153–166. [Google Scholar] [CrossRef] [PubMed]
- Fearon, K.; Strasser, F.; Anker, S.D.; Bosaeus, I.; Bruera, E.; Fainsinger, R.L.; Jatoi, A.; Loprinzi, C.; MacDonald, N.; Mantovani, G.; et al. Definition and classification of cancer cachexia: An international consensus. Lancet Oncol. 2011, 12, 489–495. [Google Scholar] [CrossRef]
- Lieffers, J.R.; Mourtzakis, M.; Hall, K.D.; McCargar, L.J.; Prado, C.M.; Baracos, V.E. A viscerally driven cachexia syndrome in patients with advanced colorectal cancer: Contributions of organ and tumor mass to whole-body energy demands. Am. J. Clin. Nutr. 2009, 89, 1173–1179. [Google Scholar] [CrossRef]
- Ravasco, P.; Monteiro-Grillo, I.; Vidal, P.M.; Camilo, M.E. Dietary counseling improves patient outcomes: A prospective, randomized, controlled trial in colorectal cancer patients undergoing radiotherapy. J. Clin. Oncol. 2005, 23, 1431–1438. [Google Scholar] [CrossRef] [PubMed]
- Bapuji, S.B.; Sawatzky, J.A. Understanding weight loss in patients with colorectal cancer: A human response to illness. Oncol. Nurs. Forum 2010, 37, 303–310. [Google Scholar] [CrossRef]
- Houten, L.; Reilley, A.A. An investigation of the cause of death from cancer. J. Surg. Oncol. 1980, 13, 111–116. [Google Scholar] [CrossRef] [PubMed]
- Benny Klimek, M.E.; Aydogdu, T.; Link, M.J.; Pons, M.; Koniaris, L.G.; Zimmers, T.A. Acute inhibition of myostatin-family proteins preserves skeletal muscle in mouse models of cancer cachexia. Biochem. Biophys. Res. Commun. 2010, 391, 1548–1554. [Google Scholar] [CrossRef]
- Gomes-Marcondes, M.C.; Tisdale, M.J. Induction of protein catabolism and the ubiquitin-proteasome pathway by mild oxidative stress. Cancer Lett. 2002, 180, 69–74. [Google Scholar] [CrossRef]
- Powers, S.K.; Kavazis, A.N.; McClung, J.M. Oxidative stress and disuse muscle atrophy. J. Appl. Phys. 2007, 102, 2389–2397. [Google Scholar]
- Powers, S.K.; Kavazis, A.N.; DeRuisseau, K.C. Mechanisms of disuse muscle atrophy: Role of oxidative stress. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2005, 288, 337–344. [Google Scholar] [CrossRef] [PubMed]
- Reid, M.B.; Moylan, J.S. Beyond atrophy: Redox mechanisms of muscle dysfunction in chronic inflammatory disease. J. Physiol. 2011, 589, 2171–2179. [Google Scholar] [CrossRef]
- Siddiqui, R.A.; Hassan, S.; Harvey, K.A.; Rasool, T.; Das, T.; Mukerji, P.; DeMichele, S. Attenuation of proteolysis and muscle wasting by curcumin C3 complex in MAC16 colon tumour-bearing mice. Br. J. Nutr. 2009, 102, 967–975. [Google Scholar] [CrossRef]
- Lambert, K.; Coisy-Quivy, M.; Bisbal, C.; Sirvent, P.; Hugon, G.; Mercier, J.; Avignon, A.; Sultan, A. Grape polyphenols supplementation reduces muscle atrophy in a mouse model of chronic inflammation. Nutrition 2015, 31, 275–283. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Yang, G.; Liang, X.; Zhu, M.; Du, M. Grape seed extract prevents skeletal muscle wasting in interleukin 10 knockout mice. BMC Complement. Altern. Med. 2014, 14, 162. [Google Scholar] [CrossRef]
- Dorchies, O.M.; Wagner, S.; Buetler, T.M.; Ruegg, U.T. Protection of dystrophic muscle cells with polyphenols from green tea correlates with improved glutathione balance and increased expression of 67LR, a receptor for (-)-epigallocatechin gallate. BioFactors 2009, 35, 279–294. [Google Scholar] [CrossRef]
- Meador, B.M.; Mirza, K.A.; Tian, M.; Skelding, M.B.; Reaves, L.A.; Edens, N.K.; Pereira, S.L. The green tea polyphenol Epigallocatechin-3-Gallate (EGCg) attenuates skeletal muscle atrophy in a rat model of sarcopenia. J. Frailty Aging 2015, 4, 209–215. [Google Scholar]
- Wang, H.; Lai, Y.J.; Chan, Y.L.; Li, T.L.; Wu, C.J. Epigallocatechin-3-gallate effectively attenuates skeletal muscle atrophy caused by cancer cachexia. Cancer Lett. 2011, 305, 40–49. [Google Scholar] [CrossRef]
- Liu, J. Pharmacology of oleanolic acid and ursolic acid. J. Ethnopharmacol. 1995, 49, 57–68. [Google Scholar] [CrossRef]
- Kunkel, S.D.; Suneja, M.; Ebert, S.M.; Bongers, K.S.; Fox, D.K.; Malmberg, S.E.; Alipour, F.; Shields, R.K.; Adams, C.M. mRNA expression signatures of human skeletal muscle atrophy identify a natural compound that increases muscle mass. Cell Metab. 2011, 8, 627–638. [Google Scholar] [CrossRef]
- Kunkel, S.D.; Elmore, C.J.; Bongers, K.S.; Ebert, S.M.; Fox, D.K.; Dyle, M.C.; Bullard, S.A.; Adams, C.M. Ursolic acid increases skeletal muscle and brown fat and decreases diet-induced obesity, glucose intolerance and fatty liver disease. PLoS One 2012, 7, e39332. [Google Scholar] [CrossRef]
- Stacewicz-Sapuntzakis, M. Dried plums and their products: Composition and health effects--an updated review. Crit. Rev. Food Sci. Nutr. 2013, 53, 277–302. [Google Scholar] [CrossRef]
- Mirza, F.; Lorenzo, J.; Drissi, H.; Lee, F.Y.; Soung, D.Y. Dried plum alleviates symptoms of inflammatory arthritis in TNF transgenic mice. J. Nutr. Biochem. 2018, 52, 54–61. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.-O.; Jeong, S.W.; Lee, C.Y. Antioxidant capacity of phenolic phytochemicals from various cultivars of plums. Food Chem. 2003, 81, 321–326. [Google Scholar] [CrossRef]
- Ramos, S.; Alia, M.; Bravo, L.; Goya, L. Comparative effects of food-derived polyphenols on the viability and apoptosis of a human hepatoma cell line (HepG2). J. Agric. Food Chem. 2005, 53, 1271–1280. [Google Scholar] [CrossRef] [PubMed]
- Yu, M.H.; Im, H.G.; Kim, H.I.; Lee, I.S. Induction of apoptosis by immature plum in human hepatocellular carcinoma. J. Med. Food 2009, 12, 518–527. [Google Scholar] [CrossRef]
- Yu, M.H.; Gwon, I.H.; Gyu, L.S.; Kim, D.I.; Jeong, S.H.; Lee, I.S. Inhibitory effect of immature plum on PMA-induced MMP-9 expression in human hepatocellular carcinoma. Nat. Prod. Res. 2009, 23, 704–718. [Google Scholar] [CrossRef] [PubMed]
- Rendina, E.; Hembree, K.D.; Davis, M.R.; Marlow, D.; Clarke, S.L.; Halloran, B.P.; Lucas, E.A.; Smith, B.J. Dried plum’s unique capacity to reverse bone loss and alter bone metabolism in postmenopausal osteoporosis model. PLoS One 2013, 8, e60569. [Google Scholar] [CrossRef]
- Arjmandi, B.H.; Johnson, S.A.; Pourafshar, S.; Navaei, N.; George, K.S.; Hooshmand, S.; Sheau, C.; Chai, S.C.; Akhavan, N.S. Bone-protective effects of dried plum in postmenopausal women: Efficacy and possible mechanisms. Nutrients 2017, 9, 496. [Google Scholar] [CrossRef] [PubMed]
- Hooshmand, S.; Brisco, J.R.; Arjmandi, B.H. The effect of dried plum on serum levels of receptor activator of NF-κB ligand, osteoprotegerin and sclerostin in osteopenic postmenopausal women: A randomised controlled trial. Br. J. Nutr. 2014, 112, 55–60. [Google Scholar] [CrossRef]
- Halloran, B.P.; Wronski, T.J.; VonHerzen, D.C.; Chu, V.; Xia, X.; Pingel, J.E.; Williams, A.A.; Smith, B.J. Dietary dried plum increases bone mass in adult and aged male mice. J. Nutr. 2010, 140, 1781–1787. [Google Scholar] [CrossRef] [PubMed]
- Niketic-Aleksic, G.K.; Hrazdina, G. Quantitative analysis of the anthocyanin content in grape juices and wines. LWT-FOOD. SCI. TECHNOL. 1972, 5, 163–165. [Google Scholar]
- Yu, L.; Haley, S.; Perret, J.; Harris, M. Antioxidant properties of extracts from hard winter wheat. Food Chem. 2002, 78, 457–461. [Google Scholar] [CrossRef]
- Parry, J.W.; Su, L.; Moore, J.; Cheng, Z.; Luther, M.; Jaladanki, R.; Wang, J.; Yu, L. Chemical Compositions, Antioxidative Capacities, and Anti-Proliferative Activities of Selected Fruit Seed Flours. J. Agric. Food Chem. 2006, 54, 3773–3778. [Google Scholar] [CrossRef]
- Cheng, Z.; Moore, J.; Yu, L. High-throughput relative DPPH radical scavenging capacity assay. J. Agric. Food Chem. 2006, 54, 7429–7436. [Google Scholar] [CrossRef] [PubMed]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evens, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Bio. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Siddiqui, R.A.; Harvey, K.A.; Xu, Z.; Natarajan, S.K.; Davisson, V.J. Characterization of lovastatin-docosahexaenoate anticancer properties against breast cancer cells. Bioorg. Med. Chem. 2014, 22, 1899–1908. [Google Scholar] [CrossRef]
- Yuan, L.; Hani, J.; Mmeng, Q.; Xi, Q.; Zhuang, Q.; Jiang, Y.; Han, Y.; Zhang, B.; Fang, Z.; Wu, G. Muscle-specific E3 ubiquitin ligases are involved in muscle atrophy of cancer cachexia: An in vitro and in vivo study. Oncol. Rept. 2015, 33, 2261–2268. [Google Scholar] [CrossRef]
- Nakatani, N.; Kayano, S.; Kikuzaki, H.; Sumino, K.; Katagiri, K.; Mitani, T. Identification, quantitative determination, and antioxidative activities of chlorogenic acid isomers in prune (Prunus domestica L.). J Agric. Food Chem 2000, 48, 5512–5516. [Google Scholar] [CrossRef]
- Graef, J.L.; Rendina-Ruedya, E.; Crocketta, E.K.; Ouyanga, P.; King, J.B.; Cichewiczb, R.H.; Lucasa, E.A.; Smitha, B.J. Select polyphenolic fractions from dried plum enhance osteoblast activity through BMP-2 signaling. J. Nutr. Biochem. 2018, 55, 59–67. [Google Scholar] [CrossRef]
- Graef, J.L.; Ouyanga, P.; Wanga, Y.; Rendina-Ruedya, E.; Lernerc, M.R.; Marlowb, D.; Lucasa, E.A.; Smith, B.J. Dried plum polyphenolic extract combined with vitamin K and potassium restores trabecular and cortical bone in osteopenic model of postmenopausal bone loss. J. Funct. Foods 2018, 42, 262–270. [Google Scholar] [CrossRef] [PubMed]
- Ferreyra, M.L.F.; Rius, S.P.; Casati, P. Flavonoids: Biosynthesis, biological functions, and biotechnological applications. Front Plant Sci. 2012, 3, 222. [Google Scholar]
- Bener, M.; Ozyurek, M.; Guçlu, K.; Apak, P. Optimization of microwave-assisted extraction of curcumin from Curcuma longa L. (turmeric) and evaluation of antioxidant activity in multi-test systems. Rec. Nat. Prod. 2016, 10, 542–554. [Google Scholar]
- Pekal, A.; Drozdz, P.; Pyrzynska, K. Comparison of the antioxidant properties of commonly consumed commercial teas. Int. J. Food Prop. 2012, 15, 1101–1109. [Google Scholar] [CrossRef]
- Forester, S.C.; Lambert, J.D. Antioxidant effects of green tea. Mol. Nutr. Food Res. 2011, 55, 844–854. [Google Scholar] [CrossRef] [PubMed]
- Arfan, M.; Khan, R.; Rybarczyk, A.; Amarowicz, R. Antioxidant activity of mulberry fruit extracts. Int. J. Mol. Sci. 2012, 13, 2472–2480. [Google Scholar] [CrossRef] [PubMed]
- Gil, M.I.; Tomas-Barberan, F.A.; Hess-Pierce, B.; Holcroft, D.M.; Kader, A.A. Antioxidant activity of pomegranate juice and its relationship with phenolic composition and processing. J. Agric. Food Chem. 2000, 48, 4581–4589. [Google Scholar] [CrossRef]
- Deyhim, F.; Stoecker, B.J.; Brusewitz, G.H.; Devareddy, L.; Arjmandi, B.H. Dried plum reverses bone loss in an osteopenic rat model of osteoporosis. Menopause 2005, 2, 755–762. [Google Scholar] [CrossRef]
- Franklin, M.; Bu, S.Y.; Lerner, M.R.; Lancaster, E.A.; Bellmer, D.; Marlow, D.; Lightfoot, S.A.; Arjmandi, B.H.; Brackett, D.J.; Lucas, E.A.; et al. Dried plum prevents bone loss in a male osteoporosis model via IGF-I and the RANK pathway. Bone 2006, 39, 1331–1342. [Google Scholar] [CrossRef]
- Vizzotto, M.; Porter, W.; Byrne, D.; Cisneros-Zevallos, L. Polyphenols of selected peach and plum genotypes reduce cell viability and inhibit proliferation of breast cancer cells while not affecting normal cells. Food Chem. 2014, 164, 363–370. [Google Scholar] [CrossRef]
- Cohen, S.; Nathan, J.A.; Goldberg, A.L. Muscle wasting in disease: Molecular mechanisms and promising therapies. Nat. Rev. Drug Discov. 2015, 14, 58–74. [Google Scholar] [CrossRef]
- Florini, J.R.; Ewton, D.Z.; Coolican, S.A. Growth hormone and the insulin-like growth factor system in myogenesis. Endocr. Rev. 1996, 17, 481–517. [Google Scholar]
- Coleman, M.E.; DeMayo, F.; Yin, K.C.; Lee, H.M.; Geske, R.; Montgomery, C.; Schwartz, R.J. Myogenic vector expression of insulin-like growth factor 1 stimulates muscle cell differentiation and myofiber hypertrophy in transgenic mice. J. Biol. Chem. 1995, 270, 12109–12116. [Google Scholar] [CrossRef] [PubMed]
- Jennische, E.; Skottner, A.; Hansson, H.A. Satellite cells express the trophic factor IGF-1 in regenerating skeletal muscle cell. Acta Physiol. Scand. 1987, 129, 9–15. [Google Scholar] [CrossRef]
- Jannische, E.; Hansson, H.A. Regenerating skeletal muscle cells express insulin-like growth factor I. Acta Physiol. Scand. 1987, 130, 327–332. [Google Scholar] [CrossRef]
- Arjmandi, B.H.; Khalil, D.A.; Lucas, E.A.; Georgis, A.; Stoecker, B.J.; Hardin, C.; Payton, M.E.; Wild, R.A. Dried plums improve indices of bone formation in postmenopausal women. J. Womens Health Gend. Based Med. 2002, 11, 61–68. [Google Scholar] [CrossRef] [PubMed]
- Smith, B.J.; Bu, S.Y.; Wang, Y.; Rendina, E.; Lim, Y.F.; Marlow, D.; Clarke, S.L.; Cullen, D.M.; Lucas, E.A. A comparative study of the bone metabolic response to dried plum supplementation and PTH treatment in adult, osteopenic ovariectomized rat. Bone 2014, 58, 151–159. [Google Scholar] [CrossRef]
- Costelli, P.; Muscaritoli, M.; Bossola, M.; Penna, F.; Reffo, P.; Bonetto, A.; Busquets, S.; Bonelli, G.; Lopez-Soriano, F.J.; Doglietto, G.B.; et al. IGF-1 is downregulated in experimental cancer cachexia. Am. J. Physiol. Regul. Integr. Comp Physiol. 2006, 291, R674–R683. [Google Scholar] [CrossRef]
- Schiaffino, S.; Mammucari, C. Regulation of skeletal muscle growth by the IGF1-Akt/PKB pathway: Insights from genetic models. Skeletal Muscle 2011. [Google Scholar] [CrossRef] [PubMed]
- Eley, H.L.; Russell, S.T.; Tisdale, M.J. Effect of branched-chain amino acids on muscle atrophy in cancer cachexia. Biochem. J. 2007, 407, 113–120. [Google Scholar] [CrossRef] [PubMed]
- Bu, S.Y.; Lerner, M.; Stoecker, B.J.; Boldrin, E.; Brackett, D.J.; Lucas, E.A.; Smith, B.J. Dried plum polyphenols inhibit osteoclastogenesis by downregulating NFATc1 and inflammatory mediators. Calcif. Tissue Int. 2008, 82, 475–488. [Google Scholar] [CrossRef] [PubMed]
- Hooshmand, S.; Kumar, A.; Zhang, J.Y.; Johnson, S.A.; Chai, S.C.; Bahram, H.; Arjmandi, B.H. Evidence for anti-inflammatory and antioxidative properties of dried plum polyphenols in macrophage RAW 264.7 cells. Food Funct. 2015, 6, 1719–1725. [Google Scholar] [CrossRef]
- Rendina, E.; Lima, Y.F.; Marlowb, D.; Wanga, Y.; Clarkea, S.L.; Kuvibidilaa, S.; Lucasa, E.A.; Brenda, J.; Smith, B.J. Dietary supplementation with dried plum prevents ovariectomy-induced bone loss while modulating the immune response in C57BL/6J mice. J. Nutr. Biochem. 2012, 23, 60–68. [Google Scholar] [CrossRef]
- Banerjee, N.; Kim, H.; Talcott, S.T.; Turner, N.D.; Byrne, D.H.; Mertens-Talcott, S.U. Plum polyphenols inhibit colorectal aberrant crypt foci formation in rats: Potential role of the miR-143/protein kinase B/mammalian target of rapamycin axis. Nutr. Res. 2016, 36, 1105–1113. [Google Scholar] [CrossRef] [PubMed]
- Noratto, G.; Martino, H.S.D.; Simbo, S.; Byrne, D.; Mertens-Talcott, S.U. Consumption of polyphenol-rich peach and plum juice prevents risk factors for obesity-related metabolic disorders and cardiovascular disease in Zucker rats. J. Nutr. Biochem. 2015, 26, 633–641. [Google Scholar] [CrossRef]
- Guttridge, D.C.; Mayo, M.W.; Madrid, L.V.; Wang, C.-Y.; Baldwin, A.S., Jr. NF-κB-induced loss of MyoD messenger RNA: Possible role in muscle decay and cachexia. Science 2000, 289, 2363–2366. [Google Scholar] [CrossRef] [PubMed]
- Aulino, P.; Berardi, E.; Cardillo, V.M.; Rizzuto, E.; Perniconi, B.; Ramina, C.; Padula, F.; Spugnini, E.P.; Baldi, A.; Faiola, F.; et al. Molecular, cellular and physiological characterization of the cancer cachexia-inducing C26 colon carcinoma in mouse. BMC Cancer 2010, 10, 363. [Google Scholar] [CrossRef]
- Tian, M.; Kliewer, K.L.; Asp, M.L.; Stout, M.B.; Belury, M.A. c9t11-Conjugated linoleic acid-rich oil fails to attenuate wasting in colon-26 tumor-induced late-stage cancer cachexia in male CD2F1 mice. Mol. Nutr. Food Res. 2010, 55, 268–277. [Google Scholar] [CrossRef]
- Siddiqui, R.; Pandya, D.; Harvey, K.; Zaloga, G.P. Nutrition modulation of cachexia/proteolysis. Nutr. Clin. Pract. 2006, 21, 55–67. [Google Scholar] [CrossRef]
- Siddiqui, R.A.; Williams, J.F. Tentative identification of the toxohormones of cancer cachexia: Roles of vasopressin, prostaglandin E2 and cachectic-TNF. Biochem. Int. 1990, 20, 787–797. [Google Scholar]
- Tisdale, M.J. Mechanisms of Cancer Cachexia. Physiol Rev. 2009, 89, 381–410. [Google Scholar] [CrossRef]
- Lorite, M.J.; Smith, H.J.; Arnold, J.A.; Morris, A.; Thompson, M.G.; Tisdale, M.J. Activation of ATP-ubiquitin-dependent proteolysis in skeletal muscle in vivo and murine myoblasts in vitro by a proteolysis-inducing factor (PIF). Brit. J. Cancer 2001, 85, 297–302. [Google Scholar] [CrossRef] [PubMed]
- Baltgalvis, K.A.; Berger, F.G.; Peña, M.M.O.; Davis, J.M.; White, J.P.; Carson, J.A. Activity level, apoptosis, and development of cachexia in Apc mice. J. Appl. Physiol. 2010, 109, 1155–1161. [Google Scholar] [CrossRef] [PubMed]
| Component | Concentration (g per 100 g) n = 3 | Flavonoid Type (USDA) | Analytical Method |
|---|---|---|---|
| Anthocyanins | 0.391 ± 0.020 (rsd = 5.1%) | Anthocyanidin | Colorimetric |
| 3-chlorogenic acid | 1.76 ± 0.01 (rsd = 0.6%) | Hydroxycinnamic acid | LC/UV * |
| Rutin | 1.12 ± 0.01 (rsd = 0.6%) | Flavanol | LC/UV * |
| Quercetin (free) | 0.718 ± 0.005 (rsd = 0.7%) | Flavanol | LC/UV * |
| Gallic acid (free) | 0.381 ± 0.004 (rsd = 1.1%) | Hydroxybenzoic | LC/UV * |
| Proanthocyanidins | 60 ± 10 (rsd < 2%) | Flavan-3-ol | LC/UV * |
| Assays | Units | Mean ± SD |
|---|---|---|
| Total Phenolic Content (TPC) | mg/g | 542.44 ± 24.75 |
| Total Flavonoid Content (TFC) | mg/g | 520.00 ± 40.10 |
| Anti-oxidant activity (DPPH) | μM Trolox Equivalent/g | 3375 ± 90 |
| Oxygen Scavenging Activity (ABTS) | μM Trolox Equivalent/g | 4250 ± 250 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alsolmei, F.A.; Li, H.; Pereira, S.L.; Krishnan, P.; Johns, P.W.; Siddiqui, R.A. Polyphenol-Enriched Plum Extract Enhances Myotubule Formation and Anabolism while Attenuating Colon Cancer-induced Cellular Damage in C2C12 Cells. Nutrients 2019, 11, 1077. https://doi.org/10.3390/nu11051077
Alsolmei FA, Li H, Pereira SL, Krishnan P, Johns PW, Siddiqui RA. Polyphenol-Enriched Plum Extract Enhances Myotubule Formation and Anabolism while Attenuating Colon Cancer-induced Cellular Damage in C2C12 Cells. Nutrients. 2019; 11(5):1077. https://doi.org/10.3390/nu11051077
Chicago/Turabian StyleAlsolmei, Faten A., Haiwen Li, Suzette L. Pereira, Padmavathy Krishnan, Paul W. Johns, and Rafat A. Siddiqui. 2019. "Polyphenol-Enriched Plum Extract Enhances Myotubule Formation and Anabolism while Attenuating Colon Cancer-induced Cellular Damage in C2C12 Cells" Nutrients 11, no. 5: 1077. https://doi.org/10.3390/nu11051077
APA StyleAlsolmei, F. A., Li, H., Pereira, S. L., Krishnan, P., Johns, P. W., & Siddiqui, R. A. (2019). Polyphenol-Enriched Plum Extract Enhances Myotubule Formation and Anabolism while Attenuating Colon Cancer-induced Cellular Damage in C2C12 Cells. Nutrients, 11(5), 1077. https://doi.org/10.3390/nu11051077





