Rectal and Vaginal Eradication of Streptococcus agalactiae (GBS) in Pregnant Women by Using Lactobacillus salivarius CECT 9145, A Target-specific Probiotic Strain
Abstract
1. Introduction
2. Material and Methods
2.1. Microbiological Analysis of Vaginal Swabs Obtained from Pregnant and Non-pregnant Women
2.2. Antimicrobial Activity of the Lactobacilli Strains against GBS
2.3. Production of Specific Antimicrobials (Bacteriocins, Lactic Acid, Hydrogen Peroxide) by the Lactobacilli Strains
2.4. Coaggregation and Co-culture Assays
2.5. Survival After In Vitro Exposure to Saliva and Gastrointestinal-Like Conditions
2.6. Adhesion to Caco-2, HT-29 and Vaginal Cells and to Mucin
2.7. Sensitivity to Antibiotics
2.8. Hemolysis, Formation of Biogenic Amines and Degradation of Mucin
2.9. Acute and Repeated Dose (4-Weeks) Oral Toxicity Studies in a Rat Model
2.10. Efficacy of L. salivarius CECT 9145 to Eradicate GBS from the Intestinal and Vaginal Tracts of Pregnant Women: A Pilot Clinical Trial
3. Results
3.1. Microbiological Analysis of Vaginal Swabs Obtained from Pregnant and Non-Pregnant Women
3.2. Antimicrobial Activity of the Lactobacilli Strains Against GBS and the Production of Potential Antimicrobial Compounds
3.3. Survival After In Vitro Exposure to Saliva and Gastrointestinal-Like Conditions
3.4. Adhesion to Caco-2, HT-29 and Vaginal Cells and to Mucin
3.5. Sensitivity to Antibiotics
3.6. Hemolysis, the Formation of Biogenic Amines and the Degradation of Mucin
3.7. Acute and Repeated Dose (4 Weeks) Oral Toxicity Studies in a Rat Model
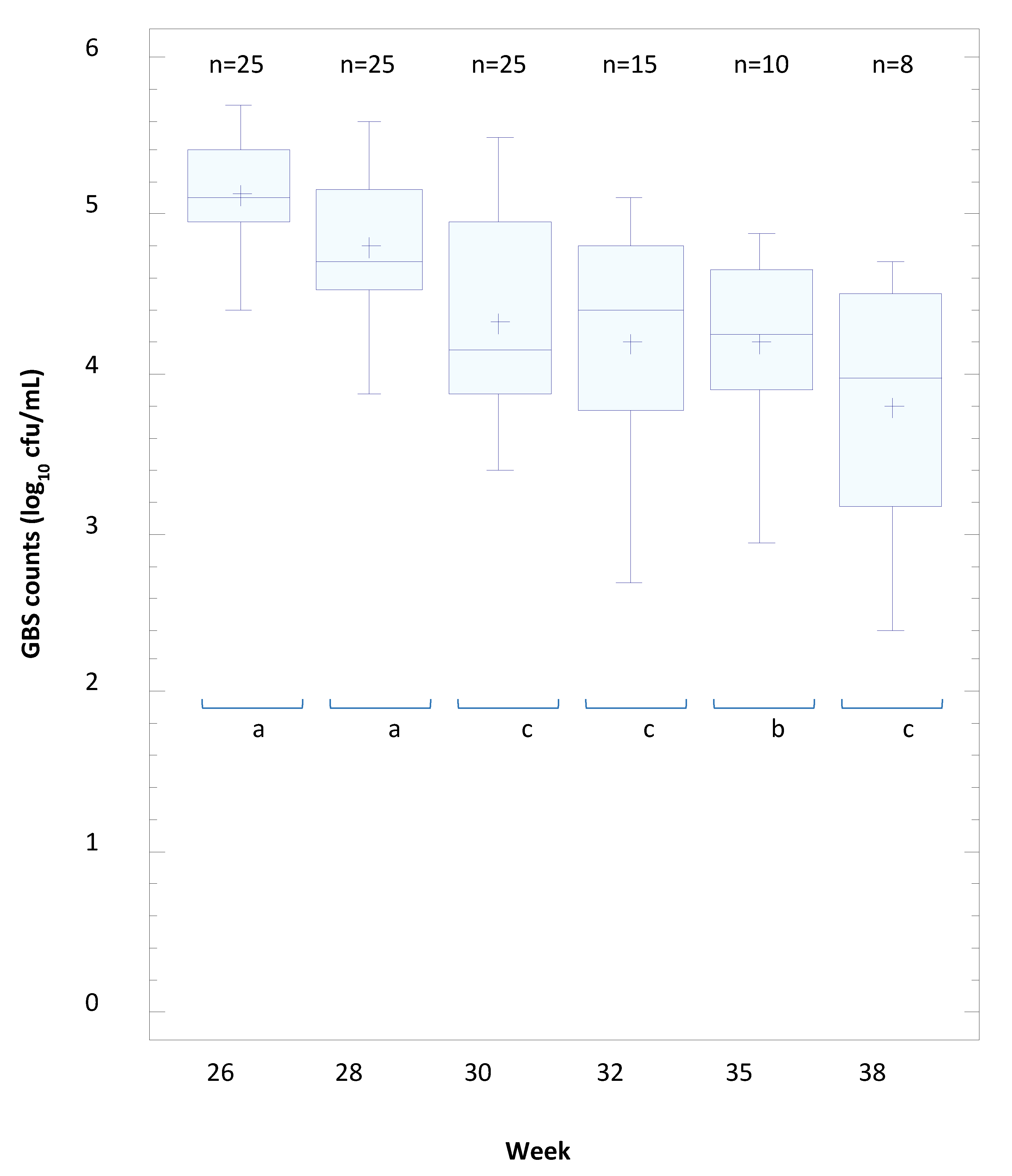
3.8. The Efficacy of L. salivarius CECT 9145 to Eradicate GBS from the Intestinal and Vaginal Tracts of Pregnant Women: A Pilot Clinical Trial
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Romero, R.; Dey, S.K.; Fisher, S.J. Preterm labor: One syndrome, many causes. Science 2014, 345, 760–765. [Google Scholar] [CrossRef]
- Stoll, B.J.; Hansen, N.I.; Sanchez, P.J.; Faix, R.G.; Poindexter, B.B.; Van Meurs, K.P.; Bizzarro, M.J.; Goldberg, R.N.; Frantz, I.D., III; Hale, E.C.; et al. Early onset neonatal sepsis: The burden of group B streptococcal and E. coli disease continues. Pediatrics 2011, 127, 817–826. [Google Scholar] [CrossRef] [PubMed]
- Le Doare, K.; Heath, P.T. An overview of global GBS epidemiology. Vaccine 2013, 31 (Suppl. 4), D7–D12. [Google Scholar] [CrossRef] [PubMed]
- Lawn, J.E.; Bianchi-Jassir, F.; Russell, N.J.; Kohli-Lynch, M.; Tann, C.J.; Hall, J.; Madrid, L.; Baker, C.J.; Bartlett, L.; Cutland, C.; et al. Group B streptococcal disease worldwide for pregnant women, stillbirths, and children: Why, what, and how to undertake estimates? Clin. Infect. Dis. 2017, 65 (Suppl. 2), S89–S99. [Google Scholar] [CrossRef] [PubMed]
- Russell, N.J.; Seale, A.C.; O’Driscoll, M.; O’Sullivan, C.; Bianchi-Jassir, F.; Gonzalez-Guarin, J.; Lawn, J.E.; Baker, C.J.; Bartlett, L.; Cutland, C.; et al. Maternal colonization with group B Streptococcus and serotype distribution worldwide: Systematic review and meta-analyses. Clin. Infect. Dis. 2017, 65 (Suppl. 2), S100–S111. [Google Scholar] [CrossRef]
- Vornhagen, J.; Adams Waldorf, K.M.; Rajagopal, L. Perinatal group B streptococcal infections: Virulence factors, immunity, and prevention strategies. Trends Microbiol. 2017, 25, 919–931. [Google Scholar] [CrossRef]
- Puopolo, K.M.; Madoff, L.C.; Eichenwald, E.C. Early-onset group B streptococcal disease in the era of maternal screening. Pediatrics 2005, 115, 1240–1246. [Google Scholar] [CrossRef] [PubMed]
- Stade, B.; Shah, V.; Ohlsson, A. Vaginal chlorhexidine during labour to prevent early-onset neonatal group B streptococcal infection. Cochrane Database Syst. Rev. 2004. [Google Scholar] [CrossRef]
- Kobayashi, M.; Schrag, S.J.; Alderson, M.R.; Madhi, S.A.; Baker, C.J.; Sobanjo-Ter Meulen, A. WHO consultation on group B Streptococcus vaccine development: Report from a meeting held on 27–28 April 2016. Vaccine 2016. [Google Scholar] [CrossRef]
- Akker-van Marle, M.E.; Rijnders, M.E.B.; Dommelen, P.; Fekkes, M.; Wouwe, J.P.; Amelink-Verburg, M.P.; Verkerk, P.H. Cost-effectiveness of different treatment strategies with intrapartum antibiotic prophylaxis to prevent early-onset group B streptococcal disease. BJOG 2005, 112, 820–826. [Google Scholar] [CrossRef] [PubMed]
- Baecher, L.; Grobman, W. Prenatal antibiotic treatment does not decrease group B Streptococcus colonization at delivery. Int. J. Gynaecol. Obstet. 2008, 101, 125–128. [Google Scholar] [CrossRef]
- Chu, Y.W.; Tse, C.; Tsang, G.K.; So, D.K.; Fung, J.T.; Lo, J.Y. Invasive group B Streptococcus isolates showing reduced susceptibility to penicillin in Hong Kong. J. Antimicrob. Chemother. 2007, 60, 1407–1409. [Google Scholar] [CrossRef] [PubMed]
- Dahesh, S.; Hensler, M.E.; Van Sorge, N.M.; Gertz, R.E.; Schrag, S.; Nizet, V.; Beall, B.W. Point mutation in the group B streptococcal pbp2x gene conferring decreased susceptibility to beta-lactam antibiotics. Antimicrob. Agents Chemother. 2008, 52, 2915–2918. [Google Scholar] [CrossRef] [PubMed]
- Gao, K.; Guan, X.; Zeng, L.; Qian, J.; Zhu, S.; Deng, Q.; Zhong, H.; Pang, S.; Gao, F.; Wang, J.; et al. An increasing trend of neonatal invasive multidrug-resistant group B streptococcus infections in southern China, 2011–2017. Infect. Drug Resist. 2018, 11, 2561–2569. [Google Scholar] [CrossRef]
- Arboleya, S.; Sánchez, B.; Solís, G.; Fernández, N.; Suárez, M.; Hernández-Barranco, A.; Milani, C.; Margolles, A.; de los Reyes-Gavilán, C.; Ventura, M.; et al. Impact of prematurity and perinatal antibiotics on the developing intestinal microbiota: A functional inference study. Int. J. Mol. Sci. 2016, 17, 649. [Google Scholar] [CrossRef]
- Cotten, C.M. Adverse consequences of neonatal antibiotic exposure. Curr. Opin. Pediatr. 2016, 28, 141–149. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, S.; Kobayashi, T.; Songjinda, P.; Tateyama, A.; Tsubouchi, M.; Kiyohara, C.; Shirakawa, T.; Sonomoto, K.; Nakayama, J. Influence of antibiotic exposure in the early postnatal period on the development of intestinal microbiota. FEMS Immunol. Med. Microbiol. 2009, 56, 80–87. [Google Scholar] [CrossRef]
- Aloisio, I.; Quagliariello, A.; de Fanti, S.; Luiselli, D.; de Filippo, C.; Albanese, D.; Corvaglia, L.T.; Faldella, G.; Di Gioia, D. Evaluation of the effects of intrapartum antibiotic prophylaxix on newborn intestinal microbiota using a sequencing approach targeted to multi hypervariate 16S rDNA regions. Appl. Microbiol. Biotechnol. 2016, 100, 5537–5546. [Google Scholar] [CrossRef]
- Cox, L.M.; Yamanishi, S.; Sohn, J.; Alekseyenko, A.V.; Leung, J.M.; Cho, I.; Kim, S.G.; Li, H.; Gao, Z.; Mahana, D.; et al. Altering the intestinal microbiota during a critical developmental window has lasting metabolic consequences. Cell 2014, 158, 705–721. [Google Scholar] [CrossRef]
- Kullen, M.J.; Sanozky-Dawes, R.B.; Crowell, D.C.; Klaenhammer, T.R. Use of DNA sequence of variable regions of the 16SrRNA gene for rapid and accurate identification of bacteria in the Lactobacillus acidophilus complex. J. Appl. Microbiol. 2000, 89, 511–518. [Google Scholar] [CrossRef]
- Marín, M.; Arroyo, R.; Espinosa-Martos, I.; Fernández, L.; Rodríguez, J.M. Identification of emerging human mastitis pathogens by MALDI-TOF and assessment of their antibiotic resistance patterns. Front. Microbiol. 2017, 8, 1258. [Google Scholar] [CrossRef]
- Ruiz-Barba, J.L.; Maldonado, A.; Jiménez-Díaz, R. Small-scale total DNA extraction from bacteria and yeast for PCR applications. Anal. Biochem. 2005, 347, 333–335. [Google Scholar] [CrossRef]
- Magnusson, J.; Schnürer, J. Lactobacillus coryniformis subsp. coryniformis strain Si3 produces a broad-spectrum proteinaceous antifungal compound. Appl. Environ. Microbiol. 2001, 67, 1–5. [Google Scholar] [CrossRef]
- Dodd, H.M.; Horn, N.; Zhang, H.; Gasson, M.J. A lactococcal expression system for engineered nisins. Appl. Environ. Microbiol. 1992, 58, 3683–3693. [Google Scholar]
- Martín, R.; Olivares, M.; Marín, M.L.; Fernández, L.; Xaus, J.; Rodríguez, J.M. Probiotic potential of 3 Lactobacilli strains isolated from breast milk. J. Hum. Lact. 2005, 21, 8–17. [Google Scholar] [CrossRef]
- Song, Y.L.; Kato, N.; Matsumiy, Y.; Lu, C.X.; Kato, H.; Watanabe, K. Identification of an hydrogen peroxide production by fecal and vaginal lactobacilli isolated from Japanese women and newborn infants. J. Clin. Microbiol. 1999, 37, 3062–3064. [Google Scholar]
- Yap, P.S.; Gilliland, S.E. Comparison of newly isolated strains of Lactobacillus delbrueckii susp. lactis for hydrogen peroxide production at 5 °C. J. Dairy Sci. 2000, 83, 628–632. [Google Scholar] [CrossRef]
- Younes, J.A.; van der Mei, H.C.; van den Heuvel, E.; Busscher, H.J.; Reid, G. Adhesion forces and coaggregation between vaginal staphylococci and lactobacilli. PLoS ONE 2012, 7, e36917. [Google Scholar] [CrossRef]
- Marteau, P.; Minekus, M.; Havenaar, R.; Huis In’t Veld, J.H.J. Survival of lactic acid bacteria in a dynamic model of the stomach and small intestine: Validation and the effects of bile. J. Dairy Sci. 1997, 80, 1031–1037. [Google Scholar] [CrossRef]
- Conway, P.L.; Gorbach, S.L.; Goldin, B.R. Survival of lactic acid bacteria in the human stomach and adhesion to intestinal cells. J. Dairy Sci. 1987, 70, 1–12. [Google Scholar] [CrossRef]
- Coconnier, M.H.; Klaenhammer, T.R.; Kernéis, S.; Bernet, M.F.; Servin, A.L. Protein-mediated adhesion of Lactobacillus acidophilus BG2FO4 on human enterocyte and mucus-secreting cell lines in culture. Appl. Environ. Microbiol. 1992, 58, 2034–2039. [Google Scholar]
- Boris, S.; Suárez, J.E.; Vázquez, F.; Barbés, C. Adherence of human vaginal lactobacilli to vaginal epithelial cells and interaction with uropathogens. Infect. Immun. 1998, 66, 1985–1989. [Google Scholar]
- Cohen, P.S.; Laux, D.C. Bacterial adhesion to and penetration of intestinal mucus in vitro. Methods Enzymol. 1995, 253, 309–314. [Google Scholar]
- Klare, I.; Konstabel, C.; Müller-Bertling, S.; Reissbrodt, R.; Huys, G.; Vancanneyt, M.; Swings, J.; Goossens, H.; Witte, W. Evaluation of new broth media for microdilution antibiotic susceptibility testing of lactobacilli, lactococci, pediococci, and bifidobacteria. Appl. Environ. Microbiol. 2005, 71, 8982–8986. [Google Scholar] [CrossRef]
- Langa, S.; Maldonado-Barragán, A.; Delgado, S.; Martín, R.; Martín, V.; Jiménez, E.; Ruíz-Barba, J.L.; Mayo, B.; Connor, R.I.; Suárez, J.E.; et al. Characterization of Lactobacillus salivarius CECT 5713, a strain isolated from human milk: From genotype to phenotype. Appl. Microbiol. Biotechnol. 2012, 94, 1279–1287. [Google Scholar] [CrossRef]
- EFSA. Guidance on the characterisation of microorganisms used as feed additives or as production organisms. EFSA J. 2018, 16, e05206. [Google Scholar]
- Bover-Cid, S.; Holzapfel, W.H. Improved screening procedure for biogenic amine production by lactic acid bacteria. Int. J. Food Microbiol. 1999, 53, 33–41. [Google Scholar] [CrossRef]
- Zhou, J.S.; Gopal, P.K.; Hill, H.S. Potential probiotic lactic acid bacteria Lactobacillus rhamnosus (HN001), Lactobacillus acidophilus (HN017) and Bifidobacterium lactis (HN019) do not degrade gastric mucin in vitro. Int. J. Food Microbiol. 2001, 63, 81–90. [Google Scholar] [CrossRef]
- Anadón, A.; Martínez, M.A.; Ares, I.; Ramos, E.; Martínez-Larrañaga, M.R.; Contreras, M.M.; Ramos, M.; Recio, I. Acute and repeated dose (4 weeks) oral toxicity studies of two antihypertensive peptides, RYLGY and AYFYPEL, that correspond to fragments (90–94) and (143–149) from alpha(s1)-casein. Food Chem. Toxicol. 2010, 48, 1836–1845. [Google Scholar]
- Lara-Villoslada, F.; Sierra, S.; Martín, R.; Delgado, S.; Rodríguez, J.M.; Olivares, M.; Xaus, J. Safety assessment of two probiotic strains, Lactobacillus coryniformis CECT5711 and Lactobacillus gasseri CECT5714. J. Appl. Microbiol. 2007, 103, 175–184. [Google Scholar] [CrossRef]
- Hansen, S.M.; Uldbjerg, N.; Kilian, M.; Sorensen, U.B.S. Dynamics of Streptococcus agalactiae colonization in women during and after pregnancy and in their infants. J. Clin. Microbiol. 2004, 42, 83–89. [Google Scholar] [CrossRef]
- Brzychczy-Wloch, M.; Pabian, W.; Majewska, E.; Zuk, M.G.; Kielbik, J.; Gosiewski, T.; Bulanda, M.G. Dynamics of colonization with group B streptococci in relation to normal flora in women during subsequent trimesters of pregnancy. New Microbiol. 2014, 37, 307–319. [Google Scholar]
- Rick, A.M.; Aguilar, A.; Cortes, R.; Gordillo, R.; Melgar, M.; Samayoa-Reyes, G.; Frank, D.N.; Asturias, E.J. Group B streptococci colonization in pregnant guatemalan women: Prevalence, risk factors, and vaginal microbiome. Open Forum Infect. Dis. 2017, 4, ofx020. [Google Scholar] [CrossRef]
- Rosen, G.H.; Randis, T.M.; Desai, P.V.; Sapra, K.J.; Ma, B.; Gajer, P.; Humphrys, M.S.; Ravel, J.; Gelber, S.E.; Ratner, A.J. Group B Streptococcus and the vaginal microbiota. J. Infect. Dis. 2017, 216, 744–751. [Google Scholar] [CrossRef]
- Cassidy-Bushrow, A.E.; Sitarik, A.; Levin, A.M.; Lynch, S.V.; Havstad, S.; Ownby, D.R.; Johnson, C.C.; Wegienka, G. Maternal group B Streptococcus and the infant gut microbiota. J. Dev. Orig. Health Dis. 2016, 7, 45–53. [Google Scholar] [CrossRef]
- Patras, K.A.; Nizet, V. Group B streptococcal maternal colonization and neonatal disease: Molecular mechanisms and preventative approaches. Front. Pediatr. 2018, 6, 27. [Google Scholar] [CrossRef]
- Bokulich, N.A.; Chung, J.; Battaglia, T.; Henderson, N.; Jay, M.; Li, H.; Lieber, A.; Wu, F.; Perez-Perez, G.I.; Chen, Y.; et al. Antibiotics, birth mode, and diet shape microbiome maturation during early life. Sci. Transl. Med. 2016, 8, 343ra382. [Google Scholar] [CrossRef]
- Boris, S.; Barbés, C. Role played by lactobacilli in controlling the population of vaginal pathogens. Microbes Infect. 2000, 2, 543–546. [Google Scholar] [CrossRef]
- Reid, G.; Burton, J. Use of Lactobacillus to prevent infection by pathogenic bacteria. Microbes Infect. 2002, 4, 319–324. [Google Scholar] [CrossRef]
- Ravel, J.; Gajer, P.; Abdo, Z.; Schneider, G.M.; Koenig, S.S.; McCulle, S.L.; Karlebach, S.; Gorle, R.; Russell, J.; Tacket, C.O.; et al. Vaginal microbiome of reproductive-age women. Proc. Natl. Acad. Sci. USA 2011, 108 (Suppl. 1), 4680–4687. [Google Scholar] [CrossRef]
- Ma, B.; Forney, L.J.; Ravel, J. Vaginal microbiome: Rethinking health and diseases. Ann. Rev. Microbiol. 2012, 66, 371–389. [Google Scholar] [CrossRef]
- Reid, G.; Kumar, H.; Khan, A.I.; Rautava, S.; Tobin, J.; Salminen, S. The case in favour of probiotics before, during and after pregnancy: Insights from the first 1500 days. Benef. Microbes 2016, 3, 1–10. [Google Scholar]
- Dunne, C.; Murphy, L.; Flynn, S.; O’Mahony, L.; O’Halloran, S.; Feeney, M.; Morrissey, D.; Thornton, G.; Fitzgerald, G.; Daly, C.; et al. Probiotics: From myth to reality. Demonstration of functionality in animal models of disease and in human clinical trials. Antonie van Leeuwenhoek 1999, 76, 279–292. [Google Scholar] [CrossRef]
- Mattila-Sandholm, T.; Blum, S.; Collins, J.K.; Crittenden, R.; de Vos, W.M.; Dunne, C.; Fondén, R.; Grenov, G.; Isolauri, E.; Kiely, B.; et al. Probiotics: Towards demonstrating efficacy. Trends Food Sci. Technol. 1999, 10, 393–399. [Google Scholar] [CrossRef]
- Dunne, C.; O’Mahony, L.; Murphy, L.; Thornton, G.; Morrissey, D.; Halloran, S.; Feeney, M.; Flynn, S.; Fitzgerald, G.; Daly, C.; et al. In vitro selection criteria for probiotic bacteria of human origin: Correlation with in vivo findings. Am. J. Clin. Nutr. 2001, 73, 386S–392S. [Google Scholar] [CrossRef]
- McCarthy, J.; O’Mahony, L.; O’Callaghan, L.; Sheil, B.; Vaughan, E.E.; Fitzsimons, N.; Fitzgibbon, J.; O’Sullivan, G.C.; Kiely, B.; Collins, J.K.; et al. Double blind; placebo controlled trial of two probiotic strains in interleukin 10 knockout mice and mechanistic link with cytokine balance. Gut 2003, 52, 975–980. [Google Scholar] [CrossRef]
- Dunne, C.; Kelly, P.; O’Halloran, S.; Soden, D.; Bennett, M.; von Wright, A.; Vilpponen-Salmela, T.; Kiely, B.; O’Mahony, L.; Collins, J.K.; et al. Mechanisms of adherence of a probiotic Lactobacillus strain during and after in vivo assessment in ulcerative colitis patients. Microb. Ecol. Health Dis. 2004, 16, 96–104. [Google Scholar] [CrossRef][Green Version]
- Sheil, B.; McCarthy, J.; O’Mahony, L.; Bennett, M.W.; Ryan, P.; Fitzgibbon, J.; Kiely, B.; Collins, J.K.; Shanahan, F. Is the mucosal route of administration essential for probiotic function? Subcutaneous administration is associated with attenuation of murine colitis and arthritis. Gut 2004, 53, 694–700. [Google Scholar] [CrossRef]
- Martín, R.; Jiménez, E.; Olivares, M.; Marín, M.; Fernández, L.; Xaus, J.; Rodríguez, J.M. Lactobacillus salivarius CECT 5713, a potential probiotic strain isolated from infant feces and breast milk of a mother-child pair. Int. J. Food Microbiol. 2006, 112, 35–43. [Google Scholar] [CrossRef]
- Olivares, M.; Díaz-Ropero, M.P.; Martín, R.; Rodríguez, J.M.; Xaus, J. Antimicrobial potential of four Lactobacillus strains isolated from breast milk. J. Appl. Microbiol. 2006, 101, 72–79. [Google Scholar] [CrossRef]
- Pérez-Cano, F.J.; Dong, H.; Yaqoob, P. In vitro immunomodulatory activity of Lactobacillus fermentum CECT5716 and Lactobacillus salivarius CECT5713: Two probiotic strains isolated from human breast milk. Inmunobiology 2010, 12, 996–1004. [Google Scholar] [CrossRef] [PubMed]
- Neville, B.A.; O’Toole, P.W. Probiotic properties of Lactobacillus salivarius and closely related Lactobacillus species. Future Microbiol. 2010, 5, 759–774. [Google Scholar] [CrossRef] [PubMed]
- Messaoudi, S.; Manai, M.; Kergourlay, G.; Prévost, H.; Connil, N.; Chobert, J.M.; Dousset, X. Lactobacillus salivarius: Bacteriocin and probiotic activity. Food Microbiol. 2013, 36, 296–304. [Google Scholar] [CrossRef] [PubMed]
- Sun, E.; Ren, F.; Liu, S.; Ge, S.; Zhang, M.; Guo, H.; Jiang, L.; Zhang, H.; Zhao, L. Complete genome sequence of Lactobacillus salivarius Ren, a probiotic strain with anti-tumor activity. J. Biotechnol. 2015, 210, 57–58. [Google Scholar] [CrossRef]
- Kang, C.H.; Han, S.H.; Kim, Y.; Paek, N.S.; So, J.S. In vitro probiotic properties of Lactobacillus salivarius mg242 isolated from human vagina. Probiotics Antimicrob. Proteins 2018, 10, 343–349. [Google Scholar] [CrossRef] [PubMed]
- Pino, A.; Bartolo, E.; Caggia, C.; Cianci, A.; Randazzo, C.L. Detection of vaginal lactobacilli as probiotic candidates. Sci. Rep. 2019, 9, 3355. [Google Scholar] [CrossRef] [PubMed]
- Hill, C.; Guarner, F.; Reid, G.; Gibson, G.R.; Merenstein, D.J.; Pot, B.; Morelli, L.; Canani, R.B.; Flint, H.J.; Salminen, S.; et al. Expert consensus document. The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 506–514. [Google Scholar] [CrossRef]
- Raftis, E.J.; Salvetti, E.; Torriani, S.; Felis, G.E.; O’Toole, P.W. Genomic diversity of Lactobacillus salivarius. Appl. Environ. Microbiol. 2011, 77, 954–965. [Google Scholar] [CrossRef]
- Fernández, L.; Cárdenas, N.; Arroyo, R.; Manzano, S.; Jiménez, E.; Martín, V.; Rodríguez, J.M. Prevention of infectious mastitis by oral administration of Lactobacillus salivarius PS2 during late pregnancy. Clin. Infect. Dis. 2016, 62, 568–573. [Google Scholar] [CrossRef]
- Arroyo, R.; Martin, V.; Maldonado, A.; Jimenez, E.; Fernández, L.; Rodríguez, J.M. Treatment of infectious mastitis during lactation: Antibiotics versus oral administration of lactobacilli isolated from breast milk. Clin. Infect. Dis. 2010, 50, 1551–1558. [Google Scholar] [CrossRef]
- Martín, R.; Soberón, N.; Vázquez, F.; Suárez, J.E. Vaginal microbiota: Composition, protective role, associated pathologies, and therapeutic perspectives. Enferm. Infecc. Microbiol. Clin. 2008, 26, 160–167. [Google Scholar] [CrossRef] [PubMed]
- Ruiz, F.O.; Gerbaldo, G.; Garcia, M.J.; Giordano, W.; Pascual, L.; Barberis, I.L. Synergistic effect between two bacteriocin-like inhibitory substances produced by lactobacilli strains with inhibitory activity for Streptococcus agalactiae. Curr. Microbiol. 2012, 64, 349–356. [Google Scholar] [CrossRef] [PubMed]
- Charlier, C.; Cretenet, M.; Even, S.; Le Loir, Y. Interactions between Staphylococcus aureus and lactic acid bacteria: An old story with new perspectives. Int. J. Food Microbiol. 2009, 131, 30–39. [Google Scholar] [CrossRef]
- Açikgöz, Z.C.; Gamberzade, S.; Göçer, S.; Ceylan, P. Inhibitor effect of vaginal lactobacilli on group B streptococci. Mikrobiyoloji Bulteni 2005, 39, 17–23. [Google Scholar]
- Borges, S.; Silva, J.; Teixeira, P. The role of lactobacilli and probiotics in maintaining vaginal health. Arch. Gynecol. Obstet. 2014, 289, 479–489. [Google Scholar] [CrossRef] [PubMed]
- Ocaña, V.S.; Pesce De Ruiz Holgado, A.A.; Nader-Macías, M.E. Characterization of a bacteriocin-like substance produced by a vaginal Lactobacillus salivarius strain. Appl. Environ. Microbiol. 1999, 65, 5631–5635. [Google Scholar]
- Cárdenas, N.; Martín, V.; Arroyo, R.; López, M.; Carrera, M.; Badiola, C.; Jiménez, E.; Rodríguez, J.M. Prevention of recurrent acute otitis media in children through the use of Lactobacillus salivarius PS7, a target-specific probiotic strain. Nutrients 2019, 11, 376. [Google Scholar] [CrossRef]
- Martín, R.; Delgado, S.; Maldonado, A.; Jiménez, E.; Olivares, M.; Fernández, L.; Sobrino, O.J.; Rodríguez, J.M. Isolation of lactobacilli from sow milk and evaluation of their probiotic potential. J. Dairy Res. 2009, 76, 418–425. [Google Scholar] [CrossRef]
- De Gregorio, P.R.; Juárez Tomás, M.S.; Leccese Terraf, M.C.; Nader-Macías, M.E. In vitro and in vivo effects of beneficial vaginal lactobacilli on pathogens responsible for urogenital tract infections. J. Med. Microbiol. 2014, 63, 685–696. [Google Scholar] [CrossRef]
- Bodaszewska, M.; Brzychczy-Włoch, M.; Gosiewski, T.; Adamski, P.; Strus, M.; Heczko, P.B. Evaluation of group B streptococcus susceptibility to lactic acid bacteria strains. Medycyna Doswiadczalna i Mikrobiologia 2010, 62, 153–161. [Google Scholar]
- Maldonado, J.; Lara-Villoslada, F.; Sierra, S.; Sempere, L.; Gómez, M.; Rodríguez, J.M.; Boza, J.; Xaus, J.; Olivares, M. Safety and tolerance of the human milk probiotic strain Lactobacillus salivarius CECT5713 in 6-month-old children. Nutrition 2010, 26, 1082–1087. [Google Scholar] [CrossRef]
- Vázquez-Fresno, R.; Llorach, R.; Marinic, J.; Tulipani, S.; Garcia-Aloy, M.; Espinosa-Martos, I.; Jiménez, E.; Rodríguez, J.M.; Andres-Lacueva, C. Urinary metabolomic fingerprinting after consumption of a probiotic strain in women with mastitis. Pharmacol. Res. 2014, 87, 160–165. [Google Scholar] [CrossRef]
- Espinosa-Martos, I.; Jiménez, E.; de Andrés, J.; Rodríguez-Alcalá, L.M.; Tavárez, S.; Manzano, S.; Fernández, L.; Alonso, E.; Fontecha, J.; Rodríguez, J.M. Milk and blood biomarkers associated to the clinical efficacy of a probiotic for the treatment of infectious mastitis. Benef. Microbes 2016, 7, 305–318. [Google Scholar] [CrossRef]
- Danielsen, M.; Wind, A. Susceptibility of Lactobacillus spp. to antimicrobial agents. Int. J. Food Microbiol. 2003, 26, 1–11. [Google Scholar] [CrossRef]
- Gueimonde, M.; Sánchez, B.; de los Reyes-Gavilán, C.G.; Margolles, A. Antibiotic resistance in probiotic bacteria. Front. Microbiol. 2013, 4, 1–6. [Google Scholar] [CrossRef]
- Bryan, L.E.; Kwan, S. Mechanisms of aminoglycoside resistance of anaerobic bacteria and facultative bacteria grown anaerobically. J. Antimicrob. Chemother. 1981, 8, S1–S8. [Google Scholar] [CrossRef]
- Handwerger, S.; Pucci, M.J.; Volk, K.J.; Liu, J.; Lee, M.S. Vancomycin-resistant Leuconostoc mesenteroides and Lactobacillus casei synthesize cytoplasmic peptidoglycan precursors that terminate in lactate. J. Bacteriol. 1994, 176, 260–264. [Google Scholar] [CrossRef][Green Version]
- Marsalková, S.; Cízek, M.; Vasil, M.; Bomba, A.; Nad’, P.; Datelinka, I.; Jonecová, Z.; Rimková, S.; Kalinácová, V.; Styriak, I. Testing two Lactobacillus plantarum and Lactobacillus acidophilus strains for their suitability as a lipoid probiotic. Berliner und Munchener Tierarztliche Wochenschrift 2004, 117, 145–147. [Google Scholar]
- Lee, Y. Characterization of Weissella kimchii PL9023 as a potential probiotic for women. FEMS Microbiol. Lett. 2005, 250, 157–162. [Google Scholar] [CrossRef]
- Zárate, G.; Nader-Macias, M.E. Influence of probiotic vaginal lactobacilli on in vitro adhesion of urogenital pathogens to vaginal epithelial cells. Lett. Appl. Microbiol. 2006, 43, 174–180. [Google Scholar] [CrossRef]
- Ermolenko, E.I.; Chernysh, A.I.; Martsinkovskaia, I.V.; Suvorov, A.N. Influence of probiotic enterococci on the growth of Streptococcus agalactiae. Zhurnal Mikrobiol. Epidemiol. Immunobiol. 2007, 5, 73–77. [Google Scholar]
- Tsapieva, A.; Duplik, N.; Suvorov, A. Structure of plantaricin locus of Lactobacillus plantarum 8P-A3. Benef. Microbes 2011, 2, 255–261. [Google Scholar] [CrossRef]
- Aloisio, I.; Mazzola, G.; Corvaglia, L.T.; Tonti, G.; Faldella, G.; Biavati, B.; Di Gioia, D. Influence of intrapartum antibiotic prophylaxis against group B Streptococcus on the early newborn gut composition and evaluation of the anti-Streptococcus activity of Bifidobacterium strains. Appl. Microbiol. Biotechnol. 2014, 98, 6051–6060. [Google Scholar] [CrossRef]
- De Gregorio, P.R.; Juárez Tomas, M.S.; Leccese Terraf, M.C.; Nader-Macias, M.E. Preventive effect of Lactobacillus reuteri CRL1324 on Group B streptococcus vaginal colonization in an experimental mouse model. J. Appl. Microbiol. 2015, 118, 1034–1047. [Google Scholar] [CrossRef]
- Patras, K.A.; Wescombe, P.A.; Rosler, B.; Hale, J.D.; Tagg, J.R.; Doran, K.S. Streptococcus salivarius K12 limits group B Streptococcus vaginal colonization. Infect. Immun. 2015, 83, 3438–3444. [Google Scholar] [CrossRef]
- Ho, M.; Chang, Y.Y.; Chang, W.C.; Lin, H.C.; Wang, M.H.; Lin, W.C.; Chiu, T.H. Oral Lactobacillus rhamnosus GR-1 and Lactobacillus reuteri RC-14 to reduce Group B Streptococcus colonization in pregnant women: A randomized controlled trial. Taiwan J. Obstet. Gynecol. 2016, 55, 515–518. [Google Scholar] [CrossRef]
- Olsen, P.; Williamson, M.; Traynor, V.; Georgiou, C. The impact of oral probiotics on vaginal Group B Streptococcal colonisation rates in pregnant women: A pilot randomised control study. Women Birth 2018, 31, 31–37. [Google Scholar] [CrossRef]
- Quagliariello, V.; Masarone, M.; Armenia, E.; Giudice, A.; Barbarisi, M.; Caraglia, M.; Barbarisi, A.; Persico, M. Chitosan-coated liposomes loaded with butyric acid demonstrate anticancer and anti-inflammatory activity in human hepatoma HepG2 cells. Oncol. Rep. 2019, 41, 1476–1486. [Google Scholar] [CrossRef]
- Ciani, O.; Arendsen, E.; Romancik, M.; Lunik, R.; Costantini, E.; Di Biase, M.; Morgia, G.; Fragalà, E.; Roman, T.; Bernat, M.; et al. Intravesical administration of combined hyaluronic acid (HA) and chondroitin sulfate (CS) for the treatment of female recurrent urinary tract infections: A European multicentre nested case-control study. BMJ Open. 2016, 6, e009669. [Google Scholar] [CrossRef]

| Strain | pH | L –lactic Acid | D-lactic Acid | Hydrogen Peroxide |
|---|---|---|---|---|
| L. salivarius V3III-1 | 4.00 | 9.66 ± 0.57 | Nd | Nd |
| L. salivarius CECT 9145 | 4.01 | 10.03 ± 0.60 | Nd | 7.29 ± 0.69 |
| L. salivarius V7II-1 | 4.02 | 9.82 ± 0.69 | Nd | Nd |
| L. salivarius V7II-62 | 4.01 | 9.76 ± 0.54 | Nd | Nd |
| L. salivarius V7IV-1 | 3.85 | 10.47 ± 0.58 | Nd | 7.46 ± 0.58 |
| L. salivarius V7IV-60 | 4.02 | 9.72 ± 0.63 | Nd | Nd |
| L. salivarius V8III-62 | 4.04 | 9.91 ± 0.55 | Nd | Nd |
| L. salivarius V11I-60 | 4.03 | 9.84 ± 0.43 | Nd | Nd |
| L. salivarius V11III-60 | 4.07 | 9.61 ± 0.47 | Nd | Nd |
| L. salivarius V11IV-60 | 4.03 | 10.02 ± 0.62 | Nd | Nd |
| L. salivarius CECT 5713 | 3.93 | 10.26 ± 0.62 | Nd | - |
| L. salivarius (Strain) | S. agalactiae (Strain) | 0 h | 6 h | 24 h |
|---|---|---|---|---|
| V3III-1 | RC5 | 7.10 | 6.44 | Nd |
| RC6 | 7.24 | 7.04 | Nd | |
| V2I-80 | 7.10 | 7.04 | Nd | |
| V14I-63 | 7.27 | 7.10 | Nd | |
| CECT 9145 | RC5 | 7.04 | 4.48 | Nd |
| RC6 | 7.23 | Nd | Nd | |
| V2I-80 | 7.10 | 4.70 | Nd | |
| V14I-63 | 7.34 | Nd | Nd | |
| V7II-1 | RC5 | 7.15 | 7.27 | Nd |
| RC6 | 7.15 | 6.70 | Nd | |
| V2I-80 | 7.04 | 7.10 | Nd | |
| V14I-63 | 7.35 | 5.65 | Nd | |
| V7II-62 | RC5 | 7.24 | 7.04 | Nd |
| RC6 | 6.98 | 7.49 | Nd | |
| V2I-80 | 7.35 | 7.92 | Nd | |
| V14I-63 | 7.10 | 6.93 | Nd | |
| V7IV-1 | RC5 | 7.32 | 7.58 | Nd |
| RC6 | 7.34 | 6.90 | Nd | |
| V2I-80 | 7.15 | 7.38 | Nd | |
| V14I-63 | 7.23 | 6.04 | Nd | |
| V7IV-60 | RC5 | 7.24 | 7.32 | Nd |
| RC6 | 7.32 | 8.06 | Nd | |
| V2I-80 | 7.04 | 7.15 | Nd | |
| V14I-63 | 7.35 | 8.34 | Nd | |
| V8III-62 | RC5 | 7.15 | 7.90 | Nd |
| RC6 | 7.34 | 7.23 | Nd | |
| V2I-80 | 7.24 | 6.90 | Nd | |
| V14I-63 | 7.20 | 8.77 | Nd | |
| V11I-60 | RC5 | 7.31 | 7.44 | Nd |
| RC6 | 7.01 | 6.94 | Nd | |
| V2I-80 | 7.23 | 7.07 | Nd | |
| V14I-63 | 6.93 | 6.60 | Nd | |
| V11 III-60 | RC5 | 7.27 | 6.44 | Nd |
| RC6 | 6.95 | 6.88 | Nd | |
| V2I-80 | 7.28 | 6.52 | Nd | |
| V14I-63 | 7.37 | 6.85 | Nd | |
| RC5 | 7.26 | 6.74 | Nd | |
| V11IV-60 | RC6 | 7.42 | 6.60 | Nd |
| V2I-80 | 7.10 | 6.60 | Nd | |
| V14I-63 | 7.06 | 5.32 | Nd | |
| RC5 | 7.20 | 9.32 | 9.34 | |
| Control cultures (no L. salivarius strain) | RC6 | 7.31 | 9.20 | 9.27 |
| V2I-80 | 7.04 | 9.15 | 9.23 | |
| V14I-63 | 7.10 | 9.02 | 9.15 |
| Strain | % Total * |
|---|---|
| L. salivarius V3III-1 | 30.2 a |
| L. salivarius CECT 9145 | 64.3 b |
| L. salivarius V7II-1 | 59.8 b |
| L. salivarius V7II-62 | 50.5 b |
| L. salivarius V7IV-1 | 48.1 b |
| L. salivarius V7IV-60 | 53.3 b |
| L. salivarius V8III-62 | 41.3 c |
| L. salivarius V11I-60 | 40.8 c |
| L. salivarius V11III-60 | 41.1 c |
| L. salivarius V11IV-60 | 42.3 c |
| L. salivarius CELA2 | 64.4 b |
| Strain | HT-29 a | Caco-2 a | Vaginal Cells b | Adhesion c |
|---|---|---|---|---|
| L. salivarius V3III-1 | 877.3 ± 303.2 | 259.1 ± 67.1 | + | 9.3 ± 2.0 |
| L. salivarius CECT 9145 | 905.2 ± 297.0 | 345.1 ± 72.8 | +++ | 10.9 ± 1.8 |
| L. salivarius V7II-1 | 900.5 ± 336.2 | 297.8 ± 84.5 | ++ | 8.9 ± 1.9 |
| L. salivarius V7II-62 | 911.7 ± 250.9 | 321.5 ± 80.2 | ++ | 9.0 ± 1.6 |
| L. salivarius V7IV-1 | 884.0 ± 226.3 | 252.3 ± 67.1 | ++ | 8.5 ± 1.2 |
| L. salivarius V7IV-60 | 799.7 ± 210.1 | 255.9 ± 60.3 | ++ | 9.6 ± 1.7 |
| L. salivarius V8III-62 | 623.4 ± 200.2 | 108.7 ± 24.3 | + | 3.3 ± 0.7 |
| L. salivarius V11I-60 | 593.2 ± 191.5 | 121.6 ± 22.0 | + | 2.9 ± 0.8 |
| L. salivarius V11III-60 | 612.4 ± 188.2 | 153.2 ± 26.7 | + | 2.4 ± 1.0 |
| L. salivarius V11IV-60 | 601.6 ± 172.0 | 159.5 ± 23.4 | + | 3.4 ± 0.8 |
| Antibiotic a | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Strain | GEN | KAN | STP | NEO | TET | ERY | CLI | CHL | AMP | PEN | VAN | VIR | LIN | TRM | CIP | RIF |
| V3III-1 | 4 | 64 | 32 | 8 | 2 | 0.12 | 0.5 | 2 | 0.5 | 0.12 | >128 | 0.5 | 0.5 | 0.5 | 2 | 0.5 |
| CECT 9145 | 4 | 256 | 32 | 8 | 2 | 0.12 | 0.5 | 2 | 0.5 | 0.12 | >128 | 0.5 | 1 | 0.25 | 4 | 1 |
| V7II-1 | 4 | 128 | 32 | 4 | 2 | 0.12 | 0.5 | 4 | 0.5 | 0.12 | >128 | 0.5 | 0.5 | 0.5 | 2 | 0.25 |
| V7II-62 | 2 | 128 | 32 | 8 | 2 | 0.25 | 0.5 | 2 | 0.5 | 0.25 | >128 | 0.25 | 1 | 0.25 | 2 | 0.5 |
| V7IV-1 | 8 | 256 | 32 | 4 | 2 | 0.12 | 0.5 | 2 | 0.5 | 0.25 | >128 | 0.5 | 1 | 0.5 | 2 | 0.5 |
| V7IV-60 | 8 | 128 | 32 | 8 | 2 | 0.12 | 0.4 | 4 | 0.5 | 0.25 | >128 | 0.5 | 1 | 0.5 | 2 | 0.5 |
| V8III-62 | 8 | 128 | 32 | 2 | 2 | 0.25 | 0.5 | 4 | 0.5 | 0.25 | >128 | 1 | 1 | 0.5 | 2 | 0.5 |
| V11I-60 | 4 | 128 | 32 | 8 | 2 | 0.12 | 0.5 | 2 | 0.5 | 0.25 | >128 | 1 | 1 | 0.5 | 2 | 0.5 |
| V11III-60 | 8 | 256 | 32 | 4 | 2 | 0.12 | 0.5 | 2 | 0.5 | 0.25 | >128 | 0.5 | 1 | 0.5 | 2 | 0.5 |
| V11IV-60 | 4 | 128 | 32 | 8 | 2 | 0.12 | 0.5 | 2 | 0.5 | 0.25 | >128 | 1 | 1 | 0.5 | 2 | 0.5 |
| Breakpoint b | 16 | 64 (R) | 64 | nr | 8 | 1 | 4 | 4 | 4 | nr | nr (R) | nr | nr | nr | nr | nr |
| Initial GBS Status | Probiotic Intake | GBS Status | Rectal Swabs (Week) | Vaginal Swabs (Week) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Negative | NO | 12-26 | 28 a | 32 b | 36-38 | 12-26 | 28 a | 32 b | 36–38 | |||||
| (n = 18) | GBS-positive | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |||||
| GBS-negative | 18 | 17 | 16 | 18 | 18 | 17 | 16 | 18 | ||||||
| GBS-negative (%) | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | ||||||
| Positive | NO | 14–17 | 28 b | 32 a | 36–38 | 14–17 | 28 b | 32 a | 36–38 | |||||
| (n = 14) | GBS-positive | 14 | 12 | 13 | 14 | 14 | 12 | 13 | 14 | |||||
| GBS-negative | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | ||||||
| GBS-negative (%) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | ||||||
| Positive | YES | 26 | 28 | 30 | 32 | 35 | 38 | 26 | 28 | 30 | 32 | 35 | 38 | |
| (n = 25) | GBS-positive | 25 | 25 | 21 | 12 | 9 | 7 | 25 | 25 | 25 | 15 | 10 | 8 | |
| GBS-negative | 0 | 0 | 4 | 13 | 16 | 18 | 0 | 0 | 0 | 10 | 15 | 17 | ||
| GBS-negative (%) | 0 | 0 | 16 | 52 | 64 | 72 | 0 | 0 | 0 | 40 | 60 | 68 | ||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martín, V.; Cárdenas, N.; Ocaña, S.; Marín, M.; Arroyo, R.; Beltrán, D.; Badiola, C.; Fernández, L.; Rodríguez, J.M. Rectal and Vaginal Eradication of Streptococcus agalactiae (GBS) in Pregnant Women by Using Lactobacillus salivarius CECT 9145, A Target-specific Probiotic Strain. Nutrients 2019, 11, 810. https://doi.org/10.3390/nu11040810
Martín V, Cárdenas N, Ocaña S, Marín M, Arroyo R, Beltrán D, Badiola C, Fernández L, Rodríguez JM. Rectal and Vaginal Eradication of Streptococcus agalactiae (GBS) in Pregnant Women by Using Lactobacillus salivarius CECT 9145, A Target-specific Probiotic Strain. Nutrients. 2019; 11(4):810. https://doi.org/10.3390/nu11040810
Chicago/Turabian StyleMartín, Virginia, Nivia Cárdenas, Sara Ocaña, María Marín, Rebeca Arroyo, David Beltrán, Carlos Badiola, Leónides Fernández, and Juan M. Rodríguez. 2019. "Rectal and Vaginal Eradication of Streptococcus agalactiae (GBS) in Pregnant Women by Using Lactobacillus salivarius CECT 9145, A Target-specific Probiotic Strain" Nutrients 11, no. 4: 810. https://doi.org/10.3390/nu11040810
APA StyleMartín, V., Cárdenas, N., Ocaña, S., Marín, M., Arroyo, R., Beltrán, D., Badiola, C., Fernández, L., & Rodríguez, J. M. (2019). Rectal and Vaginal Eradication of Streptococcus agalactiae (GBS) in Pregnant Women by Using Lactobacillus salivarius CECT 9145, A Target-specific Probiotic Strain. Nutrients, 11(4), 810. https://doi.org/10.3390/nu11040810





