Anti-Obesity Effects of Tocotrienols and Bran in High-Fat Diet-Treated Mice
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Diets
2.2. Selection of Whole Grain Wheat Samples
2.3. Measurement of Vitamin E Content
2.4. Total Serum Cholesterol Content
2.5. Measurement of Antioxidative Enzyme Activities
2.6. Western Blotting
2.7. Analysis of Lipid Peroxidation
2.8. Statistical Analysis
3. Results
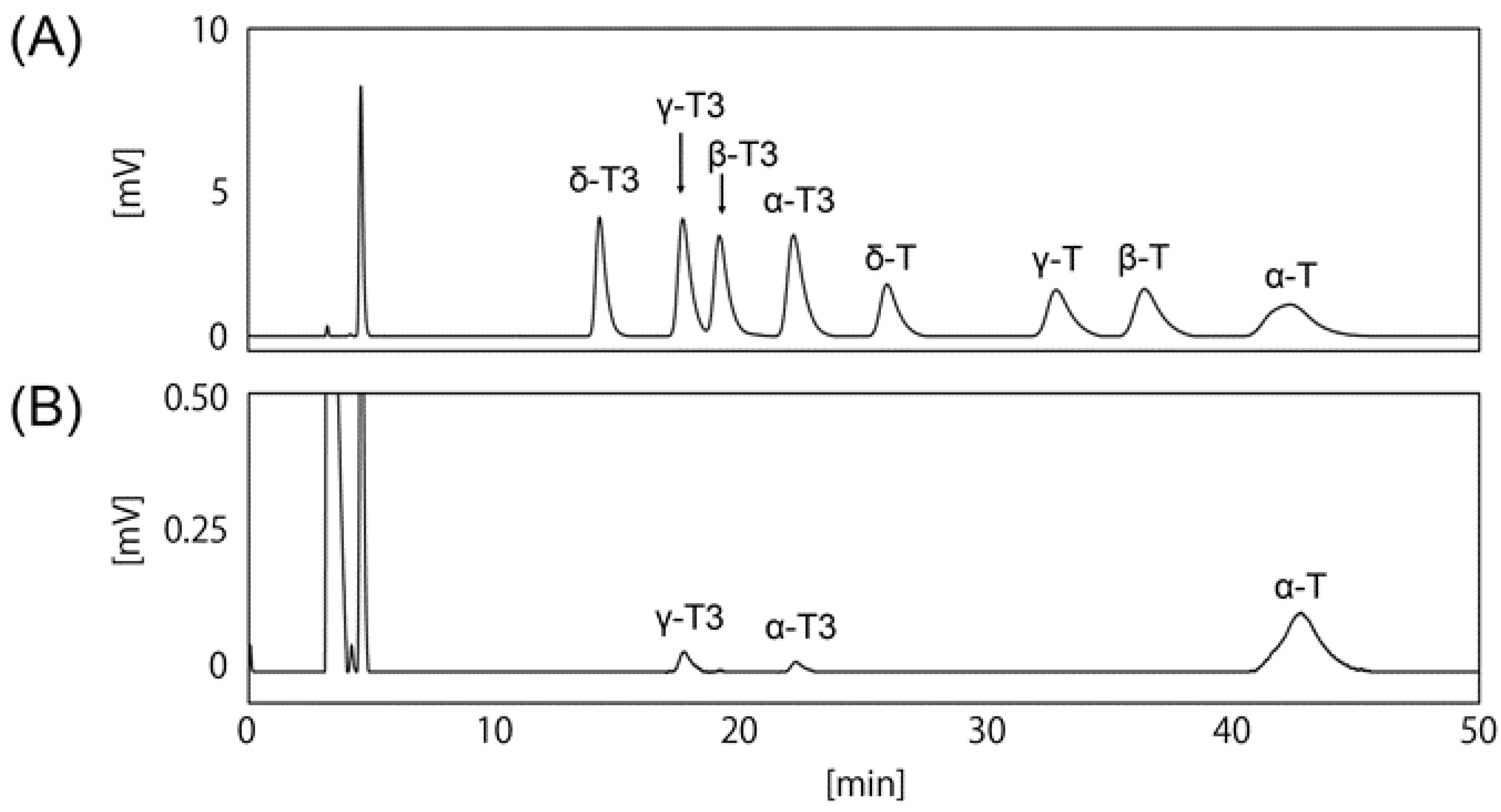
3.1. Determination of HPLC Condition
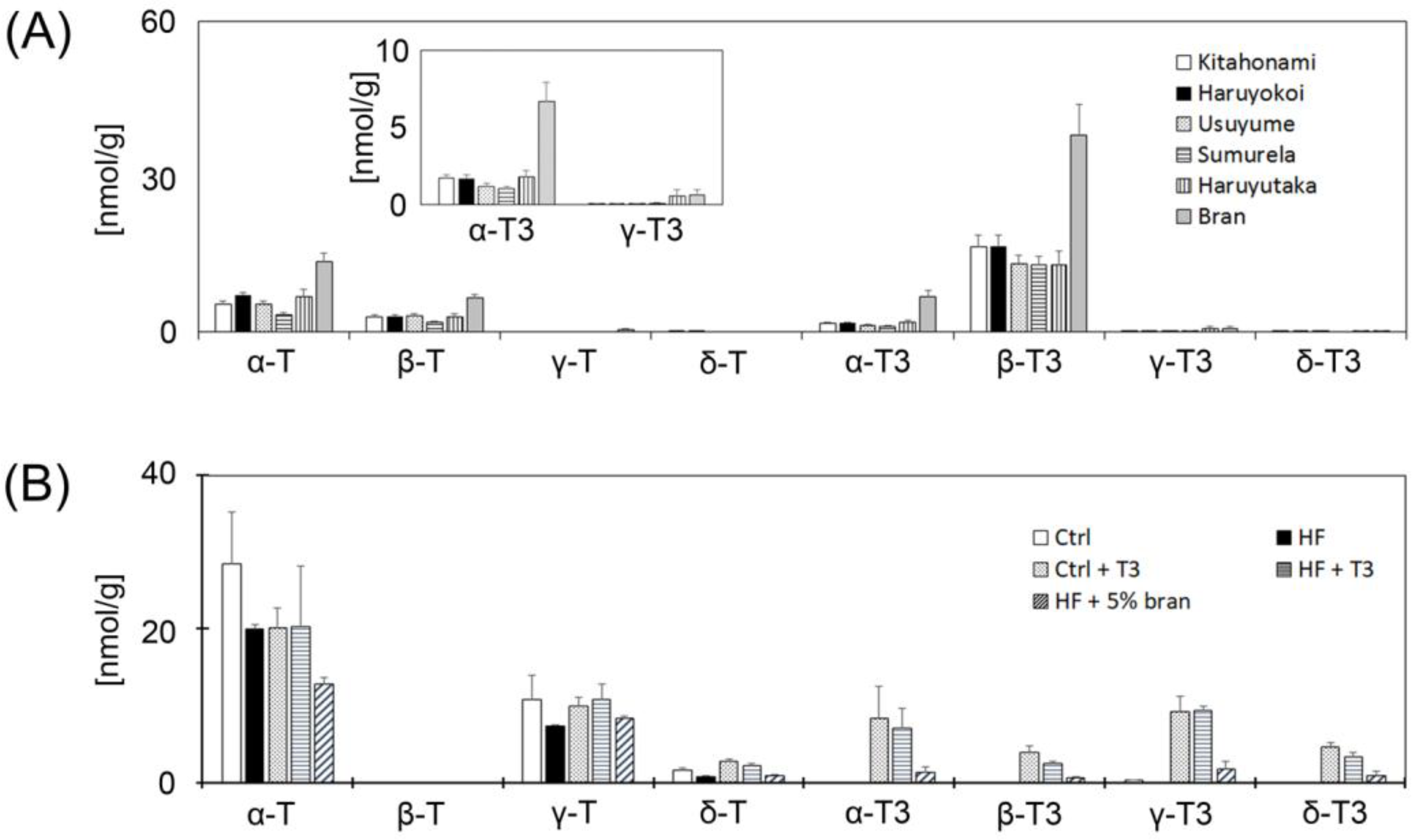
3.2. Measurement of Vitamin E Contents of Whole Grain Wheats, Bran and Control Diet
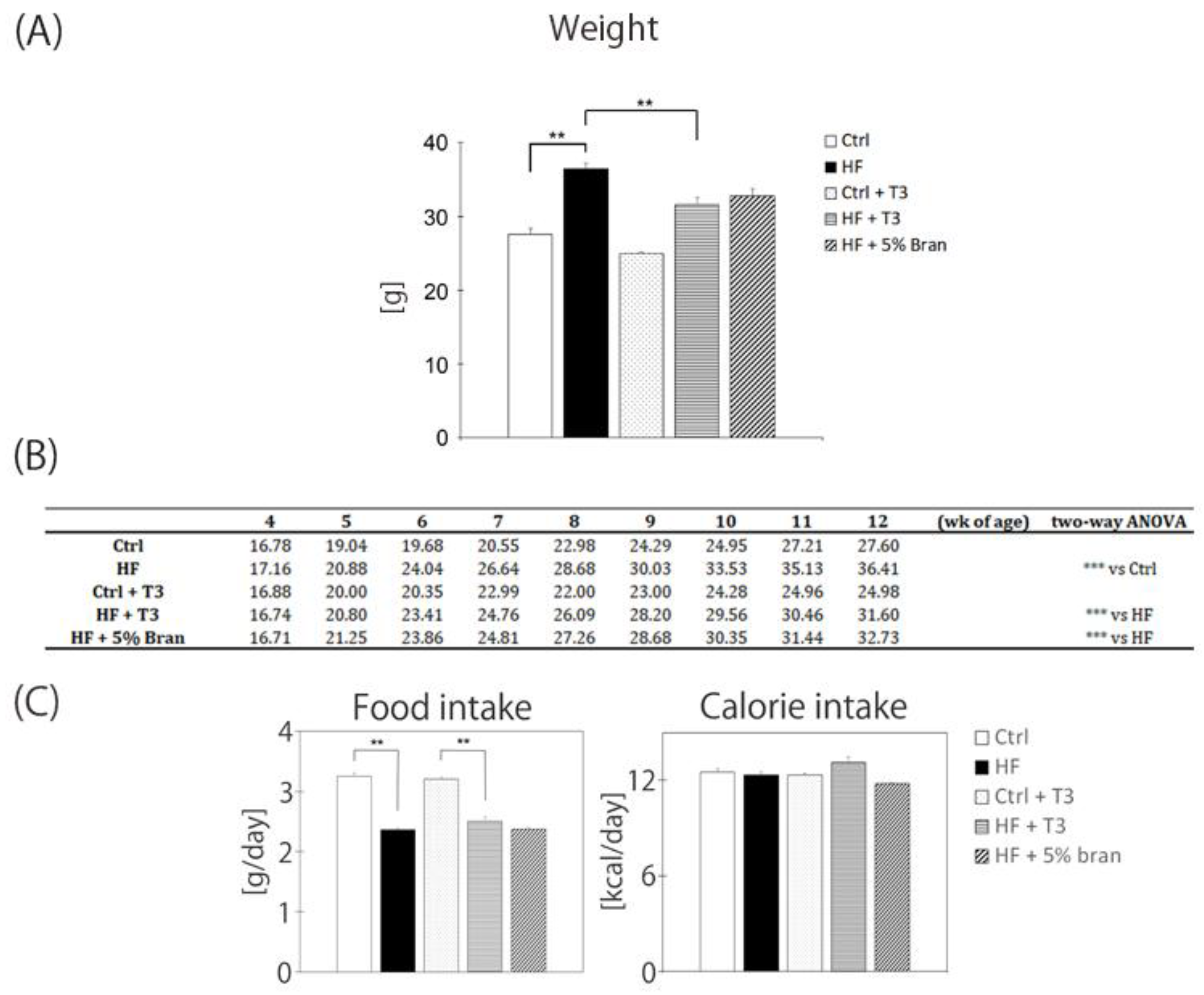
3.3. T3s and Bran Inhibited Body Weight Gain in High-Fat Diet-Treated Mice
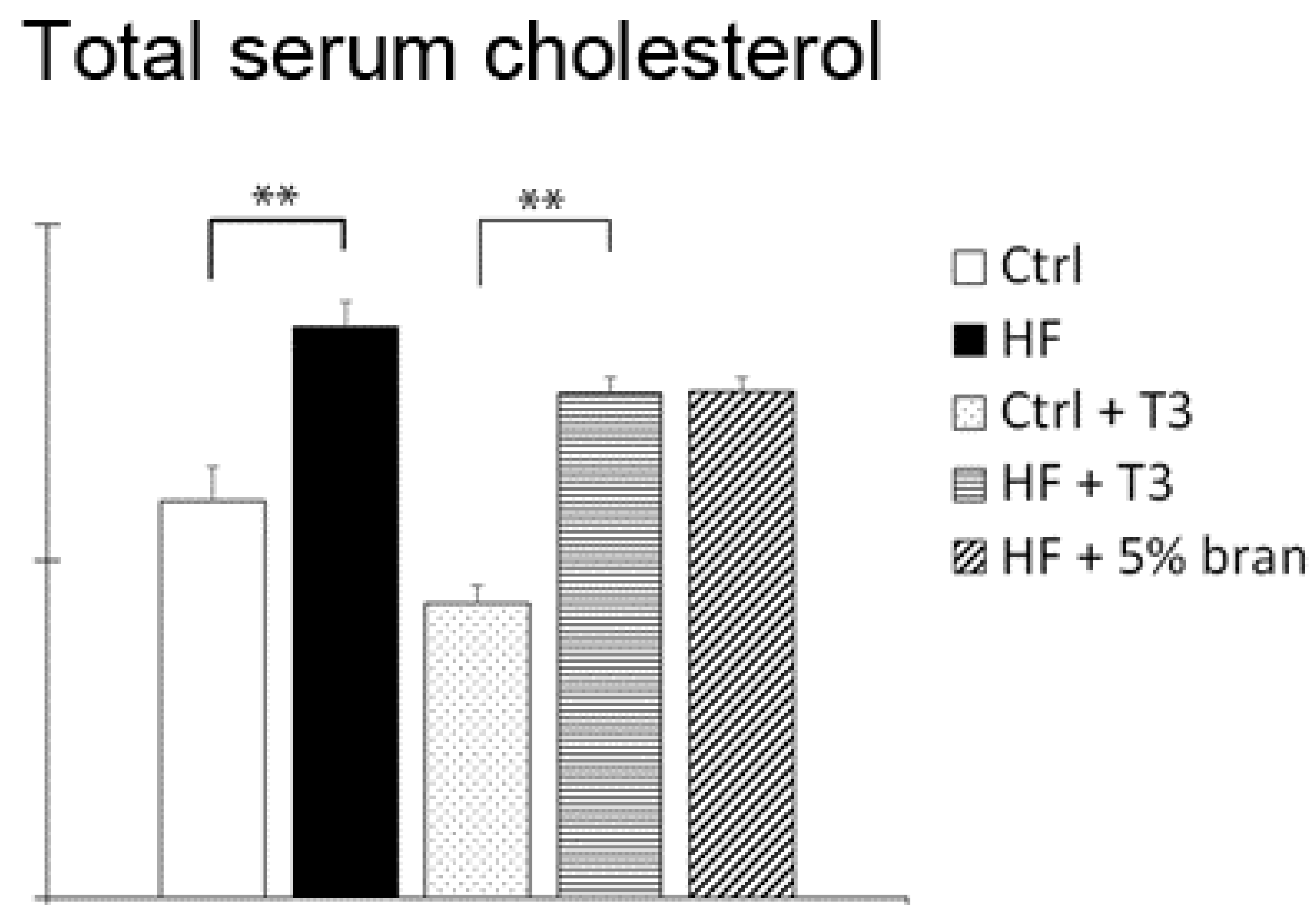
3.4. T3s and Bran Significantly Decreased Serum Cholesterol Levels
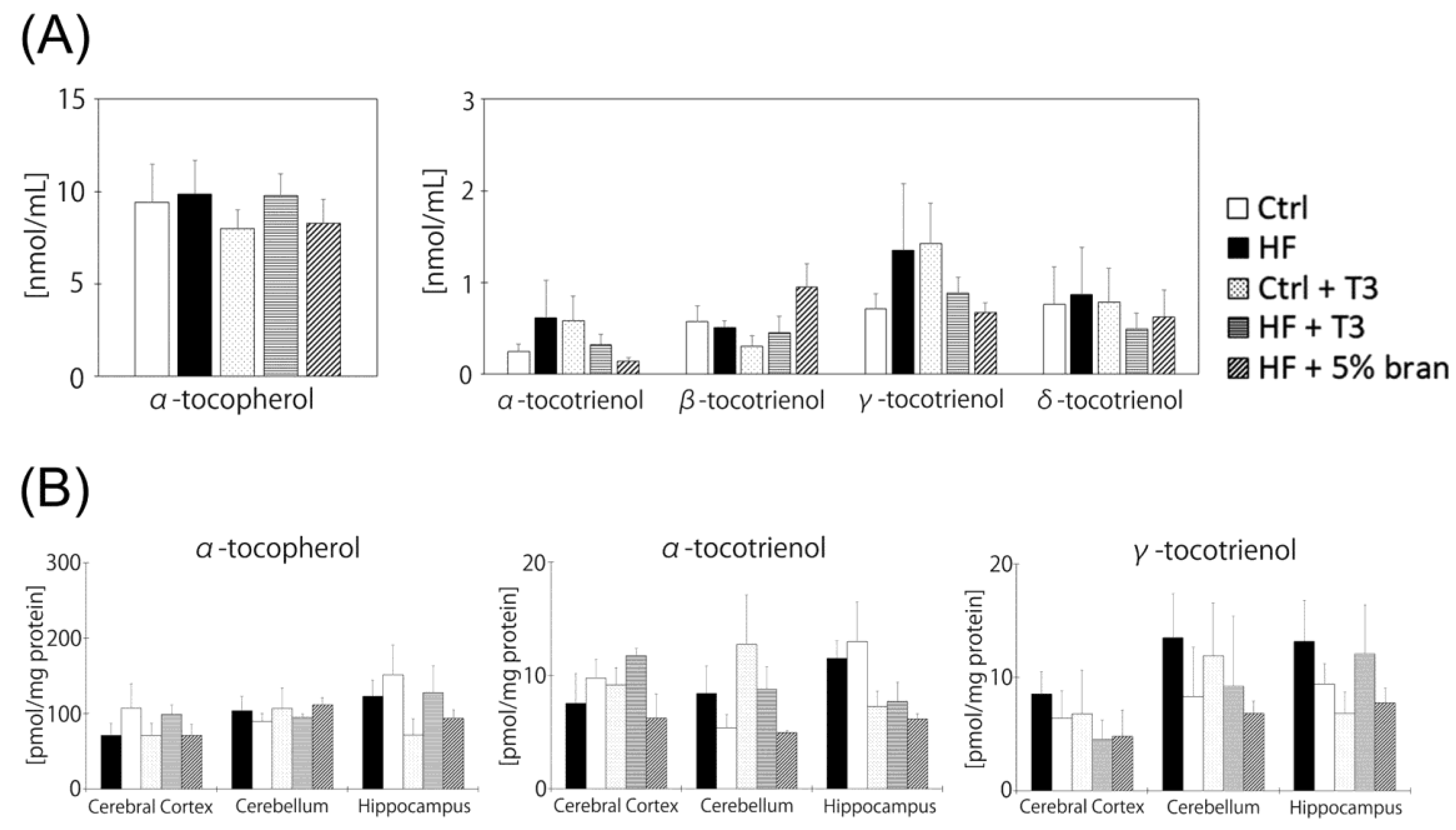
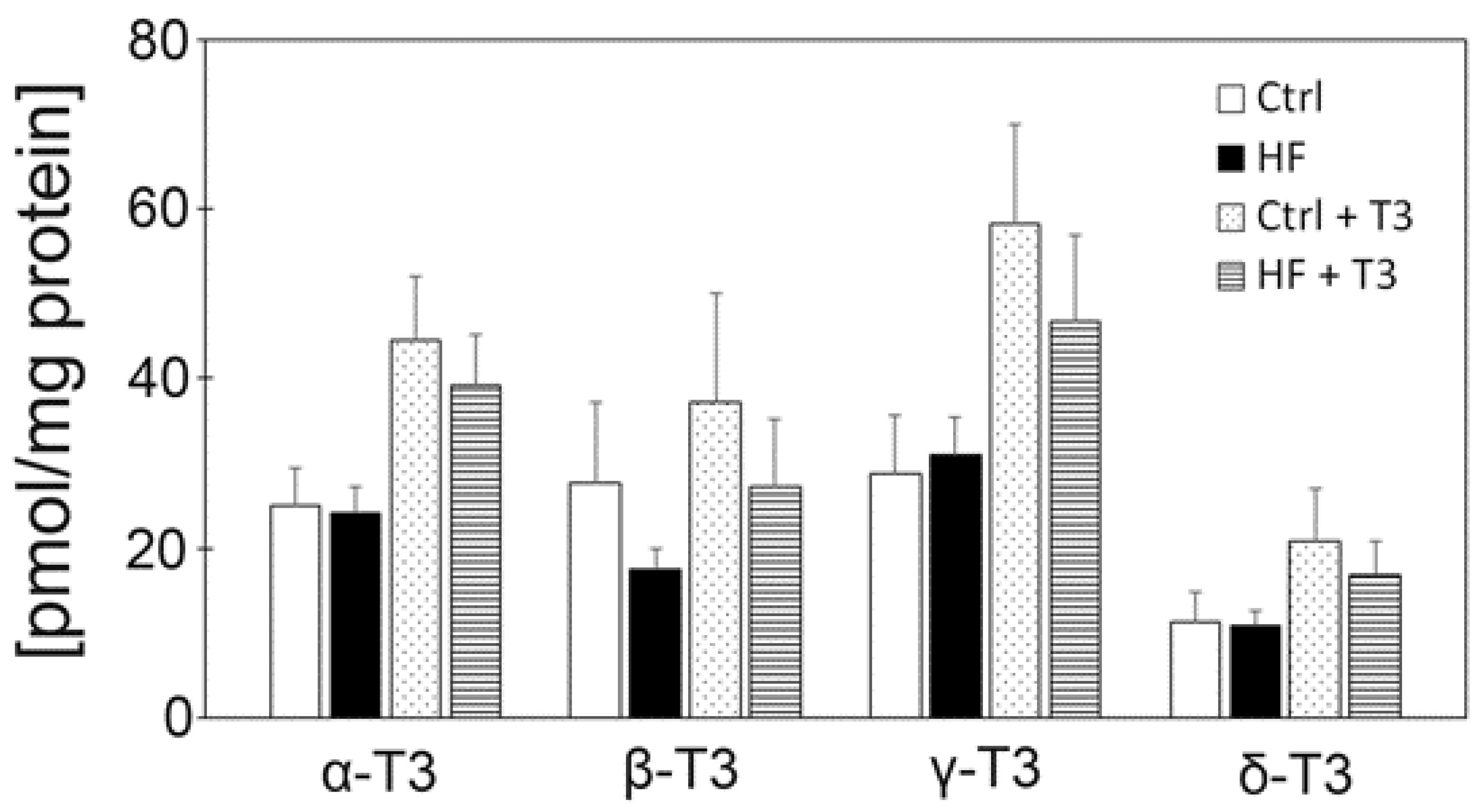
3.5. Vitamin E Contents of Mouse Brains Did Not Significantly Differ between Diet Groups
3.6. Changes in Antioxidant Enzyme Activities and Protein Expressions in the Mouse Brain
4. Discussion
4.1. Bran Contains High Levels of T3s and Attenuates Body Weight Gain
4.2. Tocotrienols Do Not Reach the Brain or Affect the Brain Antioxidant Network
4.3. Relationship between Brain Oxidation and T3 Levels
5. Conclusions
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Expert Panel Members; Jensen, M.D.; Ryan, D.H.; Donato, K.A.; Apovian, C.M.; Ard, J.D.; Comuzzie, A.G.; Hu, F.B.; Hubbard, V.S.; Jakicic, J.M.; et al. Executive summary: Guidelines (2013) for the management of overweight and obesity in adults: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the Obesity Society published by the Obesity Society and American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Based on a systematic review from the Obesity Expert Panel. Obesity (Silver Spring) 2013, 22 (Suppl. 2), S5–S39. [Google Scholar]
- The GBD 2015 Obesity Collaborators. Health effects of overweight and obesity in 195 countries over 25 years. N. Eng. J. Med. 2017, 377, 13–27. [Google Scholar] [CrossRef] [PubMed]
- Singh, G.M.; Danaei, G.; Farzadfar, F.; Stevens, G.A.; Woodward, M.; Wormser, D.; Kaptoge, S.; Whitlock, G.; Qiao, Q.; Lewington, S.; et al. The age-specific quantitative effects of metabolic risk factors on cardiovascular diseases and diabetes: A pooled analysis. PLoS ONE 2013, 8, e65174. [Google Scholar] [CrossRef]
- Koskinen, J.; Magnussen, C.G.; Sinako, A.; Woo, J.; Urbina, E.; Jacobs, D.R., Jr.; Steinberger, J.; Prineas, R.; Sabin, M.A.; Burns, T.; et al. Childhood age and associations between childhood metabolic syndrome and adult risk for metabolic syndrome, type 2 diabetes mellitus and carotid intima media thickness: The international childhood cardiovascular cohort consortium. J. Am. Heart Assoc. 2017, 6, e005632. [Google Scholar] [CrossRef] [PubMed]
- Cohen, J.B. Hypertension in obesity and the impact of weight loss. Curr. Cardiol. Rep. 2017, 19, 98. [Google Scholar] [CrossRef]
- Ingram, D.K.; de Cabo, R. Calorie restriction in rodents: Caveats to consider. Ageing Res. Rev. 2017, 39, 15–28. [Google Scholar] [CrossRef] [PubMed]
- Simpson, S.J.; Le Couteur, D.G.; Raubenheimer, D.; Solon-Biet, S.M.; Cooney, G.J.; Cogger, V.C.; Fontana, L. Dietary protein, aging and nutritional geometry. Ageing Res. Rev. 2017, 39, 78–86. [Google Scholar] [CrossRef] [PubMed]
- Wahl, D.; Cogger, V.C.; Solon-Biet, S.M.; Waern, R.V.; Gokarn, R.; Pulpitei, T.; Cabo Rd Mattson, M.P.; Raubenheimer, D.; Simpson, S.J.; Le Couteur, D.G. Nutritional strategies to optimise cognitive function in the aging brain. Ageing Res. Rev. 2016, 31, 80–92. [Google Scholar] [CrossRef]
- Mattison, J.A.; Colman, R.J.; Beasley, T.M.; Allison, D.B.; Kemnitz, J.W.; Roth, G.S.; Ingram, D.K.; Weindruch, E.; de Cabo RAnderson, R.M. Calorie restriction improves health and survival of rhesus monkeys. Nat. Commun. 2017, 8, 14063. [Google Scholar] [CrossRef]
- Jimenez-Gomez, Y.; Mattison, J.A.; Pearson, K.J.; Martin-Montalvo, A.; Palacious, H.H.; Sossong, A.M.; Ward, T.M.; Younts, C.M.; Lewis, K.; Allaed, J.S.; et al. Resveratrol improves adipose insulin signaling and reduces the inflammatory response in adipose tissue of rhesus monkeys on high-fat, high-sugar diet. Cell Metab. 2013, 18, 533–545. [Google Scholar] [CrossRef]
- Scheele, C.; Nielsen, S. Metabolic regulation and the anti-obesity perspectives of human brown fat. Redox Biol. 2017, 12, 770–775. [Google Scholar] [CrossRef] [PubMed]
- Westbrook, R.; Bonkowski, M.S.; Arum, O.; Strader, A.D.; Bartke, A. Metabolic alterations due to calorie restriction and every other day feeding in normal and growth hormone receptor knockout mice. J. Gerontol. A Biol. Sci. Med. Sci. 2014, 69, 25–33. [Google Scholar] [CrossRef] [PubMed]
- Harman, D. Aging; a theory based on free radical and radiation chemistry. J. Gerontol. 1956, 11, 298–300. [Google Scholar] [CrossRef]
- Butterfield, D.A. The 2013 SFRBM discovery award: Selected discoveries from the butterfield laboratory of oxidative stress and its sequela in brain in cognitive disorders exemplified by Alzheimer disease and chemotherapy induced cognitive impairment. Free Radic. Biol. Med. 2014, 74, 157–174. [Google Scholar] [CrossRef]
- Seet, R.C.; Lee, C.Y.; Lim, E.C.; Tan, J.J.; Quek, A.M.; Chong, W.L.; Looi, W.F.; Huang, S.H.; Wang, H.; Chan, Y.H.; et al. Oxidative damage in Parkinson disease: Measurement using accurate biomarkers. Free Radic. Biol. Med. 2010, 48, 560–566. [Google Scholar] [CrossRef]
- Fukui, K.; Takatsu, H.; Koike, T.; Urano, S. Hydrogen peroxide induces neurite degeneration: Prevention by tocotrienols. Free Radic. Res. 2011, 45, 681–691. [Google Scholar] [CrossRef]
- Fukui, K.; Ushiki, K.; Takatsu, H.; Koike, T.; Urano, S. Tocotrienols prevent hydrogen peroxide-induced axon and dendrite degeneration in cerebellar granule cells. Free Radic. Res. 2012, 46, 184–193. [Google Scholar] [CrossRef] [PubMed]
- Burdeos, G.C.; Nakagawa, K.; Kimura, F.; Miyazawa, T. Tocotrienol attenuates tryglyceride accumulation in HepG2 cells and F344 rats. Lipids 2012, 47, 471–481. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, J.K.; Gupta, S. Tocotrienol-rich fraction of palm oil induces cell cycle arrest and apoptosis selectively in human prostate cancer cells. Biochem. Biophys. Res. Commun. 2006, 346, 447–453. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Kang, I.; Fang, X.; Wang, W.; Lee, M.A.; Hollins, R.R.; Marshall, M.R.; Chung, S. Gamma-tocotrienol attenuates high-fat diet-induced obesity and insulin resistance by inhibiting adipose inflammation and M1 macrophage recruitment. Int. J. Obes. (Lond.) 2014, 39, 438–446. [Google Scholar] [CrossRef] [PubMed]
- Wong, W.Y.; Poudyal, H.; Ward, L.C.; Brown, L. Tocotrienols reverse cardiovascular, metabolic and liver changes in high carbohydrate, high fat diet-fed rats. Nutrients 2012, 4, 1527–1541. [Google Scholar] [CrossRef]
- Burdeos, G.C.; Nakagawa, K.; Abe, T.; Kimura, F.; Miyazawa, T. Tocotrienol modulates crucial lipid methabolism-related genes in differentiated 3T3-L1 preadypocytes. Food Funct. 2014, 5, 2221–2227. [Google Scholar] [CrossRef]
- Onodera, K.; Omoi, N.; Fukui, K.; Hayasaka, T.; Shinkai, T.; Suzuki, S.; Abe, K.; Urano, S. Oxidative damage of rat cerebral cortex and hippocampus, and changes in antioxidative defense systems caused by hyperoxia. Free Radic. Res. 2003, 37, 367–372. [Google Scholar] [CrossRef]
- Ohkawa, H.; Ohishi, N.; Yagi, K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal. Biochem. 1979, 95, 351–358. [Google Scholar] [CrossRef]
- Park, H.Y.; Lee, K.W.; Choi, H.D. Rice bran constituents: Immunomodulatory and therapeutic activities. Food Funct. 2017, 8, 935–943. [Google Scholar] [CrossRef]
- Ahsan, H.; Ahad, A.; Siddiqui, W.A. A review of characterization of tocotrienols from plant oils and foods. J. Chem. Biol. 2015, 8, 45–59. [Google Scholar] [CrossRef]
- Qureshi, A.A.; Burger, W.C.; Peterson, D.M.; Elson, C.E. The structure of an inhibitor of cholesterol biosynthesis isolated from barely. J. Biol. Chem. 1986, 261, 10544–10550. [Google Scholar]
- Kaneai, N.; Sumitani, K.; Fukui, K.; Koike, T.; Takatsu, H.; Urano, S. Tocotrienol improves learning and memory deficit of aged rats. J. Clin. Biochem. Nutr. 2016, 58, 114–121. [Google Scholar] [CrossRef]
- Chalasani, N.; Younossi, Z.; Lavine, J.E.; Diehi, A.M.; Brunt, E.M.; Cusi, K.; Charlton, M.; Sanyal, A.J. The diagnosis and management of non-alcoholic fatty liver disease: Practice Guideline by the American Association for the Study of Liver Diseases, American College of Gastroenterology, and the American Gastroenterological Association. Hepatology 2012, 55, 2005–2023. [Google Scholar] [CrossRef]

© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fukui, K.; Shirai, M.; Ninuma, T.; Kato, Y. Anti-Obesity Effects of Tocotrienols and Bran in High-Fat Diet-Treated Mice. Nutrients 2019, 11, 830. https://doi.org/10.3390/nu11040830
Fukui K, Shirai M, Ninuma T, Kato Y. Anti-Obesity Effects of Tocotrienols and Bran in High-Fat Diet-Treated Mice. Nutrients. 2019; 11(4):830. https://doi.org/10.3390/nu11040830
Chicago/Turabian StyleFukui, Koji, Masashi Shirai, Takeyuki Ninuma, and Yugo Kato. 2019. "Anti-Obesity Effects of Tocotrienols and Bran in High-Fat Diet-Treated Mice" Nutrients 11, no. 4: 830. https://doi.org/10.3390/nu11040830
APA StyleFukui, K., Shirai, M., Ninuma, T., & Kato, Y. (2019). Anti-Obesity Effects of Tocotrienols and Bran in High-Fat Diet-Treated Mice. Nutrients, 11(4), 830. https://doi.org/10.3390/nu11040830




