Glutamine Therapy Reduces Inflammation and Extracellular Trap Release in Experimental Acute Respiratory Distress Syndrome of Pulmonary Origin
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics Statement
2.2. Animal Preparation and Experimental Protocol
2.3. Total and Differential Cell Counts in BALF
2.4. Identification of ETs in BALF: Quantification of cf-DNA, Elastase, and Myeloperoxidase Activity
2.5. Enzyme-Linked Immunosorbent Assay (ELISA)
2.6. Lung Mechanics
2.7. Lung Histology
2.8. Detection of ETs (cf-DNA) in Vitro
2.9. Reactive Oxygen Species Measurement
2.10. Determination of Glutathione Reductase and Glutathione Peroxidase Activity
2.11. Statistical Analysis
3. Results
3.1. In Vivo Experiments
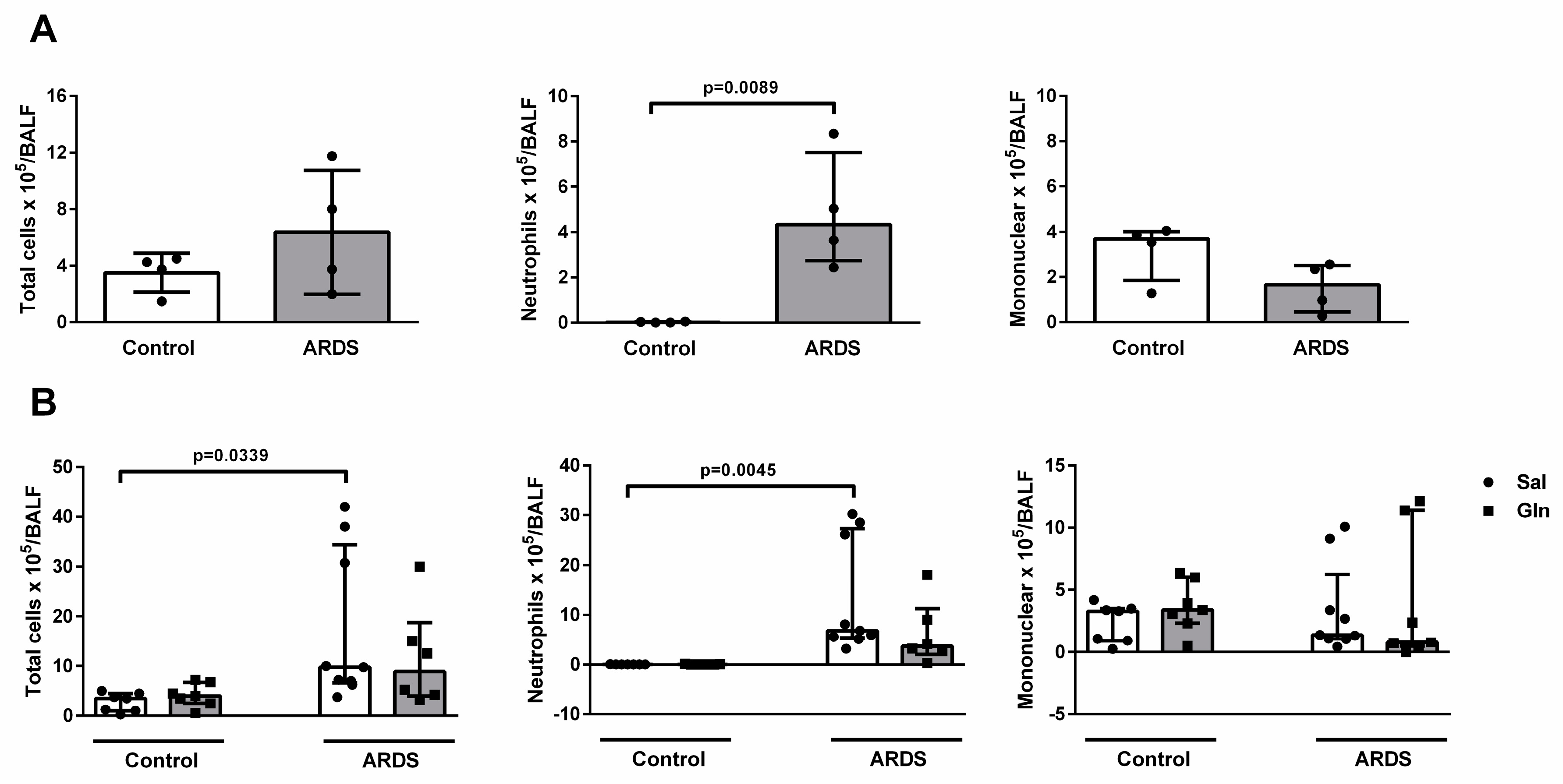
3.1.1. Leukocyte Recruitment in BALF
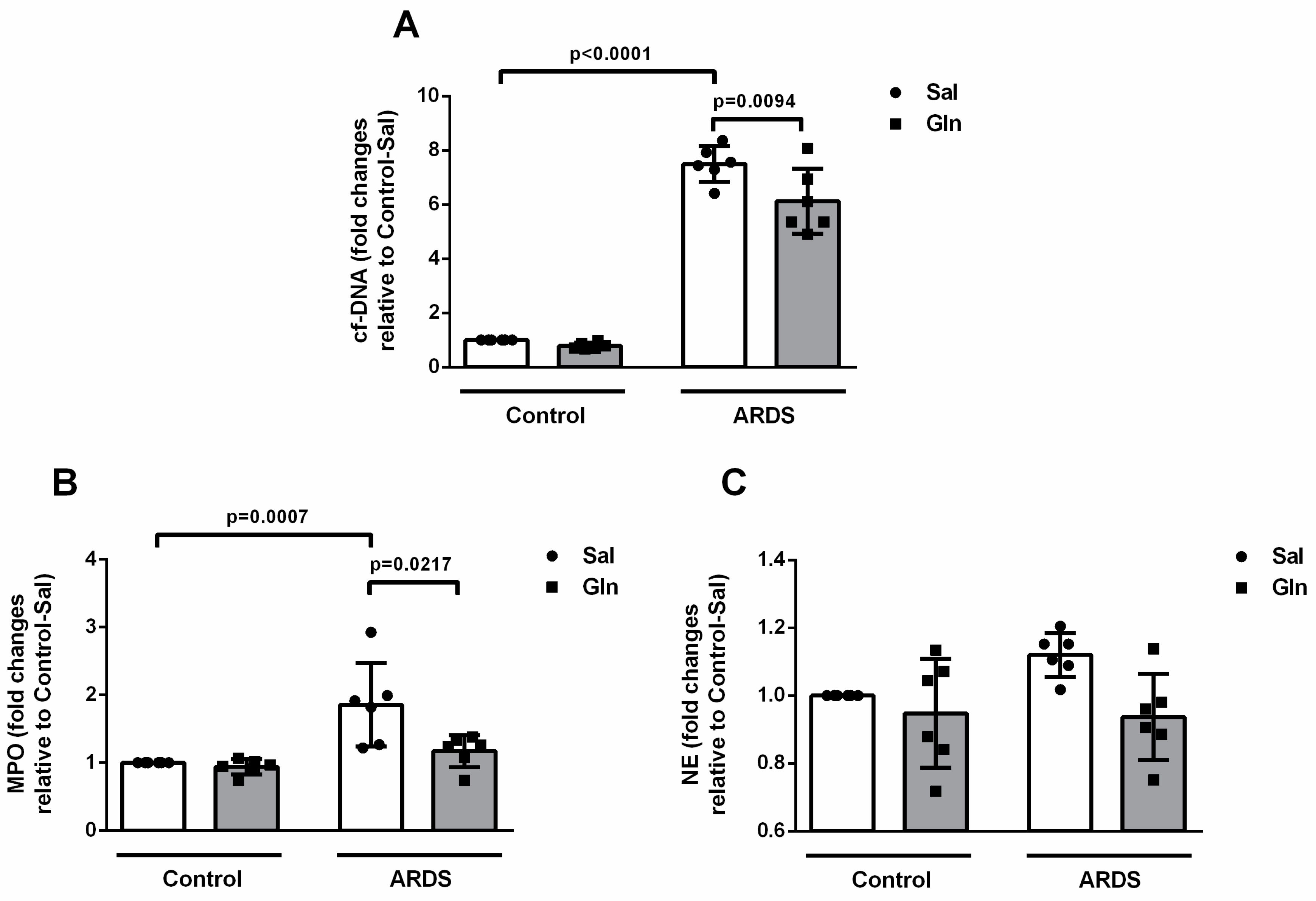
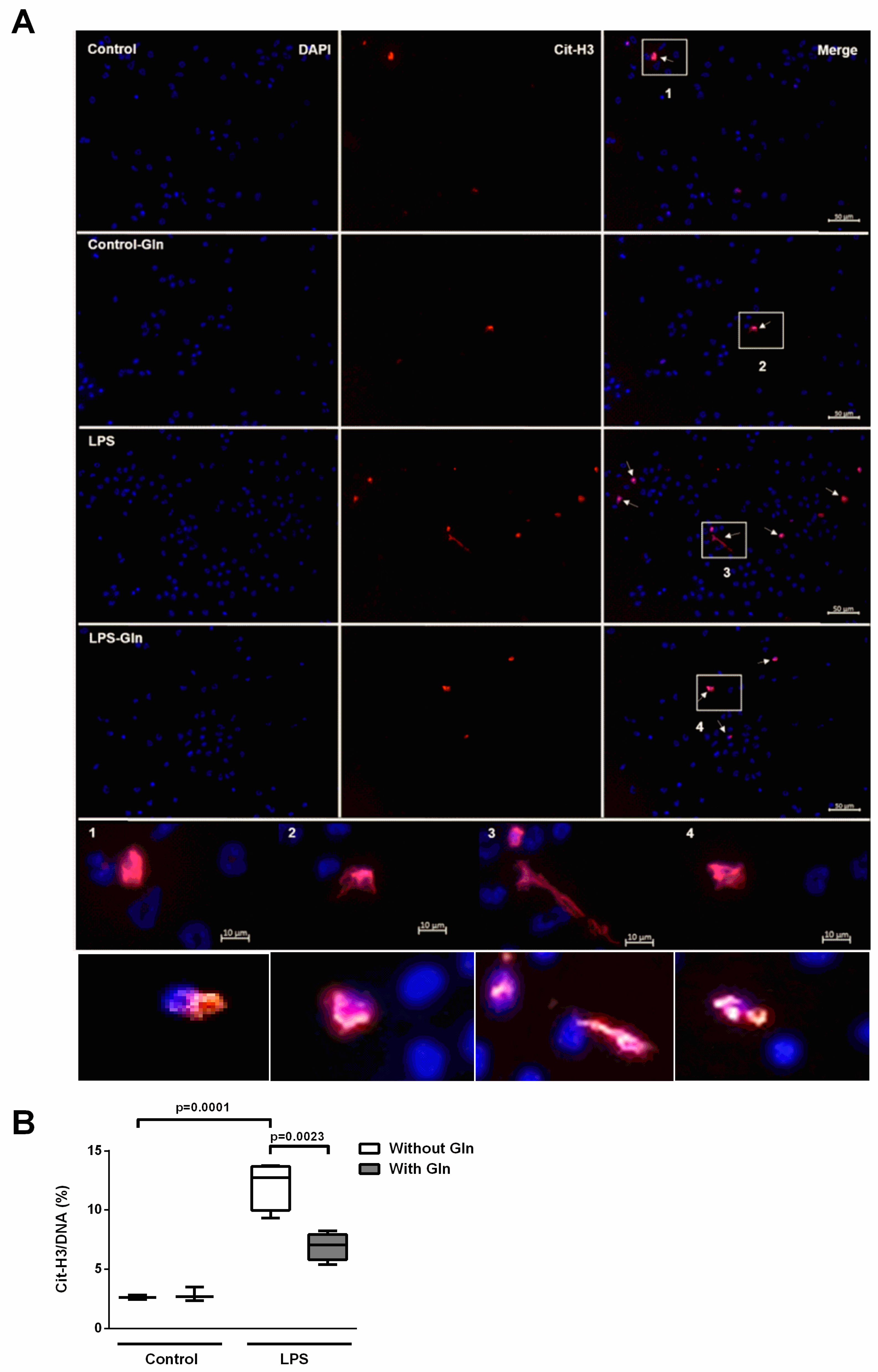
3.1.2. Glutamine Treatment Reduced ET Formation
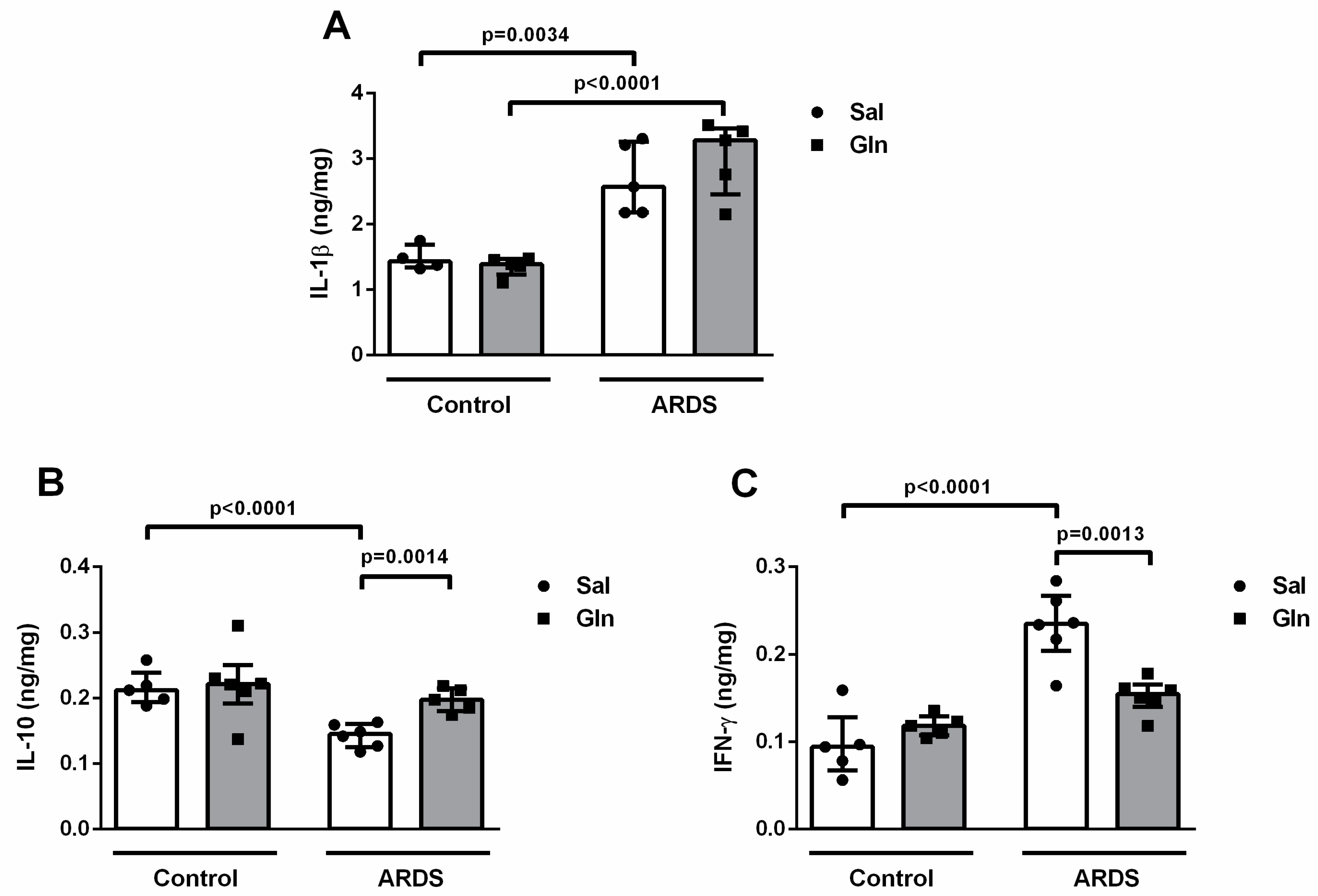
3.1.3. Lung Cytokine Modulation by Glutamine Treatment
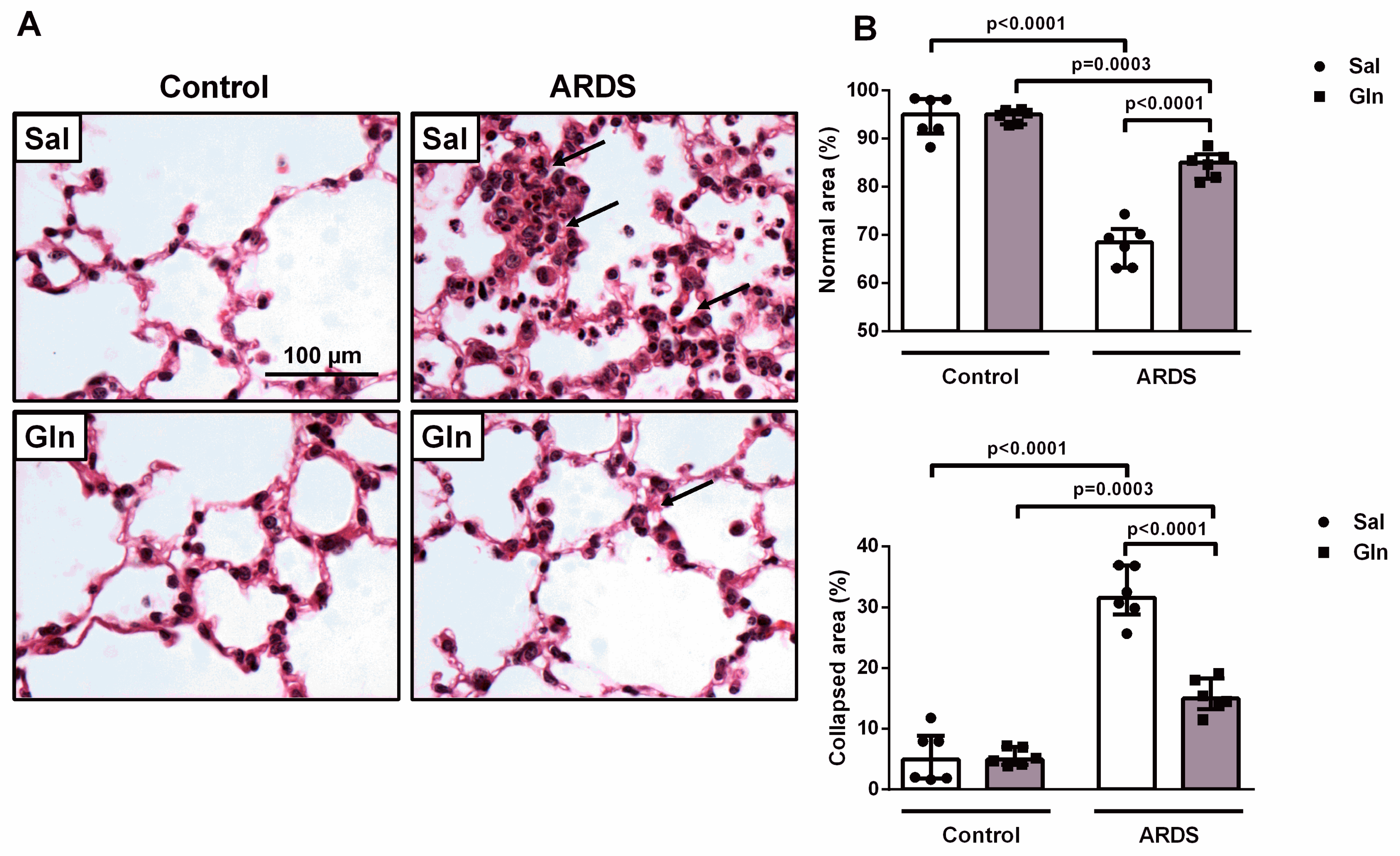
3.1.4. Effect of Glutamine on Lung Function and Histology in Pulmonary ARDS
3.2. In Vitro Experiments
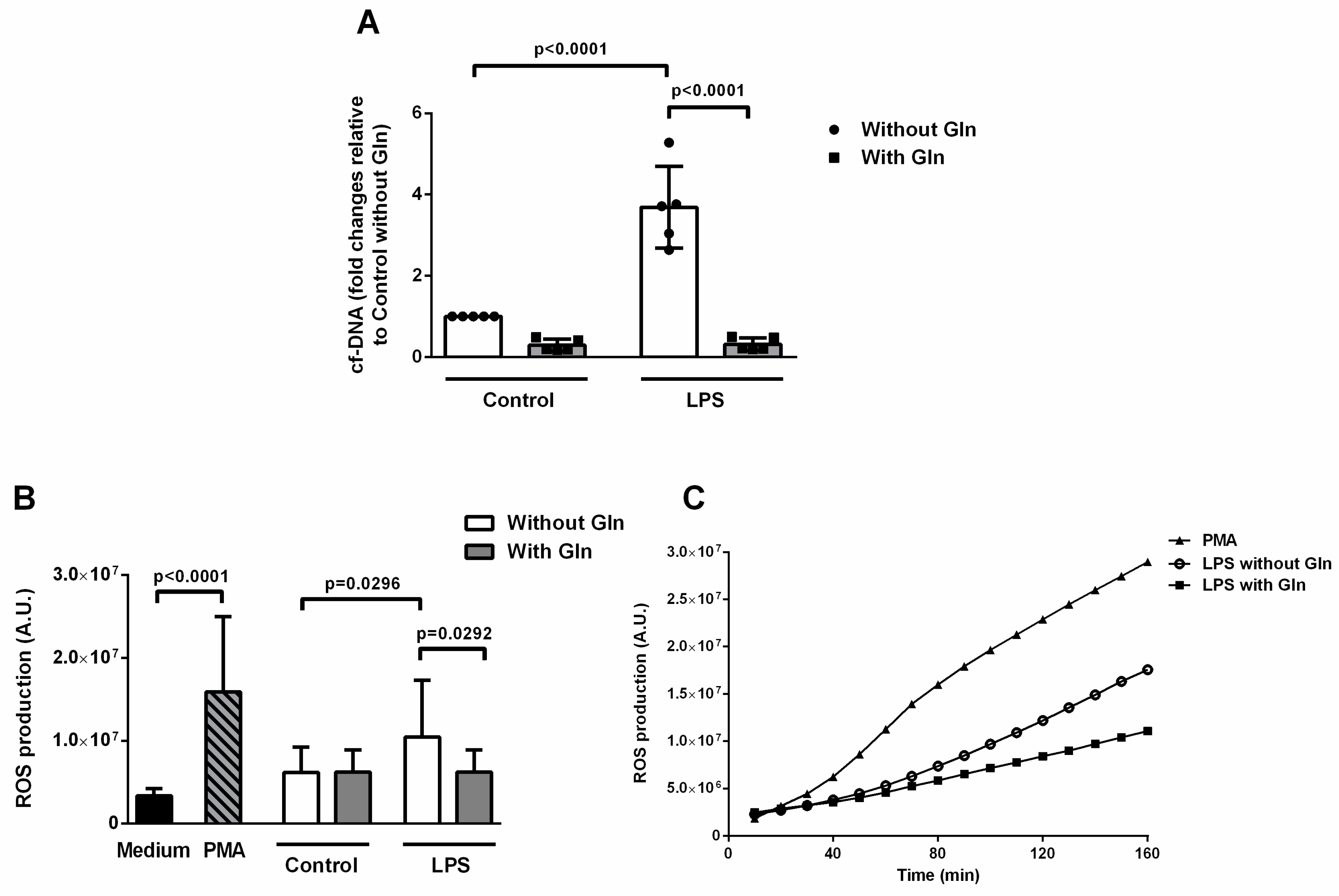
3.2.1. Glutamine Treatment Reduced ETs and ROS Formation Induced by LPS in BALF Cells
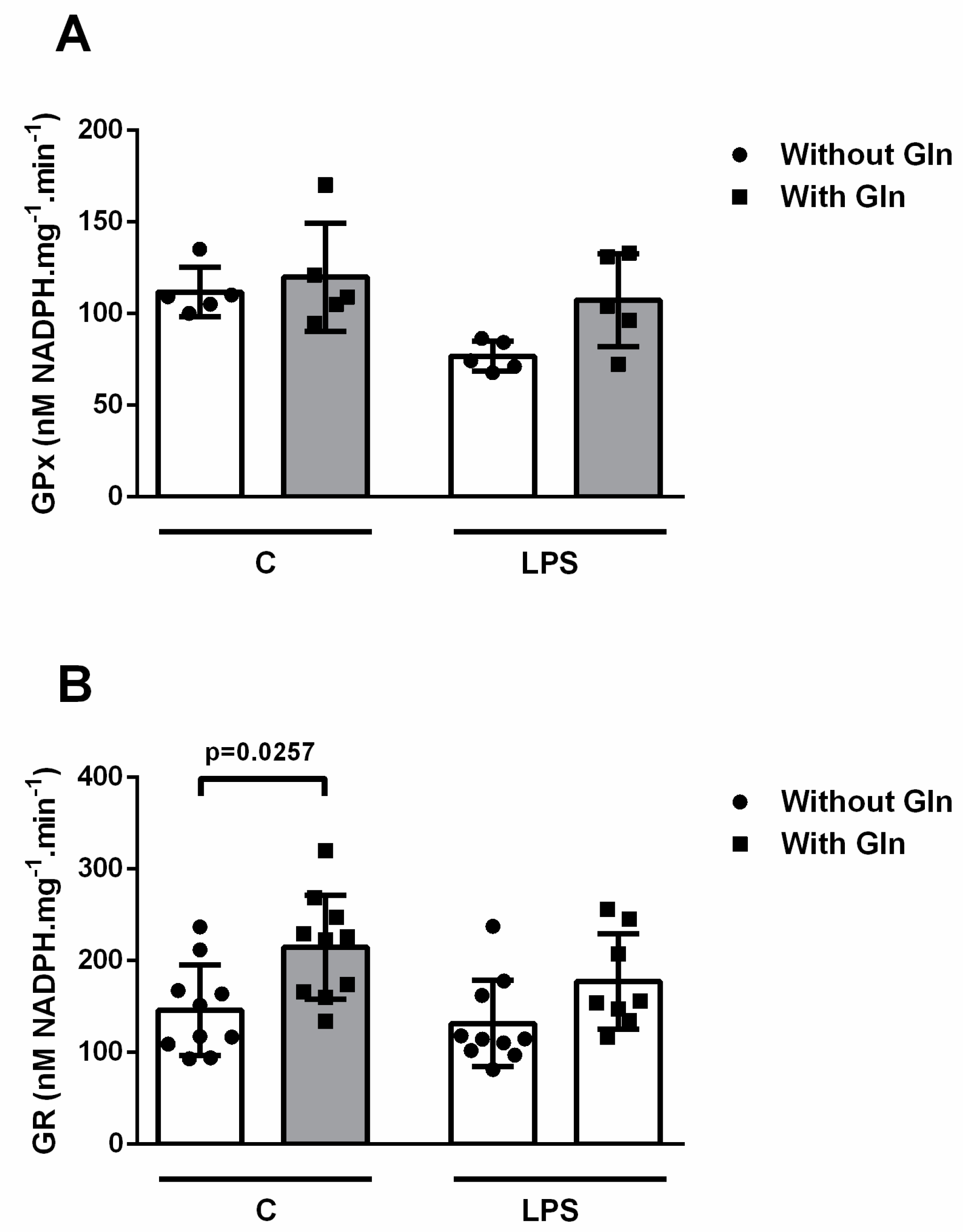
3.2.2. Glutathione Reductase and Glutathione Peroxidase Activities
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bellani, G.; Laffey, J.G.; Pham, T.; Fan, E.; Brochard, L.; Esteban, A.; Gattinoni, L.; van Haren, F.; Larsson, A.; McAuley, D.F.; et al. Epidemiology, patterns of care, and mortality for patients with acute respiratory distress syndrome. JAMA 2016, 315, 788–800. [Google Scholar] [CrossRef]
- ARDS Definition Task Force; Ranieri, V.M.; Rubenfeld, G.D.; Thompson, B.T.; Fergunson, N.D.; Caldwell, E.; Fan, E.; Camporota, L.; Slutsky, A.S. Acute respiratory distress syndrome: The Berlin Definition. JAMA 2012, 307, 2526–2533. [Google Scholar]
- Han, S.; Mallampalli, R.K. Correction: The Acute Respiratory Distress Syndrome: From Mechanism to Translation. J. Immunol. 2015, 194, 5569. [Google Scholar] [CrossRef] [PubMed]
- Williams, A.E.; Chambers, R.C. The mercurial nature of neutrophils: Still an enigma in ARDS? Am. J. Physiol. Lung Cell. Mol. Physiol. 2014, 306, L217–L230. [Google Scholar] [CrossRef]
- Williams, A.R.; José, R.J.; Mercer, P.F.; Brealey, D.; Parekh, D.; Thickett, D.R.; O’Kane, C.; McAuley, D.F.; Chambers, R.C. Evidence for chemokine synergy during neutrophil migration in ARDS. Thorax 2017, 72, 66–73. [Google Scholar] [CrossRef]
- Juss, J.K.; House, D.; Amour, A.; Begg, M.; Herre, J.; Storisteanu, D.M.L.; Hoenderdos, K.; Bradley, G.; Lennon, M.; Summers, C.; et al. Acute Respiratory Distress Syndrome Neutrophils Have a Distinct Phenotype and Are Resistant to Phosphoinositide 3-Kinase Inhibition. Am. J. Respir. Crit. Care Med. 2016, 194, 961–973. [Google Scholar] [CrossRef]
- Brinkmann, V. Neutrophil Extracellular Traps Kill Bacteria. Science 2004, 303, 1532–1535. [Google Scholar] [CrossRef]
- Yang, H.; Biermann, M.H.; Brauner, J.M.; Liu, Y.; Zhao, Y.; Herrmann, M. New Insights into Neutrophil Extracellular Traps: Mechanisms of Formation and Role in Inflammation. Front. Immunol. 2016, 7, 12. [Google Scholar] [CrossRef] [PubMed]
- Cortjens, B.; van Woensel, J.B.; Bem, R.A. Neutrophil extracellular traps in respiratory disease: Guided anti-microbial traps or toxic webs? Paediatr. Respir. Rev. 2017, 21, 54–61. [Google Scholar] [CrossRef]
- Zhang, F.; Wang, X.; Pan, L.; Wang, W.; Li, N.; Li, J. Glutamine attenuates lipopolysaccharide-induced acute lung injury. Nutrition 2009, 25, 692–698. [Google Scholar] [CrossRef] [PubMed]
- Lai, C.-C.; Liu, W.-L.; Chen, C.-M. Glutamine Attenuates Acute Lung Injury Caused by Acid Aspiration. Nutrients 2014, 6, 3101–3116. [Google Scholar] [CrossRef] [PubMed]
- Hou, Y.-C.; Pai, M.-H.; Chiu, W.-C.; Hu, Y.-M.; Yeh, S.-L. Effects of dietary glutamine supplementation on lung injury induced by lipopolysaccharide administration. Am. J. Physiol. Lung Cell. Mol. Physiol. 2009, 296, 288–295. [Google Scholar] [CrossRef][Green Version]
- Fernandez-Bustamante, A.; Agazio, A.; Wilson, P.; Elkins, N.; Domaleski, L.; He, Q.; Baer, K.A.; Moss, A.F.D.; Wischmeyer, P.E.; Repine, J.E. Brief Glutamine Pretreatment Increases Alveolar Macrophage CD163/Heme Oxygenase-1/p38-MAPK Dephosphorylation Pathway and Decreases Capillary Damage but Not Neutrophil Recruitment in IL-1/LPS-Insufflated Rats. PLoS ONE 2015, 10, e0130764. [Google Scholar] [CrossRef]
- Guimarães-Costa, A.B.; Nascimento, M.T.C.; Wardini, A.B.; Pinto-Da-Silva, L.H.; Saraiva, E.M.; Guimarã Es-Costa, A.B. ETosis: A Microbicidal Mechanism beyond Cell Death. J. Parasitol. Res. 2012, 2012, 1–11. [Google Scholar] [CrossRef]
- Oliveira, G.P.; Silva, J.D.; Marques, P.S.; Gonçalves-De-Albuquerque, C.F.; Santos, H.L.; Vascocellos, A.P.; Takiya, C.M.; Morales, M.M.; Pelosi, P.; Mócsai, A.; et al. The Effects of Dasatinib in Experimental Acute Respiratory Distress Syndrome Depend on Dose and Etiology. Cell. Physiol. Biochem. 2015, 36, 1644–1658. [Google Scholar] [CrossRef]
- Jean, D.; Rézaiguia-Delclaux, S.; Delacourt, C.; Leclercq, R.; Lafuma, C.; Brun-Buisson, C.; Harf, A.; Delclaux, C. Protective Effect of Endotoxin Instillation on Subsequent Bacteria-induced Acute Lung Injury in Rats. Am. J. Respir. Crit. Care Med. 1998, 158, 1702–1708. [Google Scholar] [CrossRef]
- Silva, J.D.; De Oliveira, G.P.; Samary, C.D.S.; Araujo, C.C.; Padilha, G.D.A.; Filho, F.C.E.S.; Da Silva, R.T.; Einicker-Lamas, M.; Morales, M.M.; Capelozzi, V.L.; et al. Respiratory and Systemic Effects of LASSBio596 Plus Surfactant in Experimental Acute Respiratory Distress Syndrome. Cell. Physiol. Biochem. 2016, 38, 821–835. [Google Scholar] [CrossRef]
- Gardinassi, L.G.; DeSouza-Vieira, T.S.; da Silva, N.O.; Garcia, G.R.; Borges, V.M.; Campos, R.N.S.; de Almeida, R.P.; de Miranda Santos, I.K.F.; Saraiva, E.M. Molecular signatures of neutrophil extracelular traps in human visceral leishmaniasis. Parasit Vectors 2017, 10, 285. [Google Scholar] [CrossRef] [PubMed]
- Bates, J.H.; Rossi, A.; Milic-Emili, J. Analysis of the behavior of the respiratory system with constant inspiratory flow. J. Appl. Physiol. 1985, 58, 1840–1848. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.S.; Chan, H.W.; Yu, L.C. Glutathione peroxidase and glutathione reductase activities are partially responsible for determining the susceptibility of cells to oxidase stress. Toxicology 2006, 226, 126–130. [Google Scholar] [CrossRef]
- Liu, S.; Su, X.; Pan, P.; Zhang, L.; Hu, Y.; Tan, H.; Wu, D.; Liu, B.; Li, H.; Li, H.; et al. Neutrophil extracellular traps are indirectly triggered by lipopolysaccharide and contribute to acute lung injury. Sci. Rep. 2016, 6, 37252. [Google Scholar] [CrossRef]
- Lefrançais, E.; Mallavia, B.; Zhuo, H.; Calfee, C.S.; Looney, M.R. Maladaptive role of neutrophil extracellular traps in pathogen-induced lung injury. JCI Insight 2018, 3, 98178. [Google Scholar] [CrossRef]
- Yildiz, C.; Palaniyar, N.; Otulakowski, G.; Khan, M.A.; Post, M.; Kuebler, W.M.; Tanswell, K.; Belcastro, R.; Masood, A.; Engelberts, D.; et al. Mechanical Ventilation Induces Neutrophil Extracellular Trap Formation. Anesthesiology 2015, 122, 864–875. [Google Scholar] [CrossRef]
- Papayannopoulos, V.; Metzler, K.D.; Hakkim, A.; Zychlinsky, A. Neutrophil elastase and myeloperoxidase regulate the formation of neutrophil extracellular traps. J. Cell Biol. 2010, 191, 677–691. [Google Scholar] [CrossRef]
- Doster, R.S.; Rogers, L.M.; Gaddy, J.A.; Aronoff, D.M. Macrophage extracellular traps: A scoping review. J. Innate Immun. 2018, 10, 3–13. [Google Scholar] [CrossRef]
- Martinelli, S.; Urosevic, M.; Daryadel, A.; Oberholzer, P.A.; Baumann, C.; Fey, M.F.; Dummer, R.; Simon, H.-U.; Yousefi, S. Induction of Genes Mediating Interferon-dependent Extracellular Trap Formation during Neutrophil Differentiation. J. Biol. Chem. 2004, 279, 44123–44132. [Google Scholar] [CrossRef]
- Saitoh, T.; Komano, J.; Saitoh, Y.; Misawa, T.; Takahama, M.; Kozaki, T.; Uehata, T.; Iwasaki, H.; Omori, H.; Yamaoka, S.; et al. Neutrophil Extracellular Traps Mediate a Host Defense Response to Human Immunodeficiency Virus-1. Cell Host Microbe 2012, 12, 109–116. [Google Scholar] [CrossRef]
- Yamada, M.; Gomez, J.C.; Chugh, P.E.; Lowell, C.A.; Dinauer, M.C.; Dittmer, D.P.; Doerschuk, C.M. Interferon-γ production by neutrophils during bacterial pneumonia in mice. Am. J. Respir. Crit. Care Med. 2011, 183, 1391–1401. [Google Scholar] [CrossRef]
- Gomez, J.C.; Yamada, M.; Martin, J.R.; Dang, H.; Brickey, W.J.; Bergmeier, W.; Dinauer, M.C.; Doerschuk, C.M. Mechanisms of Interferon-γ Production by Neutrophils and Its Function during Streptococcus pneumoniae Pneumonia. Am. J. Respir. Cell Mol. Biol. 2015, 52, 349–364. [Google Scholar] [CrossRef]
- Chuang, Y.-C.; Shaw, H.-M.; Chen, C.-C.; Pan, H.-J.; Lai, W.-C.; Huang, H.-L. Short-term glutamine supplementation decreases lung inflammation and the receptor for advanced glycation end-products expression in direct acute lung injury in mice. BMC Pulm. Med. 2014, 14, 115. [Google Scholar] [CrossRef]
- Roth, E. Nonnutritive effects of glutamine. J. Nutr. 2008, 138, 2025S–2031S. [Google Scholar] [CrossRef] [PubMed]
- Wong, M.H.; Johnson, M.D. Differential Response of Primary Alveolar Type I and Type II Cells to LPS Stimulation. PLoS ONE 2013, 8, e55545. [Google Scholar] [CrossRef] [PubMed]
- Neeli, I.; Khan, S.N.; Radic, M. Histone Deimination As a Response to Inflammatory Stimuli in Neutrophils. J. Immunol. 2008, 180, 1895–1902. [Google Scholar] [CrossRef]
- Pan, B.; Alam, H.B.; Chong, W.; Mobley, J.; Liu, B.; Deng, Q.; Liang, Y.; Wang, Y.; Chen, E.; Wang, T.; et al. CitH3: a reliable blood biomarker for diagnosis and treatment of endotoxic shock. Sci. Rep. 2017, 7, 8972. [Google Scholar] [CrossRef]
- Leshner, M.; Wang, S.; Lewis, C.; Zheng, H.; Chen, X.A.; Santy, L.; Wang, Y. PAD4 mediated histone hypercitrullination induces heterochromatin decondensation and chromatin unfolding to form neutrophil extracellular trap-like structures. Front. Immunol. 2012, 3, 307. [Google Scholar] [CrossRef]
- Kaplan, M.J.; Radic, M. Neutrophil extracellular traps (NETs): Double-edged swords of innate immunity1. J. Immunol. 2012, 189, 2689–2695. [Google Scholar] [CrossRef]
- Remijsen, Q.; Vanden, B.T.; Wirawan, E.; Asselbergh, E.; Parthoens, E.; De Rycke, R.; Noppen, S.; Delforge, M.; Willems, J.; Vandenabeele, P. Neutrophil extracellular trap cell death requires both autophagy and superoxide generation. Cell Res. 2011, 21, 290–304. [Google Scholar] [CrossRef] [PubMed]
- Rocha, J.D.B.; Nascimento, M.T.C.; Decote-Ricardo, D.; Côrte-Real, S.; Morrot, A.; Heise, N.; Nunes, M.P.; Previato, J.O.; Mendonça-Previato, L.; Dosreis, G.A.; et al. Capsular polysaccharides from Cryptococcus neoformans modulate production of neutrophil extracellular traps (NETs) by human neutrophils. Sci. Rep. 2015, 5, 8008. [Google Scholar] [CrossRef]
- Khan, M.A.; Farahvash, A.; Douda, D.N.; Licht, J.-C.; Grasemann, H.; Sweezey, N.; Palaniyar, N. JNK Activation Turns on LPS- and Gram-Negative Bacteria-Induced NADPH Oxidase-Dependent Suicidal NETosis. Sci. Rep. 2017, 7, 3409. [Google Scholar] [CrossRef] [PubMed]
- Tadie, J.-M.; Bae, H.-B.; Jiang, S.; Park, D.W.; Bell, C.P.; Yang, H.; Pittet, J.-F.; Tracey, K.J.; Thannickal, V.J.; Abraham, E.; et al. HMGB1 promotes neutrophil extracellular trap formation through interactions with Toll-like receptor 4. Am. J. Physiol. Cell. Mol. Physiol. 2013, 304, L342–L349. [Google Scholar] [CrossRef]
- Kwon, W.Y.; Suh, G.J.; Kim, K.S.; Jo, Y.H.; Lee, J.H.; Kim, K.; Jung, S.K. Glutamine attenuates acute lung injury by inhibition of high mobility group box protein-1 expression during sepsis. Br. J. Nutr. 2010, 103, 890–898. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hamiel, C.R.; Pinto, S.; Hau, A.; Wischmeyer, P.E. Glutamine enhances heat shock protein 70 expression via increased hexosamine biosynthetic pathway activity. Am. J. Physiol. Physiol. 2009, 297, C1509–C1519. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Yu, Y.; Gorshkov, B.; Haigh, S.; Bordán, Z.; Weintraub, D.; Rudic, R.D.; Chakraborty, T.; Barman, S.A.; Verin, A.D.; et al. Hsp70 Suppresses Mitochondrial Reactive Oxygen Species and Preserves Pulmonary Microvascular Barrier Integrity Following Exposure to Bacterial Toxins. Front. Immunol. 2018, 9, 9. [Google Scholar] [CrossRef] [PubMed]
- Singleton, K.D.; Wischmeyer, P.E. Glutamine’s protection against sepsis and lung injury is dependent on heat shock protein 70 expression. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2007, 292, R1839–R1845. [Google Scholar] [CrossRef] [PubMed]
- Hou, Y.C.; Chiu, W.C.; Yeh, C.L.; Yeh, S.L. Glutamine modulated lipopolysaccharide-induced activation of NF-kB via the Akt/mTOR pathway in lung epithelial cells. Am. J. Physiol. Lung Cell. Mol. Physiol. 2012, 302, L174–L183. [Google Scholar] [CrossRef] [PubMed]
- Rossaint, J.; Herter, J.M.; Van Aken, H.; Napirei, M.; Doring, Y.; Weber, C.; Soehnlein, O.; Zarbock, A. Synchronized integrin engagement and chemokine activation is crucial in neutrophil extracellular trap-mediated sterile inflammation. Blood 2014, 123, 2573–2584. [Google Scholar] [CrossRef]

© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Oliveira, G.P.; Kitoko, J.Z.; de Souza Lima-Gomes, P.; Rochael, N.C.; de Araújo, C.C.; Lugon, P.N.; dos Santos, H.L.; Martins, E.G.L.; Ornellas, F.M.; de Oliveira, H.D.; et al. Glutamine Therapy Reduces Inflammation and Extracellular Trap Release in Experimental Acute Respiratory Distress Syndrome of Pulmonary Origin. Nutrients 2019, 11, 831. https://doi.org/10.3390/nu11040831
de Oliveira GP, Kitoko JZ, de Souza Lima-Gomes P, Rochael NC, de Araújo CC, Lugon PN, dos Santos HL, Martins EGL, Ornellas FM, de Oliveira HD, et al. Glutamine Therapy Reduces Inflammation and Extracellular Trap Release in Experimental Acute Respiratory Distress Syndrome of Pulmonary Origin. Nutrients. 2019; 11(4):831. https://doi.org/10.3390/nu11040831
Chicago/Turabian Stylede Oliveira, Gisele Pena, Jamil Zola Kitoko, Phillipe de Souza Lima-Gomes, Natália Cadaxo Rochael, Carla Cristina de Araújo, Pâmella Nowaski Lugon, Heloísa Lopes dos Santos, Eduarda Gabrielle Lopes Martins, Felipe Mateus Ornellas, Helena D’Anunciação de Oliveira, and et al. 2019. "Glutamine Therapy Reduces Inflammation and Extracellular Trap Release in Experimental Acute Respiratory Distress Syndrome of Pulmonary Origin" Nutrients 11, no. 4: 831. https://doi.org/10.3390/nu11040831
APA Stylede Oliveira, G. P., Kitoko, J. Z., de Souza Lima-Gomes, P., Rochael, N. C., de Araújo, C. C., Lugon, P. N., dos Santos, H. L., Martins, E. G. L., Ornellas, F. M., de Oliveira, H. D., Morales, M. M., Olsen, P. C., Galina, A., Silva, P. L., Saraiva, E. M., Pelosi, P., & Rocco, P. R. M. (2019). Glutamine Therapy Reduces Inflammation and Extracellular Trap Release in Experimental Acute Respiratory Distress Syndrome of Pulmonary Origin. Nutrients, 11(4), 831. https://doi.org/10.3390/nu11040831





