The Role of Mineral and Trace Element Supplementation in Exercise and Athletic Performance: A Systematic Review
Abstract
:1. Introduction
2. Methods
2.1. Search Strategy
2.2. Study Selection and Data Extraction
2.3. Eligibility Criteria
2.4. Quality Assessment and Risk of Bias Tool
3. Results
3.1. Study Characteristics
3.2. Study Quality
4. Discussion
4.1. Iron
4.2. Calcium
4.3. Magnesium
4.4. Phosphate
4.5. Zinc
4.6. Sodium
4.7. Selenium
4.8. Chromium
4.9. Boron
4.10. Multi Minerals
5. Limitations
6. Conclusions
7. Key Points
- Iron and Magnesium supplementation have the best quality evidence for improvements to markers and outcomes related to exercise capacity and athletic performance.
- In NAID females, oral supplementation of 100 mg day−1 ferrous sulfate or providing elemental Fe between 15–60 mg·day−1 over six-to-eight weeks may be adequate to elect performance adaptations in 3000 m running time, running velocity to lactate threshold, onset of blood lactate accumulation, quadriceps MVC fatigue resistance, post-fatiguing MVC, 4 km rowing time trial energy efficiency (kcal), preventing exercise-induced Fe loss, Hb, VO2peak, improving 15 km cycling and two mile running time trial, quicker recovery post exercise and indicators of mood. Approximately 100 mg·day−1 elemental Fe over 11 weeks has been shown to result in adaptations in markers of dynamic and absolute strength.
- 300–500 mg·day−1 of Magnesium in the short-term (~1–4 weeks) may have a positive influence on functional dynamic measures of muscle performance (CMJ, 1RM, fatigue resistance) and longer-term (>7 weeks) benefits on quadricep torque measurements.
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Appendix A
| Section/Topic | # | Checklist Item | Reported on Page # |
| TITLE | |||
| Title | 1 | Identify the report as a systematic review, meta-analysis, or both. | 1 |
| ABSTRACT | |||
| Structured summary | 2 | Provide a structured summary including, as applicable: background; objectives; data sources; study eligibility criteria, participants, and interventions; study appraisal and synthesis methods; results; limitations; conclusions and implications of key findings; systematic review registration number. | 1 |
| INTRODUCTION | |||
| Rationale | 3 | Describe the rationale for the review in the context of what is already known. | 2 onwards |
| Objectives | 4 | Provide an explicit statement of questions being addressed with reference to participants, interventions, comparisons, outcomes, and study design (PICOS). | 2 onwards |
| METHODS | |||
| Protocol and registration | 5 | Indicate if a review protocol exists, if and where it can be accessed (e.g., Web address), and, if available, provide registration information including registration number. | 2–4 |
| Eligibility criteria | 6 | Specify study characteristics (e.g., PICOS, length of follow-up) and report characteristics (e.g., years considered, language, publication status) used as criteria for eligibility, giving rationale. | 4 |
| Information sources | 7 | Describe all information sources (e.g., databases with dates of coverage, contact with study authors to identify additional studies) in the search and date last searched. | 3–4 |
| Search | 8 | Present full electronic search strategy for at least one database, including any limits used, such that it could be repeated. | 3–4 |
| Study selection | 9 | State the process for selecting studies (i.e., screening, eligibility, included in systematic review, and, if applicable, included in the meta-analysis). | 4 |
| Data collection process | 10 | Describe method of data extraction from reports (e.g., piloted forms, independently, in duplicate) and any processes for obtaining and confirming data from investigators. | 4 |
| Data items | 11 | List and define all variables for which data were sought (e.g., PICOS, funding sources) and any assumptions and simplifications made. | NA |
| Risk of bias in individual studies | 12 | Describe methods used for assessing risk of bias of individual studies (including specification of whether this was done at the study or outcome level), and how this information is to be used in any data synthesis. | 4 |
| Summary measures | 13 | State the principal summary measures (e.g., risk ratio, difference in means). | NA |
| Synthesis of results | 14 | Describe the methods of handling data and combining results of studies, if done, including measures of consistency (e.g., I2) for each meta-analysis. | NA |
| Risk of bias across studies | 15 | Specify any assessment of risk of bias that may affect the cumulative evidence (e.g., publication bias, selective reporting within studies). | 4- |
| Additional analyses | 16 | Describe methods of additional analyses (e.g., sensitivity or subgroup analyses, meta-regression), if done, indicating which were pre-specified. | NA |
| RESULTS | |||
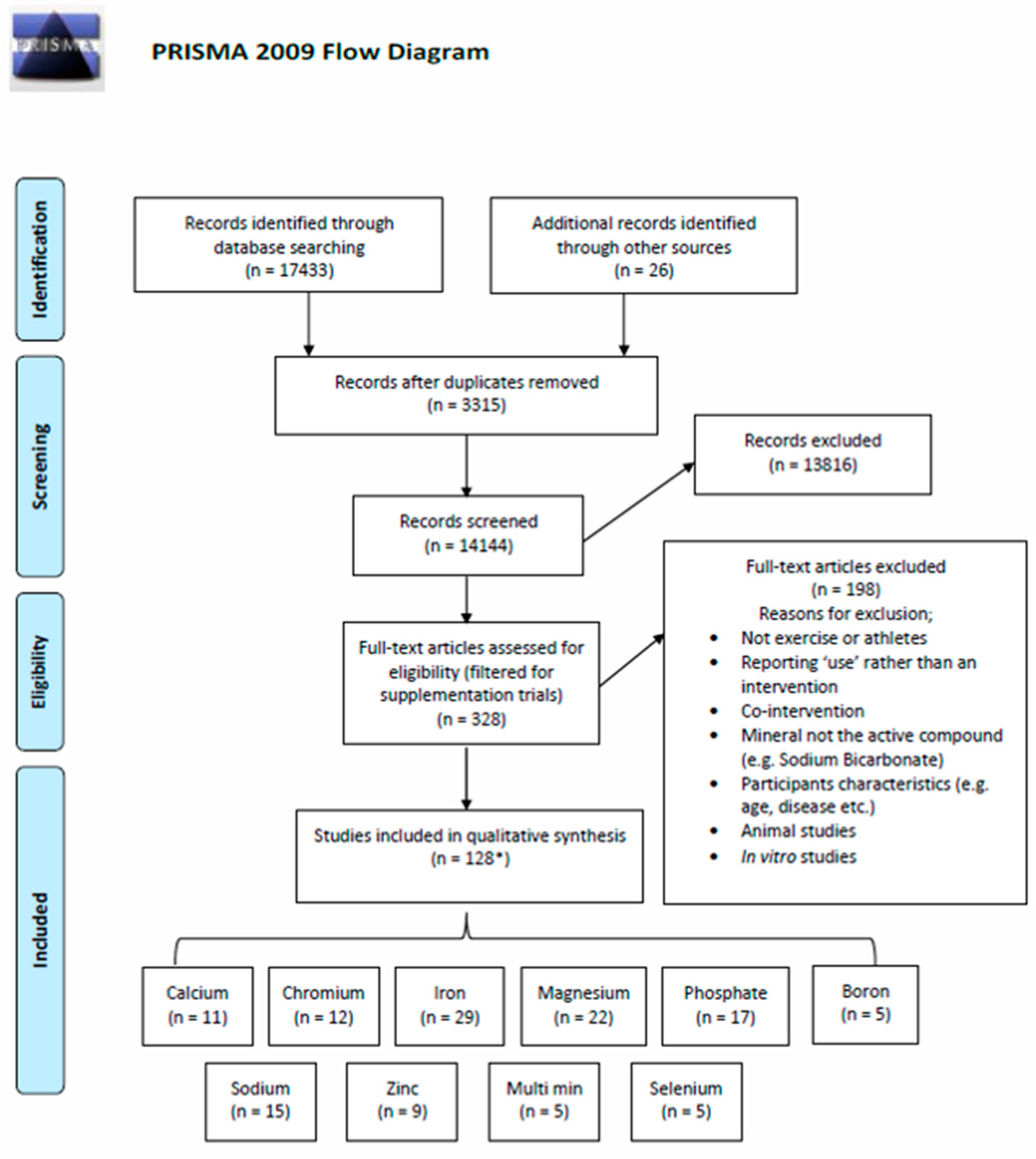
| Study selection | 17 | Give numbers of studies screened, assessed for eligibility, and included in the review, with reasons for exclusions at each stage, ideally with a flow diagram. | 5 |
| Study characteristics | 18 | For each study, present characteristics for which data were extracted (e.g., study size, PICOS, follow-up period) and provide the citations. | 5 |
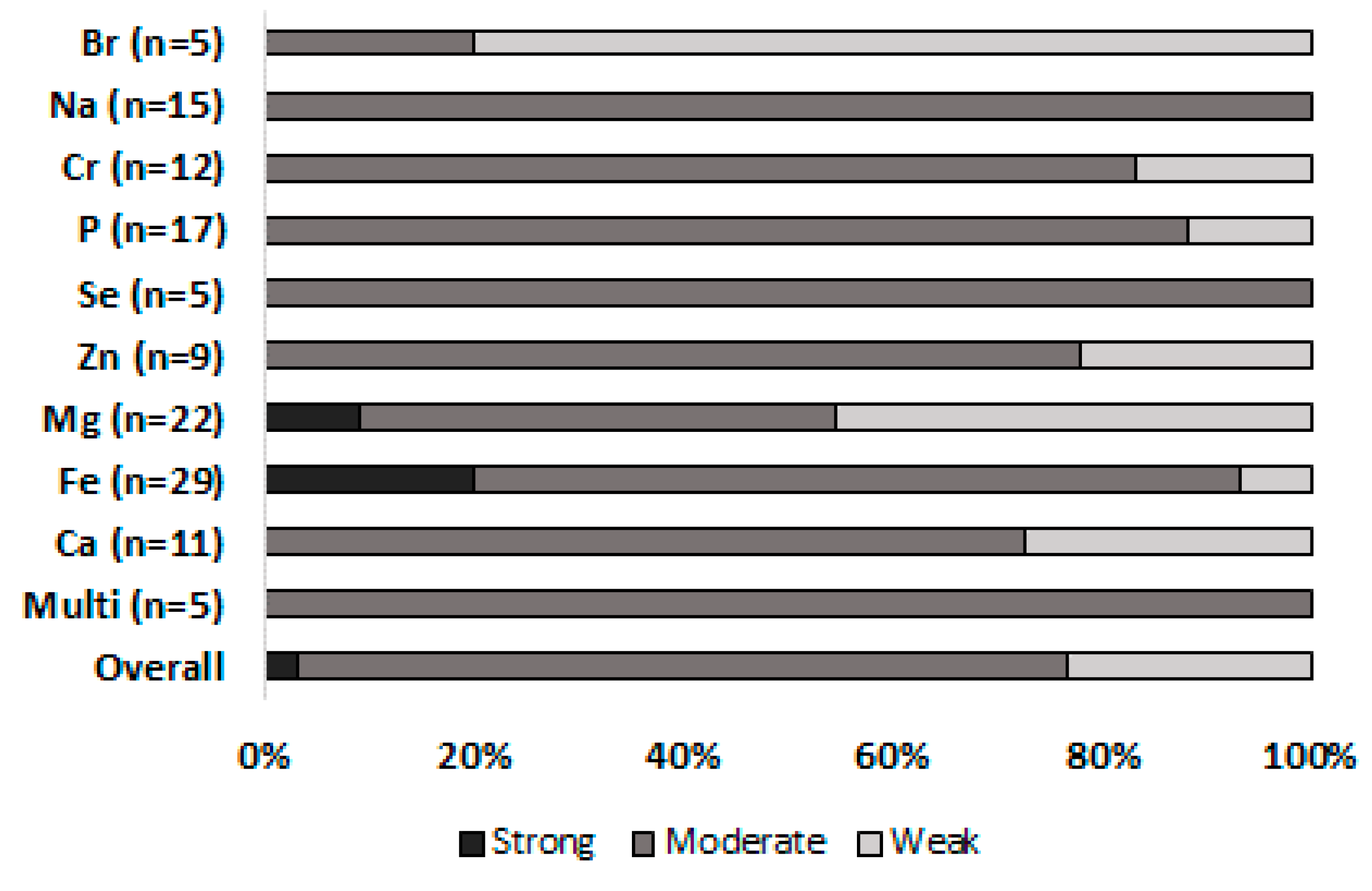
| Risk of bias within studies | 19 | Present data on risk of bias of each study and, if available, any outcome level assessment (see item 12). | Figure 2 |
| Results of individual studies | 20 | For all outcomes considered (benefits or harms), present, for each study: (a) simple summary data for each intervention group (b) effect estimates and confidence intervals, ideally with a forest plot. | Supplementary Table S1 |
| Synthesis of results | 21 | Present results of each meta-analysis done, including confidence intervals and measures of consistency. | NA |
| Risk of bias across studies | 22 | Present results of any assessment of risk of bias across studies (see Item 15). | Supplementary Table S1 |
| Additional analysis | 23 | Give results of additional analyses, if done (e.g., sensitivity or subgroup analyses, meta-regression [see Item 16]). | NA |
| DISCUSSION | |||
| Summary of evidence | 24 | Summarize the main findings including the strength of evidence for each main outcome; consider their relevance to key groups (e.g., healthcare providers, users, and policy makers). | 5–25 |
| Limitations | 25 | Discuss limitations at study and outcome level (e.g., risk of bias), and at review-level (e.g., incomplete retrieval of identified research, reporting bias). | 25 |
| Conclusions | 26 | Provide a general interpretation of the results in the context of other evidence, and implications for future research. | 25–26 |
| FUNDING | |||
| Funding | 27 | Describe sources of funding for the systematic review and other support (e.g., supply of data); role of funders for the systematic review. | 26 |
Appendix B
References
- Lazarte, C.E.; Carlsson, N.G.; Almgren, A.; Sandberg, A.S.; Granfeldt, Y. Phytate, zinc, iron and calcium content of common Bolivian food, and implications for mineral bioavailability. J. Food Compos. Anal. 2015, 39, 111–119. [Google Scholar] [CrossRef]
- Gibson, R.S.; Bailey, K.B.; Gibbs, M.; Ferguson, E.L. A review of phytate, iron, zinc, and calcium concentrations in plant-based complementary foods used in low-income countries and implications for bioavailability. Food Nutr. Bull. 2010, 31, S134–S146. [Google Scholar] [CrossRef] [PubMed]
- Gupta, U.; Gupta, S. Sources and deficiency diseases of mineral nutrients in human health and nutrition: A review. Pedosphere 2014, 24, 13–38. [Google Scholar] [CrossRef]
- Asemi, Z.; Jamilian, M.; Mesdaghinia, E.; Esmaillzadeh, A. Effects of selenium supplementation on glucose homeostasis, inflammation, and oxidative stress in gestational diabetes: Randomized, double-blind, placebo-controlled trial. Nutrition (Burbank, Los Angeles County, Calif.) 2015, 31, 1235–1242. [Google Scholar] [CrossRef] [PubMed]
- Oropeza-Moe, M.; Wisloff, H.; Bernhoft, A. Selenium deficiency associated porcine and human cardiomyopathies. J. Trace Elem. Med. Biol. 2015, 31, 148–156. [Google Scholar] [CrossRef]
- Del Gobbo, L.C.; Imamura, F.; Wu, J.H.Y.; de Oliveira-Otto, M.C.; Chiuve, S.E.; Mozaffarian, D. Circulating and dietary magnesium and risk of cardiovascular disease: A systematic review and meta-analysis of prospective studies. Am. J. Clin. Nutr. 2013, 98, 160–173. [Google Scholar] [CrossRef]
- Cheungpasitporn, W.; Thongprayoon, C.; Mao, M.A.; Srivali, N.; Ungprasert, P.; Varothai, N.; Sanguankeo, A.; Kittanamongkolchai, W.; Erickson, S.B. Hypomagnesaemia linked to depression: A systematic review and meta-analysis. Intern. Med. J. 2015, 45, 436–440. [Google Scholar] [CrossRef] [PubMed]
- Fang, X.; Han, H.; Li, M.; Liang, C.; Fan, Z.; Aaseth, J.; He, J.; Montgomery, S.; Cao, Y. Dose-response relationship between dietary magnesium intake and risk of type 2 diabetes mellitus: A systematic review and meta-regression analysis of prospective cohort studies. Nutrients 2016, 8, 739. [Google Scholar] [CrossRef]
- Joosten, M.M.; Gansevoort, R.T.; Bakker, S.J. Low plasma magnesium and risk of developing chronic kidney disease: Results from the PREVEND Study. Kidney Int. 2015, 87, 1262–1263. [Google Scholar] [CrossRef] [PubMed]
- Shaikh, M.N.; Malapati, B.R.; Gokani, R.; Patel, B.; Chatriwala, M. Serum magnesium and vitamin D levels as indicators of asthma severity. Pulm. Med. 2016, 2016, 1–5. [Google Scholar] [CrossRef]
- Rude, R.K.; Singer, F.R.; Gruber, H.E. Skeletal and hormonal effects of magnesium deficiency. J. Am. Coll. Nutr. 2009, 28, 131–141. [Google Scholar] [CrossRef]
- Kunutsor, S.K.; Whitehouse, M.R.; Blom, A.W.; Laukkanen, J.A. Low serum magnesium levels are associated with increased risk of fractures: A long-term prospective cohort study. Eur. J. Epidemiol. 2017, 32, 593–603. [Google Scholar] [CrossRef]
- Zhang, Y.; Xun, P.; Wang, R.; Mao, L.; He, K. Can Magnesium Enhance Exercise Performance? Nutrients 2017, 9, 946. [Google Scholar] [CrossRef]
- Speich, M.; Pineau, A.; Ballereau, F. Minerals, trace elements and related biological variables in athletes and during physical activity. Clin. Chim. Acta 2001, 312, 1–11. [Google Scholar] [CrossRef]
- Williams, M.H. Dietary supplements and sports performance: Minerals. J. Int. Soc. Sport Nutr. 2005, 2, 43–49. [Google Scholar] [CrossRef] [PubMed]
- Kreider, R.B.; Wilborn, C.D.; Taylor, L.; Campbell, B.; Almada, A.L.; Collins, R.; Cooke, M.; Earnest, C.P.; Greenwood, M.; Kalman, D.S. ISSN exercise and sport nutrition review: Research and recommendations. J. Int. Soc. Sports Nutr. 2010, 7–50, 7. [Google Scholar] [CrossRef]
- Misner, B. Food alone may not provide sufficient micronutrients for preventing deficiency. J. Int. Soc. Sports Nutr. 2006, 3, 51–55. [Google Scholar] [CrossRef]
- Kerksick, C.M.; Wilborn, C.D.; Roberts, M.D.; Smith-Ryan, A.; Kleiner, S.M.; Jager, R.; Collins, R.; Cooke, M.; Davis, J.N.; Galvan, E.; et al. ISSN exercise & sports nutrition review update: Research & recommendations. J. Int. Soc. Sports Nutr. 2018, 15, 38. [Google Scholar] [PubMed]
- Bailey, R.L.; West, K.P.; Black, R.E. The epidemiology of global micronutrient deficiencies. Ann. Nutr. Metab. 2015, 66, 22–33. [Google Scholar] [CrossRef] [PubMed]
- Kumssa, D.B.; Joy, E.J.; Ander, E.L.; Watts, M.J.; Young, S.D.; Walker, S.; Broadley, M.R. Dietary calcium and zinc deficiency risks are decreasing but remain prevalent. Sci. Rep. 2015, 5, 10974. [Google Scholar] [CrossRef]
- Gröber, U.; Schmidt, J.; Kisters, K. Magnesium in Prevention and Therapy. Nutrients 2015, 7, 8199–8226. [Google Scholar] [CrossRef] [PubMed]
- Moshfegh, A.; Goldman, J.; Ahuja, J.; Rhodes, D.; LaComb, R. What We Eat in America, NHANES 2005–2006: Usual Nutrient Intakes from Food and Water Compared to 1997 Dietary Reference Intakes for Vitamin D, Calcium, Phosphorus, and Magnesium; USDA: Washington, DC, USA, 2009. Available online: http://www.ars.usda.gov/ba/bhnrc/fsrg (accessed on 18 February 2018).
- Calton, J.B. Prevalence of micronutrient deficiency in popular diet plans. J. Int. Soc. Sports Nutr. 2010, 7, 24. [Google Scholar] [CrossRef]
- Engel, M.; Kern, H.; Brenna, J.T.; Mitmesser, S. Micronutrient gaps in three commercial weight-loss diet plans. Nutrients 2018, 10, 108. [Google Scholar] [CrossRef] [PubMed]
- Kristensen, N.B.; Madsen, M.L.; Hansen, T.H.; Allin, K.H.; Hoppe, C.; Fagt, S.; Lausten, M.S.; Gobel, R.J.; Vestergaard, H.; Hansen, T.; et al. Intake of macro- and micronutrients in Danish vegans. Nutr. J. 2015, 14, 115. [Google Scholar] [CrossRef]
- Castro-Quezada, I.; Roman-Vinas, B.; Serra-Majem, L. The Mediterranean diet and nutritional adequacy: A review. Nutrients 2014, 6, 231–248. [Google Scholar] [CrossRef]
- Birkenhead, K.L.; Slater, G. A Review of Factors Influencing Athletes’ Food Choices. Sports Med. 2015, 45, 1511–1522. [Google Scholar] [CrossRef] [PubMed]
- Volpe, S.L. Magnesium and the athlete. Curr. Sports Med. Rep. 2015, 14, 279–283. [Google Scholar] [CrossRef] [PubMed]
- Maynar, M.; Llerena, F.; Bartolome, I.; Alves, J.; Robles, M.C.; Grijota, F.J.; Munoz, D. Seric concentrations of copper, chromium, manganesum, nickel and selenium in aerobic, anaerobic and mixed professional sportsmen. J. Int. Soc. Sports Nutr. 2018, 15, 8. [Google Scholar] [CrossRef]
- Maynar, M.; Munoz, D.; Alves, J.; Barrientos, G.; Grijota, F.J.; Robles, M.C.; Llerena, F. Influence of an Acute Exercise Until Exhaustion on Serum and Urinary Concentrations of Molybdenum, Selenium, and Zinc in Athletes. Biol. Trace Elem. Res. 2018, 186, 1327–1329. [Google Scholar] [CrossRef]
- Wardenaar, F.C.; Ceelen, I.J.; Van Dijk, J.W.; Hangelbroek, R.W.; Van Roy, L.; Van der Pouw, B.; De Vries, J.H.; Mensink, M.; Witkamp, R.F. Nutritional supplement use by dutch elite and sub-elite athletes: Does receiving dietary counseling make a difference? Int. J. Sport Nutr. Exerc. Metab. 2017, 27, 32–42. [Google Scholar] [CrossRef]
- Harrington, J.; Perry, I.; Lutomski, J.; Morgan, K.; McGee, H.; Shelley, E.; Watson, D.; Barry, M. LÁN 2007: Survey of Lifestyle, A itudes and Nutrition in Ireland. Dietary Habits of the Irish Population. Dep Health Child 2008. Available online: http://epubs.rcsi.ie/psycholrep/6 (accessed on 18 February 2018).
- Wierniuk, A.; Wlodarek, D. Estimation of energy and nutritional intake of young men practicing aerobic sports. Roczniki Panstwowego Zakladu Higieny 2013, 64, 143–148. [Google Scholar]
- Heaney, S.; O’Connor, H.; Gifford, J.; Naughton, G. Comparison of strategies for assessing nutritional adequacy in elite female athletes’ dietary intake. Int. J. Sport Nutr. Exer. Metab. 2010, 20, 245–256. [Google Scholar] [CrossRef]
- Wardenaar, F.; Brinkmans, N.; Ceelen, I.; Van Rooij, B.; Mensink, M.; Witkamp, R.; De Vries, J. Micronutrient Intakes in 553 Dutch Elite and Sub-Elite Athletes: Prevalence of Low and High Intakes in Users and Non-Users of Nutritional Supplements. Nutrients 2017, 9, 142. [Google Scholar] [CrossRef]
- Mir-Marques, A.; Cervera, M.L.; de la Guardia, M. Mineral analysis of human diets by spectrometry methods. TrAC Trend Anal. Chem. 2016, 82, 457–467. [Google Scholar] [CrossRef]
- Freeland-Graves, J.H.; Sanjeevi, N.; Lee, J.J. Global perspectives on trace element requirements. J. Trace Elem. Med. Biol. 2015, 31, 135–141. [Google Scholar] [CrossRef]
- Larson-Meyer, D.E.; Woolf, K.; Burke, L. Assessment of Nutrient Status in Athletes and the Need for Supplementation. Int. J. Sport Nutr. Exerc. Metab. 2018, 28, 139–158. [Google Scholar] [CrossRef] [PubMed]
- Lee, N. A review of magnesium, iron, and zinc supplementation effects on athletic performance. Korean J. Phys. Edu. 2017, 56, 797–806. [Google Scholar] [CrossRef]
- Knapik, J.J.; Steelman, R.A.; Hoedebecke, S.S.; Austin, K.G.; Farina, E.K.; Lieberman, H.R. Prevalence of dietary supplement use by athletes: Systematic review and meta-analysis. Sports Med. 2016, 46, 103–123. [Google Scholar] [CrossRef] [PubMed]
- Chu, A.; Holdaway, C.; Varma, T.; Petocz, P.; Samman, S. Lower serum zinc concentration despite higher dietary zinc intake in athletes: A systematic review and meta-analysis. Sports Med. 2018, 48, 327–336. [Google Scholar] [CrossRef] [PubMed]
- Burden, R.J.; Morton, K.; Richards, T.; Whyte, G.P.; Pedlar, C.R. Is iron treatment beneficial in, iron-deficient but non-anaemic (IDNA) endurance athletes? a systematic review and meta-analysis. Br. J. Sports Med. 2015, 49, 1389–1397. [Google Scholar] [CrossRef] [PubMed]
- Rubeor, A.; Goojha, C.; Manning, J.; White, J. Does iron supplementation improve performance in iron-deficient nonanemic athletes? Sports Health 2018. Ahead of print. [Google Scholar] [CrossRef]
- Peeling, P.; Binnie, M.J.; Goods, P.S.R.; Sim, M.; Burke, L.M. Evidence-based supplements for the enhancement of athletic performance. Int. J. Sport Nutr. Exerc. Metab. 2018, 28, 178–187. [Google Scholar] [CrossRef]
- Newhouse, I.J.; Finstad, E.W. The effects of magnesium supplementation on exercise performance. Clin. J. Sport Med. 2000, 10, 195–200. [Google Scholar] [CrossRef]
- Sieja, K.; von Mach-Szczypińska, J.; Kois, N.; Ler, P.; Piechanowska, K.; Stolarska, M. Influence of selenium on oxidative stress in athletes. Cent. Eur. J. Sport Sci. Med. 2016, 14, 87–92. [Google Scholar]
- Rayssiguier, Y.; Guezennec, C.Y.; Durlach, J. New experimental and clinical data on the relationship between magnesium and sport. Magn. Res. 1990, 3, 93–102. [Google Scholar]
- Armstrong, L.E.; Maresh, C.M. Vitamin and mineral supplements as nutritional aids to exercise performance and health. Nutr. Rev. 1996, 54, S149–S158. [Google Scholar] [CrossRef] [PubMed]
- Liberati, A.; Altman, D.G.; Tetzlaff, J.; Mulrow, C.; Gotzsche, P.C.; Ioannidis, J.P.; Clarke, M.; Devereaux, P.J.; Kleijnen, J.; Moher, D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: Explanation and elaboration. PLos Med. 2009, 6, e1000100. [Google Scholar] [CrossRef] [PubMed]
- O’Connor, D.; Green, S.; Higgins, J.P. Defining the Review Question and Developing Criteria for Including Studies; Cochrane Book Series; Wiley Online Library: Hoboken, NJ, USA, 2008; pp. 81–94. [Google Scholar]
- Shea, K.L.; Barry, D.W.; Sherk, V.D.; Hansen, K.C.; Wolfe, P.; Kohrt, W.M. Calcium supplementation and parathyroid hormone response to vigorous walking in postmenopausal women. Med. Sci. Sports Exerc. 2014, 46, 2007–2013. [Google Scholar] [CrossRef] [PubMed]
- Moëzzi, N.; Peeri, M.; Matin, H. Effects of zinc, magnesium and vitamin B6 supplementation on hormones and performance in weightlifters. Ann. Biol. Res. 2013, 4, 163–168. [Google Scholar]
- Thomas, B.; Ciliska, D.; Dobbins, M.; Micucci, S. A process for systematically reviewing the literature: Providing the research evidence for public health nursing interventions. Worldv. Evid. Based Nurs. 2004, 1, 176–184. [Google Scholar] [CrossRef]
- DellaValle, D.M.; Haas, J.D. Iron supplementation improves energetic efficiency in iron-depleted female rowers. Med. Sci. Sports Exerc. 2014, 46, 1204–1215. [Google Scholar] [CrossRef] [PubMed]
- Villanueva, J.; Soria, M.; Gonzalez-Haro, C.; Ezquerra, L.; Nieto, J.L.; Escanero, J.F. Oral iron treatment has a positive effect on iron metabolism in elite soccer players. Biol. Trace Elem. Res. 2011, 142, 398–406. [Google Scholar] [CrossRef] [PubMed]
- Blee, T.; Goodman, C.; Dawson, B.; Stapff, A. The effect of intramuscular iron injections on serum ferritin levels and physical performance in elite netballers. J. Sci. Med. Sport 1999, 2, 311–321. [Google Scholar] [CrossRef]
- Powell, P.D.; Tucker, A. Iron supplementation and running performance in female cross-country runners. Int. J. Sports Med. 1991, 12, 462–467. [Google Scholar] [CrossRef] [PubMed]
- Ashenden, M.J.; Pyne, D.B.; Parisotto, R.; Dobson, G.P.; Hahn, A.G. Can reticulocyte parameters be of use in detecting iron deficient erythropoiesis in female athletes? J. Sports Med. Phys. Fit. 1999, 39, 140–146. [Google Scholar]
- Haymes, E.M.; Puhl, J.L.; Temples, T.E. Training for cross-country skiing and iron status. Med. Sci. Sports Exerc. 1986, 18, 162–167. [Google Scholar] [CrossRef] [PubMed]
- Govus, A.D.; Garvican-Lewis, L.A.; Abbiss, C.R.; Peeling, P.; Gore, C.J. Pre-altitude serum ferritin levels and daily oral iron supplement dose mediate iron parameter and hemoglobin mass responses to altitude exposure. PLoS ONE 2015, 10, e0135120. [Google Scholar] [CrossRef] [PubMed]
- Flynn, M.G.; Mackinnon, L.; Gedge, V.; Fahlman, M.; Brickman, T. Influence of iron status and iron supplements on natural killer cell activity in trained women runners. Int. J. Sports Med. 2003, 24, 217–222. [Google Scholar] [CrossRef] [PubMed]
- Hinton, P.S.; Sinclair, L.M. Iron supplementation maintains ventilatory threshold and improves energetic efficiency in iron-deficient nonanemic athletes. Eur. J. Clin. Nutr. 2007, 61, 30–39. [Google Scholar] [CrossRef]
- LaManca, J.J.; Haymes, E.M. Effects of iron repletion on VO2max, endurance, and blood lactate in women. Med. Sci. Sports Exerc. 1993, 25, 1386–1392. [Google Scholar] [CrossRef]
- Dressendorfer, R.H.; Keen, C.L.; Wade, C.E.; Claybaugh, J.R.; Timmis, G.C. Development of runner’s anemia during a 20-day road race: Effect of iron supplements. Int. J. Sports Med. 1991, 12, 332–336. [Google Scholar] [CrossRef] [PubMed]
- Friedmann, B.; Jost, J.; Rating, T.; Weller, E.; Werle, E.; Eckardt, K.U.; Bartsch, P.; Mairbaurl, H. Effects of iron supplementation on total body hemoglobin during endurance training at moderate altitude. Int. J. Sports Med. 1999, 20, 78–85. [Google Scholar] [CrossRef]
- Jensen, C.A.; Weaver, C.M.; Sedlock, D.A. Iron supplementation and iron status in exercising young women. J. Nutr. Biochem. 1991, 2, 368–373. [Google Scholar] [CrossRef]
- Fogelholm, M.; Jaakkola, L.; Lampisjarvi, T. Effects of iron supplementation in female athletes with low serum ferritin concentration. Int. J. Sports Med. 1992, 13, 158–162. [Google Scholar] [CrossRef] [PubMed]
- Klingshirn, L.A.; Pate, R.R.; Bourque, S.P.; Davis, J.M.; Sargent, R.G. Effect of iron supplementation on endurance capacity in iron-depleted female runners. Med. Sci. Sports Exerc. 1992, 24, 819–824. [Google Scholar] [CrossRef]
- Hegenauer, J.; Strause, L.; Saltman, P.; Dann, D.; White, J.; Green, R. Transitory hematologic effects of moderate exercise are not influenced by iron supplementation. Eur. J. Appl. Physiol. Occup. Physiol. 1983, 52, 57–61. [Google Scholar] [CrossRef]
- Magazanik, A.; Weinstein, Y.; Abarbanel, J.; Lewinski, U.; Shapiro, Y.; Inbar, O.; Epstein, S. Effect of an iron supplement on body iron status and aerobic capacity of young training women. Eur. J. Appl. Physiol. Occup. Physiol. 1991, 62, 317–323. [Google Scholar] [CrossRef]
- Hinton, P.S.; Giordano, C.; Brownlie, T.; Haas, J.D. Iron supplementation improves endurance after training in iron-depleted, nonanemic women. J. Appl. Physiol. 2000, 88, 1103–1111. [Google Scholar] [CrossRef]
- McClung, J.P.; Karl, J.P.; Cable, S.J.; Williams, K.W.; Nindl, B.C.; Young, A.J.; Lieberman, H.R. Randomized, double-blind, placebo-controlled trial of iron supplementation in female soldiers during military training: Effects on iron status, physical performance, and mood. Am. J. Clin. Nutr. 2009, 90, 124–131. [Google Scholar] [CrossRef]
- Yoshida, T.; Udo, M.; Chida, M.; Ichioka, M.; Makiguchi, K. Dietary iron supplement during severe physical training in competitive female distance runners. Res. Sport Med. 1990, 1, 279–285. [Google Scholar] [CrossRef]
- Brutsaert, T.D.; Hernandez-Cordero, S.; Rivera, J.; Viola, T.; Hughes, G.; Haas, J.D. Iron supplementation improves progressive fatigue resistance during dynamic knee extensor exercise in iron-depleted, nonanemic women. Am. J. Clin. Nutr. 2003, 77, 441–448. [Google Scholar] [CrossRef]
- Mielgo-Ayuso, J.; Zourdos, M.C.; Calleja-Gonzalez, J.; Urdampilleta, A.; Ostojic, S. Iron supplementation prevents a decline in iron stores and enhances strength performance in elite female volleyball players during the competitive season. Appl. Physiol. Nutr. Metab. Physiol. Appl. Nutr. Metab. 2015, 40, 615–622. [Google Scholar] [CrossRef]
- Ohira, Y.; Edgerton, V.R.; Gardner, G.W.; Senewiratne, B.; Barnard, R.J.; Simpson, D.R. Work capacity, heart rate and blood lactate responses to iron treatment. Br. J. Haematol. 1979, 41, 365–372. [Google Scholar] [CrossRef]
- Garvican, L.A.; Saunders, P.U.; Cardoso, T.; Macdougall, I.C.; Lobigs, L.M.; Fazakerley, R.; Fallon, K.E.; Anderson, B.; Anson, J.M.; Thompson, K.G.; et al. Intravenous iron supplementation in distance runners with low or suboptimal ferritin. Med. Sci. Sports Exerc. 2014, 46, 376–385. [Google Scholar] [CrossRef] [PubMed]
- Peeling, P.; Blee, T.; Goodman, C.; Dawson, B.; Claydon, G.; Beilby, J.; Prins, A. Effect of iron injections on aerobic-exercise performance of iron-depleted female athletes. Int. J. Sport Nutr. Exerc. Metab. 2007, 17, 221–231. [Google Scholar] [CrossRef] [PubMed]
- Woods, A.; Garvican-Lewis, L.A.; Saunders, P.U.; Lovell, G.; Hughes, D.; Fazakerley, R.; Anderson, B.; Gore, C.J.; Thompson, K.G. Four weeks of IV iron supplementation reduces perceived fatigue and mood disturbance in distance runners. PLoS ONE 2014, 9, e108042. [Google Scholar] [CrossRef]
- Townsend, R.; Elliott-Sale, K.J.; Pinto, A.J.; Thomas, C.; Scott, J.P.; Currell, K.; Fraser, W.D.; Sale, C. Parathyroid hormone secretion Is controlled by both ionized calcium and phosphate during exercise and recovery in men. J. Clin. Endocrinol. Metab. 2016, 101, 3231–3239. [Google Scholar] [CrossRef] [PubMed]
- Martin, B.R.; Davis, S.; Campbell, W.W.; Weaver, C.M. Exercise and calcium supplementation: Effects on calcium homeostasis in sportswomen. Med. Sci. Sports Exerc. 2007, 39, 1481–1486. [Google Scholar] [CrossRef] [PubMed]
- Frontera, W.R.; Ochala, J. Skeletal muscle: A brief review of structure and function. Calcif. Tissue Int. 2015, 96, 183–195. [Google Scholar] [CrossRef] [PubMed]
- Pu, F.; Chen, N.; Xue, S. Calcium intake, calcium homeostasis and health. Food Sci. Hum. Well 2016, 5, 8–16. [Google Scholar] [CrossRef]
- Zorbas, Y.G.; Petrov, K.L.; Kakurin, V.J.; Kuznetsov, N.A.; Charapakhin, K.P.; Alexeyev, I.D.; Denogradov, S.D. Calcium supplementation effect on calcium balance in endurance-trained athletes during prolonged hypokinesia and ambulatory conditions. Biol. Trace Elem. Res. 2000, 73, 231–250. [Google Scholar] [CrossRef]
- Zorbas, Y.G.; Kakurin, V.J.; Kuznetsov, N.A.; Yarullin, V.L.; Andreyev, I.D.; Charapakhin, K.P. Measurements in calcium-supplemented athletes during and after hypokinetic and ambulatory conditions. Clin. Biochem. 2000, 33, 393–404. [Google Scholar] [CrossRef]
- Kohrt, W.M.; Wherry, S.J.; Wolfe, P.; Sherk, V.D.; Wellington, T.; Swanson, C.M.; Weaver, C.M.; Boxer, R.S. Maintenance of serum ionized calcium during exercise attenuates parathyroid hormone and bone resorption responses. J. Bone Miner. Res. Off. J. Am. Soc. Bone Miner. Res. 2018, 33, 1326–1334. [Google Scholar] [CrossRef] [PubMed]
- Guillemant, J.; Accarie, C.; Peres, G.; Guillemant, S. Acute effects of an oral calcium load on markers of bone metabolism during endurance cycling exercise in male athletes. Calcif. Tissue Int. 2004, 74, 407–414. [Google Scholar] [CrossRef]
- Visser, M.; Deeg, D.J.; Lips, P. Low vitamin D and high parathyroid hormone levels as determinants of loss of muscle strength and muscle mass (sarcopenia): The Longitudinal Aging Study Amsterdam. J. Clin. Endocrinol. Metab. 2003, 88, 5766–5772. [Google Scholar] [CrossRef] [PubMed]
- Passeri, E.; Bugiardini, E.; Sansone, V.A.; Valaperta, R.; Costa, E.; Ambrosi, B.; Meola, G.; Corbetta, S. Vitamin D, parathyroid hormone and muscle impairment in myotonic dystrophies. J. Neurol. Sci. 2013, 331, 132–135. [Google Scholar] [CrossRef]
- Sikjaer, T.; Rolighed, L.; Hess, A.; Fuglsang-Frederiksen, A.; Mosekilde, L.; Rejnmark, L. Effects of PTH(1-84) therapy on muscle function and quality of life in hypoparathyroidism: Results from a randomized controlled trial. Osteoporos. Int. 2014, 25, 1717–1726. [Google Scholar] [CrossRef]
- Haakonssen, E.C.; Ross, M.L.; Knight, E.J.; Cato, L.E.; Nana, A.; Wluka, A.E.; Cicuttini, F.M.; Wang, B.H.; Jenkins, D.G.; Burke, L.M. The effects of a calcium-rich pre-exercise meal on biomarkers of calcium homeostasis in competitive female cyclists: A randomised crossover trial. PLoS ONE 2015, 10, e0123302. [Google Scholar] [CrossRef] [PubMed]
- Cinar, V.; Baltaci, A.K.; Mogulkoc, R.; Kilic, M. Testosterone levels in athletes at rest and exhaustion: Effects of calcium supplementation. Biol. Trace Elem. Res. 2009, 129, 65–69. [Google Scholar] [CrossRef]
- Cinar, V.; Baltaci, A.K.; Mogulkoc, R. Effect of exhausting exercise and calcium supplementation on potassium, magnesium, copper, zinc and calcium levels in athletes. Pak. J. Med. Sci. 2009, 25, 238–242. [Google Scholar]
- Cinar, V.; Mogulkoc, R.; Baltaci, A.K.; Bostanci, O. Effects of calcium supplementation on glucose and insulin levels of athletes at rest and after exercise. Biol. Trace Elem. Res. 2010, 133, 29–33. [Google Scholar] [CrossRef]
- Cinar, V.; Mogulkoc, R.; Baltaci, A.K. Calcium supplementation and 4-week exercise on blood parameters of athletes at rest and exhaustion. Biol. Trace Elem. Res. 2010, 134, 130–135. [Google Scholar] [CrossRef]
- Cinar, V.; Cakmakci, O.; Mogulkoc, R.; Baltaci, A.K. Effects of exhaustion and calcium supplementation on adrenocorticotropic hormone and cortisol levels in athletes. Biol. Trace Elem. Res. 2009, 127, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Farrell, P.A.; Garthwaite, T.L.; Gustafson, A.B. Plasma adrenocorticotropin and cortisol responses to submaximal and exhaustive exercise. J. Appl. Physiol. Respir. Environ. Exerc. Physiol. 1983, 55, 1441–1444. [Google Scholar] [CrossRef] [PubMed]
- Burt, M.G.; Mangelsdorf, B.L.; Srivastava, D.; Petersons, C.J. Acute effect of calcium citrate on serum calcium and cardiovascular function. J. Bone Miner. Res. Off. J. Am. Soc. Bone Miner. Res. 2013, 28, 412–418. [Google Scholar] [CrossRef]
- Rios, E. Calcium-induced release of calcium in muscle: 50 years of work and the emerging consensus. J. Gener. Physiol. 2018, 150, 521–537. [Google Scholar] [CrossRef] [PubMed]
- Welch, A.A.; Kelaiditi, E.; Jennings, A.; Steves, C.J.; Spector, T.D.; MacGregor, A. Dietary magnesium Is positively associated with skeletal muscle power and indices of muscle mass and may attenuate the association between circulating C-Reactive Protein and muscle mass in women. J. Bone Miner. Res. Off. J. Am. Soc. Bone Miner. Res. 2016, 31, 317–325. [Google Scholar] [CrossRef]
- Welch, A.A.; Skinner, J.; Hickson, M. Dietary magnesium may be protective for aging of bone and skeletal muscle in middle and younger older age men and women: Cross-sectional findings from the UK biobank cohort. Nutrients 2017, 9, 1189. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Chen, C.; Liu, W.; Zhou, T.; Xun, P.; He, K.; Chen, P. The effect of magnesium supplementation on muscle fitness: A meta-analysis and systematic review. Magn. Res. 2017, 30, 120–132. [Google Scholar]
- Brilla, L.R.; Haley, T.F. Effect of magnesium supplementation on strength training in humans. J. Am. Coll. Nutr. 1992, 11, 326–329. [Google Scholar] [CrossRef]
- Dmitrasinovic, G.; Pesic, V.; Stanic, D.; Plecas-Solarovic, B.; Dajak, M.; Ignjatovic, S. ACTH, cortisol and IL-6 Levels in athletes following magnesium supplementation. J. Med. Biochem. 2016, 35, 375–384. [Google Scholar] [CrossRef]
- Kass, L.S.; Skinner, P.; Poeira, F. A pilot study on the effects of magnesium supplementation with high and low habitual dietary magnesium intake on resting and recovery from aerobic and resistance exercise and systolic blood pressure. J. Sports Sci. Med. 2013, 12, 144–150. [Google Scholar] [PubMed]
- Finstad, E.W.; Newhouse, I.J.; Lukaski, H.C.; McAuliffe, J.E.; Stewart, C.R. The effects of magnesium supplementation on exercise performance. Med. Sci. Sports Exerc. 2001, 33, 493–498. [Google Scholar] [CrossRef] [PubMed]
- Setaro, L.; Santos-Silva, P.R.; Nakano, E.Y.; Sales, C.H.; Nunes, N.; Greve, J.M.; Colli, C. Magnesium status and the physical performance of volleyball players: Effects of magnesium supplementation. J. Sports Sci. 2014, 32, 438–445. [Google Scholar] [CrossRef]
- Zorbas, Y.G.; Kakurin, V.J.; Afonin, V.B.; Charapakhin, K.P.; Denogradov, S.D. Magnesium supplements’ effect on magnesium balance in athletes during prolonged restriction of muscular activity. Kidney Blood Press. Res. 1999, 22, 146–153. [Google Scholar] [CrossRef]
- Molina-Lopez, J.; Molina, J.M.; Chirosa, L.J.; Florea, D.; Saez, L.; Millan, E.; Planells, E. Association between erythrocyte concentrations of magnesium and zinc in high-performance handball players after dietary magnesium supplementation. Magn. Res. 2012, 25, 79–88. [Google Scholar]
- Cordova Martinez, A.; Fernandez-Lazaro, D.; Mielgo-Ayuso, J.; Seco Calvo, J.; Caballero Garcia, A. Effect of magnesium supplementation on muscular damage markers in basketball players during a full season. Magn. Res. 2017, 30, 61–70. [Google Scholar]
- Porta, S.; Gell, H.; Pichlkastner, K.; Porta, J.; von Ehrlich, B.; Vormann, J.; Stossier, H.; Kisters, K. A system of changes of ionized blood Mg through sports and supplementation. Trace Elem. Electrolytes 2013, 30, 105–107. [Google Scholar] [CrossRef]
- Zorbas, Y.G.; Kakurin, A.G.; Kuznetsov, N.K.; Federov, M.A.; Yaroshenko, Y.Y. Magnesium loading effect on magnesium deficiency in endurance-trained subjects during prolonged restriction of muscular activity. Biol. Trace Elem. Res. 1998, 63, 149–166. [Google Scholar] [CrossRef] [PubMed]
- Terblanche, S.; Noakes, T.D.; Dennis, S.C.; Marais, D.; Eckert, M. Failure of magnesium supplementation to influence marathon running performance or recovery in magnesium-replete subjects. Int. J. Sport Nutr. 1992, 2, 154–164. [Google Scholar] [CrossRef] [PubMed]
- Weller, E.; Bachert, P.; Meinck, H.M.; Friedmann, B.; Bartsch, P.; Mairbaurl, H. Lack of effect of oral Mg-supplementation on Mg in serum, blood cells, and calf muscle. Med. Sci. Sports Exerc. 1998, 30, 1584–1591. [Google Scholar] [CrossRef]
- Darooghegi Mofrad, M.; Djafarian, K.; Mozaffari, H.; Shab-Bidar, S. Effect of magnesium supplementation on endothelial function: A systematic review and meta-analysis of randomized controlled trials. Atherosclerosis 2018, 273, 98–105. [Google Scholar] [CrossRef] [PubMed]
- Kass, L.S.; Poeira, F. The effect of acute vs chronic magnesium supplementation on exercise and recovery on resistance exercise, blood pressure and total peripheral resistance on normotensive adults. J. Int. Soc. Sports Nutr. 2015, 12, 19. [Google Scholar] [CrossRef]
- Veronese, N.; Berton, L.; Carraro, S.; Bolzetta, F.; De Rui, M.; Perissinotto, E.; Toffanello, E.D.; Bano, G.; Pizzato, S.; Miotto, F.; et al. Effect of oral magnesium supplementation on physical performance in healthy elderly women involved in a weekly exercise program: A randomized controlled trial. Am. J. Clin. Nutr. 2014, 100, 974–981. [Google Scholar] [CrossRef]
- Petrovic, J.; Stanic, D.; Dmitrasinovic, G.; Plecas-Solarovic, B.; Ignjatovic, S.; Batinic, B.; Popovic, D.; Pesic, V. Magnesium supplementation diminishes peripheral blood lymphocyte DNA oxidative damage in athletes and sedentary young man. Oxid. Med. Cell. Longev. 2016, 2016, 2019643. [Google Scholar] [CrossRef] [PubMed]
- Margaritelis, N.V.; Theodorou, A.A.; Paschalis, V.; Veskoukis, A.S.; Dipla, K.; Zafeiridis, A.; Panayiotou, G.; Vrabas, I.S.; Kyparos, A.; Nikolaidis, M.G. Adaptations to endurance training depend on exercise-induced oxidative stress: Exploiting redox interindividual variability. Acta Physiol. 2018, 222, e12898. [Google Scholar] [CrossRef]
- Hawley, J.A.; Lundby, C.; Cotter, J.D.; Burke, L.M. Maximizing cellular adaptation to endurance exercise in skeletal muscle. Cell Metab. 2018, 27, 962–976. [Google Scholar] [CrossRef]
- Jaimovich, E.; Casas, M. Evaluating the essential role of RONS in vivo in exercised human muscle. Acta Physiol. 2018, 222, e12972. [Google Scholar] [CrossRef] [PubMed]
- Cinar, V.; Polat, Y.; Baltaci, A.K.; Mogulkoc, R. Effects of magnesium supplementation on testosterone levels of athletes and sedentary subjects at rest and after exhaustion. Biol. Trace Elem. Res. 2011, 140, 18–23. [Google Scholar] [CrossRef]
- Cinar, V.; Mogulkoc, R.; Baltaci, A.K.; Nizamlioglu, M. Effect of magnesium supplementation on some plasma elements in athletes at rest and exhaustion. Biol. Trace Elem. Res. 2007, 119, 97–102. [Google Scholar] [CrossRef] [PubMed]
- Cinar, V.; Nizamlioglu, M.; Mogulkoc, R.; Baltaci, A.K. Effects of magnesium supplementation on blood parameters of athletes at rest and after exercise. Biol. Trace Elem. Res. 2007, 115, 205–212. [Google Scholar] [CrossRef]
- Cinar, V.; Mogulkoc, R.; Baltaci, A.K.; Polat, Y. Adrenocorticotropic hormone and cortisol levels in athletes and sedentary subjects at rest and exhaustion: Effects of magnesium supplementation. Biol. Trace Elem. Res. 2008, 121, 215–220. [Google Scholar] [CrossRef] [PubMed]
- Cinar, V.; Polat, Y.; Mogulkoc, R.; Nizamlioglu, M.; Baltaci, A.K. The effect of magnesium supplementation on glucose and insulin levels of tae-kwan-do sportsmen and sedentary subjects. Pak. J. Pharm. Sci. 2008, 21, 237–240. [Google Scholar] [PubMed]
- Parazzini, F.; Di Martino, M.; Pellegrino, P. Magnesium in the gynecological practice: A literature review. Magn. Res. 2017, 30, 1–7. [Google Scholar]
- Kopec, B.J.; Dawson, B.T.; Buck, C.; Wallman, K.E. Effects of sodium phosphate and caffeine ingestion on repeated-sprint ability in male athletes. J. Sci. Med. Sport 2016, 19, 272–276. [Google Scholar] [CrossRef]
- Buck, C.; Guelfi, K.; Dawson, B.; McNaughton, L.; Wallman, K. Effects of sodium phosphate and caffeine loading on repeated-sprint ability. J. Sports Sci. 2015, 33, 1971–1979. [Google Scholar] [CrossRef] [PubMed]
- Buck, C.L.; Henry, T.; Guelfi, K.; Dawson, B.; McNaughton, L.R.; Wallman, K. Effects of sodium phosphate and beetroot juice supplementation on repeated-sprint ability in females. Eur. J. Appl. Physiol. 2015, 115, 2205–2213. [Google Scholar] [CrossRef]
- Brewer, C.P.; Dawson, B.; Wallman, K.E.; Guelfi, K.J. Effect of sodium phosphate supplementation on repeated high-intensity cycling efforts. J. Sports Sci. 2015, 33, 1109–1116. [Google Scholar] [CrossRef] [PubMed]
- Folland, J.P.; Stern, R.; Brickley, G. Sodium phosphate loading improves laboratory cycling time-trial performance in trained cyclists. J. Sci. Med. Sport 2008, 11, 464–468. [Google Scholar] [CrossRef]
- Brewer, C.P.; Dawson, B.; Wallman, K.E.; Guelfi, K.J. Effect of repeated sodium phosphate loading on cycling time-trial performance and VO2peak. Int. J. Sport Nutr. Exerc. Metab. 2013, 23, 187–194. [Google Scholar] [CrossRef]
- Goss, F.; Robertson, R.; Riechman, S.; Zoeller, R.; Dabayebeh, I.D.; Moyna, N.; Boer, N.; Peoples, J.; Metz, K. Effect of potassium phosphate supplementation on perceptual and physiological responses to maximal graded exercise. Int. J. Sport Nutr. Exerc. Metab. 2001, 11, 53–62. [Google Scholar] [CrossRef]
- Kreider, R.B.; Miller, G.W.; Schenck, D.; Cortes, C.W.; Miriel, V.; Somma, C.T.; Rowland, P.; Turner, C.; Hill, D. Effects of phosphate loading on metabolic and myocardial responses to maximal and endurance exercise. Int. J. Sport Nutr. 1992, 2, 20–47. [Google Scholar] [CrossRef] [PubMed]
- Cubbon, R.M.; Thomas, C.H.; Drozd, M.; Gierula, J.; Jamil, H.A.; Byrom, R.; Barth, J.H.; Kearney, M.T.; Witte, K.K. Calcium, phosphate and calcium phosphate product are markers of outcome in patients with chronic heart failure. J. Nephrol. 2015, 28, 209–215. [Google Scholar] [CrossRef] [PubMed]
- Ye, M.; Tian, N.; Liu, Y.; Li, W.; Lin, H.; Fan, R.; Li, C.; Liu, D.; Yao, F. High Serum Phosphorus Level Is Associated with Left Ventricular Diastolic Dysfunction in Peritoneal Dialysis Patients. PLoS ONE 2016, 11, e0163659. [Google Scholar] [CrossRef] [PubMed]
- Keskek, S.O.; Sagliker, Y.; Kirim, S.; Icen, Y.K.; Yildirim, A. Low serum phosphorus level in massry’s phosphate depletion syndrome may be one of the causes of acute heart failure. J. Nutr. Sci. Vitaminol. 2015, 61, 460–464. [Google Scholar] [CrossRef]
- Amin, M.; Fawzy, A.; Hamid, M.A.; Elhendy, A. Pulmonary hypertension in patients with chronic renal failure: Role of parathyroid hormone and pulmonary artery calcifications. Chest 2003, 124, 2093–2097. [Google Scholar] [CrossRef]
- Kamiyama, Y.; Suzuki, H.; Yamada, S.; Kaneshiro, T.; Takeishi, Y. Serum phosphate levels reflect responses to cardiac resynchronization therapy in chronic heart failure patients. J. Arrhythm. 2015, 31, 38–42. [Google Scholar] [CrossRef]
- Czuba, M.; Zając, A.; Poprzecki, S.; Cholewa, J. The influence of sodium phosphate supplementation on VO2max, serum 2, 3-diphosphoglycerate level and heart rate in off-road cyclists. J. Hum. Kinet. 2008, 19, 149–164. [Google Scholar] [CrossRef]
- Czuba, M.; Zajac, A.; Poprzecki, S.; Cholewa, J.; Woska, S. Effects of sodium phosphate loading on aerobic power and capacity in off road cyclists. J. Sports Sci. Med. 2009, 8, 591–599. [Google Scholar] [PubMed]
- Cade, R.; Conte, M.; Zauner, C.; Mars, D.; Peterson, J.; Lunne, D.; Hommen, N.; Packer, D. Effects of phosphate loading on 2,3-diphosphoglycerate and maximal oxygen uptake. Med. Sci. Sports Exerc. 1984, 16, 263–268. [Google Scholar] [CrossRef] [PubMed]
- Stewart, I.; McNaughton, L.; Davies, P.; Tristram, S. Phosphate loading and the effects on VO2max in trained cyclists. Res. Q. Exerc. Sport 1990, 61, 80–84. [Google Scholar] [CrossRef] [PubMed]
- West, J.S.; Ayton, T.; Wallman, K.E.; Guelfi, K.J. The effect of 6 days of sodium phosphate supplementation on appetite, energy intake, and aerobic capacity in trained men and women. Int. J. Sport Nutr. Exerc. Metab. 2012, 22, 422–429. [Google Scholar] [CrossRef] [PubMed]
- Buck, C.L.; Dawson, B.; Guelfi, K.J.; McNaughton, L.; Wallman, K.E. Sodium phosphate supplementation and time trial performance in female cyclists. J. Sports Sci. Med. 2014, 13, 469–475. [Google Scholar] [PubMed]
- Duffy, D.J.; Conlee, R.K. Effects of phosphate loading on leg power and high intensity treadmill exercise. Med. Sci. Sports Exerc. 1986, 18, 674–677. [Google Scholar] [CrossRef] [PubMed]
- Galloway, S.D.; Tremblay, M.S.; Sexsmith, J.R.; Roberts, C.J. The effects of acute phosphate supplementation in subjects of different aerobic fitness levels. Eur. J. Appl. Physiol. Occup. Physiol. 1996, 72, 224–230. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Failla, M.L.; Deuster, P.A. Exercise-induced changes in immune function: Effects of zinc supplementation. J. Appl. Physiol. 1994, 76, 2298–2303. [Google Scholar] [CrossRef]
- Kilic, M.; Baltaci, A.K.; Gunay, M. Effect of zinc supplementation on hematological parameters in athletes. Biol. Trace Elem. Res. 2004, 100, 31–38. [Google Scholar] [CrossRef]
- Polat, Y. Effects of zinc supplementation on hematological parameters of high performance athletes. Afr. J. Pharm. Pharmacol. 2011, 5, 1436–1440. [Google Scholar] [CrossRef]
- Saeedy, M.; Bijeh, N.; Moazzami, M. The effect of six weeks of high-intensity interval training with and without zinc supplementation on aerobic power and anaerobic power in female futsal players. Int. J. Appl. Exerc. Phys. 2016, 5, 1–10. [Google Scholar]
- Davison, G.; Marchbank, T.; March, D.S.; Thatcher, R.; Playford, R.J. Zinc carnosine works with bovine colostrum in truncating heavy exercise-induced increase in gut permeability in healthy volunteers. Am. J. Clin. Nutr. 2016, 104, 526–536. [Google Scholar] [CrossRef]
- Khaled, S.; Brun, J.F.; Cassanas, G.; Bardet, L.; Orsetti, A. Effects of zinc supplementation on blood rheology during exercise. Clin. Hemorheol. Microcirc. 1999, 20, 1–10. [Google Scholar] [PubMed]
- Smith, M.M.; Lucas, A.R.; Hamlin, R.L.; Devor, S.T. Associations among hemorheological factors and maximal oxygen consumption. Is there a role for blood viscosity in explaining athletic performance? Clin. Hemorheol. Microcirc. 2015, 60, 347–362. [Google Scholar] [CrossRef] [PubMed]
- Marques, L.F.; Donangelo, C.M.; Franco, J.G.; Pires, L.; Luna, A.S.; Casimiro-Lopes, G.; Lisboa, P.C.; Koury, J.C. Plasma zinc, copper, and serum thyroid hormones and insulin levels after zinc supplementation followed by placebo in competitive athletes. Biol. Trace Elem. Res. 2011, 142, 415–423. [Google Scholar] [CrossRef]
- Bajpeyi, S.; Tanner, C.J.; Slentz, C.A.; Duscha, B.D.; McCartney, J.S.; Hickner, R.C.; Kraus, W.E.; Houmard, J.A. Effect of exercise intensity and volume on persistence of insulin sensitivity during training cessation. J. Appl. Physiol. 2009, 106, 1079–1085. [Google Scholar] [CrossRef]
- Fisher, G.; Brown, A.W.; Bohan Brown, M.M.; Alcorn, A.; Noles, C.; Winwood, L.; Resuehr, H.; George, B.; Jeansonne, M.M.; Allison, D.B. High intensity interval- vs moderate intensity- training for improving cardiometabolic health in overweight or obese males: A randomized controlled trial. PLoS ONE 2015, 10, e0138853. [Google Scholar] [CrossRef]
- McGarrah, R.W.; Slentz, C.A.; Kraus, W.E. The effect of vigorous- versus moderate-intensity aerobic exercise on insulin action. Curr. Cardiol. Rep. 2016, 18, 117. [Google Scholar] [CrossRef]
- Cruz, K.J.; Morais, J.B.; de Oliveira, A.R.; Severo, J.S.; Marreiro, D.D. The effect of zinc supplementation on insulin resistance in obese subjects: A systematic review. Biol. Trace Elem. Res. 2017, 176, 239–243. [Google Scholar] [CrossRef]
- Perseghin, G.; Burska, A.; Lattuada, G.; Alberti, G.; Costantino, F.; Ragogna, F.; Oggionni, S.; Scollo, A.; Terruzzi, I.; Luzi, L. Increased serum resistin in elite endurance athletes with high insulin sensitivity. Diabetologia 2006, 49, 1893–1900. [Google Scholar] [CrossRef] [PubMed]
- Cınar, V.; Talaghir, L.G.; Akbulut, T.; Turgut, M.; Sarıkaya, M. The effects of the zinc supplementation and weight trainings on the testosterone levels. Hum. Sport Med. 2017, 17, 58–63. [Google Scholar]
- Cinar, V.; Akbulut, T.; Sarikaya, M. Effect of zinc supplement and weight lifting exercise on thyroid hormone levels. Indian J. Physiol. Pharmacol. 2017, 61, 232–236. [Google Scholar]
- Shafiei-Neek, L.; Gaeini, A.A.; Choobineh, S. Effect of zinc and selenium supplementation on serum testosterone and plasma lactate in cyclist after an exhaustive exercise bout. Biol. Trace Elem. Res. 2011, 144, 454–462. [Google Scholar] [CrossRef]
- Sims, S.T.; Rehrer, N.J.; Bell, M.L.; Cotter, J.D. Preexercise sodium loading aids fluid balance and endurance for women exercising in the heat. J. Appl. Physiol. 2007, 103, 534–541. [Google Scholar] [CrossRef]
- Sims, S.T.; van Vliet, L.; Cotter, J.D.; Rehrer, N.J. Sodium loading aids fluid balance and reduces physiological strain of trained men exercising in the heat. Med. Sci. Sports Exerc. 2007, 39, 123–130. [Google Scholar] [CrossRef]
- Speedy, D.B.; Thompson, J.M.; Rodgers, I.; Collins, M.; Sharwood, K.; Noakes, T.D. Oral salt supplementation during ultradistance exercise. Clin. J. Sport Med. Off. J. Can. Acad. Sport Med. 2002, 12, 279–284. [Google Scholar] [CrossRef]
- Sanders, B.; Noakes, T.D.; Dennis, S.C. Sodium replacement and fluid shifts during prolonged exercise in humans. Eur. J. Appl. Physiol. 2001, 84, 419–425. [Google Scholar] [CrossRef] [PubMed]
- Zorbas, Y.G.; Petrov, K.L.; Yarullin, V.L.; Kakurin, V.J.; Popov, V.K.; Deogeneov, V.A. Effect of fluid and salt supplementation on body hydration of athletes during prolonged hypokinesia. Acta Astronaut. 2002, 50, 641–651. [Google Scholar] [CrossRef]
- Zorbas, Y.G.; Kakurin, V.J.; Kuznetsov, N.A.; Yarullin, V.L. Fluid and salt supplementation effect on body hydration and electrolyte homeostasis during bed rest and ambulation. Acta Astronaut. 2002, 50, 765–774. [Google Scholar] [CrossRef]
- Anastasiou, C.A.; Kavouras, S.A.; Arnaoutis, G.; Gioxari, A.; Kollia, M.; Botoula, E.; Sidossis, L.S. Sodium replacement and plasma sodium drop during exercise in the heat when fluid intake matches fluid loss. J. Athl. Train. 2009, 44, 117–123. [Google Scholar] [CrossRef]
- Barr, S.I.; Costill, D.L.; Fink, W.J. Fluid replacement during prolonged exercise: Effects of water, saline, or no fluid. Med. Sci. Sports Exerc. 1991, 23, 811–817. [Google Scholar] [CrossRef] [PubMed]
- Hew-Butler, T.; Loi, V.; Pani, A.; Rosner, M.H. Exercise-associated hyponatremia: 2017 Update. Front. Med. 2017, 4, 21. [Google Scholar] [CrossRef] [PubMed]
- Driller, M.; Williams, A.; Bellinger, P.; Howe, S.; Fell, J. The effects of NaHCO3 and NaCl loading on hematocrit and high-intensity cycling performance. J. Exerc. Phys. 2012, 15, 47–57. [Google Scholar]
- Earhart, E.L.; Weiss, E.P.; Rahman, R.; Kelly, P.V. Effects of oral sodium supplementation on indices of thermoregulation in trained, endurance athletes. J. Sports Sci. Med. 2015, 14, 172–178. [Google Scholar] [PubMed]
- Hew-Butler, T.D.; Sharwood, K.; Collins, M.; Speedy, D.; Noakes, T. Sodium supplementation is not required to maintain serum sodium concentrations during an Ironman triathlon. Br. J. Sports Med. 2006, 40, 255–259. [Google Scholar] [CrossRef] [PubMed]
- Cosgrove, S.D.; Black, K.E. Sodium supplementation has no effect on endurance performance during a cycling time-trial in cool conditions: A randomised cross-over trial. J. Int. Soc. Sports Nutr. 2013, 10, 30. [Google Scholar] [CrossRef]
- Aschenbach, W.; Ocel, J.; Craft, L.; Ward, C.; Spangenburg, E.; Williams, J. Effect of oral sodium loading on high-intensity arm ergometry in college wrestlers. Med. Sci. Sports Exerc. 2000, 32, 669–675. [Google Scholar] [CrossRef]
- Hamouti, N.; Del Coso, J.; Ortega, J.F.; Mora-Rodriguez, R. Sweat sodium concentration during exercise in the heat in aerobically trained and untrained humans. Eur. J. Appl. Physiol. 2011, 111, 2873–2881. [Google Scholar] [CrossRef] [PubMed]
- Zorbas, Y.G.; Federenko, Y.F.; Naexu, K.A. Effect of daily hyperhydration on fluid-electrolyte changes in endurance-trained volunteers during prolonged restriction of muscular activity. Biol. Trace Elem. Res. 1995, 50, 57–78. [Google Scholar] [CrossRef]
- Strazzullo, P.; D’Elia, L.; Kandala, N.B.; Cappuccio, F.P. Salt intake, stroke, and cardiovascular disease: Meta-analysis of prospective studies. BMJ 2009, 339, b4567. [Google Scholar] [CrossRef]
- Margaritis, I.; Tessier, F.; Prou, E.; Marconnet, P.; Marini, J.F. Effects of endurance training on skeletal muscle oxidative capacities with and without selenium supplementation. Trace Elem. Med. Biol. 1997, 11, 37–43. [Google Scholar] [CrossRef]
- Tessier, F.; Margaritis, I.; Richard, M.J.; Moynot, C.; Marconnet, P. Selenium and training effects on the glutathione system and aerobic performance. Med. Sci. Sports Exerc. 1995, 27, 390–396. [Google Scholar] [CrossRef]
- Zamora, A.J.; Tessier, F.; Marconnet, P.; Margaritis, I.; Marini, J.F. Mitochondria changes in human muscle after prolonged exercise, endurance training and selenium supplementation. Eur. J. Appl. Physiol. Occup. Physiol. 1995, 71, 505–511. [Google Scholar] [CrossRef]
- Sun, H.J.; Rathinasabapathi, B.; Wu, B.; Luo, J.; Pu, L.P.; Ma, L.Q. Arsenic and selenium toxicity and their interactive effects in humans. Environ. Int. 2014, 69, 148–158. [Google Scholar] [CrossRef]
- Nielsen, J.; Gejl, K.D.; Hey-Mogensen, M.; Holmberg, H.C.; Suetta, C.; Krustrup, P.; Elemans, C.P.H.; Ortenblad, N. Plasticity in mitochondrial cristae density allows metabolic capacity modulation in human skeletal muscle. J. Physiol. 2017, 595, 2839–2847. [Google Scholar] [CrossRef]
- Savory, L.A.; Kerr, C.J.; Whiting, P.; Finer, N.; McEneny, J.; Ashton, T. Selenium supplementation and exercise: Effect on oxidant stress in overweight adults. Obesity 2012, 20, 794–801. [Google Scholar] [CrossRef] [PubMed]
- Alfthan, G.; Eurola, M.; Ekholm, P.; Venalainen, E.R.; Root, T.; Korkalainen, K.; Hartikainen, H.; Salminen, P.; Hietaniemi, V.; Aspila, P.; et al. Effects of nationwide addition of selenium to fertilizers on foods, and animal and human health in Finland: From deficiency to optimal selenium status of the population. J. trace Elem. Med. Biol. 2015, 31, 142–147. [Google Scholar] [CrossRef] [PubMed]
- Hughes, D.J.; Duarte-Salles, T.; Hybsier, S.; Trichopoulou, A.; Stepien, M.; Aleksandrova, K.; Overvad, K.; Tjonneland, A.; Olsen, A.; Affret, A.; et al. Prediagnostic selenium status and hepatobiliary cancer risk in the European Prospective Investigation into Cancer and Nutrition cohort. Am. J. Clin. Nutr. 2016, 104, 406–414. [Google Scholar] [CrossRef]
- Alehagen, U.; Johansson, P.; Bjornstedt, M.; Rosen, A.; Post, C.; Aaseth, J. Relatively high mortality risk in elderly Swedish subjects with low selenium status. Eur. J. Clin. Nutr. 2016, 70, 91–96. [Google Scholar] [CrossRef] [PubMed]
- Pingitore, A.; Lima, G.P.; Mastorci, F.; Quinones, A.; Iervasi, G.; Vassalle, C. Exercise and oxidative stress: Potential effects of antioxidant dietary strategies in sports. Nutrition (Burbank, Los Angeles County, Calif.) 2015, 31, 916–922. [Google Scholar] [CrossRef] [PubMed]
- Marmett, B.; Nunes, R.B. Effects of chromium picolinate supplementation on control of metabolic variables: A systematic review. J. Food Nutr. Res. 2016, 4, 633–639. [Google Scholar]
- Pittler, M.H.; Stevinson, C.; Ernst, E. Chromium picolinate for reducing body weight: Meta-analysis of randomized trials. International journal of obesity and related metabolic disorders. J. Int. Assoc. Study Obes. 2003, 27, 522–529. [Google Scholar] [CrossRef] [PubMed]
- Edwards, W.; Pringle, D.; Palfrey, T.; Anderson, D. Effects of chromium picolinate supplementation on body composition in in-season division I intercollegiate female swimmers. Med. Sport. 2012, 16, 99–103. [Google Scholar] [CrossRef]
- Kaats, G.R.; Blum, K.; Fisher, J.A.; Adelman, J.A. Effects of chromium picolinate supplementation on body composition: A randomized, double-masked, placebo-controlled study. Curr. Ther. Res. 1996, 57, 747–756. [Google Scholar] [CrossRef]
- Clancy, S.P.; Clarkson, P.M.; DeCheke, M.E.; Nosaka, K.; Freedson, P.S.; Cunningham, J.J.; Valentine, B. Effects of chromium picolinate supplementation on body composition, strength, and urinary chromium loss in football players. Int. J. Sport Nutr. 1994, 4, 142–153. [Google Scholar] [CrossRef] [PubMed]
- Hallmark, M.A.; Reynolds, T.H.; DeSouza, C.A.; Dotson, C.O.; Anderson, R.A.; Rogers, M.A. Effects of chromium and resistive training on muscle strength and body composition. Med. Sci. Sports Exerc. 1996, 28, 139–144. [Google Scholar] [CrossRef] [PubMed]
- Lukaski, H.C.; Bolonchuk, W.W.; Siders, W.A.; Milne, D.B. Chromium supplementation and resistance training: Effects on body composition, strength, and trace element status of men. Am. J. Clin. Nutr. 1996, 63, 954–965. [Google Scholar] [CrossRef]
- Walker, L.S.; Bemben, M.G.; Bemben, D.A.; Knehans, A.W. Chromium picolinate effects on body composition and muscular performance in wrestlers. Med. Sci. Sports Exerc. 1998, 30, 1730–1737. [Google Scholar] [CrossRef]
- Campbell, W.W.; Joseph, L.J.; Davey, S.L.; Cyr-Campbell, D.; Anderson, R.A.; Evans, W.J. Effects of resistance training and chromium picolinate on body composition and skeletal muscle in older men. J. Appl. Physiol. 1999, 86, 29–39. [Google Scholar] [CrossRef]
- Campbell, W.W.; Joseph, L.J.; Anderson, R.A.; Davey, S.L.; Hinton, J.; Evans, W.J. Effects of resistive training and chromium picolinate on body composition and skeletal muscle size in older women. Int. J. Sport Nutr. Exerc. Metab. 2002, 12, 125–135. [Google Scholar] [CrossRef] [PubMed]
- Livolsi, J.M.; Adams, G.M.; Laguna, P.L. The effect of chromium picolinate on muscular strength and body composition in women athletes. J. Strength Cond. Res. 2001, 15, 161–166. [Google Scholar] [PubMed]
- Hasten, D.L.; Rome, E.P.; Franks, B.D.; Hegsted, M. Effects of chromium picolinate on beginning weight training students. Int. J. Sport Nutr. 1992, 2, 343–350. [Google Scholar] [CrossRef] [PubMed]
- Lefavi, R.G.; Wilson, G.D.; Keith, R.E.; Anderson, R.A.; Blessing, D.L.; Hames, C.G.; McMillan, J.L. Lipid-lowering effect of a dietary chromium (III)—Nicotinic acid complex in male athletes. Nutr. Res. 1993, 13, 239–249. [Google Scholar] [CrossRef]
- Volek, J.S.; Silvestre, R.; Kirwan, J.P.; Sharman, M.J.; Judelson, D.A.; Spiering, B.A.; Vingren, J.L.; Maresh, C.M.; Vanheest, J.L.; Kraemer, W.J. Effects of chromium supplementation on glycogen synthesis after high-intensity exercise. Med. Sci. Sports Exerc. 2006, 38, 2102–2109. [Google Scholar] [CrossRef] [PubMed]
- Vincent, J.B. New Evidence against Chromium as an Essential Trace Element. J. Nutr. 2017, 147, 2212–2219. [Google Scholar] [CrossRef]
- Nielsen, F.H. Update on human health effects of boron. Journal of trace elements in medicine and biology. Organ Soc. Miner. Trace Elem. (GMS) 2014, 28, 383–387. [Google Scholar] [CrossRef]
- Ferrando, A.A.; Green, N.R. The effect of boron supplementation on lean body mass, plasma testosterone levels, and strength in male bodybuilders. Int. J. Sport Nutr. 1993, 3, 140–149. [Google Scholar] [CrossRef] [PubMed]
- Green, N.R.; Ferrando, A.A. Plasma boron and the effects of boron supplementation in males. Environ. Health Perspect. 1994, 102, 73–77. [Google Scholar]
- Meacham, S.L.; Taper, L.J.; Volpe, S.L. Effects of boron supplementation on bone mineral density and dietary, blood, and urinary calcium, phosphorus, magnesium, and boron in female athletes. Environ. Health Perspect. 1994, 102, 79–82. [Google Scholar] [PubMed]
- Meacham, S.L.; Taper, L.J.; Volpe, S.L. Effect of boron supplementation on blood and urinary calcium, magnesium, and phosphorus, and urinary boron in athletic and sedentary women. Am. J. Clin. Nutr. 1995, 61, 341–345. [Google Scholar] [CrossRef]
- Volpe-Snyder, S.L.; Taper, L.J.; Meacham, S.L. The effect of boron supplementation on bone mineral density and hormonal status in college female athletes. Med. Exerc. Nutr. Health 1993, 2, 323–330. [Google Scholar]
- Del Coso, J.; Gonzalez-Millan, C.; Salinero, J.J.; Abian-Vicen, J.; Areces, F.; Lledo, M.; Lara, B.; Gallo-Salazar, C.; Ruiz-Vicente, D. Effects of oral salt supplementation on physical performance during a half-ironman: A randomized controlled trial. Scand. J. Med. Sci. Sports 2016, 26, 156–164. [Google Scholar] [CrossRef]
- Barry, D.W.; Hansen, K.C.; van Pelt, R.E.; Witten, M.; Wolfe, P.; Kohrt, W.M. Acute calcium ingestion attenuates exercise-induced disruption of calcium homeostasis. Med. Sci. Sports Exerc. 2011, 43, 617–623. [Google Scholar] [CrossRef] [PubMed]
- Sherk, V.D.; Wherry, S.J.; Barry, D.W.; Shea, K.L.; Wolfe, P.; Kohrt, W.M. Calcium supplementation attenuates disruptions in calcium homeostasis during exercise. Med. Sci. Sports Exerc. 2017, 49, 1437–1442. [Google Scholar] [CrossRef] [PubMed]
- Shea, B.J.; Reeves, B.C.; Wells, G.; Thuku, M.; Hamel, C.; Moran, J.; Moher, D.; Tugwell, P.; Welch, V.; Kristjansson, E.; et al. AMSTAR 2: A critical appraisal tool for systematic reviews that include randomised or non-randomised studies of healthcare interventions, or both. BMJ 2017, 358, J4008. [Google Scholar] [CrossRef] [PubMed]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Heffernan, S.M.; Horner, K.; De Vito, G.; Conway, G.E. The Role of Mineral and Trace Element Supplementation in Exercise and Athletic Performance: A Systematic Review. Nutrients 2019, 11, 696. https://doi.org/10.3390/nu11030696
Heffernan SM, Horner K, De Vito G, Conway GE. The Role of Mineral and Trace Element Supplementation in Exercise and Athletic Performance: A Systematic Review. Nutrients. 2019; 11(3):696. https://doi.org/10.3390/nu11030696
Chicago/Turabian StyleHeffernan, Shane Michael, Katy Horner, Giuseppe De Vito, and Gillian Eileen Conway. 2019. "The Role of Mineral and Trace Element Supplementation in Exercise and Athletic Performance: A Systematic Review" Nutrients 11, no. 3: 696. https://doi.org/10.3390/nu11030696
APA StyleHeffernan, S. M., Horner, K., De Vito, G., & Conway, G. E. (2019). The Role of Mineral and Trace Element Supplementation in Exercise and Athletic Performance: A Systematic Review. Nutrients, 11(3), 696. https://doi.org/10.3390/nu11030696





