Saffron (Crocus sativus L.) in Ocular Diseases: A Narrative Review of the Existing Evidence from Clinical Studies
Abstract
1. Introduction
2. Methods
3. Clinical Evidence Regarding the Impact of Oral Supplementation with Saffron or One of Its Constituents on Vision-Related Parameters in Adults with Ocular Diseases
4. Action Time-Course of Oral Saffron Supplementation
5. Safety Profile of Oral Saffron Supplementation
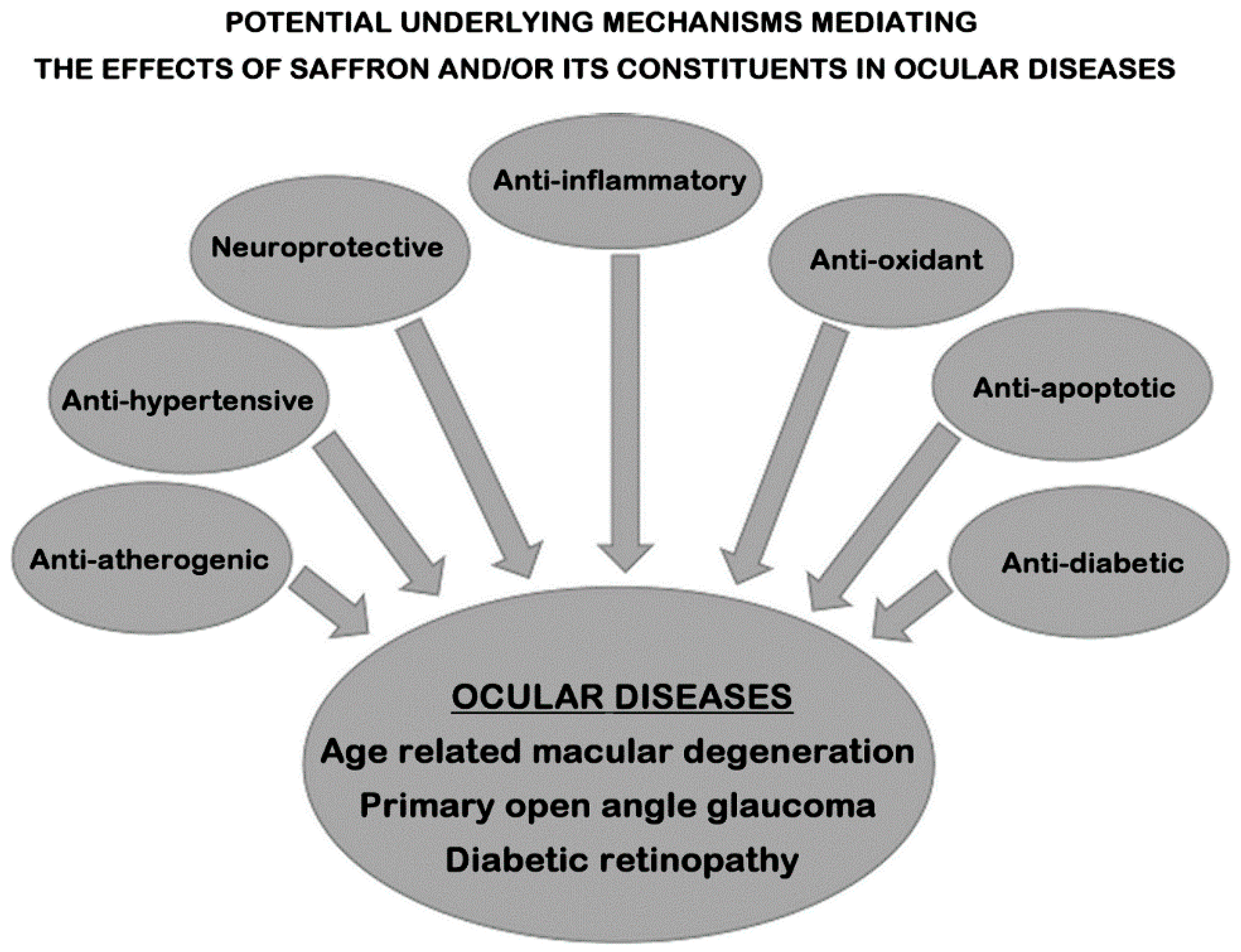
6. Proposed Mechanisms/Pathways Mediating the Effects of Saffron and/or Its Constituents in Ocular Diseases
7. Summary of the Literature
8. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Bourne, R.R.; Stevens, G.A.; White, R.A.; Smith, J.L.; Flaxman, S.R.; Price, H.; Jonas, J.B.; Keeffe, J.; Leasher, J.; Naidoo, K.; et al. Vision Loss Expert Group. Causes of vision loss worldwide, 1990–2010: A systematic analysis. Lancet Glob. Health 2013, 1, e339–e349. [Google Scholar] [CrossRef]
- Wong, T.Y.; Su, X.; Li, X.; Cheung, C.M.; Klein, R.; Cheng, C.Y.; Wong, T.Y. Global prevalence of age-related macular degeneration and disease burden projection for 2020 and 2040: A systematic review and meta-analysis. Lancet Glob. Health 2014, 2, e106–e116. [Google Scholar] [CrossRef]
- Jonas, J.B.; Aung, T.; Bourne, R.R.; Bron, A.M.; Ritch, R.; Panda-Jonas, S. Glaucoma. Lancet 2017, 390, 2183–2193. [Google Scholar] [CrossRef]
- Wong, T.Y.; Cheung, C.M.; Larsen, M.; Sharma, S.; Simo, R. Diabetic retinopathy. Nat. Rev. Dis. Primers 2016, 17, 16012. [Google Scholar] [CrossRef]
- Hernández-Zimbrón, L.F.; Zamora-Alvarado, R.; Velez-Montoya, R.; Zenteno, E.; Gulias-Cañizo, R.; Quiroz-Mercado, H.; Gonzalez-Salinas, R. Age-Related Macular Degeneration: New Paradigms for Treatment and Management of AMD. Oxid. Med. Cell. Longev. 2018, 2018, 8374647. [Google Scholar] [CrossRef] [PubMed]
- Kyrou, I.; Randeva, H.S.; Tsigos, C.; Kaltsas, G.; Weickert, M.O. Clinical Problems Caused by Obesity. In Endotext [Internet]; Feingold, K.R., Anawalt, B., Boyce, A., Chrousos, G., Dungan, K., Grossman, A., Hershman, J.M., Kaltsas, G., Koch, C., Kopp, P., et al., Eds.; MDText.com, Inc.: South Dartmouth, MA, USA, 2018. Available online: https://www.ncbi.nlm.nih.gov/books/NBK278973/ (accessed on 28 February 2019).
- Pennington, K.L.; DeAngelis, M.M. Epidemiology of age-related macular degeneration (AMD): Associations with cardiovascular disease phenotypes and lipid factors. Eye Vis. (Lond.) 2016, 3, 34. [Google Scholar] [CrossRef]
- Zhang, Q.Y.; Tie, L.J.; Wu, S.S.; Lv, P.L.; Huang, H.W.; Wang, W.Q.; Wang, H.; Ma, L. Overweight, Obesity, and Risk of Age-Related Macular Degeneration. Investig. Ophthalmol. Vis. Sci. 2016, 57, 1276–1283. [Google Scholar] [CrossRef]
- Adams, M.K.; Simpson, J.A.; Aung, K.Z.; Makeyeva, G.A.; Giles, G.G.; English, D.R.; Hopper, J.; Guymer, R.H.; Baird, P.N.; Robman, L.D. Abdominal obesity and age-related macular degeneration. Am. J. Epidemiol. 2011, 173, 1246–1255. [Google Scholar] [CrossRef]
- Chen, M.; Luo, C.; Zhao, J.; Devarajan, G.; Xu, H. Immune regulation in the aging retina. Prog. Retin. Eye Res. 2018. [Google Scholar] [CrossRef]
- Pinazo-Durán, M.D.; Gallego-Pinazo, R.; García-Medina, J.J.; Zanón-Moreno, V.; Nucci, C.; Dolz-Marco, R.; Martínez-Castillo, S.; Galbis-Estrada, C.; Marco-Ramírez, C.; López-Gálvez, M.I.; et al. Oxidative stress and its downstream signaling in aging eyes. Clin. Interv. Aging 2014, 9, 637–652. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Xu, H. Parainflammation, chronic inflammation, and age-related macular degeneration. J. Leukoc. Biol. 2015, 98, 713–725. [Google Scholar] [CrossRef]
- Ehrlich, R.; Harris, A.; Kheradiya, N.S.; Winston, D.M.; Ciulla, T.A.; Wirostko, B. Age-related macular degeneration and the aging eye. Clin. Interv. Aging 2008, 3, 473–482. [Google Scholar] [PubMed]
- Broadhead, G.K.; Grigg, J.R.; Chang, A.A.; McCluskey, P. Dietary modification and supplementation for the treatment of age-related macular degeneration. Nutr. Rev. 2015, 73, 448–462. [Google Scholar] [CrossRef] [PubMed]
- Chew, E.Y. Nutrition effects on ocular diseases in the aging eye. Investig. Ophthalmol. Vis. Sci. 2013, 54, ORSF42–ORSF47. [Google Scholar] [CrossRef] [PubMed]
- Huynh, T.P.; Mann, S.N.; Mandal, N.A. Botanical compounds: Effects on major eye diseases. Evid.-Based Complement. Altern. Med. 2013, 2013, 549174. [Google Scholar] [CrossRef] [PubMed]
- José Bagur, M.; Alonso Salinas, G.L.; Jiménez-Monreal, A.M.; Chaouqi, S.; Llorens, S.; Martínez-Tomé, M.; Alonso, G.L. Saffron: An Old Medicinal Plant and a Potential Novel Functional Food. Molecules 2017, 23, 30. [Google Scholar] [CrossRef]
- Christodoulou, E.; Kadoglou, N.P.; Kostomitsopoulos, N.; Valsami, G. Saffron: A natural product with potential pharmaceutical applications. J. Pharm. Pharmacol. 2015, 67, 1634–1649. [Google Scholar] [CrossRef] [PubMed]
- Ríos, J.L.; Recio, M.C.; Giner, R.M.; Máñez, S. An Update Review of Saffron and its Active Constituents. Phytother. Res. 1996, 10, 189–193. [Google Scholar] [CrossRef]
- Hosseini, A.; Razavi, B.M.; Hosseinzadeh, H. Saffron (Crocus sativus) petal as a new pharmacological target: A review. Iran. J. Basic Med. Sci. 2018, 21, 1091–1099. [Google Scholar] [PubMed]
- WHO. Monographs on Selected Medicinal Plants; World Health Organization: Geneva, Switzerland, 2007; Volume 3, Available online: http://apps.who.int/medicinedocs/en/m/abstract/Js14213e/ (accessed on 28 February 2019).
- Ghaffari, S.; Roshanravan, N. Saffron; An updated review on biological properties with special focus on cardiovascular effects. Biomed. Pharmacother. 2019, 109, 21–27. [Google Scholar] [CrossRef]
- Poma, A.; Fontecchio, G.; Carlucci, G.; Chichiriccò, G. Anti-inflammatory properties of drugs from saffron crocus. Antiinflamm. Anti-Allergy Agents Med. Chem. 2012, 11, 37–51. [Google Scholar] [CrossRef]
- Pourmasoumi, M.; Hadi, A.; Najafgholizadeh, A.; Kafeshani, M.; Sahebkar, A. Clinical evidence on the effects of saffron (crocus sativus L.) on cardiovascular risk factors: A systematic review meta-analysis. Pharmacol. Res. 2019, 139, 348–359. [Google Scholar] [CrossRef] [PubMed]
- Broadhead, G.K.; Chang, A.; Grigg, J.; McCluskey, P. Efficacy and Safety of Saffron Supplementation: Current Clinical Findings. Crit. Rev. Food Sci. Nutr. 2016, 56, 2767–2776. [Google Scholar] [CrossRef]
- Rahiman, N.; Akaberi, M.; Sahebkar, A.; Emami, S.A.; Tayarani-Najaran, Z. Protective effects of saffron and its active components against oxidative stress and apoptosis in endothelial cells. Microvasc. Res. 2018, 118, 82–89. [Google Scholar] [CrossRef]
- Pashirzad, M.; Shafiee, M.; Avan, A.; Ryzhikov, M.; Fiuji, H.; Bahreyni, A.; Khazaei, M.; Soleimanpour, S.; Hassanian, S.M. Therapeutic potency of crocin in the treatment of inflammatory diseases: Current status and perspective. J. Cell. Physiol. 2019, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Abou-Hany, H.O.; Atef, H.; Said, E.; Elkashef, H.A.; Salem, H.A. Crocin mediated amelioration of oxidative burden and inflammatory cascade suppresses diabetic nephropathy progression in diabetic rats. Chem. Biol. Interact. 2018, 284, 90–100. [Google Scholar] [CrossRef] [PubMed]
- Razavi, B.M.; Hosseinzadeh, H. Saffron: A promising natural medicine in the treatment of metabolic syndrome. J. Sci. Food Agric. 2017, 97, 1679–1685. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef] [PubMed]
- Liberati, A.; Altman, D.G.; Tetzlaff, J.; Mulrow, C.; Gøtzsche, P.C.; Ioannidis, J.P.; Clarke, M.; Devereaux, P.J.; Kleijnen, J.; Moher, D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: Explanation and elaboration. PLoS Med. 2009, 6, e1000100. [Google Scholar] [CrossRef] [PubMed]
- Falsini, B.; Piccardi, M.; Minnella, A.; Savastano, C.; Capoluongo, E.; Fadda, A.; Balestrazzi, E.; Maccarone, R.; Bisti, S. Influence of saffron supplementation on retinal flicker sensitivity in early age-related macular degeneration. Investig. Ophthalmol. Vis. Sci. 2010, 51, 6118–6124. [Google Scholar] [CrossRef]
- Piccardi, M.; Marangoni, D.; Minnella, A.M.; Savastano, M.C.; Valentini, P.; Ambrosio, L.; Capoluongo, E.; Maccarone, R.; Bisti, S.; Falsini, B. A Longitudinal follow-up study of saffron supplementation in early age-related macular degeneration: Sustained benefits to central retinal function. Evid.-Based Complement. Altern. Med. 2012, 2012, 429124. [Google Scholar] [CrossRef] [PubMed]
- Marangoni, D.; Falsini, B.; Piccardi, M.; Ambrosio, L.; Minnella, A.M.; Savastano, M.C.; Bisti, S.; Maccarone, R.; Fadda, A.; Mello, E.; et al. Functional effect of saffron supplementation and risk genotypes in early age-related macular degeneration: A preliminary report. J. Transl. Med. 2013, 11, 228. [Google Scholar] [CrossRef] [PubMed]
- Lashay, A.; Sadough, G.; Ashrafi, E.; Lashay, M.; Movassat, M.; Akhondzadeh, S. Short-term outcomes of saffron supplementation in patients with age-related macular degeneration: A double-blind, Placebo-controlled, randomized trial. Med. Hypothesis Discov. Innov. Ophthalmol. 2016, 5, 32. [Google Scholar] [PubMed]
- Riazi, A.; Panahi, Y.; Alishiri, A.A.; Hosseini, M.A.; Zarchi, A.A.K.; Sahebkar, A. The impact of saffron (crocus sativus) supplementation on visual function in patients with dry age-related macular degeneration. Ital. J. Med. 2017, 11, 758. [Google Scholar] [CrossRef]
- Broadhead, G.K.; Grigg, J.R.; McCluskey, P.; Hong, T.; Schlub, T.E.; Chang, A.A. Saffron Therapy for the treatment of mild/ moderate age-related macular degeneration: A randomized clinical trial. Graefe’s Arch. Clin. Exp. Ophthalmol. 2019, 257, 31–40. [Google Scholar] [CrossRef] [PubMed]
- Bonyadi, M.H.J.; Yazdani, S.; Saadat, S. The ocular hypotensive effect of saffron extractin primary open angle glaucoma: A pilot study. BMC Complement. Altern. Med. 2014, 14, 399. [Google Scholar]
- Sepahi, S.; Mohajeri, S.A.; Hosseini, S.M.; Khodaverdi, E.; Shoeibi, N.; Namdari, M.; Tabassi, S.A.S. Effects of crocin on diabetic maculopathy: A placebo-controlled randomized clinical trial. Am. J. Ophthalmol. 2018, 190, 89–98. [Google Scholar] [CrossRef]
- Di Marco, F.; Romeo, S.; Nandasena, C.; Purushothuan, S.; Adams, C.; Bisti, S.; Stone, J. The time course of action of two neuroprotectants, dietary saffron and photobiomodulation, assessed in the rat retina. Am. J. Neurodegener. Dis. 2013, 2, 208–220. [Google Scholar]
- Moshiri, M.; Vahabzadeh, M.; Hosseinzadeh, H. Clinical applications of saffron (crocus sativus) and its constituents: A review. Drug Res. 2015, 65, 287–295. [Google Scholar] [CrossRef]
- Bisti, S.; Maccarone, R.; Falsini, B. Saffron and retina: Neuroprotection and pharmacokinetics. Vis. Neurosci. 2014, 31, 355–361. [Google Scholar] [CrossRef]
- Alavizadeh, S.H.; Hosseinzadeh, H. Bioactivity assessment and toxicity of crocin: A comprehensive review. Food Chem. Toxicol. 2014, 64, 65–80. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, M.; Betti, G.; Hensel, A. Saffron in phytotherapy: Pharmacology and clinical uses. Wien. Med. Wochenschr. 2007, 157, 315–319. [Google Scholar] [CrossRef] [PubMed]
- Mohamadpour, A.H.; Ayati, Z.; Parizadeh, M.R.; Rajbai, O.; Hosseinzadeh, H. Safety Evaluation of Crocin (a constituent of saffron) Tablets in Healthy Volunteers. Iran. J. Basic Med. Sci. 2013, 16, 39–46. [Google Scholar] [PubMed]
- Modaghegh, M.H.; Shahabian, M.; Esmaeili, H.A.; Rajbai, O.; Hosseinzadeh, H. Safety evaluation of saffron (Crocus sativus) tablets in healthy volunteers. Phytomedicine 2008, 15, 1032–1037. [Google Scholar] [CrossRef] [PubMed]
- Hausenblas, H.A.; Heekin, K.; Mutchie, H.L.; Anton, S. A systematic review of randomized controlled trials examining the effectiveness of saffron (Crocus sativus L.) on psychological and behavioral outcomes. J. Integr. Med. 2015, 13, 231–240. [Google Scholar] [CrossRef]
- Ernst, E. Herbal medicinal products during pregnancy: Are they safe? BJOG 2002, 109, 227–235. [Google Scholar] [CrossRef] [PubMed]
- Gherghel, D.; Griffiths, H.R.; Hilton, E.J.; Cunliffe, I.A.; Hosking, S.L. Systemic reduction in glutathione levels occurs in patients with primary open-angle glaucoma. Investig. Ophthalmol. Vis. Sci. 2005, 46, 877–883. [Google Scholar] [CrossRef] [PubMed]
- Sung, Y.Y.; Kim, H.K. Crocin ameliorates atopic dermatitis symptoms by down regulation of Th2 response via blocking of NF-κB/STAT6 signaling pathways in mice. Nutrients 2018, 10, 1625. [Google Scholar] [CrossRef]
- Lv, B.; Huo, F.; Zhu, Z.; Xu, Z.; Dang, X.; Chen, T.; Zhang, T.; Yang, X. Crocin Upregulates CX3CR1 expression by suppressing NF-κB/YY1 signaling and inhibiting lipopolysaccharide-induced microglial activation. Neurochem. Res. 2016, 41, 1949–1957. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Kakkar, R.; Wang, J. In vivo and in vitro approach to anti-arthritic and anti-inflammatory effect of crocetin by alteration of nuclear factor-E2-related factor 2/hem oxygenase (HO)-1 and NF-κB expression. Front. Pharmacol. 2018, 9, 1341. [Google Scholar] [CrossRef]
- Giaccio, M. Crocetin from saffron: An active component of an ancient spice. Crit. Rev. Food Sci. Nutr. 2004, 44, 155–172. [Google Scholar] [CrossRef]
- Xuan, B.; Zhou, Y.H.; Li, N.; Min, Z.D.; Chiou, G.C. Effects of crocin analogs on ocular blood flow and retinal function. J. Ocul. Pharmacol. Ther. 1999, 15, 143–152. [Google Scholar] [CrossRef]
- Izzotti, A.; Saccà, S.C.; Longobardi, M.; Cartiglia, C. Sensitivity of ocular anterior chamber tissues to oxidative damage and its relevance to the pathogenesis of glaucoma. Investig. Ophthalmol. Vis. Sci. 2009, 50, 5251–5258. [Google Scholar] [CrossRef]
- Saccà, S.C.; Gandolfi, S.; Bagnis, A.; Manni, G.; Damonte, G.; Traverso, C.E.; Izzotti, A. The Outflow Pathway: A Tissue with Morphological and Functional Unity. J. Cell. Physiol. 2016, 231, 1876–1893. [Google Scholar] [CrossRef]
- Yaribeygi, H.; Zare, V.; Butler, A.E.; Barreto, G.E.; Sahebkar, A. Antidiabetic potential of saffron and its active constituents. J. Cell. Physiol. 2019, 234, 8610–8617. [Google Scholar] [CrossRef]
- Corso, L.; Cavallero, A.; Baroni, D.; Garbati, P.; Prestipino, G.; Bisti, S.; Nobile, M.; Picco, C. Saffron reduces ATP-induced retinal cytotoxicity by targeting P2X7 receptors. Purinergic Signal. 2016, 12, 161–174. [Google Scholar] [CrossRef]
- Maccarone, R.; Di Marco, S.; Bisti, S. Saffron supplement maintains morphology and function after exposure to damaging light in mammalian retina. Investig. Ophthalmol. Vis. Sci. 2008, 49, 1254–1261. [Google Scholar] [CrossRef]
- Laabich, A.; Vissvesvaran, G.P.; Lieu, K.L.; Murata, K.; McGinn, T.E.; Manmoto, C.C.; Sinclair, J.R.; Karliga, I.; Leung, D.W.; Fawzi, A.; et al. Protective effect of crocin against lue light-and white light-mediated photoreceptor cell death in bovine and primate retinal primary cell culture. Investig. Ophthalmol. Vis. Sci. 2006, 47, 3156–3163. [Google Scholar] [CrossRef]
- Waugh, N.; Loveman, E.; Colquitt, J.; Royle, P.; Yeong, J.L.; Hoad, G.; Lois, N. Treatments for dry age-related macular degeneration and Stargardt disease: A systematic review. Health Technol. Assess. 2018, 22, 1–167. [Google Scholar] [CrossRef]
- Potnuri, A.G.; Allakonda, L.; Lahkar, M. Crocin attenuates cyclophosphamide induced testicular toxicity by preserving glutathione redox system. Biomed. Pharmacother. 2018, 101, 1740180. [Google Scholar] [CrossRef]
- Xi, L.; Qian, Z.; Xu, G.; Zheng, S.; Sun, S.; Wen, N.; Sheng, L.; Shi, Y.; Zhang, Y. Beneficial impact of crocetin, a carotenoid from saffron, on insulin sensitivity in fructose-fed rats. J. Nutr. Biochem. 2007, 18, 64–72. [Google Scholar] [CrossRef]
- Asai, A.; Nakano, T.; Takahashi, M.; Nagao, A. Orally administered crocetin and crocins are absorbed into blood plasma as crocetin and its glucuronide conjugates in mice. J. Agric. Food Chem. 2005, 53, 7302–7306. [Google Scholar] [CrossRef]
- Umigai, N.; Murakami, K.; Ulit, M.V.; Antonio, L.S.; Shirotori, M.; Morikawa, H.; Nakano, T. The pharmacokinetic profile of crocetin in healthy adult human volunteers after a single oral administration. Phytomedicine 2011, 18, 575–578. [Google Scholar] [CrossRef]
- Kell, G.; Rao, A.; Beccaria, G.; Clayton, P.; Inarejos-García, A.M.; Prodanov, M. affron® a novel saffron extract (Crocus sativus L.) improves mood in healthy adults over 4 weeks in a double-blind, parallel, randomized, placebo-controlled clinical trial. Complement. Ther. Med. 2017, 33, 58–64. [Google Scholar] [CrossRef]
- Lopresti, A.L.; Drummond, P.D.; Inarejos-García, A.M.; Prodanov, M. affron®, a standardised extract from saffron (Crocus sativus L.) for the treatment of youth anxiety and depressive symptoms: A randomised, double-blind, placebo-controlled study. J. Affect. Disord. 2018, 232, 349–357. [Google Scholar] [CrossRef]
- Tóth, B.; Hegyi, P.; Lantos, T.; Szakács, Z.; Kerémi, B.; Varga, G.; Tenk, J.; Pétervári, E.; Balaskó, M.; Rumbus, Z.; et al. The Efficacy of Saffron in the Treatment of Mild to Moderate Depression: A Meta-analysis. Planta Med. 2019, 85, 24–31. [Google Scholar] [CrossRef]
- Chen, X.; Lu, L. Depression in Diabetic Retinopathy: A Review and Recommendation for Psychiatric Management. Psychosomatics 2016, 57, 465–471. [Google Scholar] [CrossRef]
- Musch, D.C.; Niziol, L.M.; Janz, N.K.; Gillespie, B.W. Trends in and Predictors of Depression Among Participants in the Collaborative Initial Glaucoma Treatment Study (CIGTS). Am. J. Ophthalmol. 2019, 197, 128–135. [Google Scholar] [CrossRef]
- European Food Safety Authority (EFSA). Food Supplements. 2019. Available online: https://www.efsa.europa.eu/en/topics/topic/food-supplements (accessed on 28 February 2019).
- Office of Dietary Supplements (ODS). Dietary Supplements. Background Information. 2011. Available online: https://ods.od.nih.gov/factsheets/dietarysupplements-healthprofessional/#disc (accessed on 28 February 2019).
| Parameters | Descriptions |
|---|---|
| Population/Participants/Problem | Adults with ocular disease |
| Intervention | Any intervention with oral administration of saffron or one of its constituents |
| Comparison | Studies with any comparator/control that incorporated a non-intervention group or studies with a pre- vs. post-intervention comparison without a comparator/control group |
| Outcomes | Vision-related outcome measures, such as visual acuity, visual field parameters, contrast sensitivity, electrophysiology parameters (ERG, fERG, mfERG), macular thickness measures, and IOP |
| Setting | Clinical studies/trials |
| Ocular Disease | Number of Subjects | Constituent Dosage | Study Design | Primary Outcome Measures—Findings | Proposed Mechanisms | Reference |
|---|---|---|---|---|---|---|
| AMD [bilateral early AMD] | N = 25 | Saffron 20 mg daily | Double-blind, placebo controlled, cross over, RCT three-month period with cross over for another three months | fERG: increased amplitude in saffron, but not in placebo group BCVA: increased (one line) in saffron, but not in placebo group | Anti-oxidant Neuroprotective | Falsini et al. (2010) [32] |
| AMD [bilateral early AMD] | N = 29 | Saffron 20 mg daily | Longitudinal interventional open-label study three monthly follow-ups over a 15-month period | fERG: increased amplitude that stabilized after three months BCVA: increased (two lines) that stabilized after three months | Anti-oxidant Neuroprotective | Piccardi et al. (2012) [33] |
| AMD [bilateral early AMD] | N = 33 | Saffron 20 mg daily | Longitudinal, 3 monthly follow-ups over a 12-month period | fERG: increased amplitude and sensitivity amplitude that stabilized after three months independent of genotype | Anti-oxidant Anti-inflammatory Neuroprotective | Marangoni et al. (2013) [34] |
| AMD [dry and wet AMD] | N = 40 | Saffron 15 mg twice daily | Placebo controlled, RCT six-month period with follow-ups at three and six months | CMT: decreased in saffron and placebo groups in wet AMD, but not in dry AMD ERG: amplitude increased in the saffron group (dry and wet AMD) compared to placebo after three months, but not six months | Neuroprotective Anti-depressant | Lashay et al. (2016) [35] |
| AMD [mild/moderate dry AMD] | N = 54 | Saffron 50 mg daily | Placebo controlled, RCT three months | CMT: unchanged BCVA: increased (one line) in saffron, but not in placebo group CS: increased in saffron, but not in placebo group | Anti-oxidant Hemorheological activity | Riazi et al. (2017) [36] |
| AMD [mild/moderate AMD] | N = 96 | Saffron 20 mg daily | Double-blind, placebo controlled, cross over, RCT three months followed by cross over into the other arm for three months | BCVA: increased in saffron group [and AREDS * + saffron], but not in placebo mfERG response density: increased in AREDS+saffron, but not in the saffron or placebo group mfERG latency: decreased in saffron group, but not in placebo group | Anti-oxidant Neuroprotective | Broadhead et al. (2019) [37] |
| POAG [clinically stable POAG] | N = 34 | Saffron 30 mg daily | Double-blind, placebo controlled RCT 1 month duration 1 month wash-out | IOP: reduction after three and four weeks compared to placebo IOP returned to pre-intervention levels after a 4-week wash out period | Antioxidant Neuroprotective | Bonyadi et al. (2014) [38] |
| DME | N = 60 (101 DME eyes) | Crocin 5 mg or 15 mg daily | Double-masked, placebo controlled, phase 2 RCT 3 months | CMT: significantly decreased after three months compared to placebo only in the 15 mg group BCVA: significantly improved after three months compared to placebo only in the 15 mg group HbA1c and FBG: significantly decreased after three months compared to placebo only in the 15 mg group | Anti-oxidant Hemorheological activity Anti-inflammatory | Sepahi et al. (2018) [39] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Heitmar, R.; Brown, J.; Kyrou, I. Saffron (Crocus sativus L.) in Ocular Diseases: A Narrative Review of the Existing Evidence from Clinical Studies. Nutrients 2019, 11, 649. https://doi.org/10.3390/nu11030649
Heitmar R, Brown J, Kyrou I. Saffron (Crocus sativus L.) in Ocular Diseases: A Narrative Review of the Existing Evidence from Clinical Studies. Nutrients. 2019; 11(3):649. https://doi.org/10.3390/nu11030649
Chicago/Turabian StyleHeitmar, Rebekka, James Brown, and Ioannis Kyrou. 2019. "Saffron (Crocus sativus L.) in Ocular Diseases: A Narrative Review of the Existing Evidence from Clinical Studies" Nutrients 11, no. 3: 649. https://doi.org/10.3390/nu11030649
APA StyleHeitmar, R., Brown, J., & Kyrou, I. (2019). Saffron (Crocus sativus L.) in Ocular Diseases: A Narrative Review of the Existing Evidence from Clinical Studies. Nutrients, 11(3), 649. https://doi.org/10.3390/nu11030649





