The Effect of an Infant Formula Supplemented with AA and DHA on Fatty Acid Levels of Infants with Different FADS Genotypes: The COGNIS Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics Statement
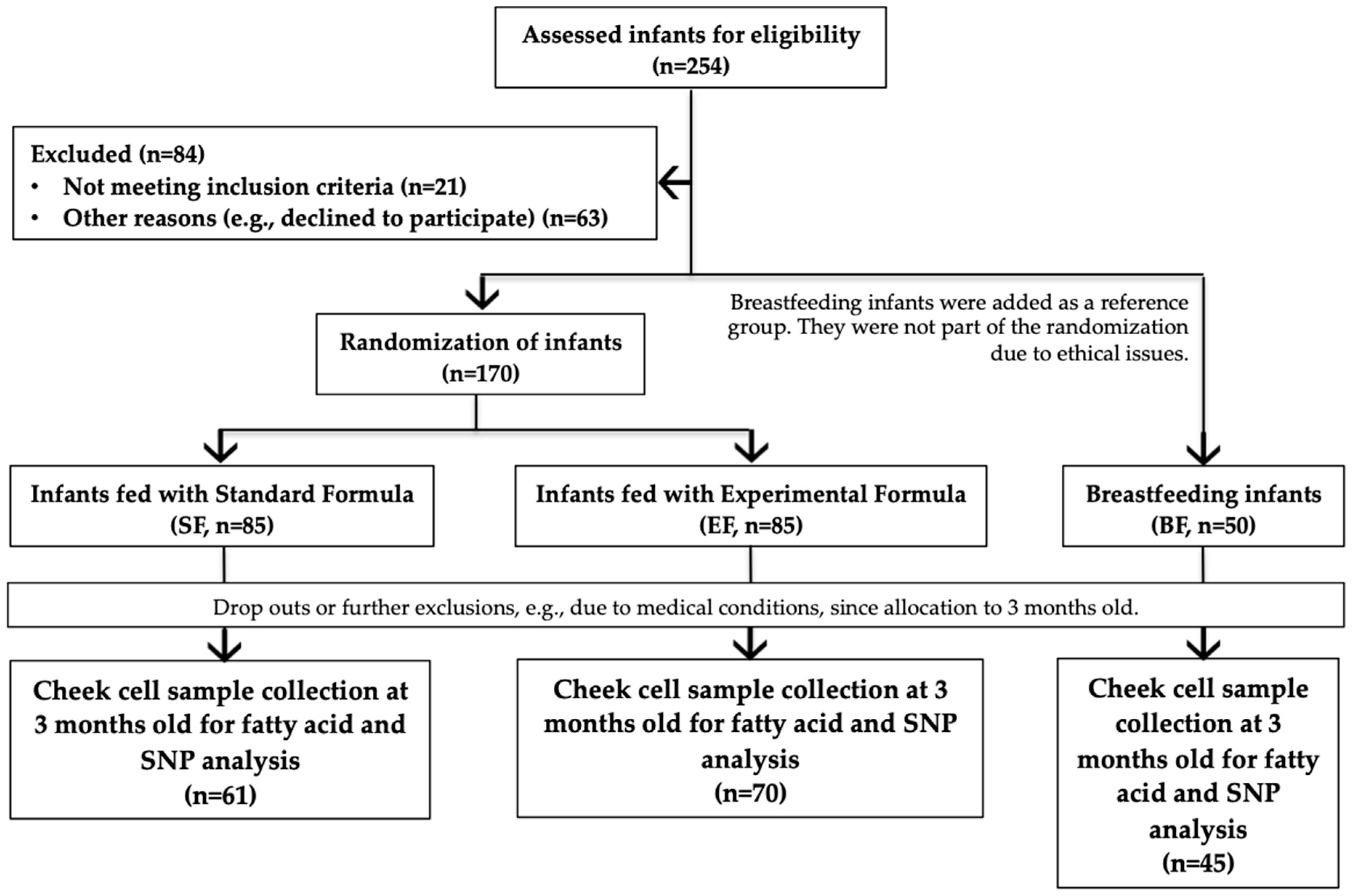
2.2. Study Population and Design
2.3. Formulas
2.4. Cheek Cell Sample Collection
2.5. Cheek Cell Fatty Acid Analysis
2.6. SNP Selection and Genotyping
2.7. Statistical Analysis
3. Results
3.1. Sample Characteristics
3.2. Associations of FADS SNPs with Fatty Acids
3.3. Fatty Acid Comparison by FADS Genotype among Feeding Practice Groups
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Abedi, E.; Sahari, M.A. Long-chain polyunsaturated fatty acid sources and evaluation of their nutritional and functional properties. Food Sci. Nutr. 2014, 2, 443–463. [Google Scholar] [CrossRef]
- Richard, C.; Lewis, E.D.; Field, C.J. Evidence for the essentiality of arachidonic and docosahexaenoic acid in the postnatal maternal and infant diet for the development of the infant’s immune system early in life. Appl. Physiol. Nutr. Metab. 2016, 41, 461–475. [Google Scholar] [CrossRef] [PubMed]
- Koletzko, B. Human milk lipids. Ann. Nutr. Metab. 2017, 69, 28–40. [Google Scholar] [CrossRef] [PubMed]
- Grote, V.; Verduci, E.; Scaglioni, S.; Vecchi, F.; Contarini, G.; Giovannini, M.; Koletzko, B.; Agostoni, C. Breast milk composition and infant nutrient intakes during the first 12 months of life. Eur. J. Clin. Nutr. 2015, 70, 1–7. [Google Scholar] [CrossRef]
- World Health Organization How to Prepare Powdered Infant Formula in Care Settings Formula Is Not Sterile. Available online: http://www.who.int/foodsafety/publications/micro/PIF_Care_en.pdf (accessed on 20 August 2008).
- Brenna, J.T.; Varamini, B.; Jensen, R.G.; Diersen-schade, D.A.; Boettcher, J.A.; Arterburn, L.M. Docosahexaenoic and arachidonic acid concentrations in humanbreast milk worldwide. Am. J. Clin. Nutr. 2007, 85, 1457–1464. [Google Scholar] [CrossRef]
- Scientific opinion on the essential composition of infant and follow-on formulae. EFSA J. 2014, 1212. [CrossRef]
- European Union Law Reglamento Delegado (UE) 2016/127 de la Comisión, de 25 de Septiembre de 2015, que Complementa el Reglamento (UE) n° 609/2013 del Parlamento Europeo y del Consejo en lo que Respecta a Los Requisitos Específicos de Composición e Información Aplicables a lo. Available online: https://eur-lex.europa.eu/legal-content/es/TXT/?uri=CELEX:32016R0127 (accesed on 11 March 2019).
- Liao, K.; Mccandliss, B.D.; Carlson, S.E.; Colombo, J.; Shaddy, D.J.; Kerling, E.H.; Lepping, R.J.; Sittiprapaporn, W.; Cheatham, C.L.; Gustafson, K.M. Event-related potential differences in children supplemented with long-chain polyunsaturated fatty acids during infancy. Dev. Sci. 2016, 1–16. [Google Scholar] [CrossRef]
- Birch, E.E.; Garfield, S.; Hoffman, D.R.; Uauy, R.; Birch, D.G. A randomized controlled trial of early dietary supply of long-chain polyunsaturated fatty acids and mental development in term infants. Dev. Med. Child Neurol. 2000, 42, 174–181. [Google Scholar] [CrossRef]
- CI, J.; Kiliaan, A.J. Long-chain polyunsaturated fatty acids (LCPUFA) from genesis to senescence: The influence of LCPUFA on neural development, aging, and neurodegeneration. Prog. Lipid Res. 2014, 53, 1–17. [Google Scholar] [CrossRef]
- Demmelmair, H.; Baumheuer, M.; Koletzko, B.; Dokoupil, K.; Kratl, G. Metabolism of U13C-labeled linoleic acid in lactating women. J. Lipid Res. 1998, 39, 1389–1396. [Google Scholar] [PubMed]
- Schaeffer, L.; Gohlke, H.; Müller, M.; Heid, I.M.; Palmer, L.J.; Kompauer, I.; Demmelmair, H.; Illig, T.; Koletzko, B.; Heinrich, J. Common genetic variants of the FADS1 FADS2 gene cluster and their reconstructed haplotypes are associated with the fatty acid composition in phospholipids. Hum. Mol. Genet. 2006, 15, 1745–1756. [Google Scholar] [CrossRef] [PubMed]
- Glaser, C.; Heinrich, J.; Koletzko, B. Role of FADS1 and FADS2 polymorphisms in polyunsaturated fatty acid metabolism. Metabolism. 2010, 59, 993–999. [Google Scholar] [CrossRef] [PubMed]
- Glaser, C.; Lattka, E.; Rzehak, P.; Steer, C.; Koletzko, B. Genetic variation in polyunsaturated fatty acid metabolism and its potential relevance for human development and health. Matern. Child Nutr. 2011, 7, 27–40. [Google Scholar] [CrossRef]
- Lattka, E.; Illig, T.; Koletzko, B.; Heinrich, J. Genetic variants of the FADS1 FADS2 gene cluster as related to essential fatty acid metabolism. Curr. Opin. Lipidol. 2010, 21, 64–69. [Google Scholar] [CrossRef] [PubMed]
- Rzehak, P.; Thijs, C.; Standl, M.; Mommers, M.; Glaser, C.; Jansen, E.; Klopp, N.; Koppelman, G.H.; Singmann, P.; Postma, D.S.; et al. Variants of the FADS1 FADS2 gene cluster, blood levels of polyunsaturated fatty acids and eczema in children within the first 2 years of life. PLoS ONE 2010, 5, e13261. [Google Scholar] [CrossRef]
- Zhang, J.Y.; Qin, X.; Liang, A.; Kim, E.; Lawrence, P.; Park, W.J.; Kothapalli, K.S.D.; Thomas Brenna, J. Fads3 modulates docosahexaenoic acid in liver and brain. Prostaglandins Leukot Essent Fat. Acids 2017, 123, 25–32. [Google Scholar] [CrossRef] [PubMed]
- Fahmida, U.; Htet, M.K.; Adhiyanto, C.; Kolopaking, R.; Yudisti, M.A.; Maududi, A.; Suryandari, D.A.; Dillon, D.; Afman, L.; Müller, M. Genetic variants of FADS gene cluster, plasma LC-PUFA levels and the association with cognitive function of under-two-year-old Sasaknese Indonesian children. Asia Pac. J. Clin. Nutr. 2015, 24, 323–328. [Google Scholar] [CrossRef]
- Morales, E.; Bustamante, M.; Gonzalez, J.R.; Guxens, M.; Torrent, M.; Mendez, M.; Garcia-Esteban, R.; Julvez, J.; Forns, J.; Vrijheid, M.; et al. Genetic variants of the FADS gene cluster and ELOVL gene family, colostrums LC-PUFA levels, breastfeeding, and child cognition. PLoS ONE 2011, 6, e17181. [Google Scholar] [CrossRef] [PubMed]
- Lattka, E.; Illig, T.; Heinrich, J.; Koletzko, B. Do FADS genotypes enhance our knowledge about fatty acid related phenotypes? Clin. Nutr. 2010, 29, 277–287. [Google Scholar] [CrossRef] [PubMed]
- Baylin, A.; Ruiz-narvaez, E.; Kraft, P.; Campos, H. α-Linolenic acid, Δ6-desaturase gene polymorphism, and the risk of nonfatal myocardial infarction. Am. J. Clin. Nutr. 2007, 85, 554–560. [Google Scholar] [CrossRef] [PubMed]
- Moltó-Puigmartí, C.; Plat, J.; Mensink, R.P.; Müller, A.; Jansen, E.; Zeegers, M.P.; Thijs, C. FADS1 FADS2 gene variants modify the association between fish intake and the docosahexaenoic acid proportions in human milk. Am. J. Clin. Nutr. 2010, 91, 1368–1376. [Google Scholar] [CrossRef] [PubMed]
- Gieger, C.; Geistlinger, L.; Altmaier, E.; De Angelis, M.H.; Kronenberg, F.; Meitinger, T.; Mewes, H.W.; Wichmann, H.E.; Weinberger, K.M.; Adamski, J.; et al. Genetics meets metabolomics: A genome-wide association study of metabolite profiles in human serum. PLoS Genet. 2008, 4, e1000282. [Google Scholar] [CrossRef] [PubMed]
- Aulchenko, Y.S.; Ripatti, S.; Lindqvist, I.; Boomsma, D.; Iris, M. Europe PMC funders group loci influencing lipid levels and coronary heart disease risk in 16 european population cohorts. Nat Genet. 2009, 41, 47–55. [Google Scholar] [CrossRef] [PubMed]
- Jensen, H.A.R.; Harsløf, L.B.S.; Nielsen, M.S.; Christensen, L.B.; Ritz, C.; Michaelsen, K.F.; Vogel, U.; Lauritzen, L. FADS single-nucleotide polymorphisms are associated with behavioral outcomes in children, and the effect varies between sexes and is dependent on PPAR genotype. Am. J. Clin. Nutr. 2014, 100, 826–832. [Google Scholar] [CrossRef] [PubMed]
- Molto-Puigmarti, C.; Jansen, E.; Heinrich, J.; Standl, M.; Mensink, R.P.; Plat, J.; Penders, J.; Mommers, M.; Koppelman, G.H.; Postma, D.S.; et al. Genetic variation in FADS genes and plasma cholesterol levels in 2-year-old infants: KOALA birth cohort study. PLoS ONE 2013, 8, e61671. [Google Scholar] [CrossRef]
- Standl, M.; Sausenthaler, S.; Lattka, E.; Koletzko, S.; Bauer, C.P.; Wichmann, H.E.; von Berg, A.; Berdel, D.; Krämer, U.; Schaaf, B.; et al. FADS gene variants modulate the effect of dietary fatty acid intake on allergic diseases in children. Clin. Exp. Allergy 2011, 41, 1757–1766. [Google Scholar] [CrossRef] [PubMed]
- Lepping, R.J.; Honea, R.A.; Martin, L.E.; Liao, K.; Choi, I.-Y.; Lee, P.; Papa, V.B.; Brooks, W.M.; Shaddy, D.J.; Carlson, S.E.; et al. Long-chain polyunsaturated fatty acid supplementation in the first year of life affects brain function, structure, and metabolism at age nine years. Dev. Psychobiol. 2018, 1–12. [Google Scholar] [CrossRef]
- de la Garza Puentes, A.; Montes Goyanes, R.; Chisaguano Tonato, A.M.; Castellote, A.I.; Moreno-Torres, R.; Campoy Folgoso, C.; López-Sabater, M.C. Evaluation of less invasive methods to assess fatty acids from phospholipid fraction: Cheek cell and capillary blood sampling. Int. J. Food Sci. Nutr. 2015, 66, 936–942. [Google Scholar] [CrossRef] [PubMed]
- Bondia, E.M.; Castellote, A.I.; Lopez, M.; Rivero, M. Determination of plasma fatty acid composition gas chromatography in neonates by gas chromatography. J. Chromatogr. B 1994, 4347, 369–374. [Google Scholar] [CrossRef]
- Hong, S.H.; Kwak, J.H. Association of polymorphisms in FADS gene with age-related changes in serum phospholipid polyunsaturated fatty acids and oxidative stress markers in middle-aged nonobese men. Clin. Interv. Aging 2013, 13, 585–596. [Google Scholar]
- Cormier, H.; Rudkowska, I.; Thifault, E.; Lemieux, S.; Couture, P.; Vohl, M.C. Polymorphisms in Fatty Acid Desaturase (FADS) gene cluster: Effects on glycemic controls following an omega-3 Polyunsaturated Fatty Acids (PUFA) supplementation. Genes (Basel) 2013, 4, 485–498. [Google Scholar] [CrossRef] [PubMed]
- Harsløf, L.B.S.; Larsen, L.H.; Ritz, C.; Hellgren, L.I.; Michaelsen, K.F.; Vogel, U.; Lauritzen, L. FADS genotype and diet are important determinants of DHA status: A cross-sectional study in Danish infants. Am. J. Clin. Nutr. 2013, 97, 1403–1410. [Google Scholar] [CrossRef] [PubMed]
- Groen-blokhuis, M.M.; Franic, S.; Van Beijsterveldt, C.E.M.; De Geus, E.; Bartels, M.; Davies, G.E.; Ehli, E.A.; Xiao, X.; Scheet, P.A.; Althoff, R.; et al. Neuropsychiatric Genetics A Prospective Study of the Effects of Breastfeeding and FADS2 Polymorphisms on Cognition and Hyperactivity/Attention Problems. Am. J. Med. Genet. 2013, 162, 457–465. [Google Scholar] [CrossRef] [PubMed]
- Al-hilal, M.; Alsaleh, A.; Maniou, Z.; Lewis, F.J.; Hall, W.L.; Sanders, T.A.B.; Dell, S.D.O. Genetic variation at the FADS1-FADS2 gene locus infl uences delta-5 desaturase activity and LC-PUFA proportions after fi sh oil supplement. J. Lipid Res. 2013, 54, 542–551. [Google Scholar] [CrossRef] [PubMed]
- Gillingham, L.G.; Harding, S.V.; Rideout, T.C.; Yurkova, N.; Cunnane, S.C.; Eck, P.K.; Jones, P.J.H. Dietary oils and FADS1–FADS2 genetic variants modulate [13C]α-linolenic acid metabolism and plasma fatty acid composition. Am. J. Clin. Nutr. 2013, 97, 195–207. [Google Scholar] [CrossRef] [PubMed]
- One, Y.S.; Ido, T.K.; Inuki, T.A.; Onoda, M.S.; Chi, I.I. Genetic variants of the fatty acid desaturase gene cluster are associated with plasma LDL cholesterol levels in japanese males. J. Nutr. Sci. Vitaminol. 2013, 59, 325–335. [Google Scholar]
- Colombo, J.; Carlson, S.E.; Cheatham, C.L.; Fitzgerald-gustafson, K.M.; Kepler, A.; Doty, T. Long Chain Polyunsaturated Fatty Acid Supplementation in Infancy Reduces Heart Rate and Positively Affects Distribution of Attention. Pediatr. Res. 2012, 70, 406–410. [Google Scholar] [CrossRef]
- Carlson, S.E.; Colombo, J. Docosahexaenoic Acid and Arachidonic Acid Nutrition in Early Development. Adv. Pediatr. 2016, 63, 453–471. [Google Scholar] [CrossRef]
- Uhl, O.; Fleddermann, M.; Hellmuth, C.; Demmelmair, H.; Koletzko, B. Phospholipid species in newborn and 4 month old infants after consumption of different formulas or breast milk. PLoS ONE 2016, 11, e0162040. [Google Scholar] [CrossRef]
- Jakobik, V.; Weck, M.; Weyermann, M.; Grallert, H.; Lattka, E.; Rzehak, P. Genetic variants in the FADS gene cluster are associated with arachidonic acid concentrations of human breast milk at 1.5 and 6 mo postpartum and influence the course of milk dodecanoic, tetracosenoic, and trans-9-octadecenoic acid concentrations. Am. J. Clin. Nutr. 2011, 93, 382–391. [Google Scholar] [CrossRef]
- Wu, Y.; Zeng, L.; Chen, X.; Xu, Y.; Ye, L.; Qin, L.; Chen, L.; Xie, L. Association of the FADS gene cluster with coronary artery disease and plasma lipid concentrations in the northern Chinese Han population. Prostaglandins Leukot. Essent. Fat. Acids 2017, 117, 11–16. [Google Scholar] [CrossRef] [PubMed]
- Hester, A.G.; Murphy, R.C.; Uhlson, C.J.; Ivester, P.; Lee, T.C.; Sergeant, S.; Miller, L.R.; Howard, T.D.; Mathias, R.A.; Chilton, F.H. Relationship between a common variant in the fatty acid desaturase (FADS) cluster and eicosanoid generation in humans. J. Biol. Chem. 2014, 289, 22482–22489. [Google Scholar] [CrossRef] [PubMed]
- Martinelli, N.; Girelli, D.; Malerba, G.; Guarini, P.; Illig, T.; Trabetti, E.; Sandri, M.; Friso, S.; Pizzolo, F.; Schaeffer, L.; et al. FADS genotypes and desaturase activity estimated by the ratio of arachidonic acid to linoleic acid are associated with inflammation and coronary artery disease. Am. J. Clin. Nutr. 2008, 88, 941–949. [Google Scholar] [CrossRef]
- Zietemann, V.; Kröger, J.; Enzenbach, C.; Jansen, E.; Fritsche, A.; Weikert, C.; Boeing, H.; Schulze, M.B. Genetic variation of the FADS1 FADS2 gene cluster and n-6 PUFA composition in erythrocyte membranes in the European Prospective Investigation into Cancer and Nutrition-Potsdam study. Br. J. Nutr. 2010, 104, 1748–1759. [Google Scholar] [CrossRef]
- Meldrum, S.J.; Li, Y.; Zhang, G.; Heaton, A.E.M.; D’Vaz, N.; Manz, J.; Reischl, E.; Koletzko, B.V.; Prescott, S.L.; Simmer, K. Can polymorphisms in the fatty acid desaturase (FADS) gene cluster alter the effects of fish oil supplementation on plasma and erythrocyte fatty acid profiles? An exploratory study. Eur. J. Nutr. 2017, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Koletzko, B.; Lattka, E.; Zeilinger, S.; Illig, T.; Steer, C. Genetic variants of the fatty acid desaturase gene cluster predict amounts of red blood cell docosahexaenoic and other polyunsaturated fatty acids in pregnant women: Findings from the Avon Longitudinal Study of Parents and Children. Am. J. Nutr. 2011, 93, 211–219. [Google Scholar] [CrossRef] [PubMed]
- Barman, M.; Nilsson, S.; Naluai, Å.T.; Sandin, A.; Wold, A.E.; Sandberg, A.S. Single nucleotide polymorphisms in the FADS gene cluster but not the ELOVL2 gene are associated with serum polyunsaturated fatty acid composition and development of allergy (in a Swedish birth cohort). Nutrients 2015, 7, 10100–10115. [Google Scholar] [CrossRef] [PubMed]
- Ding, Z.; Liu, G.-L.; Li, X.; Chen, X.-Y.; Wu, Y.-X.; Cui, C.-C.; Zhang, X.; Yang, G.; Xie, L. Association of polyunsaturated fatty acids in breast milk with fatty acid desaturase gene polymorphisms among Chinese lactating mothers. Prostaglandins Leukot. Essent. Fat. Acids 2016, 109, 66–71. [Google Scholar] [CrossRef]
- Hellstrand, S.; Ericson, U.; Gullberg, B.; Hedblad, B.; Orho-melander, M.; Sonestedt, E. Genetic Variation in FADS1 Has Little Effect on the Association between Dietary PUFA Intake and Cardiovascular Disease. J. Nutr. 2014, 144, 1356–1363. [Google Scholar] [CrossRef]
- Roke, K.; Mutch, D.M. The role of FADS1/2 polymorphisms on cardiometabolic markers and fatty acid profiles in young adults consuming fish oil supplements. Nutrients 2014, 6, 2290–2304. [Google Scholar] [CrossRef]
- Miklavcic, J.J.; Larsen, B.M.K.; Mazurak, V.C.; Scalabrin, D.M.F.; MacDonald, I.M.; Shoemaker, G.K.; Casey, L.; Van Aerde, J.E.; Clandinin, M.T. Reduction of Arachidonate Is Associated With Increase in B-Cell Activation Marker in Infants: A Randomized Trial. J. Pediatr. Gastroenterol. Nutr. 2017, 64, 446–453. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, J.; Drossard, C.; Dube, K.; Kannenberg, F.; Kunz, C.; Kalhoff, H.; Kersting, M. Dietary intake and plasma concentrations of PUFA and LC-PUFA in breastfed and formula fed infants under real-life conditions. Eur. J. Nutr. 2010, 49, 189–195. [Google Scholar] [CrossRef] [PubMed]
- Michaelsen, K.F.; Skafte, L.; Badsberg, J.H.; Jørgensen, M. Variation in macronutrients in human bank milk: Influencing factors and implications for human milk banking. J. Pediatr. Gastroenterol. Nutr. 1990, 11, 229–239. [Google Scholar] [CrossRef]
- Stam, J.; Sauer, P.J.; Boehm, G. Can we define an infant’s need from the composition of human milk? Am. J. Clin. Nutr. 2013, 98, 521S–528S. [Google Scholar] [CrossRef]
- Moon, Y.-A.; Hammer, R.E.; Horton, J.D. Deletion of ELOVL5 leads to fatty liver through activation of SREBP-1c in mice. J. Lipid Res. 2009, 50, 412–423. [Google Scholar] [CrossRef]
- Hallmann, J.; Kolossa, S.; Gedrich, K.; Celis-Morales, C.; Forster, H.; O’Donovan, C.B.; Woolhead, C.; Macready, A.L.; Fallaize, R.; Marsaux, C.F.M.; et al. Predicting fatty acid profiles in blood based on food intake and the FADS1 rs174546 SNP. Mol. Nutr. Food Res. 2015, 59, 2565–2573. [Google Scholar] [CrossRef] [PubMed]
- Field, C.J.; Van Aerde, J.E.; Robinson, L.E.; Clandinin, M.T. Effect of providing a formula supplemented with long-chain polyunsaturated fatty acids on immunity in full-term neonates. Br. J. Nutr. 2008, 99, 91–99. [Google Scholar] [CrossRef] [PubMed]
- Ward, G.R.; Huang, Y.S.; Bobik, E.; Xing, H.C.; Mutsaers, L.; Auestad, N.; Montalto, M.; Wainwright, P. Long-chain polyunsaturated fatty acid levels in formulae influence deposition of docosahexaenoic acid and arachidonic acid in brain and red blood cells of artificially reared neonatal rats. J. Nutr. 1998, 128, 2473–2487. [Google Scholar] [CrossRef] [PubMed]
- Janssen, C.I.F.; Zerbi, V.; Mutsaers, M.P.C.; de Jong, B.S.W.; Wiesmann, M.; Arnoldussen, I.A.C.; Geenen, B.; Heerschap, A.; Muskiet, F.A.J.; Jouni, Z.E.; et al. Impact of dietary n-3 polyunsaturated fatty acids on cognition, motor skills and hippocampal neurogenesis in developing C57BL/6J mice. J. Nutr. Biochem. 2015, 26, 24–35. [Google Scholar] [CrossRef] [PubMed]
- Lauritzen, L.; Sørensen, L.B.; Harsløf, L.B.; Ritz, C.; Stark, K.D.; Astrup, A.; Dyssegaard, C.B.; Egelund, N.; Michaelsen, K.F.; Damsgaard, C.T. Mendelian randomization shows sex-specific associations between long-chain PUFA-related genotypes and cognitive performance in Danish schoolchildren. Am. J. Clin. Nutr. 2017, 106, 88–95. [Google Scholar] [CrossRef]
| Standard Formula | Experimental Formula | |
|---|---|---|
| 100 mL (13.5%) | 100 mL (13.5%) | |
| Energy (kcal/kJ) | 69/288 | 68/285 |
| Proteins * (g) | 1.35 | 1.35 |
| Casein/whey (%) | 40/60 | 40/60 |
| Carbohydrates (g) | 7.97 | 7.56 |
| Lactose (g) | 7.17 | 6.82 |
| Maltodextrin (g) | 0.8 | 0.7 |
| Fat # (g) | 3.5 | 3.5 |
| Linoleic acid (LA, mg) | 579 | 569 |
| α-Linolenic acid (ALA, mg) | 49 | 49 |
| Arachidonic acid (AA, mg) | - | 15.8 |
| Docosahexaenoic acid (DHA, mg) | - | 11.2 |
| Characteristics | SF | EF | BF | p | |||
|---|---|---|---|---|---|---|---|
| N | Mean ± SD | N | Mean ± SD | N | Mean ± SD | ||
| Maternal characteristics | |||||||
| Age (years) | 61 | 30.31 ab ± 6.53 | 70 | 29.97 ± 6.22 a | 45 | 33.24 ± 5.39 b | 0.014 |
| Gestational age (months) | 61 | 39.52 ± 1.29 | 70 | 39.26 ± 1.44 | 45 | 39.4 ± 1.3 | 0.55 |
| Pre-pregnancy weight (kg) | 56 | 65.43 ± 12.92 | 63 | 64.26 ± 12.52 | 43 | 65.66 ± 10.4 | 0.81 |
| Pre-pregnancy BMI (kg/m2) (%) | 0.76 | ||||||
| Underweight | 3 | 5.45 | 8 | 12.7 | 5 | 11.63 | |
| Normal weight | 29 | 52.73 | 29 | 46.03 | 23 | 53.49 | |
| Overweight | 14 | 25.45 | 15 | 23.81 | 11 | 25.58 | |
| Obesity | 9 | 16.36 | 11 | 17.46 | 4 | 9.3 | |
| Education (%) | <0.001 | ||||||
| Primary | 14 | 22.58 | 15 | 21.43 | 1 | 2.22 | |
| Secondary | 18 | 29.03 | 25 | 35.71 | 4 | 8.89 | |
| Professional | 12 | 19.35 | 16 | 22.86 | 13 | 28.89 | |
| Bachelor degree | 18 | 29.03 | 14 | 20 | 27 | 60 | |
| Smoking during pregnancy (Yes, %) | 10 | 20.83 | 10 | 15.87 | 2 | 5.13 | 0.11 |
| Edinburgh Scale (%) | 0.42 | ||||||
| No depression | 49 | 79.03 | 53 | 76.81 | 39 | 86.67 | |
| Probable depression | 13 | 20.97 | 16 | 23.19 | 6 | 13.33 | |
| Infant characteristics | |||||||
| Sex, male (%) | 37 | 59.68 ab | 44 | 62.86 a | 18 | 40.00 b | 0.042 |
| Birth weight (kg) | 61 | 3.34 ± 0.41 | 70 | 3.32 ± 0.5 | 45 | 3.35 ± 0.42 | 0.91 |
| Birth length (cm) | 61 | 50.67 ± 2.01 | 68 | 50.72 ± 2.1 | 45 | 50.62 ± 2.39 | 0.97 |
| WAZ | 61 | 0.04 ± 0.86 | 68 | 0.02 ± 0.97 | 44 | 0.13 ± 0.86 | 0.81 |
| LAZ | 61 | 0.58 ± 1.03 | 68 | 0.59 ± 1.08 | 44 | 0.71 ± 0.98 | 0.79 |
| BMIZ | 61 | −0.39 ± 0.93 | 68 | −0.39 ± 1.04 | 44 | −0.37 ± 0.95 | 0.99 |
| Fatty Acids and Gene | SNP | M/m | Standard Formula (n = 46) (n = 46) | Experimental Formula (n = 56) (n = 56) | Breastfeeding (n = 33) (n = 33) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| β | P | βc | Pc | β | P | βc | Pc | β | P | βc | Pc | |||
| C18:2n6 (LA) | ||||||||||||||
| FADS1 | rs174537 | G/T | −0.035 | 0.818 | −0.132 | 0.408 | −0.361 | 0.006 | −0.376 | 0.005 * | −0.027 | 0.880 | −0.094 | 0.687 |
| FADS1 | rs174545 | C/G | −0.035 | 0.818 | −0.132 | 0.408 | −0.322 | 0.015 | −0.351 | 0.008 | 0.026 | 0.888 | −0.092 | 0.688 |
| FADS1 | rs174546 | C/T | −0.035 | 0.818 | −0.132 | 0.408 | −0.322 | 0.015 | −0.351 | 0.008 | −0.027 | 0.880 | −0.094 | 0.687 |
| FADS1 | rs174553 | A/G | −0.035 | 0.818 | −0.132 | 0.408 | −0.322 | 0.015 | −0.351 | 0.008 | −0.027 | 0.880 | −0.094 | 0.687 |
| FADS2 | rs1535 | A/G | −0.035 | 0.818 | −0.132 | 0.408 | −0.346 | 0.008 | −0.357 | 0.008 | −0.027 | 0.880 | −0.094 | 0.687 |
| FADS2 | rs174570 | C/T | 0.155 | 0.304 | 0.137 | 0.406 | −0.272 | 0.043 | −0.207 | 0.134 | −0.106 | 0.558 | −0.162 | 0.464 |
| C20:3n6 (DGLA) | ||||||||||||||
| FADS2 | rs174570 | C/T | −0.276 | 0.063 | −0.230 | 0.167 | −0.327 | 0.014 | −0.288 | 0.034 | −0.123 | 0.495 | −0.046 | 0.815 |
| FADS2 | rs2072114 | A/G | −0.056 | 0.709 | −0.079 | 0.632 | −0.368 | 0.005 * | −0.342 | 0.014 | −0.090 | 0.618 | −0.145 | 0.446 |
| C20:4n6 (AA) | ||||||||||||||
| FADS1 | rs174537 | G/T | −0.297 | 0.045 | −0.224 | 0.155 | −0.396 | 0.002 * | −0.440 | 0.001 * | 0.023 | 0.897 | 0.035 | 0.876 |
| FADS1 | rs174545 | C/G | −0.297 | 0.045 | −0.224 | 0.155 | −0.351 | 0.007 | −0.375 | 0.006 | 0.046 | 0.803 | 0.036 | 0.873 |
| FADS1 | rs174546 | C/T | −0.297 | 0.045 | −0.224 | 0.155 | −0.351 | 0.007 | −0.375 | 0.006 | 0.023 | 0.897 | 0.035 | 0.876 |
| FADS1 | rs174548 | C/G | −0.118 | 0.436 | −0.073 | 0.653 | −0.360 | 0.006 | −0.367 | 0.007 | 0.052 | 0.773 | 0.034 | 0.878 |
| FADS1 | rs174553 | A/G | −0.297 | 0.045 | −0.224 | 0.155 | −0.351 | 0.007 | −0.375 | 0.006 | 0.023 | 0.897 | 0.035 | 0.876 |
| FADS2 | rs1535 | A/G | −0.297 | 0.045 | −0.224 | 0.155 | −0.340 | 0.010 | −0.374 | 0.007 | 0.023 | 0.897 | 0.035 | 0.876 |
| FADS2 | rs174570 | C/T | −0.412 | 0.004 * | −0.347 | 0.030 | −0.262 | 0.051 | −0.237 | 0.096 | −0.257 | 0.148 | −0.187 | 0.379 |
| FADS2 | rs2072114 | A/G | −0.077 | 0.613 | −0.049 | 0.761 | −0.502 | <0.001 * | −0.522 | <0.001 * | −0.059 | 0.746 | −0.054 | 0.797 |
| C22:4n6 (AdA) | ||||||||||||||
| FADS1 | rs174537 | G/T | −0.350 | 0.017 | −0.408 | 0.010 | −0.346 | 0.009 | −0.365 | 0.006 | 0.015 | 0.933 | −0.042 | 0.855 |
| FADS1 | rs174545 | C/G | −0.350 | 0.017 | −0.408 | 0.010 | −0.333 | 0.011 | −0.330 | 0.014 | 0.005 | 0.979 | −0.042 | 0.856 |
| FADS1 | rs174546 | C/T | −0.350 | 0.017 | −0.408 | 0.010 | −0.333 | 0.011 | −0.330 | 0.014 | 0.015 | 0.933 | −0.042 | 0.855 |
| FADS1 | rs174548 | C/G | −0.280 | 0.059 | −0.372 | 0.022 | −0.332 | 0.012 | −0.317 | 0.016 | −0.027 | 0.883 | −0.140 | 0.542 |
| FADS1 | rs174553 | A/G | −0.350 | 0.017 | −0.408 | 0.010 | −0.333 | 0.011 | −0.330 | 0.014 | 0.015 | 0.933 | −0.042 | 0.855 |
| FADS2 | rs1535 | A/G | −0.350 | 0.017 | −0.408 | 0.010 | −0.348 | 0.008 | −0.354 | 0.009 | 0.015 | 0.933 | −0.042 | 0.855 |
| FADS2 | rs174570 | C/T | −0.222 | 0.138 | −0.244 | 0.147 | −0.374 | 0.004 * | −0.337 | 0.013 | −0.046 | 0.799 | 0.029 | 0.897 |
| FADS2 | rs2072114 | A/G | 0.060 | 0.693 | 0.025 | 0.883 | −0.362 | 0.006 | −0.302 | 0.032 | 0.011 | 0.953 | −0.019 | 0.931 |
| C22:5n6 (DPAn6) | ||||||||||||||
| FADS1 | rs174545 | C/G | −0.111 | 0.463 | −0.054 | 0.739 | −0.255 | 0.056 | −0.288 | 0.038 | 0.102 | 0.577 | 0.068 | 0.742 |
| FADS1 | rs174546 | C/T | −0.111 | 0.463 | −0.054 | 0.739 | −0.255 | 0.056 | −0.288 | 0.038 | 0.100 | 0.578 | 0.068 | 0.741 |
| FADS1 | rs174553 | A/G | −0.111 | 0.463 | −0.054 | 0.739 | −0.255 | 0.056 | −0.288 | 0.038 | 0.100 | 0.578 | 0.068 | 0.741 |
| C18:3n3 (ALA) | ||||||||||||||
| FADS2 | rs174570 | C/T | 0.303 | 0.040 | 0.279 | 0.079 | −0.040 | 0.772 | −0.042 | 0.765 | −0.061 | 0.736 | −0.089 | 0.665 |
| C20:5n3 (EPA) | ||||||||||||||
| FADS1 | rs174537 | G/T | −0.287 | 0.053 | −0.315 | 0.057 | −0.249 | 0.065 | −0.331 | 0.017 | 0.113 | 0.530 | 0.269 | 0.243 |
| FADS1 | rs174545 | C/G | −0.287 | 0.053 | −0.315 | 0.057 | −0.225 | 0.093 | 0.310 | 0.025 | 0.162 | 0.374 | 0.274 | 0.236 |
| FADS1 | rs174546 | C/T | −0.287 | 0.053 | −0.315 | 0.057 | −0.225 | 0.093 | 0.310 | 0.025 | 0.113 | 0.530 | 0.269 | 0.243 |
| FADS1 | rs174548 | C/G | −0.238 | 0.112 | −0.284 | 0.093 | −0.247 | 0.064 | −0.303 | 0.026 | 0.158 | 0.380 | 0.296 | 0.198 |
| FADS1 | rs174553 | A/G | −0.287 | 0.053 | −0.315 | 0.057 | −0.225 | 0.093 | −0.310 | 0.025 | 0.113 | 0.530 | 0.269 | 0.243 |
| GLA:LA (D6D) | ||||||||||||||
| FADS2 | rs174570 | C/T | −0.394 | 0.007 | −0.338 | 0.037 | −0.049 | 0.722 | −0.127 | 0.361 | −0.078 | 0.670 | 0.007 | 0.973 |
| DGLA:LA (D6D) | ||||||||||||||
| FADS1 | rs174537 | G/T | −0.24 | 0.010 | −0.17 | 0.28 | 0.06 | 0.62 | −0.01 | 0.94 | 0.09 | 0.62 | 0.19 | 0.38 |
| FADS1 | rs174545 | C/G | −0.24 | 0.010 | −0.17 | 0.28 | 0.04 | 0.72 | −0.03 | 0.81 | 0.07 | 0.71 | 0.19 | 0.40 |
| FADS1 | rs174546 | C/T | −0.24 | 0.010 | −0.17 | 0.28 | 0.04 | 0.72 | −0.03 | 0.81 | 0.09 | 0.62 | 0.19 | 0.38 |
| FADS1 | rs174553 | A/G | −0.24 | 0.010 | −0.17 | 0.28 | 0.04 | 0.72 | −0.03 | 0.81 | 0.09 | 0.62 | 0.19 | 0.38 |
| FADS2 | rs174570 | C/T | −0.40 | 0.006 | −0.34 | 0.032 | −0.21 | 0.12 | −0.20 | 0.15 | −0.08 | 0.64 | 0.01 | 0.93 |
| FADS2 | rs2072114 | A/G | −0.18 | 0.22 | −0.14 | 0.36 | −0.29 | 0.026 | −0.26 | 0.06 | −0.09 | 0.59 | −0.14 | 0.47 |
| AA:LA (D6D + D5D) | ||||||||||||||
| FADS1 | rs174537 | G/T | −0.36 | 0.013 | −0.28 | 0.06 | −0.22 | 0.09 | −0.26 | 0.06 | 0.04 | 0.81 | 0.09 | 0.66 |
| FADS1 | rs174545 | C/G | −0.36 | 0.013 | −0.28 | 0.06 | −0.20 | 0.13 | −0.21 | 0.13 | 0.04 | 0.83 | 0.09 | 0.67 |
| FADS1 | rs174546 | C/T | −0.36 | 0.013 | −0.28 | 0.06 | −0.20 | 0.13 | −0.21 | 0.13 | 0.04 | 0.81 | 0.09 | 0.66 |
| FADS1 | rs174548 | C/G | −0.18 | 0.24 | −0.15 | 0.36 | −0.27 | 0.045 | −0.26 | 0.06 | −0.01 | 0.95 | −0.05 | 0.83 |
| FADS1 | rs174553 | A/G | −0.36 | 0.013 | −0.28 | 0.06 | −0.20 | 0.13 | −0.21 | 0.13 | 0.04 | 0.81 | 0.09 | 0.66 |
| FADS2 | rs1535 | A/G | −0.36 | 0.013 | −0.28 | 0.06 | −0.17 | 0.18 | −0.20 | 0.14 | 0.04 | 0.81 | 0.09 | 0.66 |
| FADS2 | rs174570 | C/T | −0.47 | 0.001 * | −0.41 | 0.007 | −0.13 | 0.32 | −0.14 | 0.32 | −0.24 | 0.16 | −0.12 | 0.54 |
| FADS2 | rs2072114 | A/G | −0.16 | 0.27 | −0.12 | 0.43 | −0.43 | 0.001 * | −0.450 | 0.001 * | −0.07 | 0.67 | −0.05 | 0.79 |
| AA:DGLA (D5D) | ||||||||||||||
| FADS1 | rs174548 | C/G | −0.226 | 0.132 | −0.160 | 0.333 | −0.269 | 0.043 | −0.285 | 0.041 | −0.133 | 0.462 | −0.108 | 0.636 |
| FADS2 | rs1535 | A/G | −0.195 | 0.195 | −0.141 | 0.386 | −0.261 | 0.050 | −0.283 | 0.049 | −0.072 | 0.691 | −0.071 | 0.757 |
| C22:6n3 (DHA) | ||||||||||||||
| FADS1 | rs174537 | G/T | −0.257 | 0.085 | −0.303 | 0.054 | −0.393 | 0.003 * | −0.415 | 0.002 * | 0.057 | 0.753 | 0.176 | 0.432 |
| FADS1 | rs174545 | C/G | −0.257 | 0.085 | −0.303 | 0.054 | −0.341 | 0.010 | −0.339 | 0.013 | 0.089 | 0.629 | 0.177 | 0.429 |
| FADS1 | rs174546 | C/T | −0.257 | 0.085 | −0.303 | 0.054 | −0.341 | 0.010 | −0.339 | 0.013 | 0.057 | 0.753 | 0.176 | 0.432 |
| FADS1 | rs174548 | C/G | −0.144 | 0.339 | −0.211 | 0.192 | −0.338 | 0.010 | −0.328 | 0.015 | 0.101 | 0.577 | 0.164 | 0.463 |
| FADS1 | rs174553 | A/G | −0.257 | 0.085 | −0.303 | 0.054 | −0.341 | 0.010 | −0.339 | 0.013 | 0.057 | 0.753 | 0.176 | 0.432 |
| FADS2 | rs1535 | A/G | −0.257 | 0.085 | −0.303 | 0.054 | −0.342 | 0.009 | −0.354 | 0.010 | 0.057 | 0.753 | 0.176 | 0.432 |
| FADS2 | rs174570 | C/T | −0.233 | 0.120 | −0.258 | 0.114 | −0.265 | 0.048 | −0.238 | 0.092 | −0.352 | 0.045 | −0.274 | 0.196 |
| FADS2 | rs2072114 | A/G | 0.071 | 0.637 | 0.056 | 0.732 | −0.289 | 0.029 | −0.244 | 0.093 | −0.081 | 0.654 | −0.017 | 0.934 |
| EPA:ALA (D6D + D5D) | ||||||||||||||
| FADS1 | rs174537 | G/T | −0.37 | 0.010 | −0.35 | 0.022 | −0.13 | 0.32 | −0.19 | 0.15 | 0.08 | 0.65 | −0.04 | 0.83 |
| FADS1 | rs174545 | C/G | −0.37 | 0.010 | −0.35 | 0.022 | −0.08 | 0.52 | −0.14 | 0.30 | 0.06 | 0.71 | −0.04 | 0.84 |
| FADS1 | rs174546 | C/T | −0.37 | 0.010 | −0.35 | 0.022 | −0.08 | 0.52 | −0.14 | 0.30 | 0.08 | 0.65 | −0.04 | 0.83 |
| FADS1 | rs174553 | A/G | −0.37 | 0.010 | −0.35 | 0.022 | −0.08 | 0.52 | −0.14 | 0.30 | 0.08 | 0.65 | −0.04 | 0.83 |
| FADS2 | rs1535 | A/G | −0.37 | 0.010 | −0.35 | 0.022 | −0.07 | 0.61 | −0.13 | 0.34 | 0.08 | 0.65 | −0.04 | 0.83 |
| FADS2 | rs174570 | C/T | −0.37 | 0.010 | −0.37 | 0.019 | −0.06 | 0.61 | −0.07 | 0.58 | −0.14 | 0.43 | −0.09 | 0.65 |
| Fatty Acids and Gene | SNP | M/m | MM | Mm + mm | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Standard Formula | Experimental Formula | Breastfeeding | p | Standard Formula | Experimental Formula | Breastfeeding | p | |||||||||
| N | Mean ± SD | N | Mean ± SD | N | Mean ± SD | N | Mean ± SD | N | Mean ± SD | N | Mean ± SD | |||||
| C20:4n6 (AA) | ||||||||||||||||
| FADS1 | rs174537 | G/T | 30 | 2.0000 ± 0.64 a | 41 | 2.4900 ± 0.53 b | 18 | 2.9200 ± 0.79 b | <0.001 * | 31 | 1.7300 ± 0.53 a | 25 | 2.0000 ± 0.62 a | 20 | 2.7300 ± 0.73 b | <0.001 * |
| FADS1 | rs174545 | C/G | 30 | 2.0000 ± 0.64 a | 40 | 2.4900 ± 0.54 b | 17 | 2.9000 ± 0.81 b | <0.001 * | 31 | 1.7300 ± 0.53 a | 27 | 2.0500 ± 0.63 a | 20 | 2.7300 ± 0.73 b | <0.001 * |
| FADS1 | rs174546 | C/T | 30 | 2.0000 ± 0.64 a | 40 | 2.4900 ± 0.54 b | 18 | 2.9200 ± 0.79 b | <0.001 * | 31 | 1.7300 ± 0.53 a | 27 | 2.0500 ± 0.63 a | 20 | 2.7300 ± 0.73 b | <0.001 * |
| FADS1 | rs174548 | C/G | 33 | 1.9200 ± 0.63 a | 40 | 2.5000 ± 0.54 b | 21 | 2.8700 ± 0.78 b | <0.001 * | 28 | 1.8000 ± 0.56 a | 27 | 2.0500 ± 0.62 a | 17 | 2.7500 ± 0.73 b | <0.001 * |
| FADS1 | rs174553 | A/G | 30 | 2.0000 ± 0.64 a | 40 | 2.4900 ± 0.54 b | 18 | 2.9200 ± 0.79b | <0.001 * | 31 | 1.7300 ± 0.53 a | 27 | 2.0500 ± 0.63 a | 20 | 2.7300 ± 0.73 b | <0.001 * |
| FADS2 | rs1535 | A/G | 30 | 2.0000 ± 0.64 a | 40 | 2.4900 ± 0.54 b | 18 | 2.9200 ± 0.79 b | <0.001 * | 31 | 1.7300 ± 0.53 a | 27 | 2.0600 ± 0.63 a | 20 | 2.7300 ± 0.73 b | <0.001 * |
| FADS2 | rs174570 | C/T | 44 | 1.9600 ± 0.60 a | 53 | 2.4100 ± 0.58 b | 28 | 2.9500 ± 0.75 c | <0.001 * | 17 | 1.6200 ± 0.52 a | 13 | 1.8900 ± 0.57 ab | 10 | 2.4300 ± 0.64 b | 0.006 |
| FADS2 | rs2072114 | A/G | 47 | 1.8700 ± 0.61 a | 54 | 2.4400 ± 0.53 b | 30 | 2.8500 ± 0.76 b | <0.001 * | 14 | 1.8400 ± 0.56 a | 13 | 1.8100 ± 0.69 a | 8 | 2.6800 ± 0.75b | 0.007 |
| C22:6n3 (DHA) | ||||||||||||||||
| FADS1 | rs174537 | G/T | 30 | 0.5100 ± 0.24 a | 41 | 0.9900 ± 0.22 b | 18 | 1.2300 ± 0.42 b | <0.001 * | 31 | 0.4500 ± 0.25 a | 25 | 0.7900 ± 0.27 b | 20 | 1.1800 ± 0.46 c | <0.001 * |
| FADS1 | rs174545 | C/G | 30 | 0.5100 ± 0.24 a | 40 | 0.9900 ± 0.22 b | 17 | 1.2000 ± 0.43 b | <0.001 * | 31 | 0.4500 ± 0.25 a | 27 | 0.8200 ± 0.28 b | 20 | 1.1800 ± 0.46 c | <0.001 * |
| FADS1 | rs174546 | C/T | 30 | 0.5100 ± 0.24 a | 40 | 0.9900 ± 0.22 b | 18 | 1.2300 ± 0.42 b | <0.001 * | 31 | 0.4500 ± 0.25 a | 27 | 0.8200 ± 0.28 b | 20 | 1.1800 ± 0.46 c | <0.001 * |
| FADS1 | rs174548 | C/G | 33 | 0.4900 ± 0.24 a | 40 | 0.9900 ± 0.22 b | 21 | 1.2000 ± 0.43b | <0.001 * | 28 | 0.4600 ± 0.26 a | 27 | 0.8200 ± 0.29 b | 17 | 1.2000 ± 0.45 c | <0.001 * |
| FADS1 | rs174553 | A/G | 30 | 0.5100 ± 0.24 a | 40 | 0.9900 ± 0.22 b | 18 | 1.2300 ± 0.42 b | <0.001 * | 31 | 0.4500 ± 0.25 a | 27 | 0.8200 ± 0.28 b | 20 | 1.1800 ± 0.46 c | <0.001 * |
| FADS2 | rs1535 | A/G | 30 | 0.5100 ± 0.24 a | 40 | 0.9900 ± 0.22 b | 18 | 1.2300 ± 0.42 b | <0.001 * | 31 | 0.4500 ± 0.25 a | 27 | 0.8200 ± 0.28 b | 20 | 1.1800 ± 0.46 c | <0.001 * |
| FADS2 | rs174570 | C/T | 44 | 0.5000 ± 0.25 a | 53 | 0.9500 ± 0.22 b | 28 | 1.3000 ± 0.42 c | <0.001 * | 17 | 0.4200 ± 0.21 a | 13 | 0.7700 ± 0.33 b | 10 | 0.9200 ± 0.35 b | <0.001 * |
| FADS2 | rs2072114 | A/G | 47 | 0.4700 ± 0.24 a | 54 | 0.9600 ± 0.25 b | 30 | 1.2300 ± 0.45 b | <0.001 * | 14 | 0.5100 ± 0.28 a | 13 | 0.7600 ± 0.26 b | 8 | 1.0900 ± 0.38 c | <0.001 * |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Salas Lorenzo, I.; Chisaguano Tonato, A.M.; de la Garza Puentes, A.; Nieto, A.; Herrmann, F.; Dieguez, E.; Castellote, A.I.; López-Sabater, M.C.; Rodríguez-Palmero, M.; Campoy, C. The Effect of an Infant Formula Supplemented with AA and DHA on Fatty Acid Levels of Infants with Different FADS Genotypes: The COGNIS Study. Nutrients 2019, 11, 602. https://doi.org/10.3390/nu11030602
Salas Lorenzo I, Chisaguano Tonato AM, de la Garza Puentes A, Nieto A, Herrmann F, Dieguez E, Castellote AI, López-Sabater MC, Rodríguez-Palmero M, Campoy C. The Effect of an Infant Formula Supplemented with AA and DHA on Fatty Acid Levels of Infants with Different FADS Genotypes: The COGNIS Study. Nutrients. 2019; 11(3):602. https://doi.org/10.3390/nu11030602
Chicago/Turabian StyleSalas Lorenzo, Isabel, Aida M. Chisaguano Tonato, Andrea de la Garza Puentes, Ana Nieto, Florian Herrmann, Estefanía Dieguez, Ana I. Castellote, M. Carmen López-Sabater, Maria Rodríguez-Palmero, and Cristina Campoy. 2019. "The Effect of an Infant Formula Supplemented with AA and DHA on Fatty Acid Levels of Infants with Different FADS Genotypes: The COGNIS Study" Nutrients 11, no. 3: 602. https://doi.org/10.3390/nu11030602
APA StyleSalas Lorenzo, I., Chisaguano Tonato, A. M., de la Garza Puentes, A., Nieto, A., Herrmann, F., Dieguez, E., Castellote, A. I., López-Sabater, M. C., Rodríguez-Palmero, M., & Campoy, C. (2019). The Effect of an Infant Formula Supplemented with AA and DHA on Fatty Acid Levels of Infants with Different FADS Genotypes: The COGNIS Study. Nutrients, 11(3), 602. https://doi.org/10.3390/nu11030602






