Clustering of Metabolic Risk Components and Associated Lifestyle Factors: A Nationwide Adolescent Study in Taiwan
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Population
2.2. Questionnaire Data
2.3. Anthropometric Parameters
2.4. Clinical Variables
2.5. MetS and Its Risk Components
2.6. Statistical Analysis
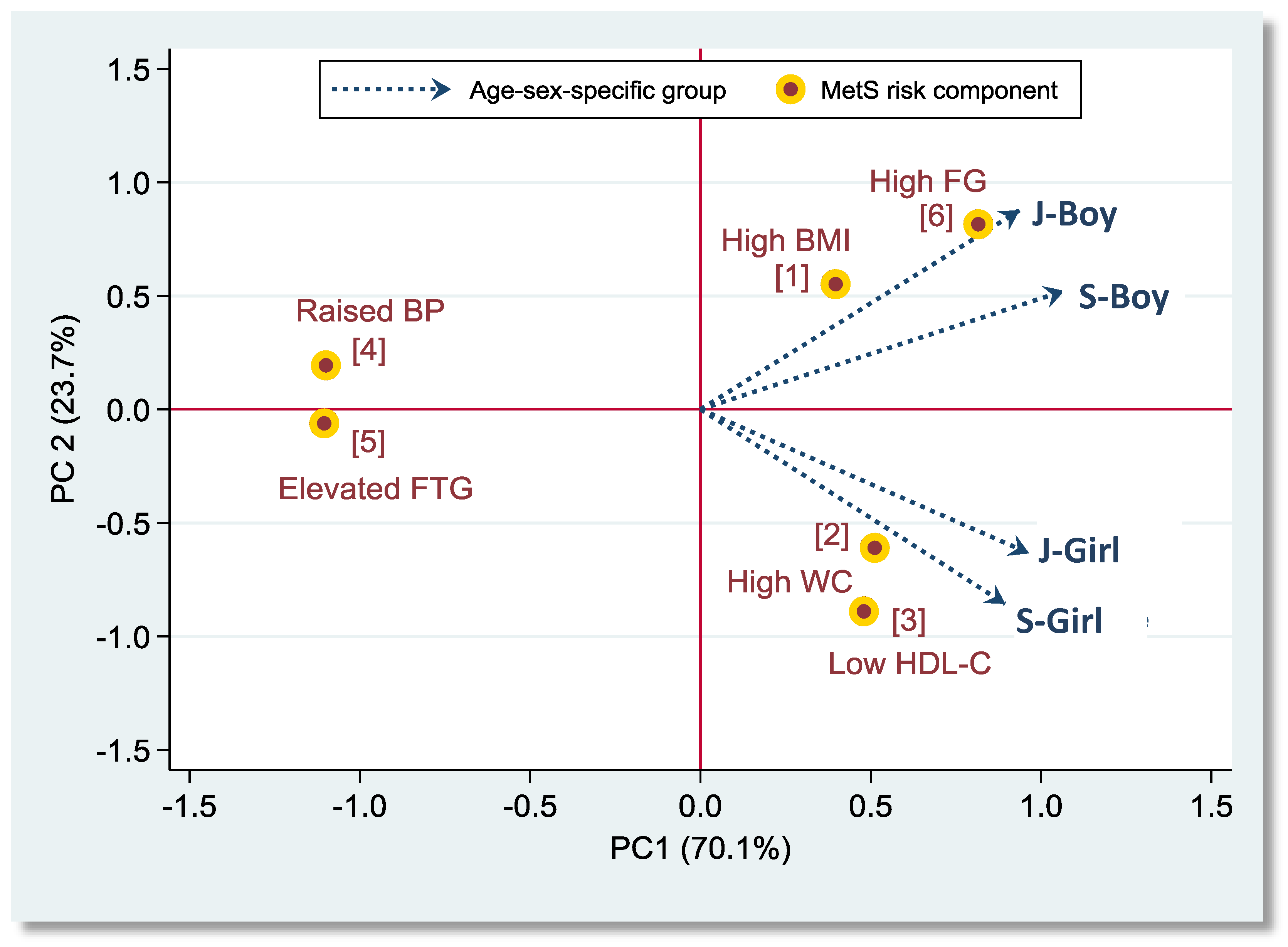
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
Abbreviation
| BMI | body mass index |
| BP | blood pressure |
| DBP | diastolic blood pressure |
| FG | fasting glucose |
| FTG | fasting triglycerides |
| HC | hip circumference |
| HDL-C | high-density lipoprotein cholesterol |
| IDF | International Diabetes Federation |
| JIS-Adult | Joint Interim Statement of MetS for adults |
| MetS | metabolic syndrome |
| NAHSIT | Nutrition and Health Survey in Taiwan |
| NCEP-ATP III | National Cholesterol Education Program, Adult Treatment Panel III |
| SBP | systolic blood pressure |
| SSB | sugar-sweetened beverage |
| T2DM | type 2 diabetes mellitus |
| TPA | Taiwan Pediatric Association |
| WC | waist circumference |
References
- Eckel, R.H.; Grundy, S.M.; Zimmet, P.Z. The metabolic syndrome. Lancet 2005, 365, 1415–1428. [Google Scholar] [CrossRef]
- Magnussen, C.G.; Koskinen, J.; Chen, W.; Thomson, R.; Schmidt, M.D.; Srinivasan, S.R.; Kivimaki, M.; Mattsson, N.; Kahonen, M.; Laitinen, T.; et al. Pediatric metabolic syndrome predicts adulthood metabolic syndrome, subclinical atherosclerosis, and type 2 diabetes mellitus but is no better than body mass index alone: The bogalusa heart study and the cardiovascular risk in young finns study. Circulation 2010, 122, 1604–1611. [Google Scholar] [CrossRef]
- Ambrosini, G.L.; Oddy, W.H.; Huang, R.C.; Mori, T.A.; Beilin, L.J.; Jebb, S.A. Prospective associations between sugar-sweetened beverage intakes and cardiometabolic risk factors in adolescents. Am. J. Clin. Nutr. 2013, 98, 327–334. [Google Scholar] [CrossRef] [PubMed]
- Chan, T.F.; Lin, W.T.; Huang, H.L.; Lee, C.Y.; Wu, P.W.; Chiu, Y.W.; Huang, C.C.; Tsai, S.; Lin, C.L.; Lee, C.H. Consumption of sugar-sweetened beverages is associated with components of the metabolic syndrome in adolescents. Nutrients 2014, 6, 2088–2103. [Google Scholar] [CrossRef] [PubMed]
- Dias Pitangueira, J.C.; Rodrigues Silva, L.; Portela de Santana, M.L.; Monteiro da Silva Mda, C.; de Farias Costa, P.R.; D’Almeida, V.; de Oliveira Assis, A.M. Metabolic syndrome and associated factors in children and adolescents of a brazilian municipality. Nutr. Hosp. 2014, 29, 865–872. [Google Scholar] [PubMed]
- Johnson, W.D.; Kroon, J.J.; Greenway, F.L.; Bouchard, C.; Ryan, D.; Katzmarzyk, P.T. Prevalence of risk factors for metabolic syndrome in adolescents: National health and nutrition examination survey (nhanes), 2001–2006. Arch. Pediatr. Adolesc. Med. 2009, 163, 371–377. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; So, W.Y. Prevalence of metabolic syndrome among korean adolescents according to the national cholesterol education program, adult treatment panel iii and international diabetes federation. Nutrients 2016, 8, 588. [Google Scholar] [CrossRef] [PubMed]
- Song, P.; Yu, J.; Chang, X.; Wang, M.; An, L. Prevalence and correlates of metabolic syndrome in chinese children: The china health and nutrition survey. Nutrients 2017, 9, 79. [Google Scholar] [CrossRef] [PubMed]
- Gaston, S.A.; Tulve, N.S.; Ferguson, T.F. Abdominal obesity, metabolic dysfunction, and metabolic syndrome in U.S. Adolescents: National health and nutrition examination survey 2011–2016. Ann. Epidemiol. 2019, 30, 30–36. [Google Scholar] [CrossRef] [PubMed]
- Lakka, T.A.; Laaksonen, D.E. Physical activity in prevention and treatment of the metabolic syndrome. Appl. Physiol. Nutr. Metab. 2007, 32, 76–88. [Google Scholar] [CrossRef] [PubMed]
- Feldeisen, S.E.; Tucker, K.L. Nutritional strategies in the prevention and treatment of metabolic syndrome. Appl. Physiol. Nutr. Metab. 2007, 32, 46–60. [Google Scholar] [CrossRef] [PubMed]
- Albert Perez, E.; Mateu Olivares, V.; Martinez-Espinosa, R.M.; Molina Vila, M.D.; Reig Garcia-Galbis, M. New insights about how to make an intervention in children and adolescents with metabolic syndrome: Diet, exercise vs. Changes in body composition. A systematic review of rct. Nutrients 2018, 10, 878. [Google Scholar] [CrossRef] [PubMed]
- Steinberger, J.; Daniels, S.R.; Eckel, R.H.; Hayman, L.; Lustig, R.H.; McCrindle, B.; Mietus-Snyder, M.L.; American Heart Association Atherosclerosis, Hypertension; Obesity in the Young Committee of the Council on Cardiovascular Disease in the Young; Council on Cardiovascular Nursing; et al. Progress and challenges in metabolic syndrome in children and adolescents: A scientific statement from the american heart association atherosclerosis, hypertension, and obesity in the young committee of the council on cardiovascular disease in the young; council on cardiovascular nursing; and council on nutrition, physical activity, and metabolism. Circulation 2009, 119, 628–647. [Google Scholar] [PubMed]
- Zimmet, P.; Alberti, K.G.; Kaufman, F.; Tajima, N.; Silink, M.; Arslanian, S.; Wong, G.; Bennett, P.; Shaw, J.; Caprio, S.; et al. The metabolic syndrome in children and adolescents—An idf consensus report. Pediatr. Diabetes 2007, 8, 299–306. [Google Scholar] [CrossRef] [PubMed]
- Cook, S.; Weitzman, M.; Auinger, P.; Nguyen, M.; Dietz, W.H. Prevalence of a metabolic syndrome phenotype in adolescents: Findings from the third national health and nutrition examination survey, 1988–1994. Arch. Pediatr. Adolesc. Med. 2003, 157, 821–827. [Google Scholar] [CrossRef] [PubMed]
- Lin, W.T.; Huang, H.L.; Huang, M.C.; Chan, T.F.; Ciou, S.Y.; Lee, C.Y.; Chiu, Y.W.; Duh, T.H.; Lin, P.L.; Wang, T.N.; et al. Effects on uric acid, body mass index and blood pressure in adolescents of consuming beverages sweetened with high-fructose corn syrup. Int. J. Obes. 2013, 37, 532–539. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.Y.; Lin, W.T.; Tsai, S.; Hung, Y.C.; Wu, P.W.; Yang, Y.C.; Chan, T.F.; Huang, H.L.; Weng, Y.L.; Chiu, Y.W.; et al. Association of parental overweight and cardiometabolic diseases and pediatric adiposity and lifestyle factors with cardiovascular risk factor clustering in adolescents. Nutrients 2016, 8, 567. [Google Scholar] [CrossRef]
- Lin, W.T.; Chan, T.F.; Huang, H.L.; Lee, C.Y.; Tsai, S.; Wu, P.W.; Yang, Y.C.; Wang, T.N.; Lee, C.H. Fructose-rich beverage intake and central adiposity, uric acid, and pediatric insulin resistance. J. Pediatr. 2016, 171, 90–96. [Google Scholar] [CrossRef]
- Kim, Y.; Je, Y. Meat consumption and risk of metabolic syndrome: Results from the korean population and a meta-analysis of observational studies. Nutrients 2018, 10, 390. [Google Scholar] [CrossRef]
- Lin, W.T.; Lin, P.C.; Lee, C.Y.; Chen, Y.L.; Chan, T.F.; Tsai, S.; Huang, H.L.; Wu, P.W.; Chin, Y.T.; Lin, H.Y.; et al. Effects of insulin resistance on the association between the circulating retinol-binding protein 4 level and clustering of pediatric cardiometabolic risk factors. Pediatr. Diabetes 2018, 19, 611–621. [Google Scholar] [CrossRef]
- Liou, T.H.; Huang, Y.C.; Chou, P. Prevalence and secular trends in overweight and obese Taiwanese children and adolescents in 1991–2003. Ann. Hum. Biol. 2009, 36, 176–185. [Google Scholar] [CrossRef]
- National Health Research Institutes. Nutrition and Health Survey in Taiwan 2010–2011. Available online: https://www.hpa.gov.tw/Pages/List.aspx?nodeid=1773 (accessed on 1 December 2018).
- Chen, C.M.; Lou, M.F.; Gau, B.S. Prevalence of impaired fasting glucose and analysis of related factors in Taiwanese adolescents. Pediatr. Diabetes 2014, 15, 220–228. [Google Scholar] [CrossRef]
- Taiwanese Food and Nutrients Databank. 1993. Available online: https://consumer.Fda.Gov.Tw/foodanalysis/ingredients.Htm (accessed on 10 October 2017). (In Chinese)
- Gau, S.F.; Soong, W.T.; Tsai, W.Y.; Chiu, Y.N. A Chinese version of a self-administered rating scale for pubertal development. Taiwan. J. Psychiatr. 1997, 11, 129–140. [Google Scholar]
- The Statement of Taiwan Pediatric Association for Child and Adolescent Metabolic Syndrome. Taiwan Pediatric Association, June 2016. Available online: https://www.Pediatr.Org.Tw/people/edu_info.Asp?Id=33 (accessed on 1 November 2018).
- Alberti, K.G.; Eckel, R.H.; Grundy, S.M.; Zimmet, P.Z.; Cleeman, J.I.; Donato, K.A.; Fruchart, J.C.; James, W.P.; Loria, C.M.; Smith, S.C., Jr.; et al. Harmonizing the metabolic syndrome: A joint interim statement of the international diabetes federation task force on epidemiology and prevention; national heart, lung, and blood institute; american heart association; world heart federation; international atherosclerosis society; and international association for the study of obesity. Circulation 2009, 120, 1640–1645. [Google Scholar] [PubMed]
- Altman, D.G. Practical Statistics for Medical Research; Chapman and Hall: London, UK, 1991. [Google Scholar]
- Lee, C.H.; Chiang, S.L.; Ko, A.M.; Hua, C.H.; Tsai, M.H.; Warnakulasuriya, S.; Ibrahim, S.O.; Sunarjo; Zain, R.B.; Ling, T.Y.; et al. Betel-quid dependence domains and syndrome associated with betel-quid ingredients among chewers: An asian multi-country evidence. Addiction 2014, 109, 1194–1204. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.H.; Ko, A.M.; Yen, C.F.; Chu, K.S.; Gao, Y.J.; Warnakulasuriya, S.; Sunarjo; Ibrahim, S.O.; Zain, R.B.; Patrick, W.K.; et al. Betel-quid dependence and oral potentially malignant disorders in six asian countries. Br. J. Psychiatry 2012, 201, 383–391. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.H.; Ko, A.M.; Yang, F.M.; Hung, C.C.; Warnakulasuriya, S.; Ibrahim, S.O.; Zain, R.B.; Ko, Y.C. Association of dsm-5 betel-quid use disorder with oral potentially malignant disorder in 6 betel-quid endemic asian populations. JAMA Psychiatry 2018, 75, 261–269. [Google Scholar] [CrossRef] [PubMed]
- Morrison, J.A.; Friedman, L.A.; Wang, P.; Glueck, C.J. Metabolic syndrome in childhood predicts adult metabolic syndrome and type 2 diabetes mellitus 25 to 30 years later. J. Pediatr. 2008, 152, 201–206. [Google Scholar] [CrossRef] [PubMed]
- Morrison, J.A.; Friedman, L.A.; Gray-McGuire, C. Metabolic syndrome in childhood predicts adult cardiovascular disease 25 years later: The princeton lipid research clinics follow-up study. Pediatrics 2007, 120, 340–345. [Google Scholar] [CrossRef]
- Magge, S.N.; Goodman, E.; Armstrong, S.C.; Committee On, N.; Section On, E.; Section On, O. The metabolic syndrome in children and adolescents: Shifting the focus to cardiometabolic risk factor clustering. Pediatrics 2017, 140, e20171603. [Google Scholar] [CrossRef]
- Elwood, P.C.; Pickering, J.E.; Fehily, A.M. Milk and dairy consumption, diabetes and the metabolic syndrome: The caerphilly prospective study. J. Epidemiol. Community Health 2007, 61, 695–698. [Google Scholar] [CrossRef] [PubMed]
- Fumeron, F.; Lamri, A.; Emery, N.; Bellili, N.; Jaziri, R.; Porchay-Balderelli, I.; Lantieri, O.; Balkau, B.; Marre, M.; Group, D.S. Dairy products and the metabolic syndrome in a prospective study, desir. J. Am. Coll. Nutr. 2011, 30, 454S–463S. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhang, D.Z. Associations of vegetable and fruit consumption with metabolic syndrome. A meta-analysis of observational studies. Public Health Nutr. 2018, 21, 1693–1703. [Google Scholar] [CrossRef] [PubMed]
- Richelsen, B. Sugar-sweetened beverages and cardio-metabolic disease risks. Curr. Opin. Clin. Nutr. Metab. Care 2013, 16, 478–484. [Google Scholar] [CrossRef] [PubMed]
- Ter Horst, K.W.; Serlie, M.J. Fructose consumption, lipogenesis, and non-alcoholic fatty liver disease. Nutrients 2017, 9, 981. [Google Scholar] [CrossRef] [PubMed]
- Chiu, S.; Mulligan, K.; Schwarz, J.M. Dietary carbohydrates and fatty liver disease: De novo lipogenesis. Curr. Opin. Clin. Nutr. Metab. Care 2018, 21, 277–282. [Google Scholar] [CrossRef] [PubMed]
- Low, W.S.; Cornfield, T.; Charlton, C.A.; Tomlinson, J.W.; Hodson, L. Sex differences in hepatic de novo lipogenesis with acute fructose feeding. Nutrients 2018, 10, 1263. [Google Scholar] [CrossRef]
- Rodriguez, L.A.; Madsen, K.A.; Cotterman, C.; Lustig, R.H. Added sugar intake and metabolic syndrome in us adolescents: Cross-sectional analysis of the national health and nutrition examination survey 2005–2012. Public Health Nutr. 2016, 19, 2424–2434. [Google Scholar] [CrossRef]
- Gui, Z.H.; Zhu, Y.N.; Cai, L.; Sun, F.H.; Ma, Y.H.; Jing, J.; Chen, Y.J. Sugar-sweetened beverage consumption and risks of obesity and hypertension in Chinese children and adolescents: A national cross-sectional analysis. Nutrients 2017, 9, 1302. [Google Scholar] [CrossRef]
- Ha, K.; Chung, S.; Lee, H.S.; Kim, C.I.; Joung, H.; Paik, H.Y.; Song, Y. Association of dietary sugars and sugar-sweetened beverage intake with obesity in Korean children and adolescents. Nutrients 2016, 8, 31. [Google Scholar] [CrossRef]
- Department of Statistics, Taiwanese Ministry of Education. Available online: https://stats.moe.gov.tw/qframe.aspx?qno=MQA1AA2 (accessed on 18 February 2019). (In Chinese)
| Factor/Category | Total | Gender | p Value 2 | Grade 1 | p Value 2 | ||
|---|---|---|---|---|---|---|---|
| Male | Female | Junior | Senior | ||||
| Study participants, number | 1920 | 949 | 971 | 990 | 930 | ||
| Design-adjusted distribution 3 | |||||||
| Demographic factors | |||||||
| Age (year), mean ± SE | 15.0 ± 0.1 | 15.0 ± 0.1 | 15.0 ± 0.1 | 0.980 | 13.7 ± 0.1 | 16.8 ± 0.03 | <0.001 |
| Study area, % | |||||||
| North | 46.0 | 46.5 | 45.5 | 0.864 | 47.6 | 43.8 | 0.339 |
| Center | 25.8 | 25.1 | 26.5 | 25.6 | 25.9 | ||
| South | 25.7 | 25.8 | 25.6 | 24.4 | 27.5 | ||
| East | 2.5 | 2.7 | 2.4 | 2.4 | 2.7 | ||
| Postpuberty, % | 85.4 | 74.9 | 97.0 | <0.001 | 77.4 | 96.6 | <0.001 |
| Lifestyle factors | |||||||
| Dietary pattern (binary), % | |||||||
| Egg, ≥1 serving/day | 40.2 | 40.9 | 39.4 | 0.580 | 37.5 | 43.9 | 0.017 |
| Milk, ≥1 serving/day | 22.5 | 25.2 | 19.5 | 0.016 | 26.2 | 17.3 | <0.001 |
| Fresh fruit, ≥1 serving/day | 29.0 | 33.6 | 24.1 | 0.001 | 34.7 | 21.3 | <0.001 |
| Meats, ≥1 serving/day | 18.7 | 18.0 | 19.5 | 0.486 | 17.4 | 20.6 | 0.128 |
| Fried food, ≥1 serving/week | 42.5 | 43.0 | 42.0 | 0.738 | 41.9 | 43.4 | 0.599 |
| Sugar-sweetened beverage,≥1 drink/week | 88.7 | 90.9 | 86.1 | 0.006 | 87.7 | 90.0 | 0.154 |
| Soda, ≥1 drink/week | 28.4 | 36.1 | 19.8 | <0.001 | 28.8 | 27.9 | 0.737 |
| Sport drink, ≥1 drink/week | 28.4 | 37.3 | 18.4 | <0.001 | 28.4 | 28.4 | 0.991 |
| Coffee, ≥1 drink/week | 17.6 | 17.1 | 18.2 | 0.596 | 14.8 | 21.6 | 0.002 |
| Tea, ≥1 drink/week | 64.6 | 65.2 | 63.9 | 0.629 | 59.3 | 72.2 | <0.001 |
| Total calories intake (kcal), mean ± SE | 2354.8 ± 27.3 | 2626.5 ± 37.1 | 2056.3 ± 37.8 | <0.001 | 2304.5 ± 38.4 | 2425.5 ± 37.7 | 0.025 |
| Physical activity (binary), % | |||||||
| Sedentary behavior, ≥60 min/day | 55.5 | 59.9 | 50.5 | 0.001 | 51.9 | 60.5 | 0.002 |
| Physical exercise, ≥30 min/day | 38.2 | 48.7 | 26.5 | <0.001 | 34.7 | 43.1 | 0.002 |
| Substance use (binary), % | |||||||
| Alcohol drinking | 12.5 | 12.8 | 12.1 | 0.721 | 8.4 | 18.2 | <0.001 |
| Cigarette smoking | 5.1 | 6.8 | 3.2 | 0.007 | 3.5 | 7.4 | 0.003 |
| MetS Components | Junior (12–15 years) | Senior (16–18 years) | All | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total | Male | Female | p1 | Total | Male | Female | p1 | Total | Male | Female | p1 | |
| Distribution, aMean ± SE 2 | ||||||||||||
| WC (cm) | 74.8 ± 0.4 | 75.4 ± 0.6 | 74.2 ± 0.5 | 0.162 | 78.3 ± 0.4 | 79.7 ± 0.6 | 76.8 ± 0.4 | <0.001 | 76.3 ± 0.3 | 77.2 ± 0.4 | 75.2 ± 0.3 | <0.001 |
| HDL-C (mg/dL) | 55.1 ± 0.5 | 53.4 ± 0.7 | 56.8 ± 0.9 | 0.005 | 55.2 ± 0.4 | 51.2 ± 0.6 | 59.7 ± 0.7 | <0.001 | 55.1 ± 0.4 | 52.4 ± 0.5 | 58.1 ± 0.6 | <0.001 |
| SBP (mmHg) | 104.4 ± 0.4 | 108.6 ± 0.6 | 99.7 ± 0.5 | <0.001 | 104.3 ± 0.4 | 109.7 ± 0.5 | 98.4 ± 0.5 | <0.001 | 104.4 ± 0.3 | 109.2 ± 0.4 | 99.1 ± 0.4 | <0.001 |
| DBP (mmHg) | 60.6 ± 0.3 | 61.1 ± 0.5 | 60.1 ± 0.5 | 0.180 | 60.0 ± 0.3 | 60.0 ± 0.4 | 59.9 ± 0.4 | 0.865 | 60.3 ± 0.2 | 60.6 ± 0.3 | 60.0 ± 0.3 | 0.228 |
| FTG (md/dL) | 71.6 ± 1.4 | 71.9 ± 2.4 | 71.2 ± 1.8 | 0.846 | 72.5 ± 1.2 | 74.8 ± 1.7 | 69.9 ± 1.7 | 0.043 | 71.9 ± 1.0 | 73.1 ± 1.6 | 70.6 ± 1.2 | 0.227 |
| FG (mg/dL) | 95.2 ± 0.3 | 96.3 ± 0.4 | 94.0 ± 0.4 | <0.001 | 94.8 ± 0.5 | 96.4 ± 0.5 | 92.9 ± 1.0 | 0.001 | 95.0 ± 0.3 | 96.3 ± 0.3 | 93.6 ± 0.5 | <0.001 |
| Prevalence, % | ||||||||||||
| Central obesity 3 | ||||||||||||
| TPA (high BMI) | 18.3 | 23.9 | 11.9 | 0.003 | 16.8 | 19.8 | 13.6 | 0.046 | 17.7 | 22.2 | 12.6 | <0.001 |
| IDF (high WC) | 15.0 | 12.5 | 17.9 | 0.026 | 22.5 | 18.3 | 27.2 | 0.002 | 18.1 | 14.9 | 21.8 | <0.001 |
| JIS-Adult (high WC) | 15.0 | 12.5 | 17.9 | 0.026 | 22.5 | 18.3 | 27.2 | 0.002 | 18.1 | 14.9 | 21.8 | <0.001 |
| Low HDL-C | ||||||||||||
| TPA/JIS-Adult | 21.3 | 12.5 | 31.3 | <0.001 | 16.5 | 12.3 | 21.2 | 0.001 | 19.3 | 12.4 | 27.1 | <0.001 |
| IDF | 9.4 | 12.5 | 5.8 | 0.005 | 16.5 | 12.3 | 21.2 | 0.001 | 12.3 | 12.4 | 12.2 | 0.484 |
| Increased BP | 3.8 | 6.4 | 1.0 | <0.001 | 2.3 | 4.0 | 0.4 | 0.003 | 3.2 | 5.4 | 0.8 | <0.001 |
| Elevated FTG | 2.8 | 2.9 | 2.6 | 0.454 | 3.2 | 4.0 | 2.4 | 0.219 | 3.0 | 3.3 | 2.5 | 0.215 |
| High FG | 24.9 | 29.1 | 20.1 | 0.043 | 18.2 | 25.2 | 10.5 | <0.001 | 22.1 | 27.5 | 16.1 | <0.001 |
| MetS/Category | Junior (12–15 years) | Senior (16–18 years) | All | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total | Male | Female | p1 | Total | Male | Female | p1 | Total | Male | Female | p1 | |
| Metabolic disorders, % | ||||||||||||
| TPA criteria | ||||||||||||
| High BMI + 1aC | 8.06 | 10.33 | 5.51 | 0.079 | 6.31 | 6.59 | 6.00 | 0.998 | 7.34 | 8.80 | 5.71 | 0.148 |
| MetS (high BMI + ≥2aC) | 4.66 | 5.48 | 3.74 | 0.521 | 3.35 | 4.37 | 2.21 | 0.089 | 4.12 | 5.03 | 3.11 | 0.169 |
| IDF criteria | ||||||||||||
| High WC + 1aC | 5.13 | 5.41 | 4.81 | 0.765 | 8.35 | 6.17 | 10.75 | 0.009 | 6.45 | 5.72 | 7.27 | 0.057 |
| MetS (high WC + ≥2aC) | 2.73 | 3.59 | 1.76 | 0.161 | 3.41 | 4.37 | 2.35 | 0.110 | 3.01 | 3.91 | 2.01 | 0.040 |
| MetS Kappa (TPA vs. IDF) | 0.690 * | 0.783 * | 0.519 * | 0.990 * | 1.000 * | 0.969 * | 0.808 * | 0.871 * | 0.686 * | |||
| JIS-Adult criteria | ||||||||||||
| 2aC | 10.02 | 9.31 | 10.82 | 0.620 | 10.07 | 9.00 | 11.25 | 0.210 | 10.04 | 9.18 | 11.00 | 0.251 |
| MetS (≥3aC) | 4.35 | 4.38 | 4.32 | 0.967 | 3.57 | 4.67 | 2.35 | 0.074 | 4.03 | 4.50 | 3.50 | 0.403 |
| MetS Kappa (TPA vs. JIS-adult) | 0.874 * | 0.836 * | 0.925 * | 0.967 * | 0.966 * | 0.969 * | 0.907 * | 0.887 * | 0.938 * | |||
| TPA-IDF-defined central adiposity + 1aC, % | 9.82 | 10.33 | 9.24 | 0.786 | 8.84 | 6.86 | 11.02 | 0.019 | 9.42 | 8.91 | 9.98 | 0.137 |
| TPA-IDF generalized MetS 2, % | 4.80 | 5.48 | 4.03 | 0.602 | 3.41 | 4.37 | 2.35 | 0.110 | 4.23 | 5.03 | 3.34 | 0.224 |
| Factor/Category | Male | Female | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| High BMI | High FG | High BMI or High FG | ≥2 MetS-aC | MetS | High WC | Low HDL-C | High WC or Low HDL-C | ≥2 MetS-aC | MetS | |
| aOR (95%CI) | aOR (95%CI) | aOR (95%CI) | aOR (95%CI) | aOR (95%CI) | aOR (95%CI) | aOR (95%CI) | aOR (95%CI) | aOR (95%CI) | aOR (95%CI) | |
| Total calories intake 1 | ||||||||||
| ≥2500 vs. <2500 kcal/day | 0.8 (0.5–1.4) | 1.4 (0.8–2.5) | 1.3 (0.8–2.1) | 0.7 (0.4–1.4) | 1.1 (0.4–2.8) | 1.5 (0.7–2.8) | 1.3 (0.6–2.6) | 1.5 (0.8–2.8) | 1.4 (0.6–3.2) | 0.6 (0.1–3.3) |
| Dietary pattern 2 | ||||||||||
| Egg | ||||||||||
| 1–6 vs. <1 serving/week | 1.2 (0.5–2.7) | 1.0 (0.5–2.3) | 1.4 (0.7–2.9) | 1.3 (0.5–3.6) | 1.1 (0.2–5.2) | 1.3 (0.5–3.6) | 1.3 (0.5–3.4) | 1.6 (0.6–4.3) | 0.8 (0.3–2.3) | 1.9 (0.2–15.9) |
| ≥7 vs. <1 serving/week | 1.2 (0.5–2.7) | 0.9 (0.4–2.0) | 1.2 (0.6–2.5) | 1.4 (0.5–4.2) | 1.3 (0.3–6.5) | 2.2 (0.8–6.1) | 1.4 (0.5–3.7) | 2.1 (0.8–5.6) | 1.5 (0.5–4.6) | 2.4 (0.3–21.3) |
| Dairy products | ||||||||||
| ≥1 vs. <1 serving/week | 0.6 (0.4–0.9) | 0.7 (0.5–1.1) | 0.6 (0.4–0.9) | 0.5 (0.3–0.8) | 0.6 (0.3–1.5) | 0.7 (0.4–1.2) | 1.0 (0.6–1.7) | 0.9 (0.6–1.5) | 0.5 (0.2–0.9) | 0.6 (0.2–2.0) |
| Fresh fruit | ||||||||||
| ≥1 vs. <1 serving/week | 0.5 (0.3–0.8) | 0.7 (0.5–1.1) | 0.6 (0.4–0.9) | 0.4 (0.2–0.7) | 0.6 (0.2–1.5) | 0.7 (0.4–1.2) | 1.1 (0.6–1.8) | 1.0 (0.6–1.6) | 0.4 (0.2–0.9) | 0.5 (0.2–1.8) |
| Meat | ||||||||||
| 1–6 vs. <1 serving/week | 1.0 (0.6–1.7) | 0.8 (0.5–1.4) | 0.9 (0.6–1.4) | 1.5 (0.8–2.9) | 2.1 (0.8–5.8) | 1.2 (0.8–1.9) | 1.0 (0.6–1.5) | 1.0 (0.6–1.5) | 1.3 (0.7–2.3) | 1.5 (0.5–4.4) |
| ≥7 vs. <1 serving/week | 1.4 (0.7–2.5) | 1.1 (0.6–1.9) | 1.2 (0.7–2.1) | 2.0 (0.9–4.2) | 1.9 (0.6–6.0) | 1.1 (0.6–2.0) | 0.9 (0.5–1.7) | 0.9 (0.5–1.5) | 1.6 (0.8–3.1) | 0.5 (0.1–2.8) |
| Fried food | ||||||||||
| 1–6 vs. <1 serving/week | 0.7 (0.5–1.1) | 0.8 (0.6–1.2) | 0.8 (0.6–1.2) | 0.9 (0.5–1.4) | 0.7 (0.3–1.6) | 1.0 (0.7–1.5) | 1.1 (0.7–1.6) | 1.0 (0.7–1.4) | 1.2 (0.8–2.0) | 1.3 (0.5–4.0) |
| ≥7 vs. <1 serving/week | 0.8 (0.3–2.1) | 1.1 (0.5–2.5) | 0.8 (0.3–1.8) | 0.8 (0.3–2.5) | 1.3 (0.3–4.7) | 0.5 (0.2–1.8) | 0.5 (0.2–1.7) | 0.5 (0.2–1.4) | 0.2 (0.02–1.3) | NA |
| Factor/Category | Male | Female | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| High BMI | High FG | High BMI or High FG | ≥2 MetS-aC | MetS | High WC | Low HDL-C | High WC or Low HDL-C | ≥2 MetS-aC | MetS | |
| aOR 1 (95% CI) | aOR 1 (95% CI) | aOR 1 (95% CI) | aOR 1 (95% CI) | aOR 1 (95% CI) | aOR 1 (95% CI) | aOR 1 (95% CI) | aOR 1 (95% CI) | aOR 1 (95% CI) | aOR 1 (95% CI) | |
| Type of beverage | ||||||||||
| Coffee | ||||||||||
| ≥1 vs. <1 drink/week | 2.1 (1.3–3.3) | 1.2 (0.8–1.9) | 1.7 (1.1–2.6) | 1.7 (0.9–3.0) | 2.3 (0.9–6.0) | 1.6 (1.0–2.6) | 1.1 (0.7–1.7) | 1.3 (0.8–1.9) | 1.6 (0.9–2.7) | 2.2 (0.7–6.8) |
| Sport drink | ||||||||||
| 1–6 vs. <1 drink/week | 1.2 (0.8–1.8) | 1.1 (0.7–1.6) | 1.2 (0.9–1.7) | 1.2 (0.7–2.0) | 0.5 (0.2–1.3) | 1.3 (0.7–2.3) | 1.1 (0.7–2.0) | 1.2 (0.7–1.9) | 1.0 (0.4–2.3) | 4.7 (1.3–17.0) |
| ≥7 vs. <1 drink/week | 1.9 (0.8–4.7) | 3.5 (1.3–9.1) | 2.8 (1.1–6.9) | 2.4 (0.8–6.8) | 3.4 (0.97–12.1) | 0.2 (0.0–1.1) | 1.2 (0.3–4.5) | 0.7 (0.2–2.6) | 0.3 (0.1–2.1) | 1.9 (0.2–18.1) |
| Soda | ||||||||||
| 1–6 vs. <1 drink/week | 0.8 (0.6–1.3) | 1.4 (0.95–2.0) | 1.1 (0.8–1.6) | 0.8 (0.5–1.4) | 0.8 (0.4–1.9) | 1.1 (0.7–1.9) | 1.3 (0.8–2.2) | 1.3 (0.8–2.0) | 1.1 (0.5–2.4) | 2.5 (0.7–9.1) |
| ≥7 vs. <1 drink/week | 2.1 (0.8–5.6) | 1.4 (0.5–3.7) | 1.4 (0.6–3.5) | 3.5 (1.2–10.0) | 4.6 (1.1–19.0) | 0.4 (0.1–3.0) | 0.9 (0.2–3.9) | 0.5 (0.1–2.1) | 0.8 (0.1–6.6) | NA |
| Tea | ||||||||||
| 1–6 vs. <1 drink/week | 1.0 (0.6–1.6) | 1.0 (0.7–1.5) | 1.1 (0.8–1.6) | 1.1 (0.6–1.9) | 0.6 (0.2–1.6) | 1.4 (0.9–2.1) | 1.1 (0.7–1.7) | 1.1 (0.8–1.6) | 1.8 (1.1–3.0) | 3.0 (1.2–7.4) |
| ≥7 vs. <1 drink/week | 1.8 (0.98–3.2) | 0.9 (0.5–1.5) | 1.5 (0.9–2.5) | 1.9 (0.9–3.8) | 1.1 (0.4–3.2) | 2.0 (1.0–3.8) | 1.4 (0.7–2.6) | 1.5 (0.9–2.6) | 2.0 (0.8–4.8) | 6.8 (1.3–36.6) |
| Physical activity | ||||||||||
| Sedentary behavior | ||||||||||
| ≥60 vs. <60 min/day | 1.1 (0.7–1.6) | 1.0 (0.7–1.4) | 1.0 (0.7–1.4) | 1.1 (0.7–1.7) | 1.5 (0.7–3.1) | 1.2 (0.8–1.8) | 0.8 (0.6–1.2) | 1.0 (0.7–1.4) | 1.1 (0.7–1.8) | 0.3 (0.1–1.1) |
| Physical exercise | ||||||||||
| ≥30 vs. <30 min/day | 1.4 (0.96–2.1) | 0.9 (0.6–1.3) | 1.1 (0.8–1.5) | 1.4 (0.9–2.2) | 1.0 (0.5–2.1) | 1.4 (0.96–2.1) | 1.2 (0.8–1.8) | 1.4 (0.96–1.9) | 1.4 (0.8–2.3) | 0.8 (0.3–2.6) |
| Factor/Category | Male | Female1 | ||||||
|---|---|---|---|---|---|---|---|---|
| High BMI | High FG | High BMI or High FG | ≥2 MetS-aC | MetS | High WC | ≥2 MetS-aC | MetS | |
| aOR 2 (95% CI) | aOR 2 (95% CI) | aOR 2 (95% CI) | aOR 2 (95% CI) | aOR 2 (95% CI) | aOR 2 (95% CI) | aOR 2 (95% CI) | aOR 2 (95% CI) | |
| Dietary pattern | ||||||||
| Dairy products or fresh fruit 3 | ||||||||
| ≥1 vs. <1 serving/week | 0.6 (0.4–0.9) | 0.9 (0.6–1.2) | 0.7 (0.5–1.0) | 0.5 (0.3–0.8) | 0.7 (0.3–1.5) | 0.8 (0.5–1.1) | 0.5 (0.3–0.9) | 0.5 (0.2–1.2) |
| Type of beverage | ||||||||
| Coffee | ||||||||
| ≥1 vs. <1 drink/week | 2.1 (1.3–3.3) | NI | 1.7 (1.1–2.5) | NI | NI | 1.5 (0.96–2.5) | NI | NI |
| Sport drink | ||||||||
| 1–6 vs. <1 drink/week | NI | 1.1 (0.7–1.6) | 1.2 (0.8–1.7) | NI | NI | NI | NI | 4.0 (1.4–12.0) |
| ≥7 vs. <1 drink/week | NI | 3.5 (1.4–9.0) | 2.7 (1.1–6.6) | NI | NI | NI | NI | 2.0 (0.2–22.8) |
| Soda | ||||||||
| 1–6 vs. <1 drink/week | NI | NI | NI | 0.8 (0.5–1.3) | 0.8 (0.4–1.9) | NI | NI | NI |
| ≥7 vs. <1 drink/week | NI | NI | NI | 3.5 (1.2–10.0) | 4.6 (1.1–18.5) | NI | NI | NI |
| Tea | ||||||||
| 1–6 vs. <1 drink/week | NI | NI | NI | NI | NI | 1.3 (0.9–2.0) | 1.8 (1.1–3.0) | 2.8 (1.1–7.0) |
| ≥7 vs. <1 drink/week | NI | NI | NI | NI | NI | 1.9 (0.97–3.6) | 2.0 (0.9–4.8) | 5.2 (1.3–20.5) |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, W.-T.; Lee, C.-Y.; Tsai, S.; Huang, H.-L.; Wu, P.-W.; Chin, Y.-T.; Seal, D.W.; Chen, T.; Chao, Y.-Y.; Lee, C.-H. Clustering of Metabolic Risk Components and Associated Lifestyle Factors: A Nationwide Adolescent Study in Taiwan. Nutrients 2019, 11, 584. https://doi.org/10.3390/nu11030584
Lin W-T, Lee C-Y, Tsai S, Huang H-L, Wu P-W, Chin Y-T, Seal DW, Chen T, Chao Y-Y, Lee C-H. Clustering of Metabolic Risk Components and Associated Lifestyle Factors: A Nationwide Adolescent Study in Taiwan. Nutrients. 2019; 11(3):584. https://doi.org/10.3390/nu11030584
Chicago/Turabian StyleLin, Wei-Ting, Chun-Ying Lee, Sharon Tsai, Hsiao-Ling Huang, Pei-Wen Wu, Yu-Ting Chin, David W. Seal, Ted Chen, Yu-Ying Chao, and Chien-Hung Lee. 2019. "Clustering of Metabolic Risk Components and Associated Lifestyle Factors: A Nationwide Adolescent Study in Taiwan" Nutrients 11, no. 3: 584. https://doi.org/10.3390/nu11030584
APA StyleLin, W.-T., Lee, C.-Y., Tsai, S., Huang, H.-L., Wu, P.-W., Chin, Y.-T., Seal, D. W., Chen, T., Chao, Y.-Y., & Lee, C.-H. (2019). Clustering of Metabolic Risk Components and Associated Lifestyle Factors: A Nationwide Adolescent Study in Taiwan. Nutrients, 11(3), 584. https://doi.org/10.3390/nu11030584





