Adaptation and Validation of the Hydration Status Questionnaire in a Spanish Adolescent-Young Population: A Cross Sectional Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Design of the Hydration Status Questionnaire in a Healthy Adolescent-Young Spanish Population
2.2. Questionnaire Analysis
2.3. Questionnaire Validation
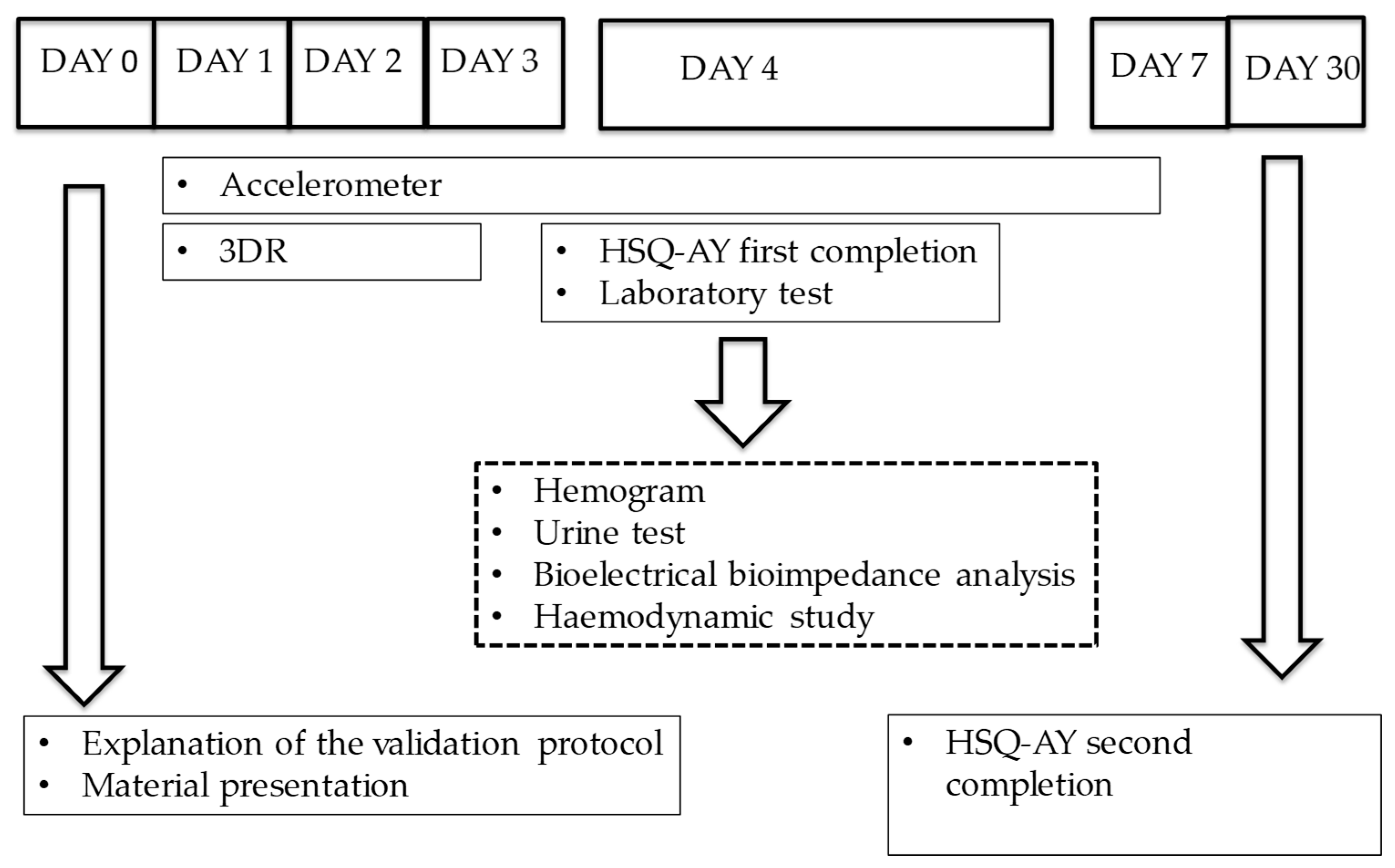
2.4. Validation Protocol
- Haematological variables: haemoglobin, haematocrit and erythrocyte were determined by capillary finger-stick whole blood with Calligari™ Analyser.
- Body composition: TBW and water percentage was estimated by BIA with Bioscan Spectrum™ Multifrequency. Individuals were weighed using a digital scale with an accuracy of 200 g (SECA™ 877). Height was measured to the nearest 0.1cm using a wall-mounted stadiometer (SECA™ 213). The anthropometric measurements were made according to the recommendations of the International Standards for Anthropometric Assessment (ISAK) [43] by level I and II accredited anthropometrists.
- Urine variables: volunteers provided a first morning urine sample in which urine pH and USG was determined using urine stick test Spinreact™ and urine colour via the Urine Colour Chart [44]. Results were compared with reference values of hydration biomarkers in first urine morning spot established by Armstrong et al. [40] (Euhydration: specific gravity = 1.023–1.025, urine colour = 4–5).
- Haemodynamic variables: pulse, SBP and DBP were determined using a digital sphygmomanometer (Omron™, M3 model).
2.5. Statistical Analysis
3. Results
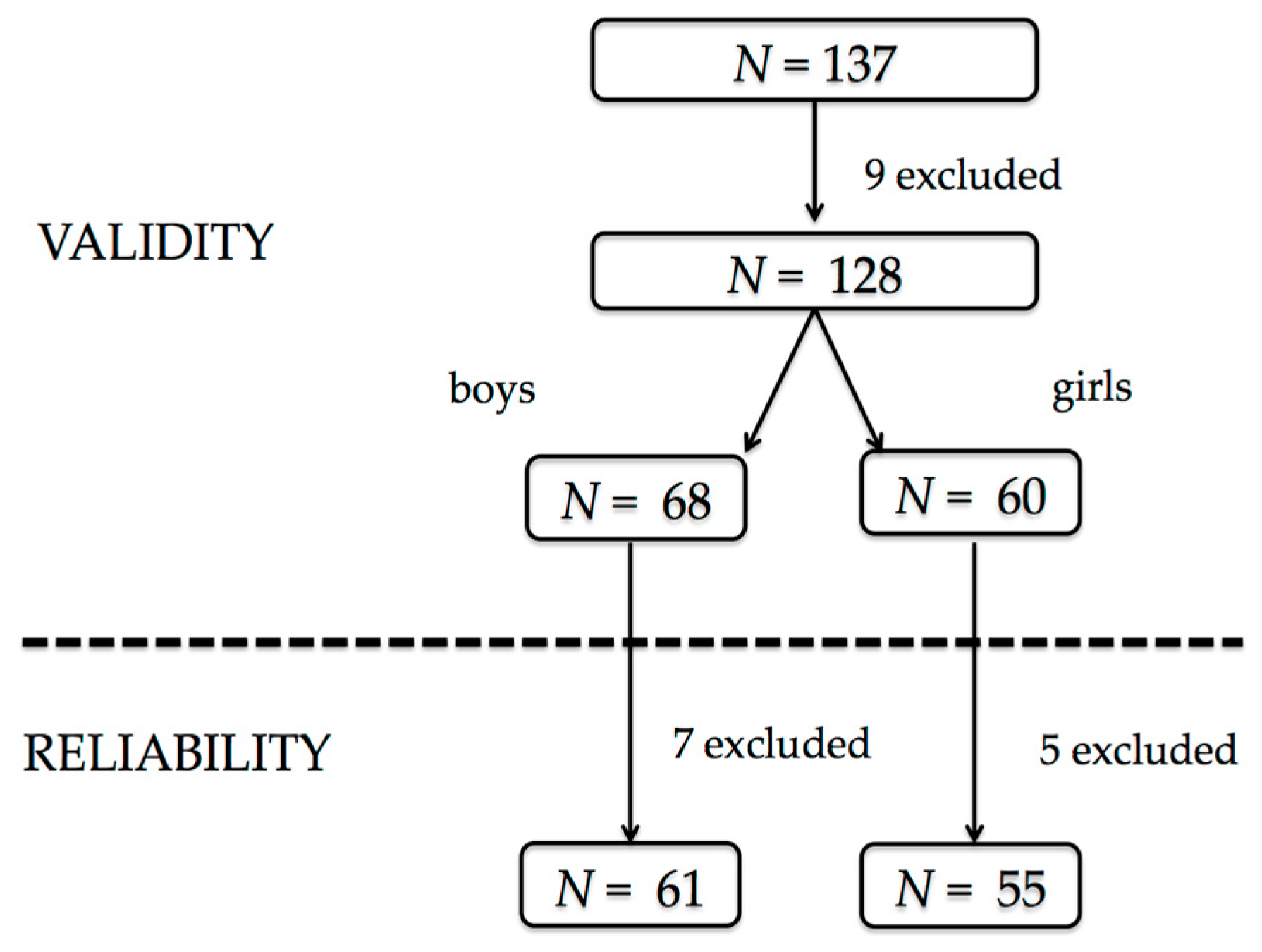
3.1. Sample Characteristics
3.2. Validity of the Questionnaire
3.3. Reliability of the Questionnaire
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Jequier, E.; Constant, F. Water as an essential nutrient: The physiological basis of hydration. Eur. J. Clin. Nutr. 2009, 64, 115–123. [Google Scholar] [CrossRef] [PubMed]
- Popkin, B.M.; D’Anci, K.E.; Rosenberg, I.H. Water, hydration, and health. Nutr. Rev. 2010, 68, 439–458. [Google Scholar] [CrossRef] [PubMed]
- Riebl, S.K.; Davy, B.M. The Hydration Equation: Update on Water Balance and Cognitive Performance. ACSMs Health Fit. J. 2013, 17, 21–28. [Google Scholar] [PubMed]
- Benton, D.; Burgess, N. The effect of the consumption of water on the memory and attention of children. Appetite 2009 53, 143–146. [CrossRef]
- Edmonds, C.J.; Burford, D. Should children drink more water?: The effects of drinking water on cognition in children. Appetite 2009, 52, 776–779. [Google Scholar] [CrossRef] [PubMed]
- Kenney, E.L.; Long, M.W.; Cradock, A.L.; Gortmaker, S.L. Prevalence of Inadequate Hydration Among US Children and Disparities by Gender and Race/Ethnicity: National Health and Nutrition Examination Survey, 2009–2012. Am. J. Public Health 2015, 105, e113–e118. [Google Scholar] [CrossRef]
- Phillips, S.M.; Sykes, D.; Gibson, N. Hydration Status and Fluid Balance of Elite European Youth Soccer Players during Consecutive Training Sessions. J. Sports Sci. Med. 2014, 13, 817–822. [Google Scholar] [PubMed]
- Castro-Sepulveda, M.; Ramirez-Campillo, R.; Abad-Colil, F.; Monje, C.; Peñailillo, L.; Cancino, J.; Zbinden-Foncea, H. Basal Mild Dehydration Increase Salivary Cortisol After a Friendly Match in Young Elite Soccer Players. Front. Physiol. 2018, 9, 1347. [Google Scholar] [CrossRef]
- Castro-Sepulveda, M.; Astudillo, J.; Letelier, P.; Zbinden-Foncea, H. Prevalence of Dehydration Before Training Sessions, Friendly and Official Matches in Elite Female Soccer Players. J. Hum. Kinet. 2016, 50, 79–84. [Google Scholar] [CrossRef]
- Bar-David, Y.; Urkin, J.; Kozminsky, E. The effect of voluntary dehydration on cognitive functions of elementary school children. Acta Paediatr. 2005, 94, 1667–1673. [Google Scholar] [CrossRef]
- Fadda, R.; Rapinett, G.; Grathwohl, D.; Parisi, M.; Fanari, R.; Calò, C.M.; Schmitt, J. Effects of drinking supplementary water at school on cognitive performance in children. Appetite 2012, 59, 730–737. [Google Scholar] [CrossRef] [PubMed]
- Warren, J.; Guelinckx, I.; Livingstone, B.; Potischman, N.; Nelson, M.; Foster, E.; Holmes, B. Challenges in the assessment of total fluid intake in children and adolescents: A discussion paper. Eur. J. Nutr. 2018, 57 (Suppl. 3), 43–51. [Google Scholar] [CrossRef] [PubMed]
- Kant, A.K.; Graubard, B.I. Contributors of water intake in US children and adolescents: Associations with dietary and meal characteristics—National Health and Nutrition Examination Survey 2005–2006. Am. J. Clin. Nutr. 2010, 92, 887–896. [Google Scholar] [CrossRef] [PubMed]
- Malik, V.S.; Pan, A.; Willett, W.C.; Hu, F.B. Sugar-sweetened beverages and weight gain in children and adults: A systematic review and meta-analysis. Am. J. Clin. Nutr. 2013, 98, 1084–1102. [Google Scholar] [CrossRef] [PubMed]
- Iglesia, I.; Guelinckx, I.; De Miguel-Etayo, P.M.; González-Gil, E.M.; Salas-Salvadó, J.; Kavouras, S.A.; Gandy, J.; Martínez, H.; Bardosono, S.; Abdollahi, M.; et al. Total fluid intake of children and adolescents: Cross-sectional surveys in 13 countries worldwide. Eur. J. Nutr. 2015, 54 (Suppl. 2), 57–67. [Google Scholar] [CrossRef] [PubMed]
- Hydration for Health. Available online: https://www.h4hinitiative.com/hydration-science/hydration-lab/water-intake-and-hydration-physiology-during-childhood/consumption#total-water-intake (accessed on 10 December 2018).
- Perrier, E.; Rondeau, P.; Poupin, M.; Le Bellego, L.; Armstrong, L.E.; Lang, F.; Stookey, J.; Tack, I.; Vergne, S.; Klein, A. Relation between urinary hydration biomarkers and total fluid intake in healthy adults. Eur. J. Clin. Nutr. 2013, 67, 939–943. [Google Scholar] [CrossRef] [PubMed]
- European Food Safety Agency (EFSA). Scientific Opinion on Dietary Reference Values for water. EFSA J. 2010, 8, 1459. [Google Scholar]
- Dougherty, K.A.; Baker, L.B.; Chow, M.; Kenney, W.L. Two percent dehydration impairs and six percent carbohydrate drink improves boys basketball skills. Med. Sci. Sports Exerc. 2006, 38, 1650–1658. [Google Scholar] [CrossRef] [PubMed]
- Fiorito, L.M.; Marini, M.; Mitchell, D.C.; Smiciklas-Wright, H.; Birch, L.L. Girls’ early sweetened carbonated beverage intake predicts different patterns of beverage and nutrient intake across childhood and adolescence. J. Am. Diet. Assoc. 2010, 110, 543–550. [Google Scholar] [CrossRef]
- Guelinckx, I.; Frémont-Marquis, A.S.; Eon, E.; Kavouras, S.A.; Armstrong, L.E. Assessing Hydration in Children: From Science to Practice. Ann. Nutr. Metab. 2015, 66, 5–9. [Google Scholar] [CrossRef]
- Shirreffs, S.M. Markers of hydration status. Eur. J. Clin. Nutr. 2003, 57 (Suppl. 2), S6–S9. [Google Scholar] [CrossRef] [PubMed]
- Perrier, E.; Vergne, S.; Klein, A.; Poupin, M.; Rondeau, P.; Le Bellego, L.; Armstrong, L.E.; Lang, F.; Stookey, J.; Tack, I. Hydration biomarkers in free-living adults with different levels of habitual fluid consumption. Br. J. Nutr. 2013, 109, 1678–1687. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, L.E. Assessing hydration status: The elusive gold standard. J. Am. Coll. Nutr. 2007, 26, 575S–584S. [Google Scholar] [CrossRef] [PubMed]
- Gandy, J.; Martinez, H.; Guelinckx, I.; Moreno, L.A.; Bardosono, S.; Salas-Salvadó, J.; Kavouras, S.A. Relevance of Assessment Methods for Fluid Intake. Ann. Nutr. Metab. 2016, 68 (Suppl. 2), 1–5. [Google Scholar] [CrossRef] [PubMed]
- Laja García, A.; Mercuur, N.; Samaniego-Vaesken, M.L.; Partearroyo, T.; Varela-Moreiras, G. Questionnaire design to evaluate water balance. Nutr. Hosp. 2015, 32 (Suppl. 2), 10310. [Google Scholar]
- Malisova, O.; Bountziouka, V.; Panagiotakos, D.B.; Zampelas, A.; Kapsokefalou, M. The water balance questionnaire: Design, reliability and validity of a questionnaire to evaluate water balance in the general population. Int. J. Food Sci. Nutr. 2012, 63, 138–144. [Google Scholar] [CrossRef] [PubMed]
- Hedrick, V.E.; Comber, D.L.; Estabrooks, P.A.; Savla, J.; Davy, B.M. The beverage intake questionnaire: Determining initial validity and reliability. J. Am. Diet. Assoc. 2010, 110, 1227–1232. [Google Scholar] [CrossRef] [PubMed]
- Murray, B. Hydration and physical performance. J. Am. Coll. Nutr. 2007, 26 (Suppl. 5), 542S–548S. [Google Scholar] [CrossRef] [PubMed]
- Moreiras, O.; Carbajal, A.; Cabrera, L.; Cuadrado, C. Tablas de Composición de Alimentos, 19th ed.; Pirámide: Madrid, Spain, 2018. [Google Scholar]
- Panagiotakos, D. Health measurement scales: Methodological issues. Open Cardiovasc. Med. J. 2009, 3, 160–165. [Google Scholar] [CrossRef] [PubMed]
- Ministerio de Ciencia e Innovación. Base de Datos Española de Composición de Alimentos (BEDCA). Available online: www.bedca.net (accessed on 20 December 2018).
- Rehrer, N.J.; Burke, L.M. Sweating losses during various sports. Aust. J. Nutr. Diet. 1996, 53, 13–16. [Google Scholar]
- Costill, D.L. Sweating: Its composition and effects on body fluids. Ann. N. Y. Acad. Sci. 1977, 301, 160–174. [Google Scholar] [CrossRef] [PubMed]
- Clarkson, P.M. The effects of exercise and heat on vitamin and requirements. In Nutritional Needs in Hot Environments; Marriott, B.M., Ed.; National Academies Press: Washington, DC, USA, 1993; pp. 137–171. [Google Scholar]
- Fischbach, F.T.; Dunning, M.B. A Manual of Laboratory & Diagnostic Tests; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2003. [Google Scholar]
- Nunnally, J.C.; Bernstein, I.H.; Berge, J.M.T. Psychometric Theory; McGraw Hill: New York, NY, USA, 1967. [Google Scholar]
- Oppliger, R.A.; Magnes, S.A.; Popowski, L.A.; Gisolfi, C.V. Accuracy of urine specific gravity and osmolality as indicators of hydration status. Int. J. Sport Nutr. Exerc. Metab. 2005, 15, 236–251. [Google Scholar] [CrossRef] [PubMed]
- Kavouras, S.A. Assessing hydration status. Curr. Opin. Clin. Nutr. Metab. Care 2002, 5, 519–524. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, L.E.; Pumerantz, A.C.; Fiala, K.A.; Roti, M.W.; Kavouras, S.A.; Casa, D.J.; Maresh, C.M. Human hydration indices: Acute and longitudinal reference values. Int. J. Sport Nutr. Exerc. Metab. 2010, 20, 145–153. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, L.E. Hydration assessment techniques. Nutr. Rev. 2005, 63, S40–S54. [Google Scholar] [CrossRef] [PubMed]
- González-Alonso, J.; Mora-Rodríguez, R.; Below, P.R.; Coyle, E.F. Dehydration markedly impairs cardiovascular function in hyperthermic endurance athletes during exercise. J. Appl. Physiol. 1985, 82, 1229–1236. [Google Scholar]
- Marfell-Jones, M.J.; Stewart, A.D.; De Ridder, J.H. International Standards for Anthropometric Assessment; Lower Hutt: North Island, New Zealand, 2011. [Google Scholar]
- Armstrong, L.E. Performing in Extreme Environments; Human Kinetics: Champaign, IL, USA, 2000. [Google Scholar]
- Armstrong, L.E.; Johnson, E.C.; Munoz, C.X.; Swokla, B.; Le Bellego, L.; Jimenez, L.; Casa, D.J.; Maresh, C.M. Hydration biomarkers and dietary fluid consumption of women. J. Acad. Nutr. Diet. 2012, 112, 1056–1061. [Google Scholar] [CrossRef]
- Armstrong, L.E.; Soto, J.A.; Hacker, F.T., Jr.; Casa, D.J.; Kavouras, S.A.; Maresh, C.M. Urinary indices during dehydration, exercise, and rehydration. Int. J. Sport Nutr. 1998, 8, 345–355. [Google Scholar] [CrossRef]
- McKenzie, A.L.; Muñoz, C.X.; Ellis, L.A.; Perrier, E.T.; Guelinckx, I.; Klein, A.; Kavouras, S.A.; Armstrong, L.E. Urine color as an indicator of urine concentration in pregnant and lactating women. Eur. J. Nutr. 2017, 56, 355–362. [Google Scholar] [CrossRef]
- McKenzie, A.L.; Muñoz, C.X.; Armstrong, L.E. Accuracy of Urine Color to Detect Equal to or Greater Than 2% Body Mass Loss in Men. J. Athl. Train. 2015, 50, 1306–1309. [Google Scholar] [CrossRef]
- Armstrong, L.E.; Maresh, C.M.; Castellani, J.W.; Bergeron, M.F.; Kenefick, R.W.; LaGasse, K.E.; Riebe, D. Urinary indices of hydration status. Int. J. Sport Nutr. Exerc. Metab. 1994, 4, 265–279. [Google Scholar] [CrossRef]
- Van Marken Lichtenbelt, W.D.; Westerterp, K.R.; Wouters, L.; Luijendijk, S.C. Validation of bioelectrical-impedance measurements as a method to estimate body-water compartments. Am. J. Clin. Nutr. 1994, 60, 159–166. [Google Scholar] [CrossRef] [PubMed]
- Van Loan, M.D.; Withers, P.; Matthie, J.; Mayclin, P.L. Use of bioimpedance spectroscopy to determine extracellular fluid, intracellular fluid, total body water, and fat-free mass. Basic Life Sci. 1993, 60, 67–70. [Google Scholar] [PubMed]
- Patel, R.V.; Matthie, J.R.; Withers, P.O.; Peterson, E.L.; Zarowitz, B.J. Estimation of total body and extracellular water using single- and multiple-frequency bioimpedance. Ann. Pharmacother. 1994, 28, 565–569. [Google Scholar] [CrossRef] [PubMed]
- Matthie, J.; Zarowitz, B.; De Lorenzo, A.; Andreoli, A.; Katzarski, K.; Pan, G.; Withers, P. Analytic assessment of the various bioimpedance methods used to estimate body water. J. Appl. Physiol. 1985, 84, 1801–1816. [Google Scholar] [CrossRef] [PubMed]
- Matias, C.N.; Júdice, P.B.; Santos, D.A.; Magalhães, J.P.; Minderico, C.S.; Fields, D.A.; Sardinha, L.B.; Silva, A.M. Suitability of Bioelectrical Based Methods to Assess Water Compartments in Recreational and Elite Athletes. J. Am. Coll. Nutr. 2016, 35, 413–421. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, L.E.; Kenefick, R.W.; Castellani, J.W.; Riebe, D.; Kavouras, S.A.; Kuznicki, J.T.; Maresh, C.M. Bioimpedance spectroscopy technique: Intra-, extracellular, and total body water. Med. Sci. Sports Exerc. 1997, 29, 1657–1663. [Google Scholar] [CrossRef] [PubMed]
- National Institute of Health. Bioelectrical impedance analysis in body composition measurement: National Institutes of Health Technology Assessment Conference Statement. Am. J. Clin. Nutr. 1996, 64, 524S–532S. [Google Scholar] [CrossRef] [PubMed]
- International Chair for Advanced Studies on Hydration. Available online: http://cieah.ulpgc.es/en/icash (accessed on 20 December 2018).
- Moreno, L.A.; Mesana, M.I.; González-Gross, M.; Gil, C.M.; Fleta, J.; Wärnberg, J.; Ruiz, J.R.; Sarría, A.; Marcos, A.; Bueno, M.; et al. Anthropometric body fat composition reference values in Spanish adolescents. The AVENA Study. Eur. J. Clin. Nutr. 2006, 60, 191–196. [Google Scholar] [CrossRef] [PubMed]
- McCarthy, H.D.; Cole, T.J.; Fry, T.; Jebb, S.A.; Prentice, A.M. Body fat reference curves for children. Int. J. Obes. (Lond.) 2006, 30, 598–602. [Google Scholar] [CrossRef] [PubMed]
- Kasper, D.L.; Braunwald, E.; Fauci, A.S.; Hauser, S.L.; Longo, D.L.; Jameson, J.L. Harrinson’s Principles of Internal Medicine, 16th ed.; McGraw-Hill: New York, NY, USA, 2004. [Google Scholar]
- Kavouras, S.A.; Johnson, E.C.; Bougatsas, D.; Arnaoutis, G.; Panagiotakos, D.B.; Perrier, E.; Klein, A. Validation of a urine color scale for assessment of urine osmolality in healthy children. Eur. J. Nutr. 2016, 55, 907–915. [Google Scholar] [CrossRef] [PubMed]
- National High Blood Pressure Education Program Working Group on High Blood Pressure in Children and Adolescents. The fourth report on the diagnosis, evaluation, and treatment of high blood pressure in children and adolescents. Pediatrics 2004, 114, 555–576. [Google Scholar] [CrossRef]
- Ferreira-Pêgo, C.; Guelinckx, I.; Moreno, L.A.; Kavouras, S.A.; Gandy, J.; Martinez, H.; Bardosono, S.; Abdollahi, M.; Nasseri, E.; Jarosz, A.; et al. Total fluid intake and its determinants: Cross-sectional surveys among adults in 13 countries worldwide. Eur. J. Nutr. 2015, 54 (Suppl. 2), 35–43. [Google Scholar] [CrossRef]
- Fenández-Alvira, J.M.; Iglesia, I.; Ferreira-Pêgo, C.; Babio, N.; Salas-Salvadó, J.; Moreno, L.A. Fluid intake in Spanish children and adolescents; a cross-sectional study. Nutr. Hosp. 2014, 29, 1163–1170. [Google Scholar] [PubMed]
- Athanasatou, A.; Malisova, O.; Kandyliari, A.; Kapsokefalou, M. Water Intake in a Sample of Greek Adults Evaluated with the Water Balance Questionnaire (WBQ) and a Seven-Day Diary. Nutrients 2016, 8, 559. [Google Scholar] [CrossRef] [PubMed]
- Nissensohn, M.; Sánchez-Villegas, A.; Galan, P.; Turrini, A.; Arnault, N.; Mistura, L.; Ortiz-Andrellucchi, A.; Edelenyi, F.S.; D’Addezio, L.; Serra-Majem, L. Beverage Consumption Habits among the European Population: Association with Total Water and Energy Intakes. Nutrients 2017, 9, 383. [Google Scholar] [CrossRef] [PubMed]
- Jensen, R.; Buffangeix, D.; Covi, G. Measuring water content of feces by the Karl Fischer method. Clin. Chem. 1976, 22, 1351–1354. [Google Scholar] [PubMed]
- Plasqui, G.; Bonomi, A.G.; Westerterp, K.R. Daily physical activity assessment with accelerometers: New insights and validation studies. Obes. Rev. 2013, 14, 451–462. [Google Scholar] [CrossRef] [PubMed]
| Males (n = 68) | Females (n = 60) | p Values | |
|---|---|---|---|
| Weight (Kg) | 58.3 (55.3–61.3) | 54.8 (52.2–57.5) | 0.084 |
| Height (cm) | 166.7 (164.5–169.0) | 161.4 (160.0–162.7) | 0.000 |
| Body fat mass (Kg) | 15.1 (13.6–16.6) | 18.2 (16.7–19.7) | 0.004 |
| Body lean mass (Kg) | 44.0 (41.9–46.1) | 36.9 (35.9–38.0) | 0.000 |
| TBW (%) | 56.0 (55.0–57.1) | 51.3 (50.1–52.5) | 0.000 |
| TBW (L) | 32.9 (31.3–34.5) | 28.1 (27.1–29.1) | 0.000 |
| Specific gravity (g/L) | 1.025 (1.024–1.027) | 1.025 (1.024–1.026) | 0.681 |
| pH | 5.3 (5.2–5.4) | 5.1 (5.1–5.2) | 0.043 |
| Urine colour | 3.5 (3.2–3.7) | 3.4 (3.2–3.9) | 0.671 |
| Haematocrit (%) | 43.1 (42.3–44.0) | 40.0 (39.1–41.0) | 0.000 |
| Erythrocyte (mill/μL) | 5.5 (4.1–6.9) | 4.4 (4.3–4.6) | 0.133 |
| Haemoglobin (g/dL) | 14.6 (14.2–15.0) | 13.7 (13.2–14.1) | 0.002 |
| SBP (mmHg) | 120.1 (116.6–123.5) | 111.9 (107.6–116.2) | 0.003 |
| DBP (mmHg) | 62.3 (60.2–64.5) | 67.1 (65.0–69.2) | 0.003 |
| Pulse (beats/min) | 70.9 (68.1–73.8) | 81.0 (78.0–84.9) | 0.000 |
| Males (n = 68) | Females (n = 60) | p Values | |
|---|---|---|---|
| Drinking water (mL/day) | 1897.6 (1683.2–2112. 1) | 1611.1 (1293.2–1929.0) | 0.138 |
| Water from beverages (mL/day) | 2713.7 (2465.0–2962.4) | 2308.8 (1983.2–2634.4) | 0.051 |
| Water from food (mL/day) | 424.0 (366.8–481.2) | 501.5 (425.3–577.8) | 0.107 |
| Water intake (mL/day) | 3137.7 (2880.7–3394.8) | 2810.3 (2465.6–3154.9) | 0.131 |
| Total water loss (mL/day) | 3811.0 (3631.1–3990.3) | 3213.5 (3030.0–3397.0) | 0.000 |
| Water balance (mL/day) | −673.3 (−975.1–−371.5) | −403.2 (−771.7–−34.7) | 0.259 |
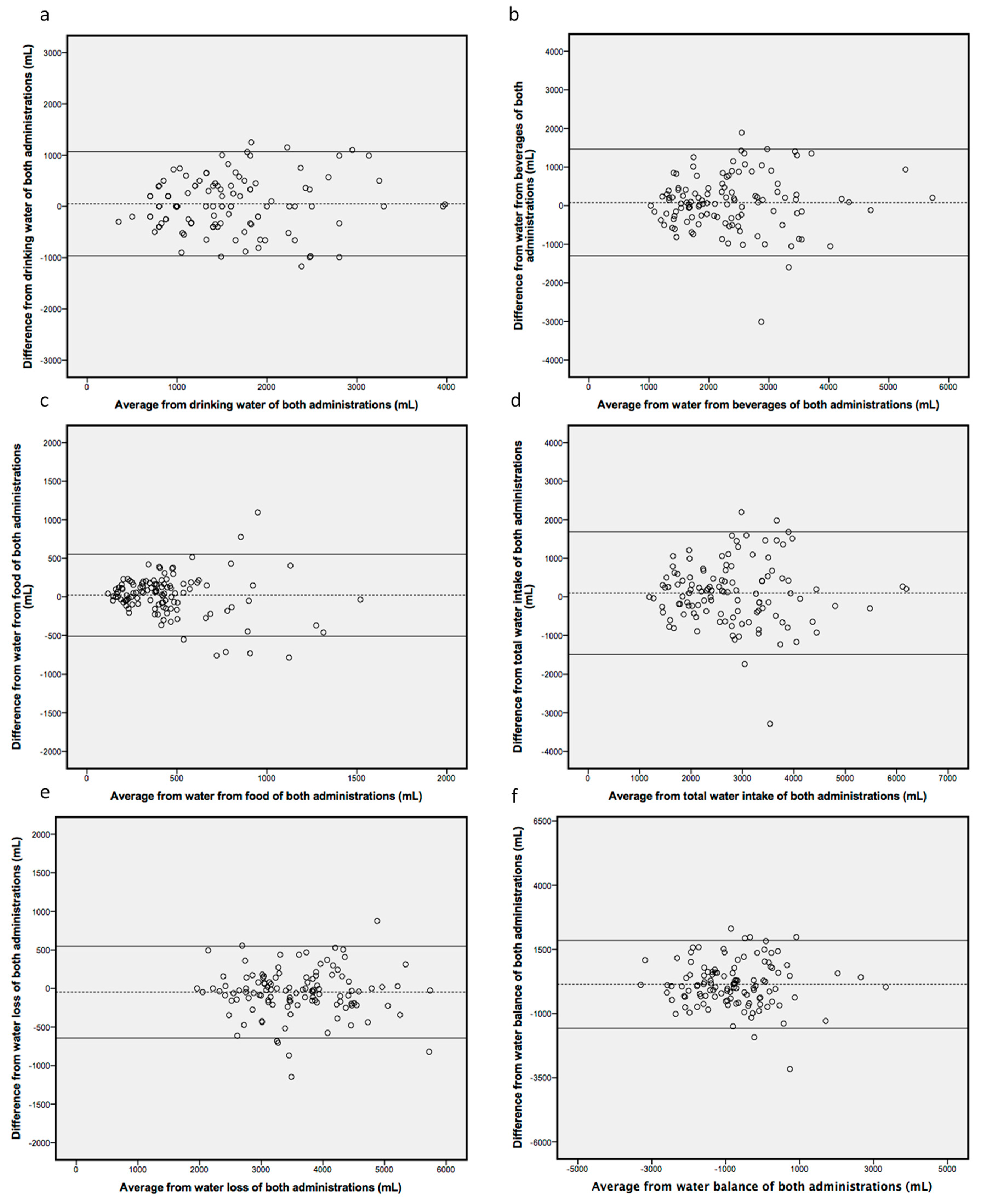
| 1st Completion (n = 128) | 2nd Completion (n = 116) | Mean Difference | p Values | Limits of Agreements | |
|---|---|---|---|---|---|
| Drinking water (mL/day) | 1628.1 (1472.2–1784.0) | 1577.0 (1429.3–1724.7) | 51.1 | 0.292 | 1068.8–966.6 |
| Water from beverages (mL/day) | 2380.0 (2203.0–2557.0) | 2300.8 (2128.4–2473.1) | 79.1 | 0.230 | 1462.2–1303.9 |
| Water from food (mL/day) | 459.0 (409.2–509.0) | 437.7 (380.0–495.3) | 21.3 | 0.398 | 551.5–508.9 |
| Water intake (mL/day) | 2839.0 (2649.1–3029.0) | 2738.5 (2545.6–2931.3) | 100.4 | 0.184 | 1685.8–1485.0 |
| Water loss (mL/day) | 3558.0 (3408.0–3708.0) | 3606.1 (3455.2–3757.0) | −48.2 | 0.090 | 547.0–643.4 |
| Water balance (mL/day) | −719.0 (−934.0–−504.0) | −855.4 (−1074.9–−636.0) | 136.4 | 0.095 | 1848.1–1575.2 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Laja García, A.I.; Samaniego-Vaesken, M.d.L.; Partearroyo, T.; Varela-Moreiras, G. Adaptation and Validation of the Hydration Status Questionnaire in a Spanish Adolescent-Young Population: A Cross Sectional Study. Nutrients 2019, 11, 565. https://doi.org/10.3390/nu11030565
Laja García AI, Samaniego-Vaesken MdL, Partearroyo T, Varela-Moreiras G. Adaptation and Validation of the Hydration Status Questionnaire in a Spanish Adolescent-Young Population: A Cross Sectional Study. Nutrients. 2019; 11(3):565. https://doi.org/10.3390/nu11030565
Chicago/Turabian StyleLaja García, Ana Isabel, Maria de Lourdes Samaniego-Vaesken, Teresa Partearroyo, and Gregorio Varela-Moreiras. 2019. "Adaptation and Validation of the Hydration Status Questionnaire in a Spanish Adolescent-Young Population: A Cross Sectional Study" Nutrients 11, no. 3: 565. https://doi.org/10.3390/nu11030565
APA StyleLaja García, A. I., Samaniego-Vaesken, M. d. L., Partearroyo, T., & Varela-Moreiras, G. (2019). Adaptation and Validation of the Hydration Status Questionnaire in a Spanish Adolescent-Young Population: A Cross Sectional Study. Nutrients, 11(3), 565. https://doi.org/10.3390/nu11030565








