The Type of Dietary Fat in an Isocaloric Breakfast Meal Does Not Modify Postprandial Metabolism in Overweight/Obese Pregnant Women
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Design
2.2. Study Participants
2.3. Study Day Procedures
2.4. Test Meals
2.5. Body Anthropometrics and Composition
2.6. Blood Sampling, Aliquoting, and Analysis
2.7. Satiety Assessment
2.8. Statistical Analysis
3. Results
3.1. Participant Characteristics
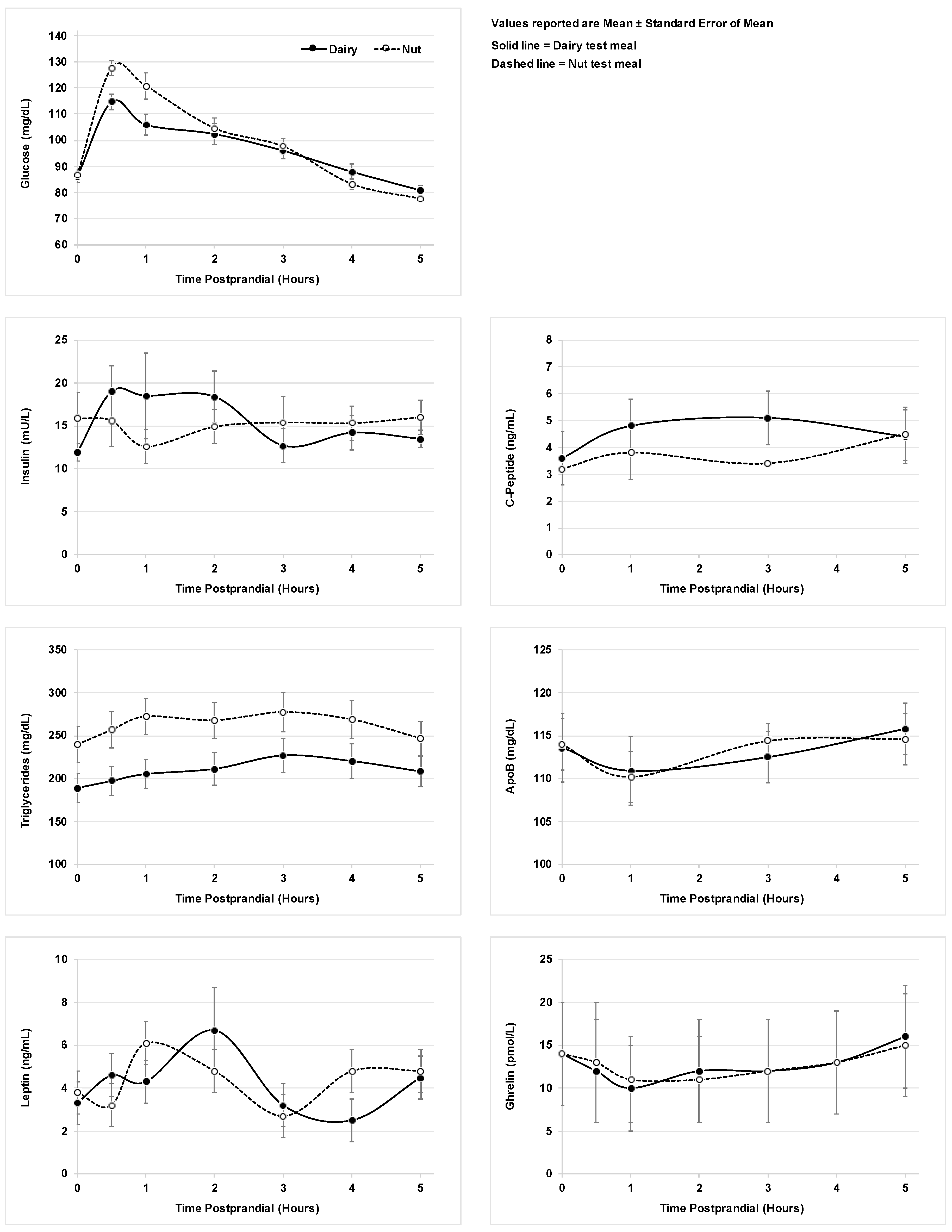
3.2. Metabolic Markers
3.3. Satiety Questionnaires and Buffet Lunch
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Flegal, K.M.; Kruszon-Moran, D.; Carroll, M.D.; Fryar, C.D.; Ogden, C.L. Trends in Obesity Among Adults in the United States, 2005 to 2014. JAMA 2016, 315, 2284–2291. [Google Scholar] [CrossRef] [PubMed]
- Bardenheier, B.H.; Imperatore, G.; Gilboa, S.M.; Geiss, L.S.; Saydah, S.H.; Devlin, H.M.; Kim, S.Y.; Gregg, E.W. Trends in Gestational Diabetes Among Hospital Deliveries in 19 U.S. States, 2000–2010. Am. J. Prev. Med. 2015, 49, 12–19. [Google Scholar] [CrossRef] [PubMed]
- Fridman, M.; Korst, L.M.; Chow, J.; Lawton, E.; Mitchell, C.; Gregory, K.D. Trends in maternal morbidity before and during pregnancy in California. Am. J. Public Health 2014, 104 (Suppl. 1), S49–S57. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, N.; Nachum, Z.; Green, M.S. The prevalence of gestational diabetes mellitus recurrence—Effect of ethnicity and parity: A metaanalysis. Am. J. Obs. Gynecol. 2015, 213, 310–317. [Google Scholar] [CrossRef] [PubMed]
- Entringer, S.; Buss, C.; Swanson, J.M.; Cooper, D.M.; Wing, D.A.; Waffarn, F.; Wadhwa, P.D. Fetal programming of body composition, obesity, and metabolic function: The role of intrauterine stress and stress biology. J. Nutr. Metab. 2012, 2012, 632548. [Google Scholar] [CrossRef] [PubMed]
- Entringer, S.; Wadhwa, P.D. Developmental programming of obesity and metabolic dysfunction: Role of prenatal stress and stress biology. Nestle Nutr. Inst. Workshop Ser. 2013, 74, 107–120. [Google Scholar] [PubMed]
- Poston, L.; Harthoorn, L.F.; Van Der Beek, E.M. Obesity in pregnancy: Implications for the mother and lifelong health of the child. A consensus statement. Pediatr. Res. 2011, 69, 175–180. [Google Scholar] [CrossRef] [PubMed]
- Gunderson, E.P.; Abrams, B. Epidemiology of gestational weight gain and body weight changes after pregnancy. Epidemiol. Rev. 2000, 22, 261–274. [Google Scholar] [CrossRef] [PubMed]
- Rong, K.; Yu, K.; Han, X.; Szeto, I.M.; Qin, X.; Wang, J.; Ning, Y.; Wang, P.; Ma, D. Pre-pregnancy BMI, gestational weight gain and postpartum weight retention: A meta-analysis of observational studies. Public Health Nutr. 2015, 18, 2172–2182. [Google Scholar] [CrossRef] [PubMed]
- Samura, T.; Steer, J.; Michelis, L.D.; Carroll, L.; Holland, E.; Perkins, R. Factors Associated with Excessive Gestational Weight Gain: Review of Current Literature. Glob. Adv. Health Med. 2016, 5, 87–93. [Google Scholar] [CrossRef] [PubMed]
- Tielemans, M.J.; Garcia, A.H.; Peralta Santos, A.; Bramer, W.M.; Luksa, N.; Luvizotto, M.J.; Moreira, E.; Topi, G.; de Jonge, E.A.; Visser, T.L.; et al. Macronutrient composition and gestational weight gain: A systematic review. Am. J. Clin. Nutr. 2016, 103, 83–99. [Google Scholar] [CrossRef] [PubMed]
- Deierlein, A.L.; Siega-Riz, A.M.; Herring, A. Dietary energy density but not glycemic load is associated with gestational weight gain. Am. J. Clin. Nutr. 2008, 88, 693–699. [Google Scholar] [CrossRef] [PubMed]
- Headen, I.E.; Davis, E.M.; Mujahid, M.S.; Abrams, B. Racial-ethnic differences in pregnancy-related weight. Adv. Nutr. 2012, 3, 83–94. [Google Scholar] [CrossRef] [PubMed]
- Somashekhar, S.P.; Gupta, P.; Ballal, S.; Parameshwaran Zaveri, S.S.; Venkatachala Udupa, K.V. Minimally invasive radioguided surgery for parathyroid adenomas (MIRP). Natl. Med. J. India 2007, 20, 13–15. [Google Scholar] [PubMed]
- Zaveri, S.; Drummond, S. The effect of including a conventional snack (cereal bar) and a nonconventional snack (almonds) on hunger, eating frequency, dietary intake and body weight. J. Hum. Nutr. Diet. 2009, 22, 461–468. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, D.J.; Kendall, C.W.; Josse, A.R.; Salvatore, S.; Brighenti, F.; Augustin, L.S.; Ellis, P.R.; Vidgen, E.; Rao, A.V. Almonds decrease postprandial glycemia, insulinemia, and oxidative damage in healthy individuals. J. Nutr. 2006, 136, 2987–2992. [Google Scholar] [CrossRef] [PubMed]
- Josse, A.R.; Kendall, C.W.; Augustin, L.S.; Ellis, P.R.; Jenkins, D.J. Almonds and postprandial glycemia—A dose-response study. Metabolism 2007, 56, 400–404. [Google Scholar] [CrossRef] [PubMed]
- Wien, M.A.; Sabate, J.M.; Ikle, D.N.; Cole, S.E.; Kandeel, F.R. Almonds vs. complex carbohydrates in a weight reduction program. Int. J. Obes. Relat. Metab. Disord. 2003, 27, 1365–1372. [Google Scholar] [CrossRef] [PubMed]
- Robertson, M.D.; Henderson, R.A.; Vist, G.E.; Rumsey, R.D. Extended effects of evening meal carbohydrate-to-fat ratio on fasting and postprandial substrate metabolism. Am. J. Clin. Nutr. 2002, 75, 505–510. [Google Scholar] [CrossRef] [PubMed]
- Wali, R.; King, J.; Mauldin, K. Dose-Dependent Effect of Almond Intake on Satiety in Overweight/Obese Pregnant Women: Analysis of Test Meals; California State University, San Jose: San Jose, CA, USA, 2012. [Google Scholar]
- Fields, D.A.; Goran, M.I.; McCrory, M.A. Body-composition assessment via air-displacement plethysmography in adults and children: A review. Am. J. Clin. Nutr. 2002, 75, 453–467. [Google Scholar] [CrossRef] [PubMed]
- McCrory, M.A.; Mole, P.A.; Gomez, T.D.; Dewey, K.G.; Bernauer, E.M. Body composition by air-displacement plethysmography by using predicted and measured thoracic gas volumes. J. Appl. Physiol. 1998, 84, 1475–1479. [Google Scholar] [CrossRef] [PubMed]
- Siri, W.E. Body composition from fluid spaces and density: Analysis of methods. 1961. Nutrition 1993, 9, 480–491. [Google Scholar] [PubMed]
- Reisenberg, A.; Mauldin, K.; Sawrey-Kubicek, L.; Lesser, M.N.R.; King, J. Pilot Study Examining Pregnancy-Specific Equations to Estimate Percent Body Fat in an Overweight/Obese Pregnant Hispanic Population. Calif. J. Health Promot. 2016, 14, 22–30. [Google Scholar]
- Rifai, N.; King, M.E. Immunoturbidimetric assays of apolipoproteins A, AI, AII, and B in serum. Clin. Chem. 1986, 32, 957–961. [Google Scholar] [PubMed]
- Smith, S.J.; Cooper, G.R.; Henderson, L.O.; Hannon, W.H. An international collaborative study on standardization of apolipoproteins A-I and B. Part, I. Evaluation of a lyophilized candidate reference and calibration material. Clin. Chem. 1987, 33, 2240–2249. [Google Scholar]
- Caulfield, M.P.; Li, S.; Lee, G.; Blanche, P.J.; Salameh, W.A.; Benner, W.H.; Reitz, R.E.; Krauss, R.M. Direct determination of lipoprotein particle sizes and concentrations by ion mobility analysis. Clin. Chem. 2008, 54, 1307–1316. [Google Scholar] [CrossRef] [PubMed]
- Cohen, A.E.; Johnston, C.S. Almond ingestion at mealtime reduces postprandial glycemia and chronic ingestion reduces hemoglobin A(1c) in individuals with well-controlled type 2 diabetes mellitus. Metabolism 2011, 60, 1312–1317. [Google Scholar] [CrossRef] [PubMed]
- Garrett Fitzmaurice, N.L.J.W. Applied Longitudinal Analysis; John Wiley & Sons: Hoboken, NJ, USA, 2004. [Google Scholar]
- Zar, J.H. Biostatistical Analysis, 5th ed.; Pearson Education: Upper Saddle River, NJ, USA, 2007. [Google Scholar]
- American Diabetes, A. 13. Management of Diabetes in Pregnancy: Standards of Medical Care in Diabetes-2018. Diabetes Care 2018, 41, S137–S143. [Google Scholar] [CrossRef] [PubMed]
- McLachlan, K.A.; O’Neal, D.; Jenkins, A.; Alford, F.P. Do adiponectin, TNFalpha, leptin and CRP relate to insulin resistance in pregnancy? Studies in women with and without gestational diabetes, during and after pregnancy. Diabetes Metab. Res. Rev. 2006, 22, 131–138. [Google Scholar] [CrossRef] [PubMed]
- Vitoratos, N.; Deliveliotou, A.; Vlahos, N.F.; Mastorakos, G.; Papadias, K.; Botsis, D.; Creatsas, G.K. Serum adiponectin during pregnancy and postpartum in women with gestational diabetes and normal controls. Gynecol. Endocrinol. 2008, 24, 614–619. [Google Scholar] [CrossRef] [PubMed]
- Butte, N.F. Carbohydrate and lipid metabolism in pregnancy: normal compared with gestational diabetes mellitus. Am. J. Clin. Nutr. 2000, 71, 1256S–1261S. [Google Scholar] [CrossRef] [PubMed]
- Kendall, C.W.; Esfahani, A.; Josse, A.R.; Augustin, L.S.; Vidgen, E.; Jenkins, D.J. The glycemic effect of nut-enriched meals in healthy and diabetic subjects. Nutr. Metab. Cardiovasc. Dis. 2011, 21 (Suppl. 1), S34–S39. [Google Scholar] [CrossRef] [PubMed]
- Soares, M.J.; Murhadi, L.L.; Kurpad, A.V.; Chan She Ping-Delfos, W.L.; Piers, L.S. Mechanistic roles for calcium and vitamin D in the regulation of body weight. Obes. Rev. 2012, 13, 592–605. [Google Scholar] [CrossRef] [PubMed]
- Monfort-Pires, M.; Delgado-Lista, J.; Gomez-Delgado, F.; Lopez-Miranda, J.; Perez-Martinez, P.; Ferreira, S.R. Impact of the Content of Fatty Acids of Oral Fat Tolerance Tests on Postprandial Triglyceridemia: Systematic Review and Meta-Analysis. Nutrients 2016, 8, 580. [Google Scholar] [CrossRef] [PubMed]
- Masson, C.J.; Mensink, R.P. Exchanging saturated fatty acids for (n-6) polyunsaturated fatty acids in a mixed meal may decrease postprandial lipemia and markers of inflammation and endothelial activity in overweight men. J. Nutr. 2011, 141, 816–821. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, D.J.; Kendall, C.W.; Marchie, A.; Parker, T.L.; Connelly, P.W.; Qian, W.; Haight, J.S.; Faulkner, D.; Vidgen, E.; Lapsley, K.G.; et al. Dose response of almonds on coronary heart disease risk factors: Blood lipids, oxidized low-density lipoproteins, lipoprotein(a), homocysteine, and pulmonary nitric oxide: A randomized, controlled, crossover trial. Circulation 2002, 106, 1327–1332. [Google Scholar] [CrossRef] [PubMed]
- Jamshed, H.; Sultan, F.A.; Iqbal, R.; Gilani, A.H. Dietary Almonds Increase Serum HDL Cholesterol in Coronary Artery Disease Patients in a Randomized Controlled Trial. J. Nutr. 2015, 145, 2287–2292. [Google Scholar] [CrossRef] [PubMed]
- Rajaram, S.; Connell, K.M.; Sabate, J. Effect of almond-enriched high-monounsaturated fat diet on selected markers of inflammation: A randomised, controlled, crossover study. Br. J. Nutr. 2010, 103, 907–912. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.Y.; Holbrook, M.; Duess, M.A.; Dohadwala, M.M.; Hamburg, N.M.; Asztalos, B.F.; Milbury, P.E.; Blumberg, J.B.; Vita, J.A. Effect of almond consumption on vascular function in patients with coronary artery disease: A randomized, controlled, cross-over trial. Nutr. J. 2015, 14, 61. [Google Scholar] [CrossRef] [PubMed]
| Test Meal | Meal Contents | Total Calories | Total Fat (% Energy) | Saturated Fat (% Energy) | Total Protein (% Energy) | Total Carb (% Energy) | Total Fiber |
|---|---|---|---|---|---|---|---|
| Dairy (Cream Cheese) | 65 g bagel 240 g apple juice 86 g cream cheese 9 g Polycose powder 8 g Beneprotein powder | 665 kcal | 31 g (42%) | 18 g (24%) | 19 g (12%) | 77 g (46%) | 2 g |
| Nut (Almonds) | 65 g bagel 240 g apple juice 56.7 g almonds | 665 kcal | 31 g (42%) | 3 g (4%) | 19 g (12%) | 77 g (46%) | 9 g |
| Parameter | Sample Collection Times Points (Hours) | Analysis Method | Sample Collected (+ Additives If Any) |
|---|---|---|---|
| Glucose | 0, 0.5, 1, 2, 3, 4, 5 | Polychemical Clinical Analyzer, MedTest DX (Cortlandt Manor, NY) | Plasma |
| Triglyceride | 0, 0.5, 1, 2, 3, 4, 5 | Polychemical Clinical Analyzer, MedTest DX (Cortlandt Manor, NY) | Plasma |
| Ghrelin | 0, 0.5, 1, 2, 3, 4, 5 | Multi-array Electrochemiluminescence (Human Ghrelin Prototype Assay), Meso Scale Discovery (Rockville, MD) | Plasma + aprotinin inhibitor |
| Insulin | 0, 0.5, 1, 2, 3, 4, 5 | Multi-array Electrochemiluminescence (Human Active GLP-1, Insulin, Glucagon, Leptin Assay Kit), Meso Scale Discovery (Rockville, MD) | Plasma + DPP-IV inhibitor + aprotinin inhibitor |
| Hemoglobin A1c | 0 | Enzymatic, Diazyme (Poway, CA) | Whole blood |
| Leptin | 0, 0.5, 1, 2, 3, 4, 5 | Multi-array Electrochemiluminescence (Human Active GLP-1, Insulin, Glucagon, Leptin Assay Kit), Meso Scale Discovery (Rockville, MD) | Plasma + DPP-IV inhibitor + aprotinin inhibitor |
| C-peptide | 0, 1, 3, 5 | Multi-array Electrochemiluminescence (Human C-Peptide Prototype Assay), Meso Scale Discovery (Rockville, MD) | Plasma |
| Apolipoprotein B | 0, 1, 3, 5 | Immunoturbidimetric assay [25,26] (Kamiya Biomedical Company, Seattle, Washington), Liasys 330 automated chemistry analyzer | Serum |
| High Molecular Weight Adiponectin | 0 | Multi-array Electrochemiluminescence (Human Adiponectin Assay Kit), Meso Scale Discovery (Rockville, MD) | Plasma |
| High-sensitivity C-Reactive Protein | 0 | Multi-array Electrochemiluminescence (C-Reactive Protein Assay), MSD (Rockville, MD) | Plasma |
| Total-cholesterol | 0 | Enzymatic end-point measurement (AMS Diagnostics, Brookfield, Connecticut), Liasys 330 automated chemistry analyzer | Plasma |
| Low Density Lipoprotein-cholesterol | 0 | Calculated by subtraction of estimated Very Low Density Lipoprotein and measured High Density Lipoprotein cholesterol from the measured total cholesterol and triglyceride in plasma | Plasma |
| High Density Lipoprotein-cholesterol | 0 | Enzymatic end-point measurement (AMS Diagnostics, Brookfield, Connecticut), Liasys 330 automated chemistry analyzer | Plasma |
| Lipid-particle size | 0 | Ion mobility (IM) analysis, electrospray utilized to create an aerosol of particles that pass through a differential mobility analyzer (DMA) coupled to a particle counter [27] | Plasma |
| Characteristic | Mean ± SD or Percentage |
|---|---|
| Weeks of gestation | 32.6 ± 1.8 |
| Weight, kg | 84.6 ± 15.2 |
| Estimated Gestational Weight Gain, kg a | 6.3 ± 4.7 |
| Body Fat, % | 41.9 ± 5.5 |
| Blood Pressure Systolic Diastolic | 104 ± 10.7 63 ± 5.9 |
| Hemoglobin A1c, % | 7.2 ± 0.6 |
| Glucose, mg/dL | 86.5 ± 7.4 |
| Insulin, mU/L | 13.9 ± 7.9 |
| Triglycerides, mg/dL | 214.4 ± 79.5 |
| Leptin, ng/mL | 3.60 ± 3.2 |
| Ghrelin, pmol/L | 14.1 ± 6.2 |
| Adiponectin, ug/mL | 7.3 ± 3.6 |
| C-reactive protein, mg/dL | 0.2 ± 0.1 |
| Total cholesterol, mg/dL High Density Lipoprotein-cholesterol, mg/dL Low Density Lipoprotein-cholesterol, mg/dL Very Low Density Lipoprotein-cholesterol, mg/dL | 230.3 ± 32 74.2 ± 21.3 115.3 ± 34.3 40.8 ± 13.6 |
| Low Density Lipoprotein Peak Diameter (Å) | 217.8 ± 5 |
| Apolipoprotein B, mg/dL | 114 ± 24.6 |
| Low Density Lipoprotein Phenotype (%) | |
| A | 52.8% |
| I | 11.1% |
| B | 36.1% |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lesser, M.N.R.; Mauldin, K.; Sawrey-Kubicek, L.; Gildengorin, V.; King, J.C. The Type of Dietary Fat in an Isocaloric Breakfast Meal Does Not Modify Postprandial Metabolism in Overweight/Obese Pregnant Women. Nutrients 2019, 11, 490. https://doi.org/10.3390/nu11030490
Lesser MNR, Mauldin K, Sawrey-Kubicek L, Gildengorin V, King JC. The Type of Dietary Fat in an Isocaloric Breakfast Meal Does Not Modify Postprandial Metabolism in Overweight/Obese Pregnant Women. Nutrients. 2019; 11(3):490. https://doi.org/10.3390/nu11030490
Chicago/Turabian StyleLesser, Mary N. R., Kasuen Mauldin, Lisa Sawrey-Kubicek, Virginia Gildengorin, and Janet C. King. 2019. "The Type of Dietary Fat in an Isocaloric Breakfast Meal Does Not Modify Postprandial Metabolism in Overweight/Obese Pregnant Women" Nutrients 11, no. 3: 490. https://doi.org/10.3390/nu11030490
APA StyleLesser, M. N. R., Mauldin, K., Sawrey-Kubicek, L., Gildengorin, V., & King, J. C. (2019). The Type of Dietary Fat in an Isocaloric Breakfast Meal Does Not Modify Postprandial Metabolism in Overweight/Obese Pregnant Women. Nutrients, 11(3), 490. https://doi.org/10.3390/nu11030490






