Effect of Cell Concentration on the Persistence in the Human Intestine of Four Probiotic Strains Administered through a Multispecies Formulation
Abstract
1. Introduction
2. Materials and Methods
2.1. Human Intervention Study
2.1.1. Study Population
2.1.2. Formulations under Study
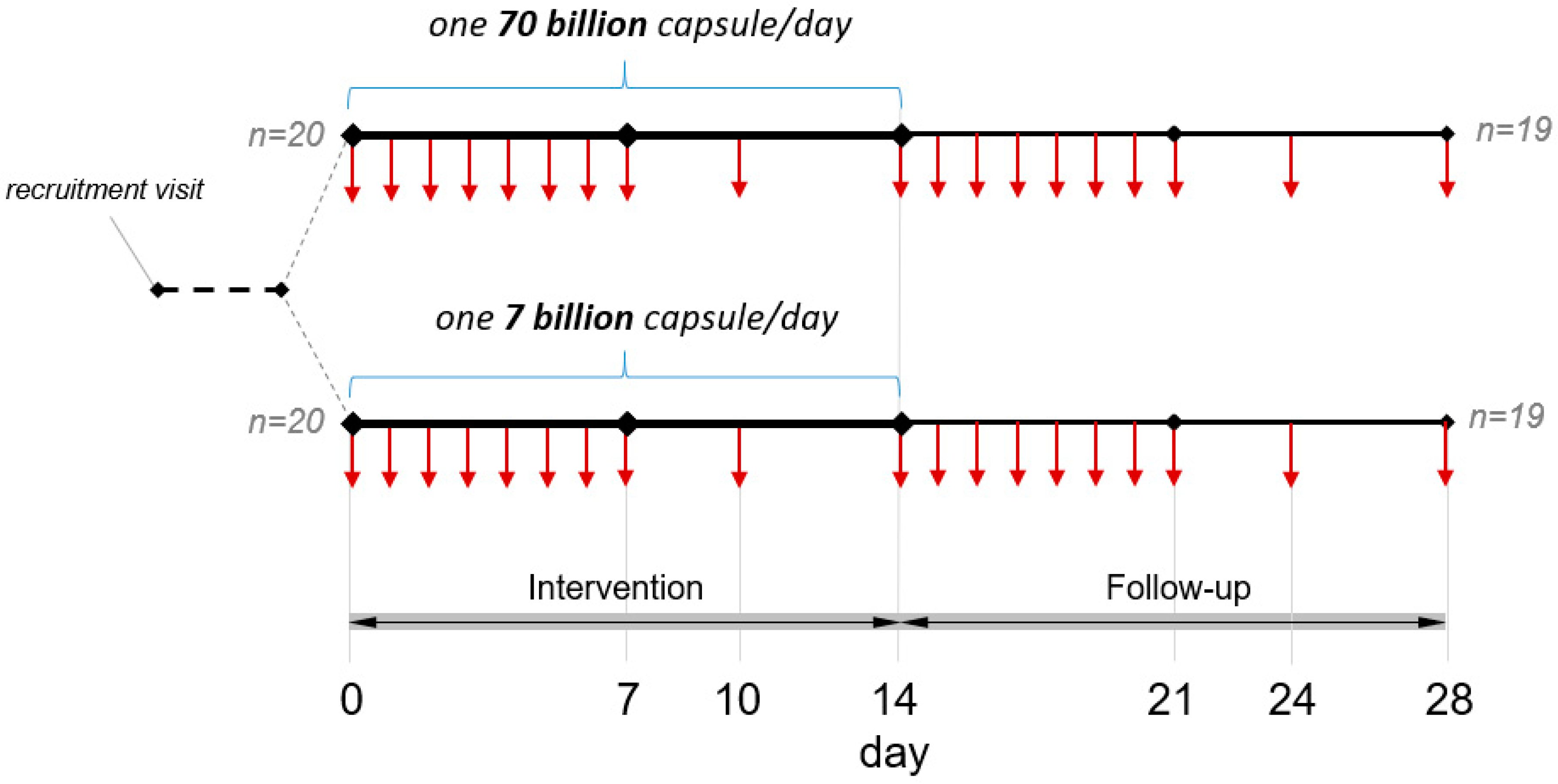
2.1.3. Intervention and Study Scheme
2.1.4. Sample Collection
2.1.5. Ethical Statement
2.1.6. Volunteer Compliance
2.2. DNA Extraction from Fecal Samples
2.3. Quantification of Bacterial Strains through Quantitative Polymerase Chain Reaction (qPCR)
2.4. Viable Recovery of Probiotic Strains
2.5. Assessment of Bacterial Cell Stability in Probiotic Capsules throughout the Study
2.6. Statistical Analysis
3. Results
3.1. Adherence to Study Protocol and Case Reports
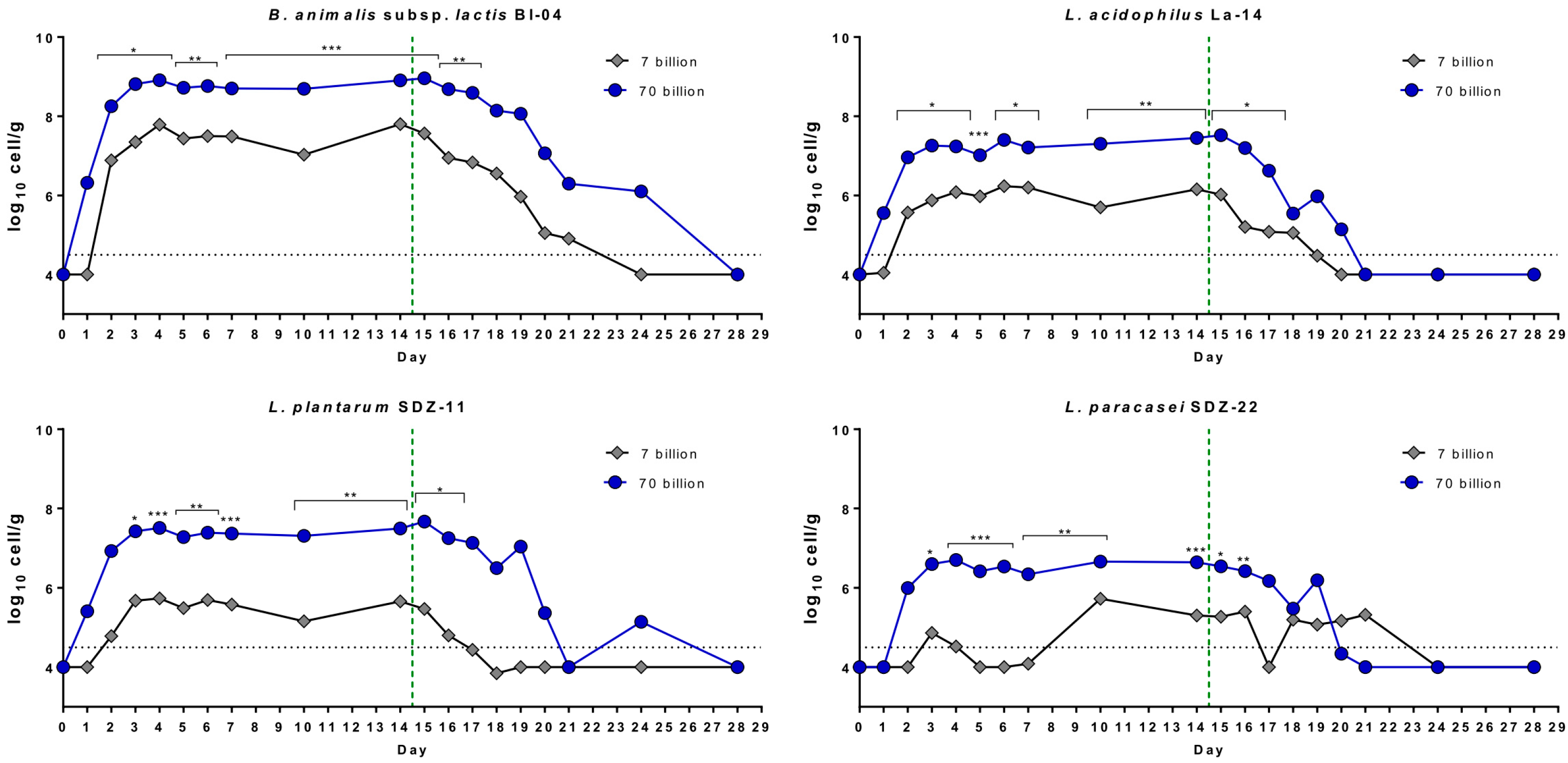
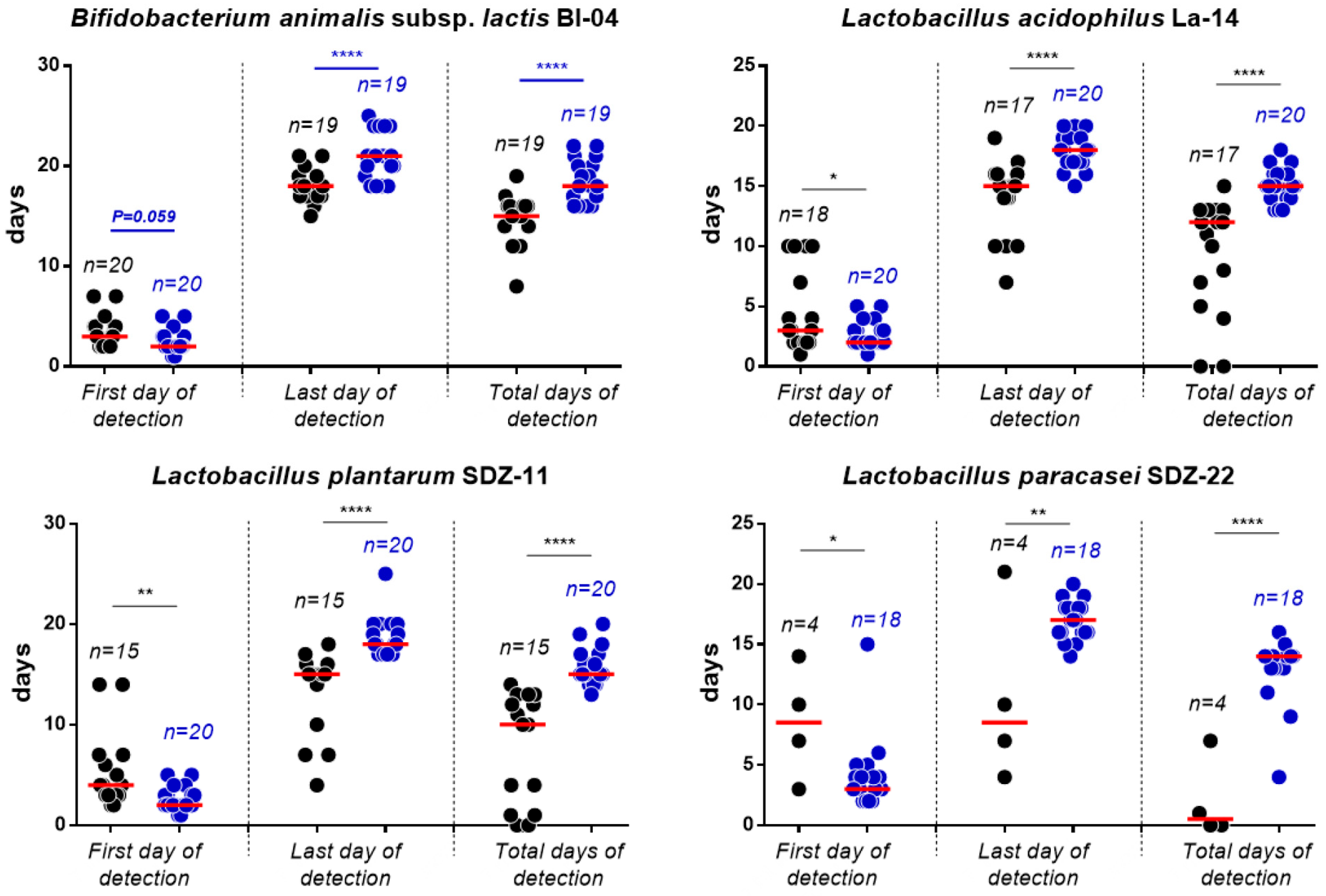
3.2. Enumeration of Probiotic Bacteria in Fecal Samples
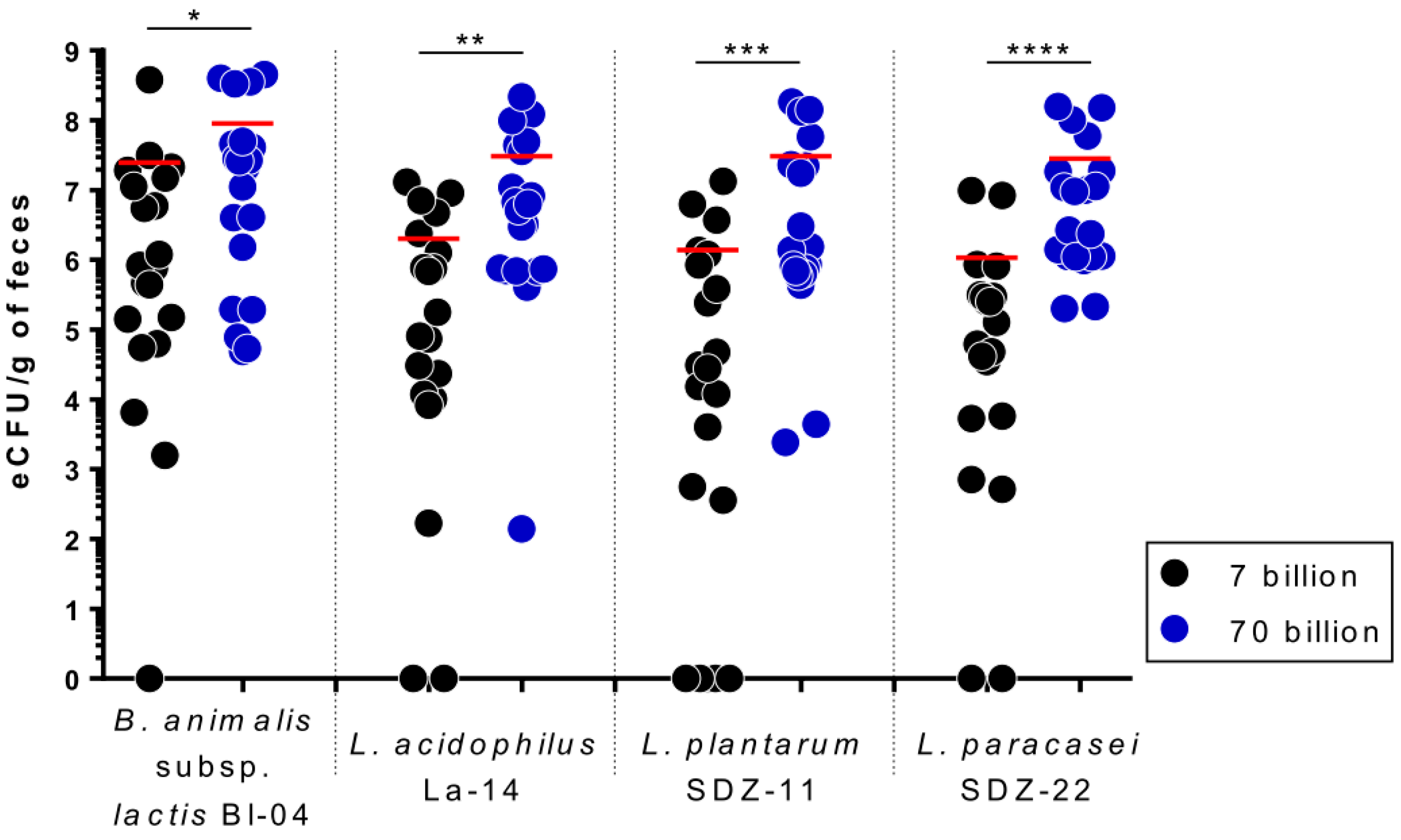
3.3. Viable Recovery of Probiotic Strains from Fecal Samples
3.4. Stability of the Probiotic Bacteria in Capsules during the Trial
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
Appendix A. Validation of Strain- and Species-Specific Primers and Preparation of Calibration Curves for Bacterial Quantification through qPCR
References
- FAO/WHO. Expert Consultation on Evaluation of Health and Nutritional Properties of Probiotics in Food Including Powder Milk with Live Lactic Acid Bacteria; Food and Agriculture Organization of the United Nations: Córdoba, Argentina, 2001. [Google Scholar]
- Hill, C.; Guarner, F.; Reid, G.; Gibson, G.R.; Merenstein, D.J.; Pot, B.; Morelli, L.; Canani, R.B.; Flint, H.J.; Salminen, S.; et al. Expert consensus document. The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 506–514. [Google Scholar] [CrossRef] [PubMed]
- Markowiak, P.; Slizewska, K. Effects of Probiotics, Prebiotics, and Synbiotics on Human Health. Nutrients 2017, 9, 1021. [Google Scholar] [CrossRef] [PubMed]
- Kolacek, S.; Hojsak, I.; Berni Canani, R.; Guarino, A.; Indrio, F.; Orel, R.; Pot, B.; Shamir, R.; Szajewska, H.; Vandenplas, Y.; et al. Commercial Probiotic Products: A Call for Improved Quality Control. A Position Paper by the ESPGHAN Working Group for Probiotics and Prebiotics. J. Pediatr. Gastroenterol. Nutr. 2017, 65, 117–124. [Google Scholar] [CrossRef]
- Bertazzoni, E.; Donelli, G.; Midtvedt, T.; Nicoli, J.; Sanz, Y. Probiotics and clinical effects: Is the number what counts? J. Chemother. 2013, 25, 193–212. [Google Scholar] [CrossRef]
- Arioli, S.; Koirala, R.; Taverniti, V.; Fiore, W.; Guglielmetti, S. Quantitative Recovery of Viable Lactobacillus paracasei CNCM I-1572 (L. casei DG(R)) after Gastrointestinal Passage in Healthy Adults. Front. Microbiol. 2018, 9, 1720. [Google Scholar] [CrossRef] [PubMed]
- Tuohy, K.M.; Pinart-Gilberga, M.; Jones, M.; Hoyles, L.; McCartney, A.L.; Gibson, G.R. Survivability of a probiotic Lactobacillus casei in the gastrointestinal tract of healthy human volunteers and its impact on the faecal microflora. J. Appl. Microbiol. 2007, 102, 1026–1032. [Google Scholar] [CrossRef] [PubMed]
- Radicioni, M.; Koirala, R.; Fiore, W.; Leuratti, C.; Guglielmetti, S.; Arioli, S. Survival of L. casei DG((R)) (Lactobacillus paracasei CNCMI1572) in the gastrointestinal tract of a healthy paediatric population. Eur. J. Nutr. 2018. [Google Scholar] [CrossRef] [PubMed]
- Ahlroos, T.; Tynkkynen, S. Quantitative strain-specific detection of Lactobacillus rhamnosus GG in human faecal samples by real-time PCR. J. Appl. Microbiol. 2009, 106, 506–514. [Google Scholar] [CrossRef]
- Stahl, B.; Barrangou, R. Complete Genome Sequence of Probiotic Strain Lactobacillus acidophilus La-14. Genome Announc. 2013, 1. [Google Scholar] [CrossRef]
- Diplock, A.T.; Aggett, P.J.; Ashwell, M.; Bornet, F.; Fern, E.B.; Roberfroid, M.B. Scientific concepts of functional foods in Europe. Consensus document. Br. J. Nutr. 1999, 81 (Suppl. 1), S1–S27. [Google Scholar]
- Holzapfel, W.H. Introduction to prebiotics and probiotics. In Probiotics in Food Safety and Human Health; Ipek Goktepe, V.K.J., Ahmedna, M., Eds.; CRC Press: Boca Raton, FL, USA, 2005; pp. 48–49. [Google Scholar]
- FAO/WHO. Guidelines for the Evaluation of Probiotics in Food; Food and Agriculture Organization of the United Nations: London, ON, USA, 2002. [Google Scholar]
- Granata, M.; Brandi, G.; Borsari, A.; Gasbarri, R.; Gioia, D.D. Synbiotic yogurt consumption by healthy adults and the elderly: The fate of bifidobacteria and LGG probiotic strain. Int. J. Food Sci. Nutr. 2013, 64, 162–168. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Chen, S.; Jin, J.; Ren, F.; Li, Y.; Qiao, Z.; Wang, Y.; Zhao, L. Survival of Lactobacillus casei strain Shirota in the intestines of healthy Chinese adults. Microbiol. Immunol. 2015, 59, 268–276. [Google Scholar] [CrossRef]
- Poutsiaka, D.D.; Mahoney, I.J.; McDermott, L.A.; Stern, L.L.; Thorpe, C.M.; Kane, A.V.; Baez-Giangreco, C.; McKinney, J.; Davidson, L.E.; Leyva, R.; et al. Selective method for identification and quantification of Bifidobacterium animalis subspecies lactis BB-12 (BB-12) from the gastrointestinal tract of healthy volunteers ingesting a combination probiotic of BB-12 and Lactobacillus rhamnosus GG. J. Appl. Microbiol. 2017, 122, 1321–1332. [Google Scholar] [CrossRef] [PubMed]
- Saxelin, M.; Lassig, A.; Karjalainen, H.; Tynkkynen, S.; Surakka, A.; Vapaatalo, H.; Jarvenpaa, S.; Korpela, R.; Mutanen, M.; Hatakka, K. Persistence of probiotic strains in the gastrointestinal tract when administered as capsules, yoghurt, or cheese. Int. J. Food Microbiol. 2010, 144, 293–300. [Google Scholar] [CrossRef]
- MacPherson, C.W.; Shastri, P.; Mathieu, O.; Tompkins, T.A.; Burguiere, P. Genome-Wide Immune Modulation of TLR3-Mediated Inflammation in Intestinal Epithelial Cells Differs between Single and Multi-Strain Probiotic Combination. PLoS ONE 2017, 12, e0169847. [Google Scholar] [CrossRef]
- Ouwehand, A.C.; Isolauri, E.; Kirjavainen, P.V.; Tolkko, S.; Salminen, S.J. The mucus binding of Bifidobacterium lactis Bb12 is enhanced in the presence of Lactobacillus GG and Lact. delbrueckii subsp. bulgaricus. Lett. Appl. Microbiol. 2000, 30, 10–13. [Google Scholar] [CrossRef] [PubMed]
- Shiou, S.R.; Yu, Y.; Guo, Y.; He, S.M.; Mziray-Andrew, C.H.; Hoenig, J.; Sun, J.; Petrof, E.O.; Claud, E.C. Synergistic protection of combined probiotic conditioned media against neonatal necrotizing enterocolitis-like intestinal injury. PLoS ONE 2013, 8, e65108. [Google Scholar] [CrossRef] [PubMed]
- Forssten, S.D.; Ouwehand, A.C. Simulating colonic survival of probiotics in single-strain products compared to multi-strain products. Microb. Ecol. Health Dis. 2017, 28, 1378061. [Google Scholar] [CrossRef] [PubMed]
- Szulinska, M.; Loniewski, I.; van Hemert, S.; Sobieska, M.; Bogdanski, P. Dose-Dependent Effects of Multispecies Probiotic Supplementation on the Lipopolysaccharide (LPS) Level and Cardiometabolic Profile in Obese Postmenopausal Women: A 12-Week Randomized Clinical Trial. Nutrients 2018, 10, 773. [Google Scholar] [CrossRef]
- Kim, J.Y.; Park, Y.J.; Lee, H.J.; Park, M.Y.; Kwon, O. Effect of Lactobacillus gasseri BNR17 on irritable bowel syndrome: A randomized, double-blind, placebo-controlled, dose-finding trial. Food Sci. Biotechnol. 2018, 27, 853–857. [Google Scholar] [CrossRef]
- Ouwehand, A.C.; DongLian, C.; Weijian, X.; Stewart, M.; Ni, J.; Stewart, T.; Miller, L.E. Probiotics reduce symptoms of antibiotic use in a hospital setting: A randomized dose response study. Vaccine 2014, 32, 458–463. [Google Scholar] [CrossRef] [PubMed]
- Turner, R.B.; Woodfolk, J.A.; Borish, L.; Steinke, J.W.; Patrie, J.T.; Muehling, L.M.; Lahtinen, S.; Lehtinen, M.J. Effect of probiotic on innate inflammatory response and viral shedding in experimental rhinovirus infection—A randomised controlled trial. Benef. Microbes 2017, 8, 207–215. [Google Scholar] [CrossRef] [PubMed]
- Muyzer, G.; de Waal, E.C.; Uitterlinden, A.G. Profiling of complex microbial populations by denaturing gradient gel electrophoresis analysis of polymerase chain reaction-amplified genes coding for 16S rRNA. Appl. Environ. Microbiol. 1993, 59, 695–700. [Google Scholar] [PubMed]
| Ingredient | Formulation 1 | Formulation 2 |
|---|---|---|
| Coating Agent | Hydroxypropyl methylcellulose | Hydroxypropyl methylcellulose |
| Bulking agent | Microcrystalline cellulose | Microcrystalline cellulose |
| Anti-caking agent | Magnesium salts of fatty acids and silicon dioxide | Magnesium salts of fatty acids and silicon dioxide |
| Coloring agent | Titanium dioxide | Titanium dioxide |
| Probiotic strains (lyophilized biomass; 109 CFUs/capsule) | ||
| Bifidobacterium animalis subsp. lactis BL-04 | 5.25 | 52.5 |
| Lactobacillus acidophilus LA-14 | 1.4 | 14 |
| Lactobacillus plantarum SDZ-11 | 0.28 | 2.8 |
| Lactobacillus paracasei SDZ-22 | 0.07 | 0.7 |
| Total probiotic cells | 7 | 70 |
| Primer Name | Primer Sequences | Target Strain | Source |
|---|---|---|---|
| Bl-04-F Bl-04-R | 5′-CTTCCCAGAAGGCCGGGT-3′ 5′-CGAGGCCACGGTGCTCATATAGA-3′ | B. animalis subsp. lactis BL-04 | [25] |
| La-14-F La-14-R | 5′-ATCGTGATTTGCATATAAATTGA-3′ 5′-ACCTTGCTTAATTTGCAAGTCT-3′ | L. acidophilus LA-14 | This study |
| SDZ-11-F SDZ-11-R | 5′-CTTGATGACTCTTCTGGGGC-3′ 5′-ACGGGAGTGATAGACGTTGAG-3′ | L. plantarum SDZ-11 | This study |
| SDZ-22-F SDZ-22-R | 5′-TTGGGTGCTATGGGAAACACA-3′ 5′-AAGTTACGCCGCCACAAAC-3′ | L. paracasei SDZ-22 | This study |
| 357F 907R | 5′-CCTACGGGAGGCAGCAG-3′ 5′-CCGTCAATTCMTTTRAGTTT-3′ | Panbacteria | [26] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Taverniti, V.; Koirala, R.; Dalla Via, A.; Gargari, G.; Leonardis, E.; Arioli, S.; Guglielmetti, S. Effect of Cell Concentration on the Persistence in the Human Intestine of Four Probiotic Strains Administered through a Multispecies Formulation. Nutrients 2019, 11, 285. https://doi.org/10.3390/nu11020285
Taverniti V, Koirala R, Dalla Via A, Gargari G, Leonardis E, Arioli S, Guglielmetti S. Effect of Cell Concentration on the Persistence in the Human Intestine of Four Probiotic Strains Administered through a Multispecies Formulation. Nutrients. 2019; 11(2):285. https://doi.org/10.3390/nu11020285
Chicago/Turabian StyleTaverniti, Valentina, Ranjan Koirala, Alessandro Dalla Via, Giorgio Gargari, Elena Leonardis, Stefania Arioli, and Simone Guglielmetti. 2019. "Effect of Cell Concentration on the Persistence in the Human Intestine of Four Probiotic Strains Administered through a Multispecies Formulation" Nutrients 11, no. 2: 285. https://doi.org/10.3390/nu11020285
APA StyleTaverniti, V., Koirala, R., Dalla Via, A., Gargari, G., Leonardis, E., Arioli, S., & Guglielmetti, S. (2019). Effect of Cell Concentration on the Persistence in the Human Intestine of Four Probiotic Strains Administered through a Multispecies Formulation. Nutrients, 11(2), 285. https://doi.org/10.3390/nu11020285








