Effects of Low-Fat and High-Fat Meals, with and without Dietary Fiber, on Postprandial Endothelial Function, Triglyceridemia, and Glycemia in Adolescents
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participant Recruitment
2.2. Study Design and Interventions
- The low-fat, low-fiber meal consisted of 31.0 g of low-fiber, wheat-based cereal (General Mills Golden Grahams); 245.0 g of non-fat milk; 124.5 g of orange juice; 65.0 g of refined, enriched-grain bread; 15.0 g of margarine; and 14.0 g of jelly.
- The low-fat, high-fiber meal consisted of 50.0 g of high-fiber, wheat-based cereal (Kellogg’s All-Bran Original); 245.0 g of non-fat milk; 124.5 g of orange juice; 68.0 g of whole-grain wheat bread; 15.0 g of margarine; and 14.0 g of jelly.
- The high-fat, low-fiber meal consisted of 88.0 g of eggs; 28.0 g of cheddar cheese; 34.0 g of turkey sausage; 244.0 g of whole milk; 124.5 g of orange juice; and 10.0 g of margarine.
- The high-fat, high-fiber meal consisted of 50.0 g of high-fiber, wheat-based cereal (Kellogg’s All-Bran Original); 88.0 g of eggs; 28.0 g of cheddar cheese; 34.0 g of turkey sausage; 244.0 g whole milk; 124.5 g of orange juice; 10.0 g of margarine; and 2.0 g sucralose sweetener.
2.3. Clinical and Biochemical Assessments
2.4. Statistical Analyses
2.4.1. Postprandial Blood Analyses
Glucose and Insulin
Triglycerides
Flow-Mediated Dilation (FMD)
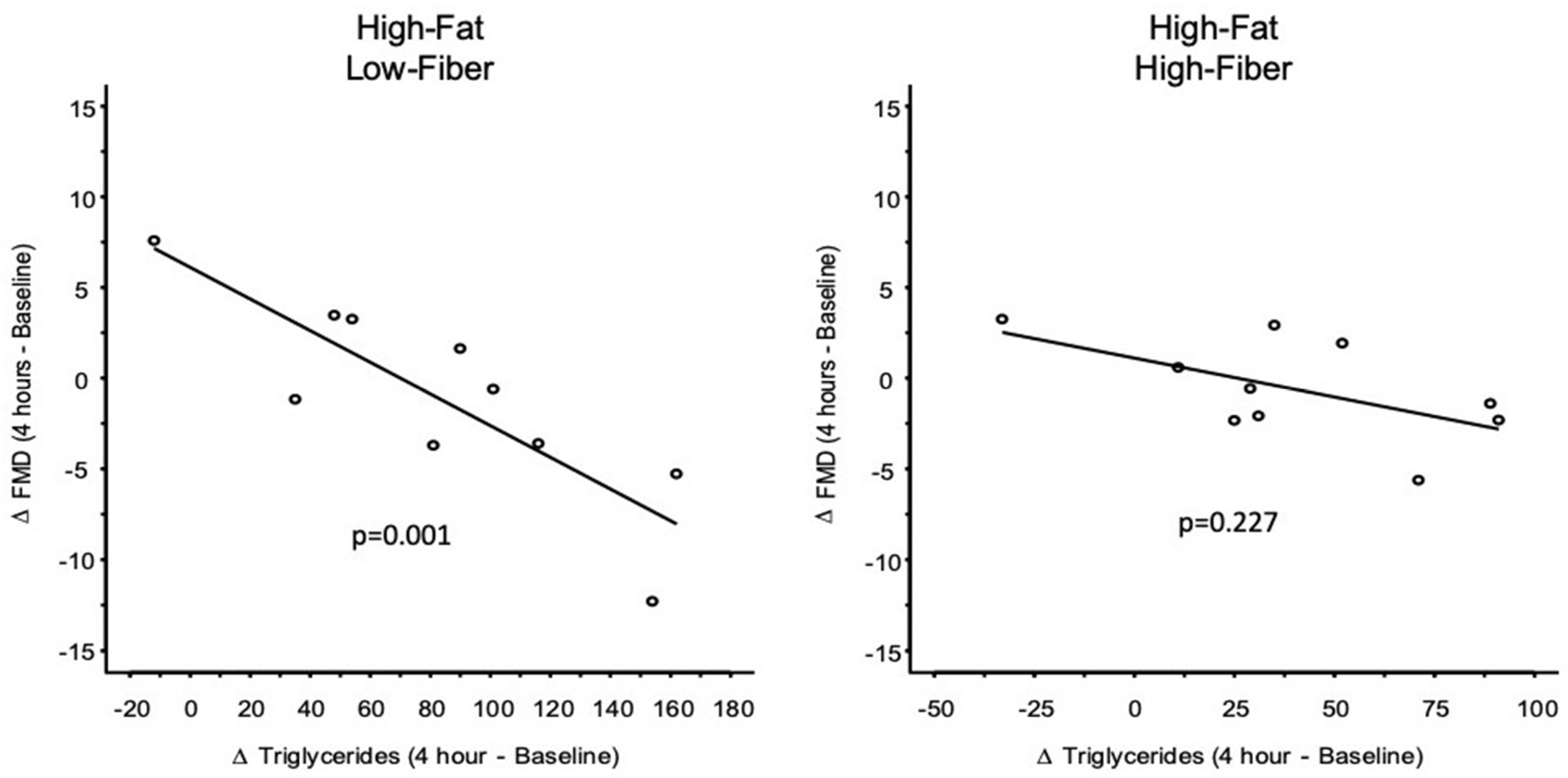
Regression Analysis
3. Results
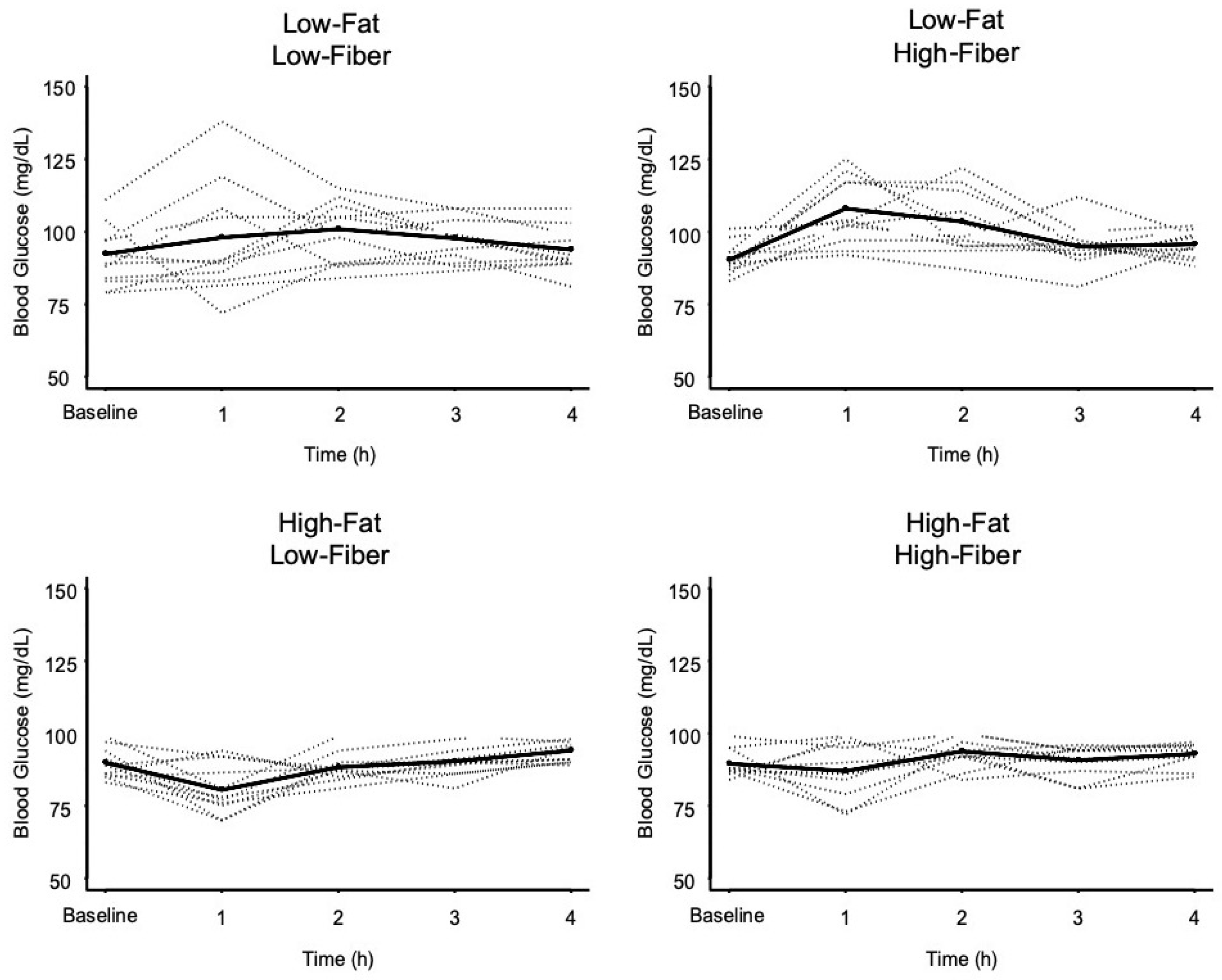
3.1. Glycemic Response Data
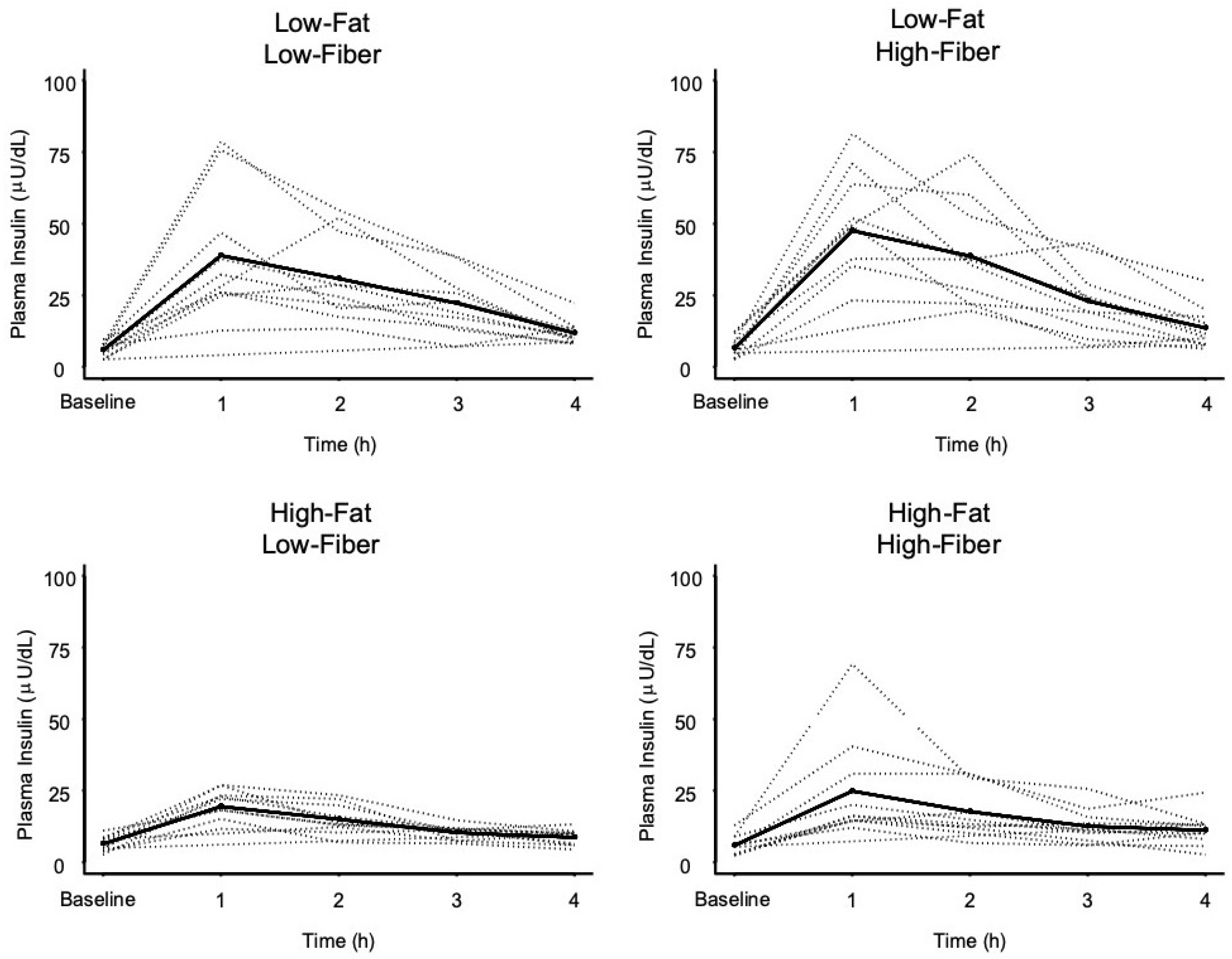
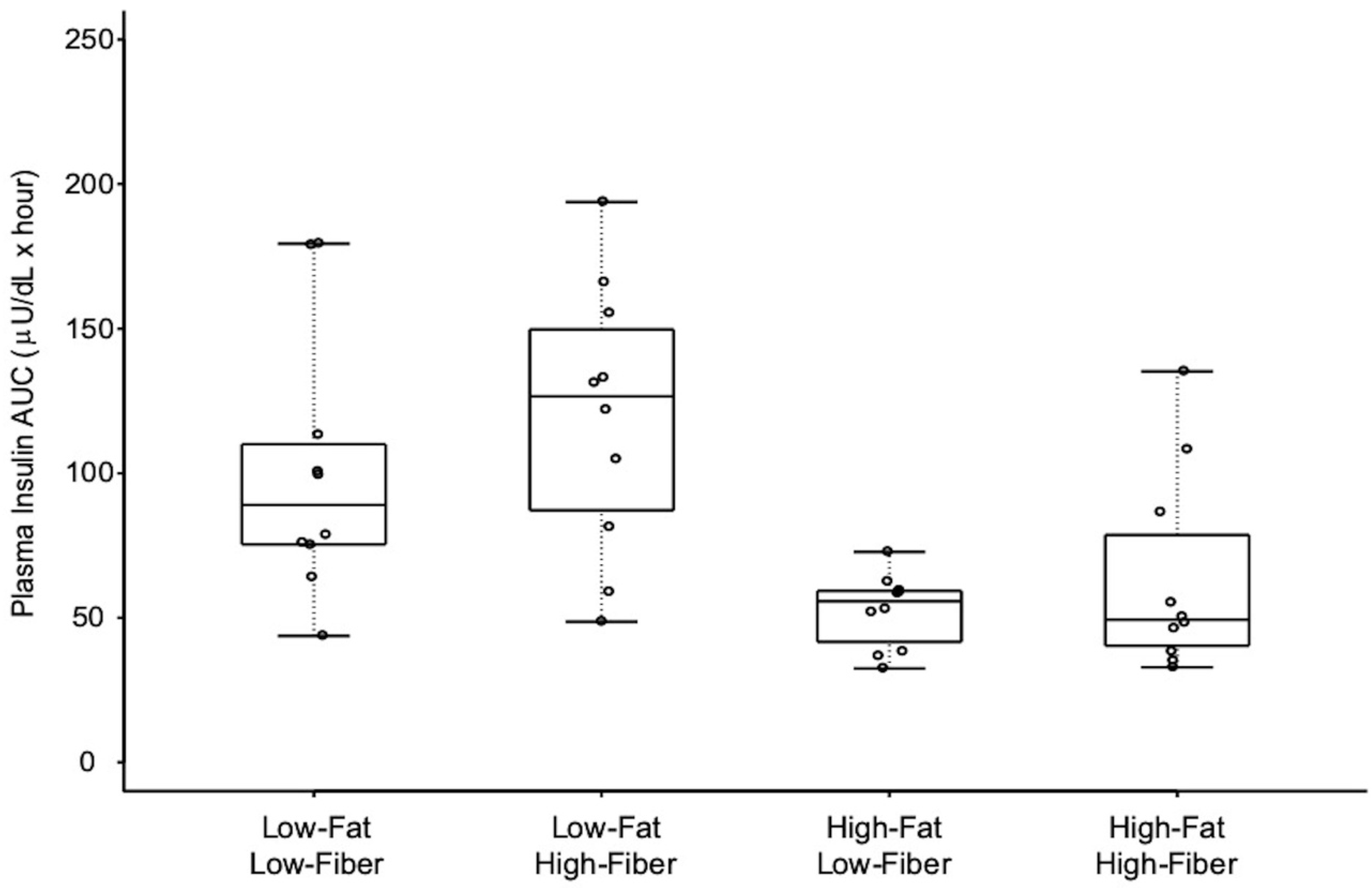
3.2. Insulin Response Data
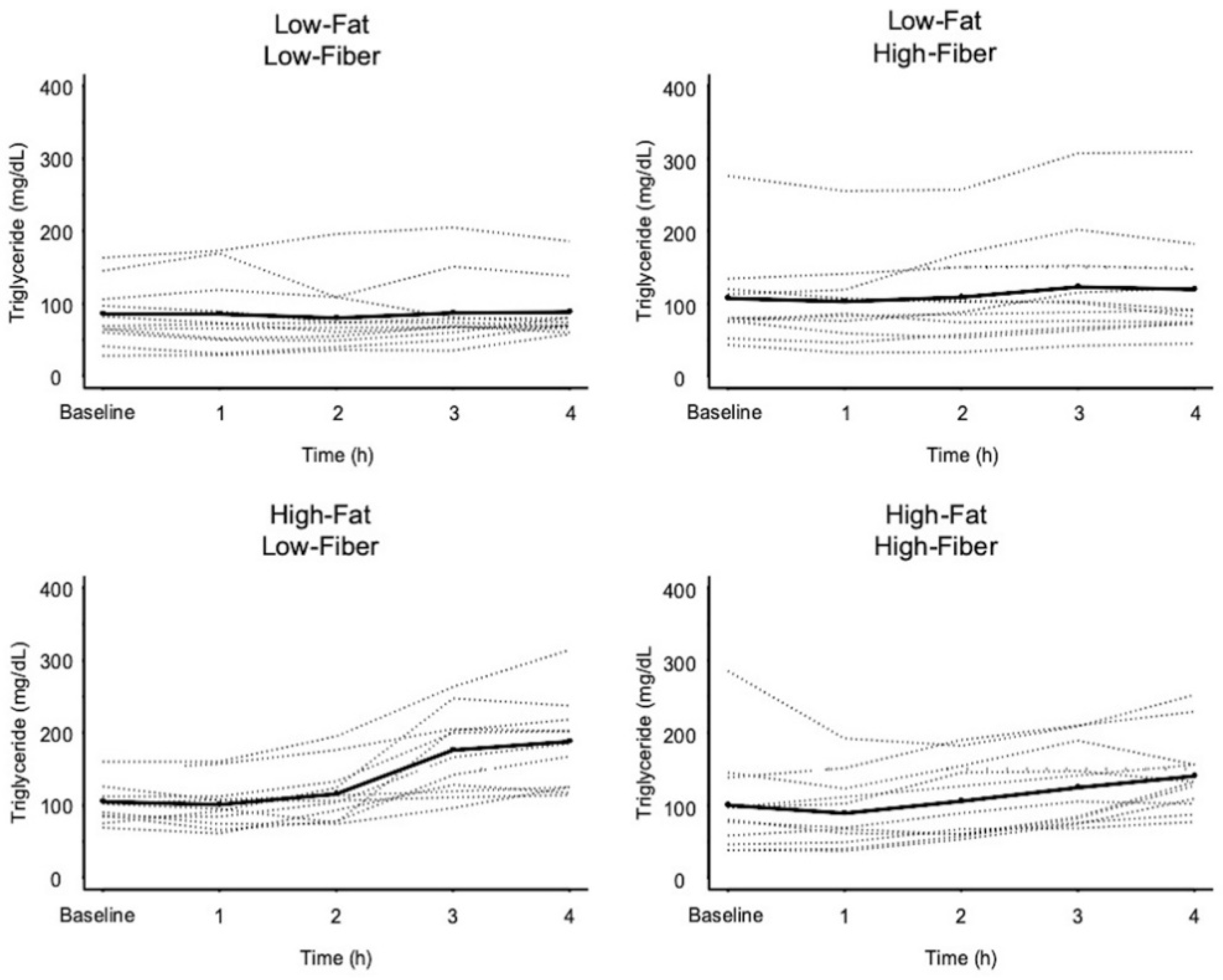
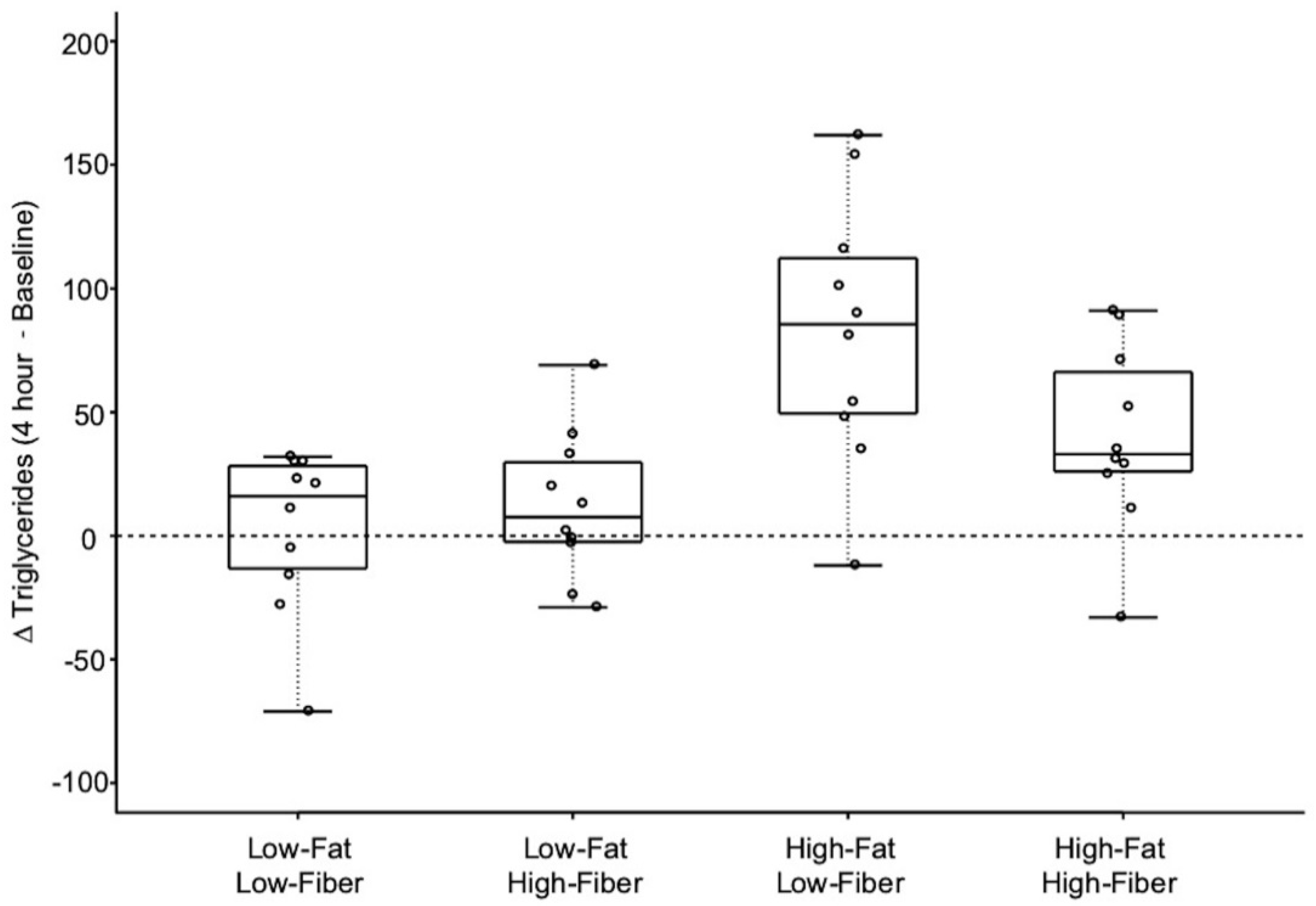
3.3. Triglyceride Response Data
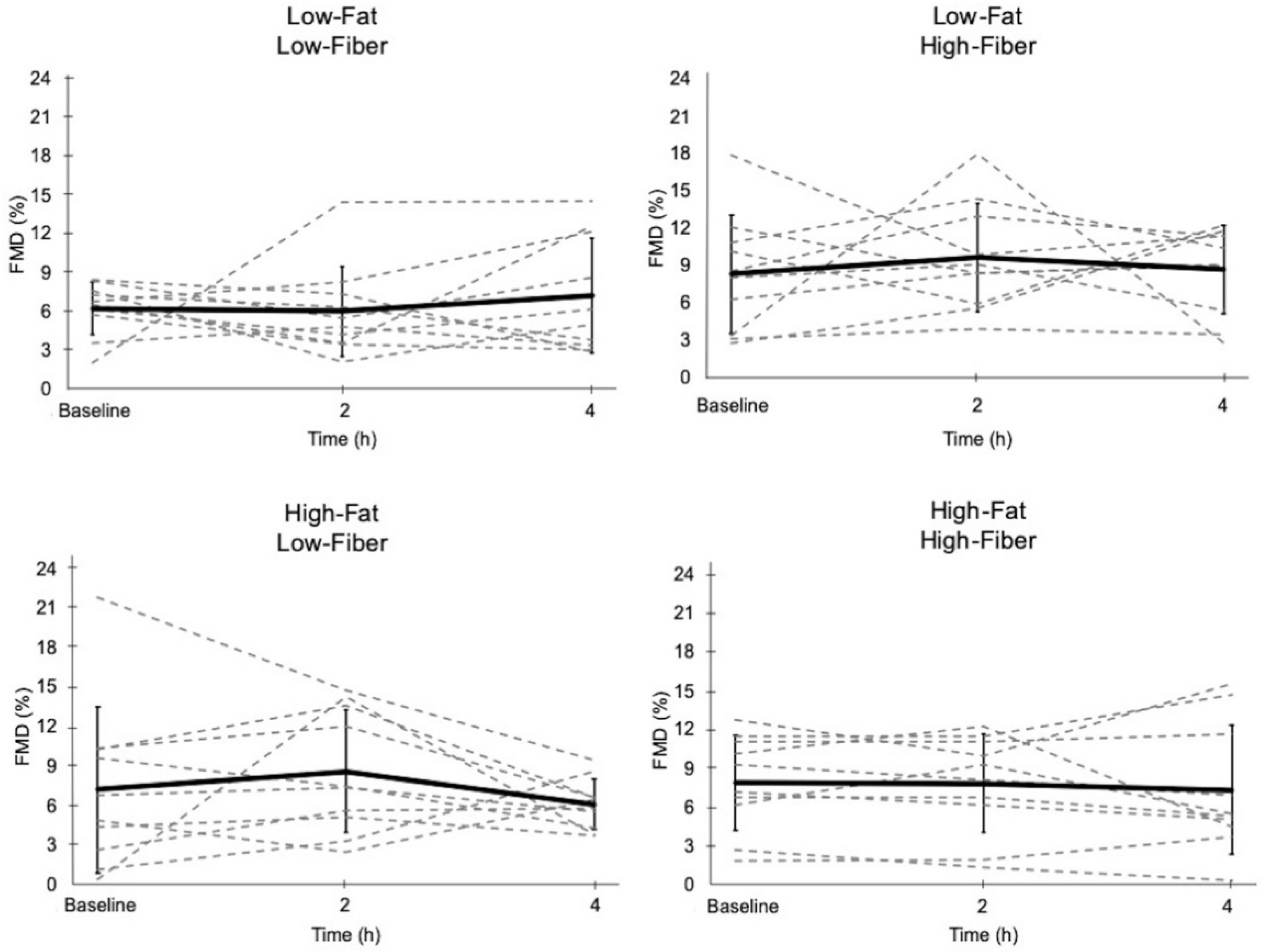
3.4. Flow-Mediated Dilation
4. Discussion
Strengths and Limitations
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Gokce, N.; Keaney, J.F.; Hunter, L.M.; Watkins, M.T.; Menzoian, J.O.; Vita, J.A. Risk stratification for postoperative cardiovascular events via noninvasive assessment of endothelial function: A prospective study. Circulation 2002, 105, 1567–1572. [Google Scholar] [CrossRef] [PubMed]
- Zilversmit, D.B. Atherogenesis: A postprandial phenomenon. Circulation 1979, 60, 473–485. [Google Scholar] [CrossRef] [PubMed]
- Irawati, D.; Mamo, J.C.L.; Slivkoff-Clark, K.M.; Soares, M.J.; James, A.P. Dietary fat and physiological determinants of plasma chylomicron remnant homoeostasis in normolipidaemic subjects: Insight into atherogenic risk. Br. J. Nutr. 2017, 117, 403–412. [Google Scholar] [CrossRef] [PubMed]
- Langsted, A.; Nordestgaard, B.G. Nonfasting versus fasting lipid profile for cardiovascular risk prediction. Pathology 2019, 51, 131–141. [Google Scholar] [CrossRef] [PubMed]
- DECODE Study Group. Is the current definition for diabetes relevant to mortality risk from all causes and cardiovascular and noncardiovascular diseases? Diabetes Care 2003, 26, 688–696. [Google Scholar] [CrossRef]
- Levitan, E.B.; Song, Y.; Ford, E.S.; Liu, S. Is Nondiabetic Hyperglycemia a Risk Factor for Cardiovascular Disease? Arch. Intern. Med. 2004, 164, 2147. [Google Scholar] [CrossRef] [PubMed]
- O’Keefe, J.H.; Gheewala, N.M.; O’Keefe, J.O. Dietary Strategies for Improving Post-Prandial Glucose, Lipids, Inflammation, and Cardiovascular Health. J. Am. Coll. Cardiol. 2008, 51, 249–255. [Google Scholar] [CrossRef] [Green Version]
- Stary, H.C. Evolution and progression of atherosclerotic lesions in coronary arteries of children and young adults. Arteriosclerosis 1989, 9, 19–32. [Google Scholar]
- Berenson, G.S.; Wattigney, W.A.; Tracy, R.E.; Newman, W.P.; Srinivasan, S.R.; Webber, L.S.; Dalferes, E.R.; Strong, J.P. Atherosclerosis of the aorta and coronary arteries and cardiovascular risk factors in persons aged 6 to 30 years and studied at necropsy (The Bogalusa Heart Study). Am. J. Cardiol. 1992, 70, 851–858. [Google Scholar] [CrossRef]
- Thom, N.J.; Early, A.R.; Hunt, B.E.; Harris, R.A.; Herring, M.P. Eating and arterial endothelial function: A meta-analysis of the acute effects of meal consumption on flow-mediated dilation. Obes. Rev. 2016, 17, 1080–1090. [Google Scholar] [CrossRef]
- Harris, R.A.; Nishiyama, S.K.; Wray, D.W.; Richardson, R.S. Ultrasound Assessment of Flow-Mediated Dilation. Hypertension 2010, 55, 1075–1085. [Google Scholar] [CrossRef]
- Inaba, Y.; Chen, J.A.; Bergmann, S.R. Prediction of future cardiovascular outcomes by flow-mediated vasodilatation of brachial artery: A meta-analysis. Int. J. Cardiovasc. Imaging 2010, 26, 631–640. [Google Scholar] [CrossRef] [PubMed]
- Bae, J.H.; Bassenge, E.; Kim, K.B.; Kim, Y.N.; Kim, K.S.; Lee, H.J.; Moon, K.C.; Lee, M.S.; Park, K.Y.; Schwemmer, M. Postprandial hypertriglyceridemia impairs endothelial function by enhanced oxidant stress. Atherosclerosis 2001, 155, 517–523. [Google Scholar] [CrossRef]
- Vogel, R.A.; Corretti, M.C.; Plotnick, G.D. Effect of a single high-fat meal on endothelial function in healthy subjects. Am. J. Cardiol. 1997, 79, 350–354. [Google Scholar] [CrossRef]
- Gaenzer, H.; Sturm, W.; Neumayr, G.; Kirchmair, R.; Ebenbichler, C.; Ritsch, A.; Föger, B.; Weiss, G.; Patsch, J.R. Pronounced postprandial lipemia impairs endothelium-dependent dilation of the brachial artery in men. Cardiovasc. Res. 2001, 52, 509–516. [Google Scholar] [CrossRef] [Green Version]
- Williams, M.J.; Sutherland, W.H.; McCormick, M.P.; de Jong, S.A.; Walker, R.J.; Wilkins, G.T. Impaired endothelial function following a meal rich in used cooking fat. J. Am. Coll. Cardiol. 1999, 33, 1050–1055. [Google Scholar] [CrossRef] [Green Version]
- Marchesi, S.; Lupattelli, G.; Schillaci, G.; Pirro, M.; Siepi, D.; Roscini, A.R.; Pasqualini, L.; Mannarino, E. Impaired flow-mediated vasoactivity during post-prandial phase in young healthy men. Atherosclerosis 2000, 153, 397–402. [Google Scholar] [CrossRef]
- Zhao, S.P.; Liu, L.; Gao, M.; Zhou, Q.C.; Li, Y.L.; Xia, B. Impairment of endothelial function after a high-fat meal in patients with coronary artery disease. Coron. Artery Dis. 2001, 12, 561–565. [Google Scholar] [CrossRef]
- Steer, P.; Sarabi, D.M.; Karlstrom, B.; Basu, S.; Berne, C.; Vessby, B.; Lind, L. The effect of a mixed meal on endothelium-dependent vasodilation is dependent on fat content in healthy humans. Clin. Sci. 2003, 105, 81–87. [Google Scholar] [CrossRef] [Green Version]
- Loader, J.; Montero, D.; Lorenzen, C.; Watts, R.; Méziat, C.; Reboul, C.; Stewart, S.; Walther, G. Acute Hyperglycemia Impairs Vascular Function in Healthy and Cardiometabolic Diseased Subjects: Systematic Review and Meta-Analysis. Arterioscler. Thromb. Vasc. Biol. 2015, 35, 2060–2072. [Google Scholar] [CrossRef]
- Sanders, T.A.; Lewis, F.J.; Goff, L.M.; Chowienczyk, P.J. RISCK Study Group SFAs do not impair endothelial function and arterial stiffness. Am. J. Clin. Nutr. 2013, 98, 677–683. [Google Scholar] [PubMed]
- Ayer, J.G.; Harmer, J.A.; Steinbeck, K.; Celermajer, D.S. Postprandial Vascular Reactivity in Obese and Normal Weight Young Adults. Obesity 2010, 18, 945–951. [Google Scholar] [CrossRef] [PubMed]
- Sedgwick, M.J.; Morris, J.G.; Nevill, M.E.; Barrett, L.A. Effect of repeated sprints on postprandial endothelial function and triacylglycerol concentrations in adolescent boys. J. Sports Sci. 2015, 33, 806–816. [Google Scholar] [CrossRef] [PubMed]
- Sedgwick, M.J.; Morris, J.G.; Nevill, M.E.; Tolfrey, K.; Nevill, A.; Barrett, L.A. Effect of exercise on postprandial endothelial function in adolescent boys. Br. J. Nutr. 2013, 110, 301–309. [Google Scholar] [CrossRef]
- Lane-Cordova, A.D.; Witmer, J.R.; Dubishar, K.; DuBose, L.E.; Chenard, C.A.; Siefers, K.J.; Myers, J.E.; Points, L.J.; Pierce, G.L. High trans but not saturated fat beverage causes an acute reduction in postprandial vascular endothelial function but not arterial stiffness in humans. Vasc. Med. 2016, 21, 429–436. [Google Scholar] [CrossRef] [Green Version]
- Brock, D.W.; Davis, C.K.; Irving, B.A.; Rodriguez, J.; Barrett, E.J.; Weltman, A.; Taylor, A.G.; Gaesser, G.A. A high-carbohydrate, high-fiber meal improves endothelial function in adults with the metabolic syndrome. Diabetes Care 2006, 29, 2313–2315. [Google Scholar] [CrossRef]
- Kondo, K.; Morino, K.; Nishio, Y.; Ishikado, A.; Arima, H.; Nakao, K.; Nakagawa, F.; Nikami, F.; Sekine, O.; Nemoto, K.; et al. Fiber-rich diet with brown rice improves endothelial function in type 2 diabetes mellitus: A randomized controlled trial. PLoS ONE 2017, 12, e0179869. [Google Scholar] [CrossRef]
- Wolk, A.; Manson, J.E.; Stampfer, M.J.; Colditz, G.A.; Hu, F.B.; Speizer, F.E.; Hennekens, C.H.; Willett, W.C. Long-term intake of dietary fiber and decreased risk of coronary heart disease among women. JAMA 1999, 281, 1998–2004. [Google Scholar] [CrossRef]
- Jensen, M.K.; Koh-Banerjee, P.; Hu, F.B.; Franz, M.; Sampson, L.; Grønbæk, M.; Rimm, E.B. Intakes of whole grains, bran, and germ and the risk of coronary heart disease in men. Am. J. Clin. Nutr. 2004, 80, 1492–1499. [Google Scholar] [CrossRef]
- Hajishafiee, M.; Saneei, P.; Benisi-Kohansal, S.; Esmaillzadeh, A. Cereal fibre intake and risk of mortality from all causes, CVD, cancer and inflammatory diseases: A systematic review and meta-analysis of prospective cohort studies. Br. J. Nutr. 2016, 116, 343–352. [Google Scholar] [CrossRef]
- Mateo Anson, N.; Aura, A.M.; Selinheimo, E.; Mattila, I.; Poutanen, K.; van den Berg, R.; Havenaar, R.; Bast, A.; Haenen, G.R.M.M. Bioprocessing of wheat bran in whole wheat bread increases the bioavailability of phenolic acids in men and exerts antiinflammatory effects ex vivo. J. Nutr. 2011, 141, 137–143. [Google Scholar] [CrossRef] [PubMed]
- Neacsu, M.; Varga, A.; Socaciu, C.; Van Camp, J. Analysis of antioxidant phytochemicals from Brussels sprout. Commun. Agric. Appl. Biol. Sci. 2004, 69, 257–260. [Google Scholar] [PubMed]
- Kern, S.M.; Bennett, R.N.; Mellon, F.A.; Kroon, P.A.; Garcia-Conesa, M.T. Absorption of hydroxycinnamates in humans after high-bran cereal consumption. J. Agric. Food Chem. 2003, 51, 6050–6055. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Jiménez, J.; Saura-Calixto, F. Literature data may underestimate the actual antioxidant capacity of cereals. J. Agric. Food Chem. 2005, 53, 5036–5040. [Google Scholar] [CrossRef]
- Cara, L.; Dubois, C.; Borel, P.; Armand, M.; Senft, M.; Portugal, H.; Pauli, A.M.; Bernard, P.M.; Lairon, D. Effects of oat bran, rice bran, wheat fiber, and wheat germ on postprandial lipemia in healthy adults. Am. J. Clin. Nutr. 1992, 55, 81–88. [Google Scholar] [CrossRef]
- Dubois, C.; Cara, L.; Borel, P.; Armand, M.; Senft, M.; Portugal, H.; Bernard, P.M.; Lafont, H.; Lairon, D. Cereal dietary fibers affect post-prandial lipoproteins in healthy human subjects. Carbohydr. Polym. 1993, 21, 189–194. [Google Scholar] [CrossRef]
- Lairon, D. Macronutrient intake and modulation on chylomicron production and clearance. Atheroscler. Suppl. 2008, 9, 45–48. [Google Scholar] [CrossRef]
- Bond, B.; Gates, P.E.; Jackman, S.R.; Corless, L.M.; Williams, C.A.; Barker, A.R. Exercise intensity and the protection from postprandial vascular dysfunction in adolescents. Am. J. Physiol. Heart Circ. Physiol. 2015, 308, H1443–H1450. [Google Scholar] [CrossRef]
- Metzig, A.M.; Schwarzenberg, S.J.; Fox, C.K.; Deering, M.M.; Nathan, B.M.; Kelly, A.S. Postprandial endothelial function, inflammation, and oxidative stress in obese children and adolescents. Obesity 2011, 19, 1279–1283. [Google Scholar] [CrossRef]
- Dengel, D.R.; Kelly, A.S.; Steinberger, J.; Sinaiko, A.R. Effect of oral glucose loading on endothelial function in normal-weight and overweight children. Clin. Sci. 2007, 112, 493–498. [Google Scholar] [CrossRef] [Green Version]
- Herrington, D.M.; Fan, L.; Drum, M.; Riley, W.A.; Pusser, B.E.; Crouse, J.R.; Burke, G.L.; McBurnie, M.A.; Morgan, T.M.; Espeland, M.A. Brachial flow-mediated vasodilator responses in population-based research: Methods, reproducibility and effects of age, gender and baseline diameter. J. Cardiovasc. Risk 2001, 8, 319–328. [Google Scholar] [CrossRef] [PubMed]
- Sorensen, K.E.; Celermajer, D.S.; Spiegelhalter, D.J.; Georgakopoulos, D.; Robinson, J.; Thomas, O.; Deanfield, J.E. Non-invasive measurement of human endothelium dependent arterial responses: Accuracy and reproducibility. Br. Heart J. 1995, 74, 247–253. [Google Scholar] [CrossRef] [PubMed]
- Corretti, M.C.; Anderson, T.J.; Benjamin, E.J.; Celermajer, D.; Charbonneau, F.; Creager, M.A.; Deanfield, J.; Drexler, H.; Gerhard-Herman, M.; Herrington, D.; et al. Guidelines for the ultrasound assessment of endothelial-dependent flow-mediated vasodilation of the brachial artery: A report of the International Brachial Artery Reactivity Task Force. J. Am. Coll. Cardiol. 2002, 39, 257–265. [Google Scholar] [CrossRef] [Green Version]
- Hallmark, R.; Patrie, J.T.; Liu, Z.; Gaesser, G.A.; Barrett, E.J.; Weltman, A. The effect of exercise intensity on endothelial function in physically inactive lean and obese adults. PLoS ONE 2014, 9, e85450. [Google Scholar] [CrossRef]
- Mah, E.; Bruno, R.S. Postprandial hyperglycemia on vascular endothelial function: Mechanisms and consequences. Nutr. Res. 2012, 32, 727–740. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Tomé, M.; Murcia, M.A.; Frega, N.; Ruggieri, S.; Jiménez, A.M.; Roses, F.; Parras, P. Evaluation of Antioxidant Capacity of Cereal Brans. J. Agric. Food Chem. 2004, 52, 4690–4699. [Google Scholar] [CrossRef]
- Westphal, S.; Taneva, E.; Kästner, S.; Martens-Lobenhoffer, J.; Bode-Böger, S.; Kropf, S.; Dierkes, J.; Luley, C. Endothelial dysfunction induced by postprandial lipemia is neutralized by addition of proteins to the fatty meal. Atherosclerosis 2006, 185, 313–319. [Google Scholar] [CrossRef] [PubMed]
- Hopping, B.N.; Erber, E.; Grandinetti, A.; Verheus, M.; Kolonel, L.N.; Maskarinec, G. Dietary Fiber, Magnesium, and Glycemic Load Alter Risk of Type 2 Diabetes in a Multiethnic Cohort in Hawaii. J. Nutr. 2010, 140, 68–74. [Google Scholar] [CrossRef]
- Schulze, M.B.; Schulz, M.; Heidemann, C.; Schienkiewitz, A.; Hoffmann, K.; Boeing, H. Fiber and Magnesium Intake and Incidence of Type 2 Diabetes. Arch. Intern. Med. 2007, 167, 956. [Google Scholar] [CrossRef]
- Bajorek, S.A.; Morello, C.M. Effects of Dietary Fiber and Low Glycemic Index Diet on Glucose Control in Subjects with Type 2 Diabetes Mellitus. Ann. Pharmacother. 2010, 44, 1786–1792. [Google Scholar] [CrossRef]
- Weickert, M.O.; Möhlig, M.; Schöfl, C.; Arafat, A.M.; Otto, B.; Viehoff, H.; Koebnick, C.; Kohl, A.; Spranger, J.; Pfeiffer, A.F.H. Cereal fiber improves whole-body insulin sensitivity in overweight and obese women. Diabetes Care 2006, 29, 775–780. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Stote, K.S.; Behall, K.M.; Spears, K.; Vinyard, B.; Conway, J.M. Glucose and insulin responses to whole grain breakfasts varying in soluble fiber, beta-glucan: A dose response study in obese women with increased risk for insulin resistance. Eur. J. Nutr. 2009, 48, 170–175. [Google Scholar] [CrossRef] [PubMed]
- Kranz, S.; Brauchla, M.; Slavin, J.L.; Miller, K.B. What do we know about dietary fiber intake in children and health? The effects of fiber intake on constipation, obesity, and diabetes in children. Adv. Nutr. 2012, 3, 47–53. [Google Scholar] [CrossRef]
- Moreno, L.A.; Tresaco, B.; Bueno, G.; Fleta, J.; Rodríguez, G.; Garagorri, J.M.; Bueno, M. Psyllium fibre and the metabolic control of obese children and adolescents. J. Physiol. Biochem. 2003, 59, 235–242. [Google Scholar] [CrossRef] [PubMed]
- Cheng, G.; Karaolis-Danckert, N.; Libuda, L.; Bolzenius, K.; Remer, T.; Buyken, A.E. Relation of dietary glycemic index, glycemic load, and fiber and whole-grain intakes during puberty to the concurrent development of percent body fat and body mass index. Am. J. Epidemiol. 2009, 169, 667–677. [Google Scholar] [CrossRef]
- Thijssen, D.H.J.; Black, M.A.; Pyke, K.E.; Padilla, J.; Atkinson, G.; Harris, R.A.; Parker, B.; Widlansky, M.E.; Tschakovsky, M.E.; Green, D.J. Assessment of flow-mediated dilation in humans: A methodological and physiological guideline. Am. J. Physiol. Circ. Physiol. 2011, 300, H2–H12. [Google Scholar] [CrossRef]
- Thijssen, D.H.J.; Bullens, L.M.; van Bemmel, M.M.; Dawson, E.A.; Hopkins, N.; Tinken, T.M.; Black, M.A.; Hopman, M.T.E.; Cable, N.T.; Green, D.J. Does arterial shear explain the magnitude of flow-mediated dilation?: A comparison between young and older humans. Am. J. Physiol. Heart Circ. Physiol. 2009, 296, H57–H64. [Google Scholar] [CrossRef]
- Walters, J.F.; Hampton, S.M.; Deanfield, J.E.; Donald, A.E.; Skene, D.J.; Ferns, G.A.A. Circadian variation in endothelial function is attenuated in postmenopausal women. Maturitas 2006, 54, 294–303. [Google Scholar] [CrossRef]
- Etsuda, H.; Takase, B.; Uehata, A.; Kusano, H.; Hamabe, A.; Kuhara, R.; Akima, T.; Matsushima, Y.; Arakawa, K.; Satomura, K.; et al. Morning attenuation of endothelium-dependent, flow-mediated dilation in healthy young men: Possible connection to morning peak of cardiac events? Clin. Cardiol. 1999, 22, 417–421. [Google Scholar] [CrossRef]

| Subject Characteristic | Mean ± SD | Range |
|---|---|---|
| Age (years) | 14.1 ± 2.6 | 10–17 |
| Weight (kg) | 48.6 ± 12.2 | 29.6–65.4 |
| Height (cm) | 163.3 ± 15.4 | 135.3–189.2 |
| Body Mass Index Percentile | 28.3 ± 16.5 | 9–50 |
| % body fat | 16.4 ± 7.2 | 8.1–28.8 |
| Blood glucose (mg/dL) | 90.6 ± 6.5 | 84.3–98.3 |
| Plasma insulin (µU/mL) | 6.2 ± 3.0 | 3.2–9.6 |
| Plasma triglycerides (mg/dL) | 99.6 ± 53.8 | 48.8–221.0 |
| Nutritional Component | Low-Fat Low-Fiber | Low-Fat High-Fiber | High-Fat Low-Fiber | High-Fat High-Fiber |
|---|---|---|---|---|
| Energy (kcal) | 545 | 546 | 550 | 680 |
| Total Fiber (g) | 3.0 | 19.3 | 0.3 | 16.3 |
| Total Carbohydrate (g) a | 95.7 68.9% | 103.7 64.1% | 24.7 17.9% | 61.7 27.7% |
| Total Fat (g) | 11.4 | 12.1 | 34.6 | 36.3 |
| Total Protein (g) | 16.7 | 21.8 | 35.0 | 41.3 |
| Glycemic Index | 61 | 53 | 42 | 48 |
| Meal | Glucose mg/dL (Mean ± SD) | Low-Fat Low-Fiber | Low-Fat High-Fiber | High-Fat Low-Fiber | High-Fat High-Fiber | |
|---|---|---|---|---|---|---|
| Baseline | 1 h | Unadjusted p-Values (Bonferroni-Adjusted p-Values) | ||||
| Low-Fat Low-Fiber | 91.9 ± 10.5 | 98.4 ± 19.3 | - | 0.108 (0.647) | 0.015 (0.090) | 0.199 (1.000) |
| Low-Fat High-Fiber | 91.1 ± 5.3 | 108.4 ± 11.7 * | - | <0.001 (<0.001) | 0.002 (0.009) | |
| High-Fat Low-Fiber | 89.8 ± 5.4 | 79.7 ± 9.6 ** | - | 0.126 (0.756) | ||
| High-Fat High-Fiber | 89.6 ± 4.9 | 87.0 ± 10.3 | - | |||
| Meal | Glucose AUC mg/dL × h (Mean ± SD) | Low-Fat Low-Fiber | Low-Fat High-Fiber | High-Fat Low-Fiber | High-Fat High-Fiber |
|---|---|---|---|---|---|
| Unadjusted p-Values (Bonferroni-Adjusted p-Values) | |||||
| Low-Fat Low-Fiber | 390 ± 36 | - | 0.454 (1.000) | 0.006 (0.035) | 0.045 (0.269) |
| Low-Fat High-Fiber | 400 ± 22 | - | <0.001 (<0.001) | 0.001 (0.003) | |
| High-Fat Low-Fiber | 351 ± 16 | - | 0.110 (0.663) | ||
| High-Fat High-Fiber | 363 ± 17 | - | |||
| Meal | Insulin µU/mL (Mean ± SD) | Low-Fat Low-Fiber | Low-Fat High-Fiber | High-Fat Low-Fiber | High-Fat High-Fiber | |
|---|---|---|---|---|---|---|
| Baseline | 1 h | Unadjusted p-Values (Bonferroni-Adjusted p-Values) | ||||
| Low-Fat Low-Fiber | 5.9 ± 2.3 | 38.6 ± 22.3 * | - | 0.682 (1.000) | 0.048 (0.289) | 0.169 (1.000) |
| Low-Fat High-Fiber | 6.8 ± 3.4 | 45.7 ± 19.5 * | - | 0.019 (0.112) | 0.071 (0.426) | |
| High-Fat Low-Fiber | 6.1 ± 2.7 | 20.4 ± 6.4 * | - | 0.407 (1.000) | ||
| High-Fat High-Fiber | 5.8 ± 3.5 | 24.8 ± 18.0 * | - | |||
| Meal | Insulin AUC µU/mL × h (Mean ± SD) | Low-Fat Low-Fiber | Low-Fat High-Fiber | High-Fat Low-Fiber | High-Fat High-Fiber |
|---|---|---|---|---|---|
| Unadjusted p-Values (Bonferroni-Adjusted p-Values) | |||||
| Low-Fat Low-Fiber | 97 ± 46 | - | 0.318 (1.000) | 0.006 (0.035) | 0.042 (0.251) |
| Low-Fat High-Fiber | 119 ± 47 | - | 0.001 (0.003) | 0.006 (0.035) | |
| High-Fat Low-Fiber | 55 ± 14 | - | 0.680 (1.000) | ||
| High-Fat High-Fiber | 64 ± 35 | - | |||
| Meal | Δ Triglycerides [4 h–Baseline] mg/dL (Mean ± SD) | Low-Fat Low-Fiber | Low-Fat High-Fiber | High-Fat Low-Fiber | High-Fat High-Fiber |
|---|---|---|---|---|---|
| Unadjusted p-Values (Bonferroni-Adjusted p-Values) | |||||
| Low-Fat Low-Fiber | 3 ± 33 | - | 0.515 (1.000) | 0.001 (0.007) | 0.030 (0.181) |
| Low-Fat High-Fiber | 12 ± 30 | - | 0.003 (0.016) | 0.083 (0.497) | |
| High-Fat Low-Fiber | 83 ± 54 * | - | 0.056 (0.336) | ||
| High-Fat High-Fiber | 40 ± 38 ** | - | |||
| Baseline Diameter (mm) | Peak Diameter (mm) | FMD (%) | ||
|---|---|---|---|---|
| Low-Fat, Low-Fiber | 0 h | 3.00 ± 0.34 | 3.19 ± 0.39 | 6.2 ± 2.1 |
| 2 h | 3.10 ± 0.32 | 3.28 ± 0.37 | 6.0 ± 3.5 | |
| 4 h | 3.11 ± 0.37 | 3.32 ± 0.36 | 7.2 ± 4.5 | |
| Low-Fat, High-Fiber | 0 h | 2.93 ± 0.43 | 3.16 ± 0.36 | 8.3 ± 4.7 |
| 2 h | 3.00 ± 0.32 | 3.28 ± 0.37 | 9.7 ± 4.4 | |
| 4 h | 3.07 ± 0.45 | 3.32 ± 0.41 | 8.7 ± 3.6 | |
| High-Fat, Low-Fiber | 0 h | 2.91 ± 0.32 | 3.11 ± 0.30 | 7.2 ± 6.3 |
| 2 h | 3.03 ± 0.34 | 3.28 ± 0.29 | 8.6 ± 4.7 | |
| 4 h | 3.14 ± 0.30 | 3.33 ± 0.35 | 6.1 ± 1.9 | |
| High-Fat, High-Fiber | 0 h | 2.91 ± 0.34 | 3.13 ± 0.31 | 7.9 ± 3.7 |
| 2 h | 3.05 ± 0.37 | 3.28 ± 0.36 | 7.8 ± 4.1 | |
| 4 h | 3.06 ± 0.36 | 3.28 ± 0.34 | 7.3 ± 5.0 |
| Meal | Intercept [95% CI] | Slope [95% CI] | p-Value |
|---|---|---|---|
| Low-Fat, Low-Fiber | 0.924 [−1.675, 3.524] | 0.029 [−0.054, 0.111] | 0.482 |
| Low-Fat, High-Fiber | 1.127 [−1.689, 3.943] | −0.060 [−0.152, 0.032] | 0.191 |
| High-Fat, Low-Fiber | 6.144 [1.204, 11.083] | −0.087 [−0.138, −0.037] | 0.001 |
| High-Fat, High-Fiber | 1.174 [−2.723, 5.071] | −0.044 [−0.117, 0.029] | 0.227 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Whisner, C.M.; Angadi, S.S.; Weltman, N.Y.; Weltman, A.; Rodriguez, J.; Patrie, J.T.; Gaesser, G.A. Effects of Low-Fat and High-Fat Meals, with and without Dietary Fiber, on Postprandial Endothelial Function, Triglyceridemia, and Glycemia in Adolescents. Nutrients 2019, 11, 2626. https://doi.org/10.3390/nu11112626
Whisner CM, Angadi SS, Weltman NY, Weltman A, Rodriguez J, Patrie JT, Gaesser GA. Effects of Low-Fat and High-Fat Meals, with and without Dietary Fiber, on Postprandial Endothelial Function, Triglyceridemia, and Glycemia in Adolescents. Nutrients. 2019; 11(11):2626. https://doi.org/10.3390/nu11112626
Chicago/Turabian StyleWhisner, Corrie M., Siddhartha S. Angadi, Nathan Y. Weltman, Arthur Weltman, Jessica Rodriguez, James T. Patrie, and Glenn A. Gaesser. 2019. "Effects of Low-Fat and High-Fat Meals, with and without Dietary Fiber, on Postprandial Endothelial Function, Triglyceridemia, and Glycemia in Adolescents" Nutrients 11, no. 11: 2626. https://doi.org/10.3390/nu11112626
APA StyleWhisner, C. M., Angadi, S. S., Weltman, N. Y., Weltman, A., Rodriguez, J., Patrie, J. T., & Gaesser, G. A. (2019). Effects of Low-Fat and High-Fat Meals, with and without Dietary Fiber, on Postprandial Endothelial Function, Triglyceridemia, and Glycemia in Adolescents. Nutrients, 11(11), 2626. https://doi.org/10.3390/nu11112626





