SIRT1-Mediated Protective Effect of Aralia elata (Miq.) Seem against High-Glucose-Induced Senescence in Human Umbilical Vein Endothelial Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. EC Culture
2.3. Cell-Viability Assay
2.4. Scratch-Wound Migration Assay
2.5. SA-β-gal Staining
2.6. Cell Cycle Analysis
2.7. Measurement of NO Production
2.8. Western Blot Analysis
2.9. Statistical Analysis
3. Results
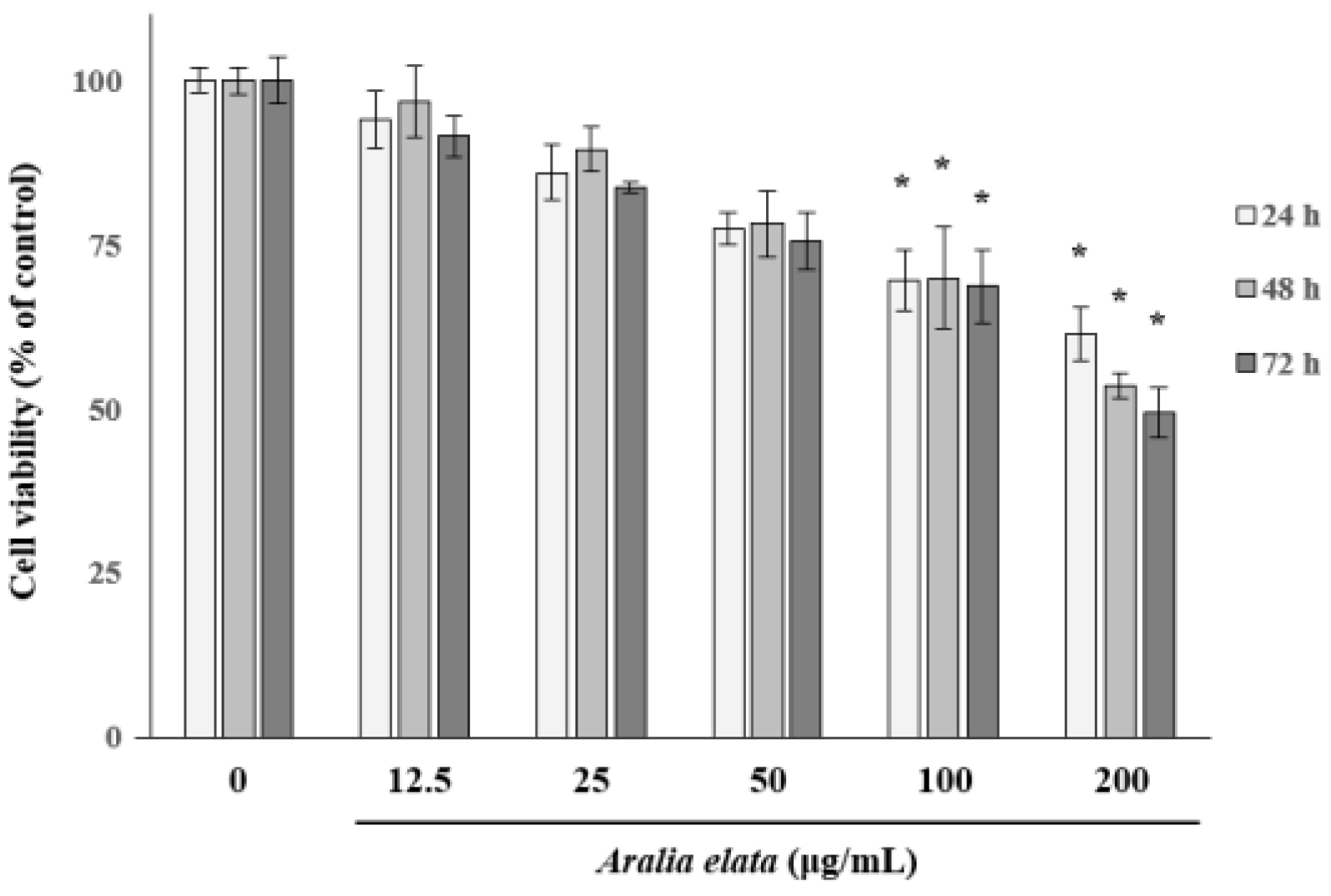
3.1. The Effect of AS on EC Viability
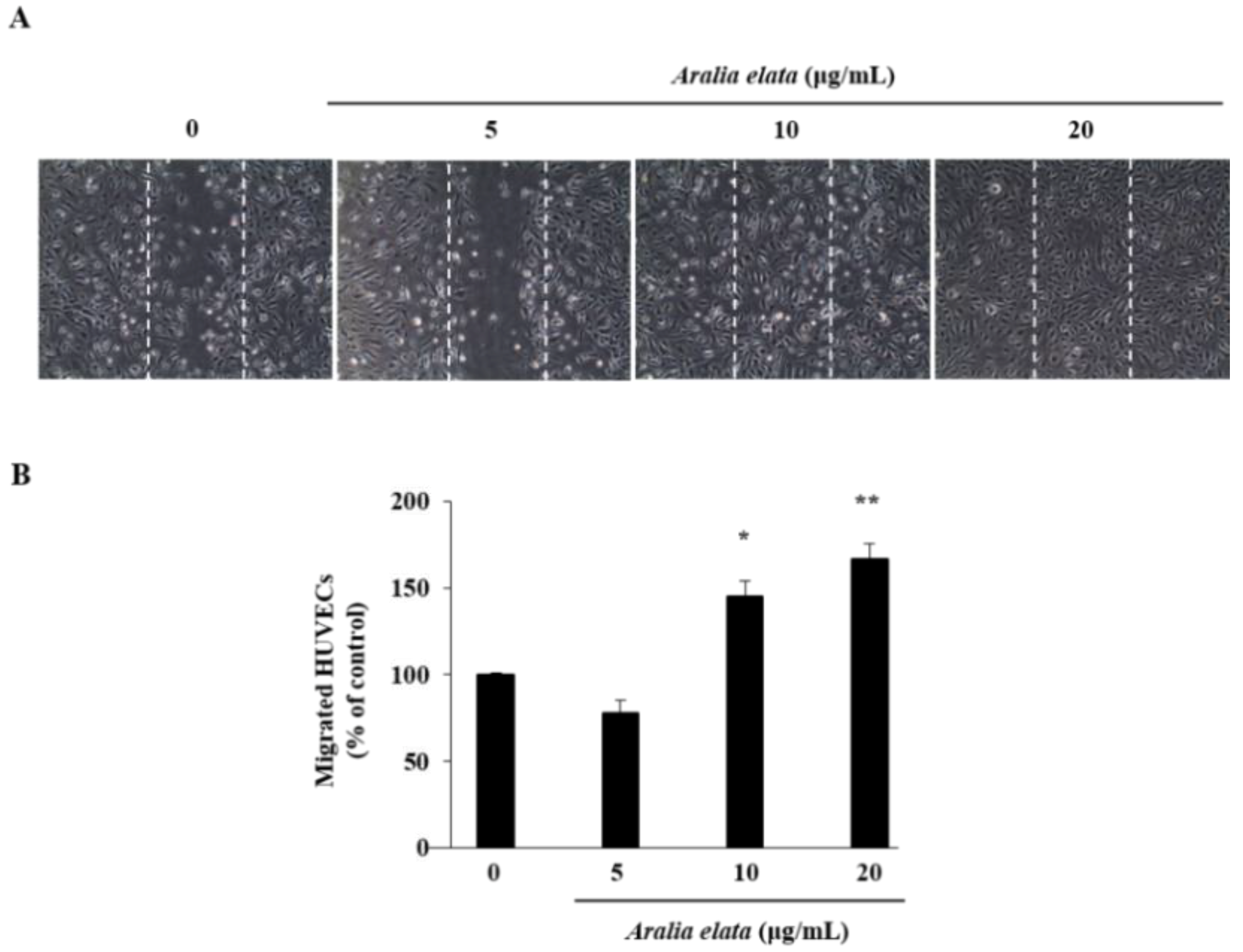
3.2. Activation of EC Migration by AS
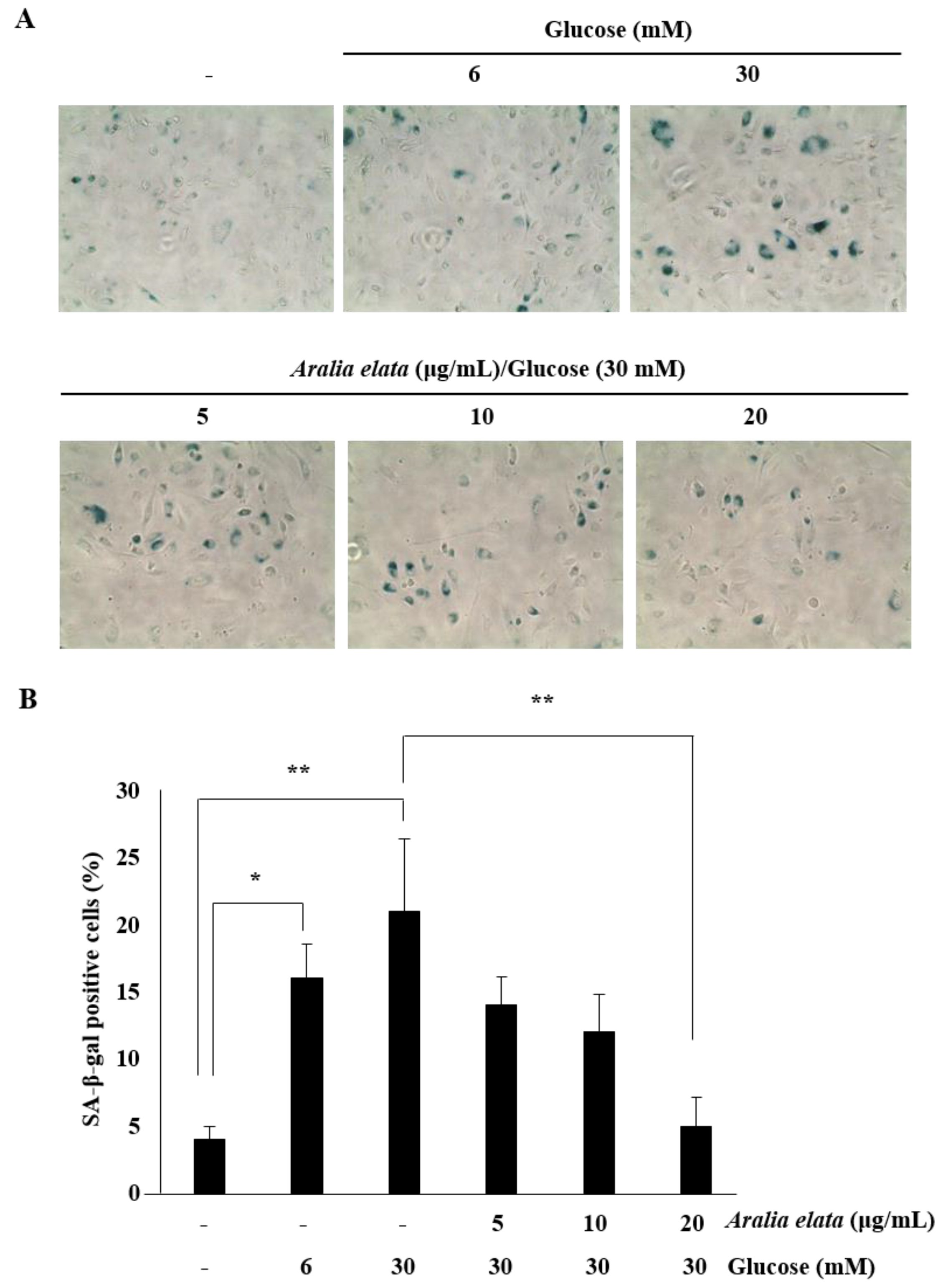
3.3. Inhibition of HG-Induced EC Senescence by AS
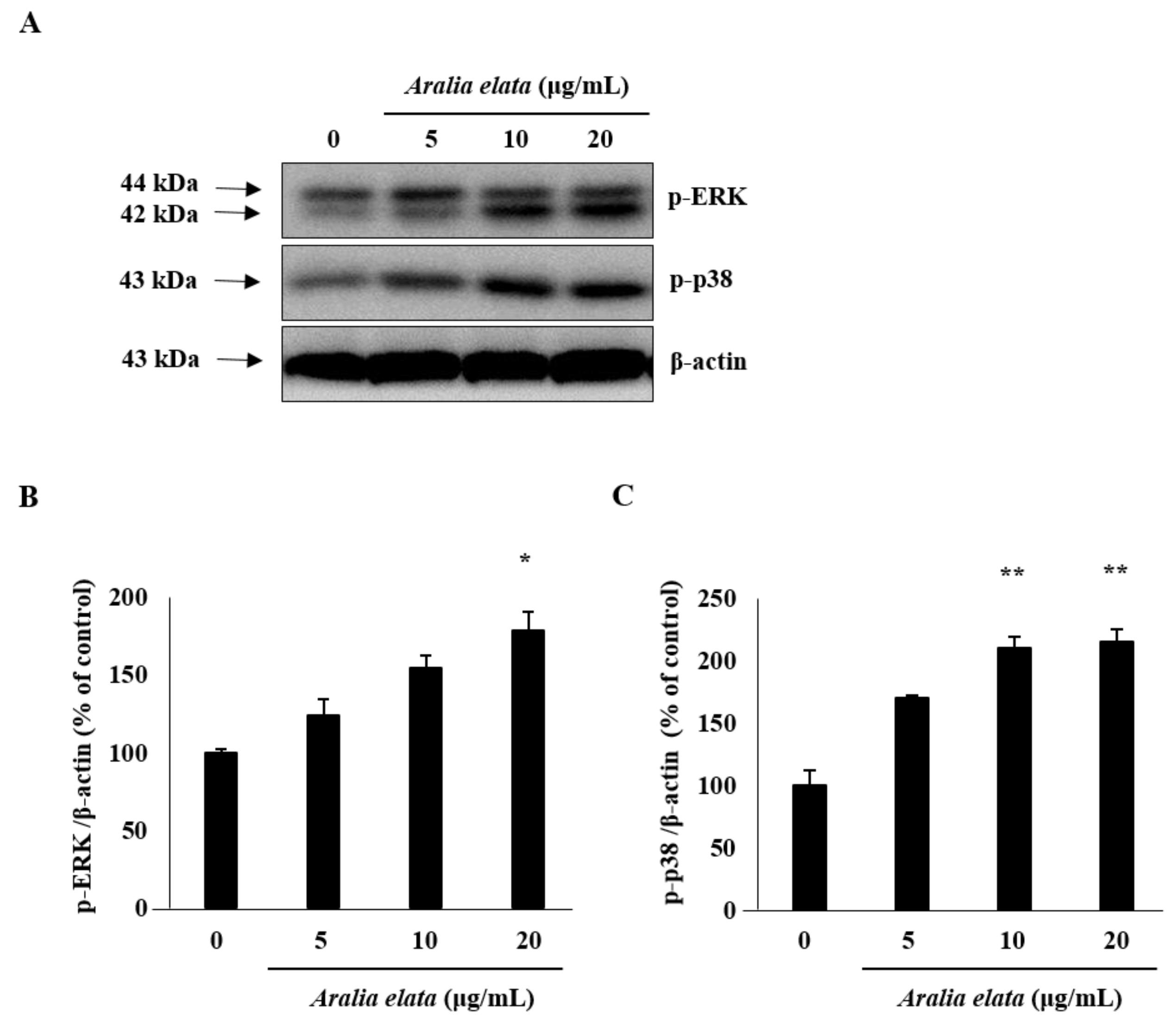
3.4. Activation of SIRT/AMPK and AKT/eNOS Expression by AS
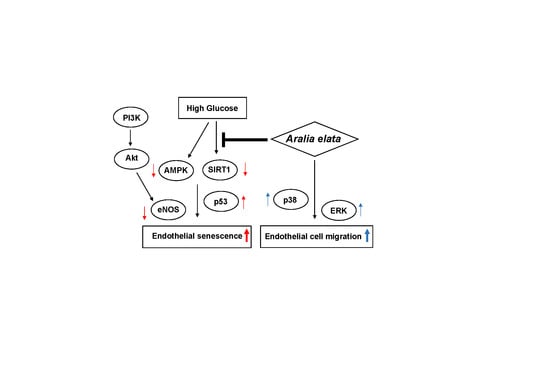
4. Discussion
5. Conclusions
Funding
Acknowledgments
Conflicts of Interest
References
- Regina, C.; Panatta, E.; Candi, E.; Melino, G.; Amelio, I.; Balistreri, C.R.; Annicchiarico-Petruzzelli, M.; Daniele, N.; Ruvolo, G. Vascular ageing and endothelial cell senescence: Molecular mechanisms of physiology and diseases. Mech. Ageing Dev. 2016, 159, 14–21. [Google Scholar] [CrossRef]
- Liu, R.; Liu, H.; Ha, Y.; Tilton, R.G.; Zhang, W. Oxidative stress induces endothelial cell senescence via downregulation of Sirt6. Biomed. Res. Int. 2014, 2014, 902842. [Google Scholar] [CrossRef]
- Tang, Y.; Xu, J.; Qu, W.; Peng, X.; Xin, P.; Yang, X.; Ying, C.; Sun, X.; Hao, L. Resveratrol reduces vascular cell senescence through attenuation of oxidative stress by SIRT1/NADPH oxidase-dependent mechanisms. J. Nutr. Biochem. 2012, 23, 1410–1416. [Google Scholar] [CrossRef] [PubMed]
- El Assar, M.; Angulo, J.; Rodríguez-Mañas, L. Oxidative stress and vascular inflammation in aging. Free Radic. Biol. Med. 2013, 65, 380–401. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Bloom, S.I.; Donato, A.J. The role of senescence, telomere dysfunction and shelterin in vascular aging. Microcirculation. 2019, 26, e12487. [Google Scholar] [CrossRef] [PubMed]
- Rogers, S.C.; Zhang, X.; Azhar, G.; Luo, S.; Wei, J.Y. Exposure to high or low glucose levels accelerates the appearance of markers of endothelial cell senescence and induces dysregulation of nitric oxide synthase. J. Gerontol. A Biol. Sci. Med. Sci. 2013, 68, 1469–1481. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Tian, F.; Wang, J.; Zhou, S.; Dong, X.; Guo, K.; Jing, J.; Zhou, Y.; Chen, Y. Donepezil attenuates high glucose-accelerated senescence in human umbilical vein endothelial cells through SIRT1 activation. Cell Stress Chaperones 2015, 20, 787–792. [Google Scholar] [CrossRef] [PubMed]
- Zhou, P.; Xie, W.; Luo, Y.; Lu, S.; Dai, Z.; Wang, R.; Sun, G.; Sun, X. Protective Effects of Total Saponins of Aralia elata (Miq.) on Endothelial Cell Injury Induced by TNF-α via Modulation of the PI3K/Akt and NF-κB Signalling Pathways. Int. J. Mol. Sci. 2019, 20, 36. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.R.; Du, Y.J.; Chen, L.; Liu, Z.G.; Pan, Y.H.; Liu, J.F.; Liu, B. Quercetin protects against high glucose-induced damage in bone marrow-derived endothelial progenitor cells. Int. J. Mol. Med. 2014, 34, 1025–1031. [Google Scholar] [CrossRef]
- Dimri, G.P.; Lee, X.; Basile, G.; Acosta, M.; Scott, G.; Roskelley, C.; Medrano, E.E.; Linskens, M.; Rubelj, I.; Pereira-Smith, O.; et al. A biomarker that identifies senescent human cells in culture and in aging skin in vivo. Proc. Natl. Acad. Sci. USA 1995, 92, 9363–9367. [Google Scholar] [CrossRef]
- Luo, J.; Nikolaev, A.Y.; Imai, S.; Chen, D.; Su, F.; Shiloh, A.; Guarente, L.; Gu, W. Negative control of p53 by Sir2alpha promotes cell survival under stress. Cell 2001, 107, 137–148. [Google Scholar] [CrossRef]
- Mortuza, R.; Chen, S.; Feng, B.; Sen, S.; Chakrabarti, S. High glucose induced alteration of SIRTs in endothelial cells causes rapid aging in a p300 and FOXO regulated pathway. PLoS ONE 2013, 8, e54514. [Google Scholar] [CrossRef]
- Donato, A.J.; Morgan, R.G.; Walker, A.E.; Lesniewski, L.A. Cellular and molecular biology of aging endothelial cells. J. Mol. Cell. Cardiol. 2015, 89, 122–135. [Google Scholar] [CrossRef] [Green Version]
- Li, C.; Reif, M.M.; Craige, S.M.; Kant, S.; Keaney, J.F. Endothelial AMPK activation induces mitochondrial biogenesis and stress adaptation via eNOS-dependent mTORC1 signaling. Nitric Oxide 2016, 55, 45–53. [Google Scholar] [CrossRef] [PubMed]
- Bai, J.; Wang, Q.; Qi, J.; Yu, H.; Wang, C.; Wang, X.; Ren, Y.; Yang, F. Promoting effect of baicalin on nitric oxide production in CMECs via activating the PI3K-AKT-eNOS pathway attenuates myocardial ischemia-reperfusion injury. Phytomedicine 2019, 63, 153035. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.H.; Chen, Y.; Gao, C.Y.; Cui, Z.T.; Yao, J.M. Protective effects of microRNA-126 on human cardiac microvascular endothelial cells against hypoxia/reoxygenation-induced injury and inflammatory response by activating PI3K/Akt/eNOS signaling pathway. Cell. Physiol. Biochem. 2017, 42, 506–518. [Google Scholar] [CrossRef]
- Nhiem, N.X.; Lim, H.Y.; Kiem, P.V.; Minh, C.V.; Thu, V.K.; Tai, B.H.; Quang, T.H.; Song, S.B.; Kim, Y.H. Oleanane-type triterpene saponins from the bark of Aralia elata and their NF-κB inhibition and PPAR activation signal pathway. Bioorg. Med. Chem. Lett. 2011, 21, 6143–6147. [Google Scholar] [CrossRef]
- Zhang, Y.; Peng, Y.; Li, L.; Zhao, L.; Hu, Y.; Hu, C.; Song, S. Studies on cytotoxic triterpene saponins from the leaves of Aralia elata. Food Chem. 2013, 138, 208–213. [Google Scholar] [CrossRef]
- Wang, M.; Xu, X.; Xu, H.; Wen, F.; Zhang, X.; Sun, H.; Yao, F.; Sun, G.; Sun, X. Effect of the total saponins of Aralia elata (Miq) Seem on cardiac contractile function and intracellular calcium cycling regulation. J. Ethnopharmacol. 2014, 155, 240–247. [Google Scholar] [CrossRef]
- Luo, Y.; Dong, X.; Yu, Y.; Sun, G.; Sun, X. Total aralosides of aralia elata (Miq) seem (TASAES) ameliorate nonalcoholic steatohepatitis by modulating IRE1α-mediated JNK and NF-κB pathways in ApoE-/-mice. J. Ethnopharmacol. 2015, 163, 241–250. [Google Scholar] [CrossRef]
- Luo, Y.; Lu, S.; Ai, Q.; Zhou, P.; Qin, M.; Sun, G.; Sun, X. SIRT1/AMPK and Akt/eNOS signaling pathways are involved in endothelial protection of total aralosides of Aralia elata (Miq) Seem against high-fat diet-induced atherosclerosis in ApoE-/-mice. Phytother. Res. 2019, 33, 768–778. [Google Scholar] [CrossRef] [PubMed]
- Shon, M.S.; Lee, Y.; Song, J.H.; Park, T.; Lee, J.K.; Kim, M.; Park, E.; Kim, G.N. Anti-aging Potential of Extracts Prepared from Fruits and Medicinal Herbs Cultivated in the Gyeongnam Area of Korea. Prev. Nutr. Food Sci. 2014, 19, 178–186. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oh, J.; Kim, G.D.; Kim, S.; Lee, S.K. Antofine, a natural phenanthroindolizidine alkaloid, suppresses angiogenesis via regulation of AKT/mTOR and AMPK pathway in endothelial cells and endothelial progenitor cells derived from mouse embryonic stem cells. Food Chem. Toxicol. 2017, 107, 201–207. [Google Scholar] [CrossRef] [PubMed]
- Kim, G.D.; Kim, G.J.; Seok, J.H.; Chung, H.M.; Chee, K.M.; Rhee, G.S. Differentiation of endothelial cells derived from mouse embryoid bodies: A possible in vitro vasculogenesis model. Toxicol. Lett. 2008, 180, 166–173. [Google Scholar] [CrossRef]
- Kim, G.D. Myricetin inhibits angiogenesis by inducing apoptosis and suppressing PI3K/Akt/mTOR signaling in endothelial cells. J. Cancer Prev. 2017, 22, 219–227. [Google Scholar] [CrossRef]
- Sikora, E.; Bielak-Zmijewska, A.; Mosieniak, G. Cellular senescence in ageing, age-related disease and longevity. Curr. Vasc. Pharmacol. 2014, 12, 698–706. [Google Scholar] [CrossRef]
- Yuan, Q.; Peng, J.; Liu, S.Y.; Wang, C.J.; Xiang, D.X.; Xiong, X.M.; Hu, C.P.; Li, Y.J. Inhibitory effect of resveratrol derivative BTM-0512 on high glucose-induced cell senescence involves dimethylaminohydrolase/asymmetric dimethylarginine pathway. Clin. Exp. Pharmacol. Physiol. 2010, 37, 630–635. [Google Scholar] [CrossRef]
- Ben-Porath, I.; Weinberg, R.A. The signals and pathways activating cellular senescence. Int. J. Biochem. Cell Biol. 2005, 37, 961–976. [Google Scholar] [CrossRef]
- Wen, Y.Y.; Yang, Z.Q.; Song, M.; Li, B.L.; Zhu, J.J.; Wang, E.H. SIAH1 induced apoptosis by activation of the JNK pathway and inhibited invasion by inactivation of the ERK pathway in breast cancer cells. Cancer Sci. 2010, 101, 73–79. [Google Scholar] [CrossRef]
- Du, M.R.; Zhou, W.H.; Yan, F.T.; Zhu, X.Y.; He, Y.Y.; Yang, J.Y.; Li, D.J. Cyclosporine A induces titin expression via MAPK/ERK signaling and improves proliferative and invasive potential of human trophoblast cells. Hum. Reprod. 2007, 22, 2528–2537. [Google Scholar] [CrossRef]
- Ota, H.; Akishita, M.; Eto, M.; Iijima, K.; Kaneki, M.; Ouchi, Y. Sirt1 modulates premature senescence-like phenotype in human endothelial cells. J. Mol. Cell. Cardiol. 2007, 43, 571–579. [Google Scholar] [CrossRef]
- Kitada, M.; Ogura, Y.; Koya, D. The protective role of Sirt1 in vascular tissue: Its relationship to vascular aging and atherosclerosis. Aging 2016, 8, 2290–2307. [Google Scholar] [CrossRef]
- Servillo, L.; D’Onofrio, N.; Longobardi, L.; Sirangelo, I.; Giovane, A.; Cautela, D.; Castaldo, D.; Giordano, A.; Balestrieri, M.L. Stachydrine ameliorates high-glucose induced endothelial cell senescence and SIRT1 downregulation. J. Cell. Biochem. 2013, 114, 2522–2530. [Google Scholar] [CrossRef]
- Xu, R.Y.; Xu, X.W.; Deng, Y.Z.; Ma, Z.X.; Li, X.R.; Zhao, L.; Qiu, L.J.; Liu, H.Y.; Chen, H.P. Resveratrol attenuates myocardial hypoxia/reoxygenation-induced cell apoptosis through DJ-1-mediated SIRT1-p53 pathway. Biochem. Biophys. Res. Commun. 2019, 514, 401–406. [Google Scholar] [CrossRef]
- Wang, S.; Miao, J.; Qu, M.; Yang, G.Y.; Shen, L. Adiponectin modulates the function of endothelial progenitor cells via AMPK/eNOS signaling pathway. Biochem. Biophys. Res. Commun. 2017, 493, 64–70. [Google Scholar] [CrossRef]
- Ou, H.C.; Chou, W.C.; Chu, P.M.; Hsieh, P.L.; Hung, C.H.; Tsai, K.L. Fucoxanthin Protects against oxLDL-Induced Endothelial Damage via Activating the AMPK-Akt-CREB-PGC1α Pathway. Mol. Nutr. Food Res. 2019, 63, e1801353. [Google Scholar] [CrossRef]
- Park, H.S.; Lim, J.H.; Kim, M.Y.; Kim, Y.; Hong, Y.A.; Choi, S.R.; Chung, S.; Kim, H.W.; Choi, B.S.; Kim, Y.S.; et al. Resveratrol increases AdipoR1 and AdipoR2 expression in type 2 diabetic nephropathy. J. Transl. Med. 2016, 14, 176. [Google Scholar] [CrossRef]
- Wang, W.; Shang, C.; Zhang, W.; Jin, Z.; Yao, F.; He, Y.; Wang, B.; Li, Y.; Zhang, J.; Lin, R. Hydroxytyrosol NO regulates oxidative stress and NO production through SIRT1 in diabetic mice and vascular endothelial cells. Phytomedicine 2019, 52, 206–215. [Google Scholar] [CrossRef]
- Osaki, M.; Oshimura, M.; Ito, H. PI3K-Akt pathway: Its functions and alterations in human cancer. Apoptosis 2004, 9, 667–676. [Google Scholar] [CrossRef]
- Mineo, C.; Shaul, P.W. Regulation of eNOS in caveolae. Adv. Exp. Med. Biol. 2012, 729, 51–62. [Google Scholar]


© 2019 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, G.D. SIRT1-Mediated Protective Effect of Aralia elata (Miq.) Seem against High-Glucose-Induced Senescence in Human Umbilical Vein Endothelial Cells. Nutrients 2019, 11, 2625. https://doi.org/10.3390/nu11112625
Kim GD. SIRT1-Mediated Protective Effect of Aralia elata (Miq.) Seem against High-Glucose-Induced Senescence in Human Umbilical Vein Endothelial Cells. Nutrients. 2019; 11(11):2625. https://doi.org/10.3390/nu11112625
Chicago/Turabian StyleKim, Gi Dae. 2019. "SIRT1-Mediated Protective Effect of Aralia elata (Miq.) Seem against High-Glucose-Induced Senescence in Human Umbilical Vein Endothelial Cells" Nutrients 11, no. 11: 2625. https://doi.org/10.3390/nu11112625
APA StyleKim, G. D. (2019). SIRT1-Mediated Protective Effect of Aralia elata (Miq.) Seem against High-Glucose-Induced Senescence in Human Umbilical Vein Endothelial Cells. Nutrients, 11(11), 2625. https://doi.org/10.3390/nu11112625