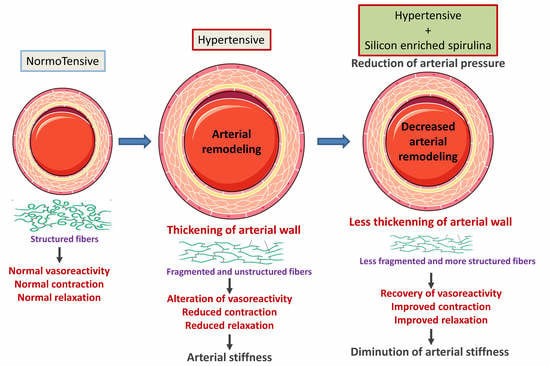
Dietary Supplementation with Silicon-Enriched Spirulina Improves Arterial Remodeling and Function in Hypertensive Rats
Abstract
:1. Introduction
2. Materials and Methods
2.1. Spirulina and Tablet Supplements
2.2. Animals and Experimental Design
2.3. Arterial Pressure
2.4. Sampling and Plasma Assay
2.5. Echocardiography
2.6. Histological Staining and Morphometric Analysis
2.7. Quantitative PCR
2.8. Vascular Reactivity
2.9. Statistical Analysis
3. Results
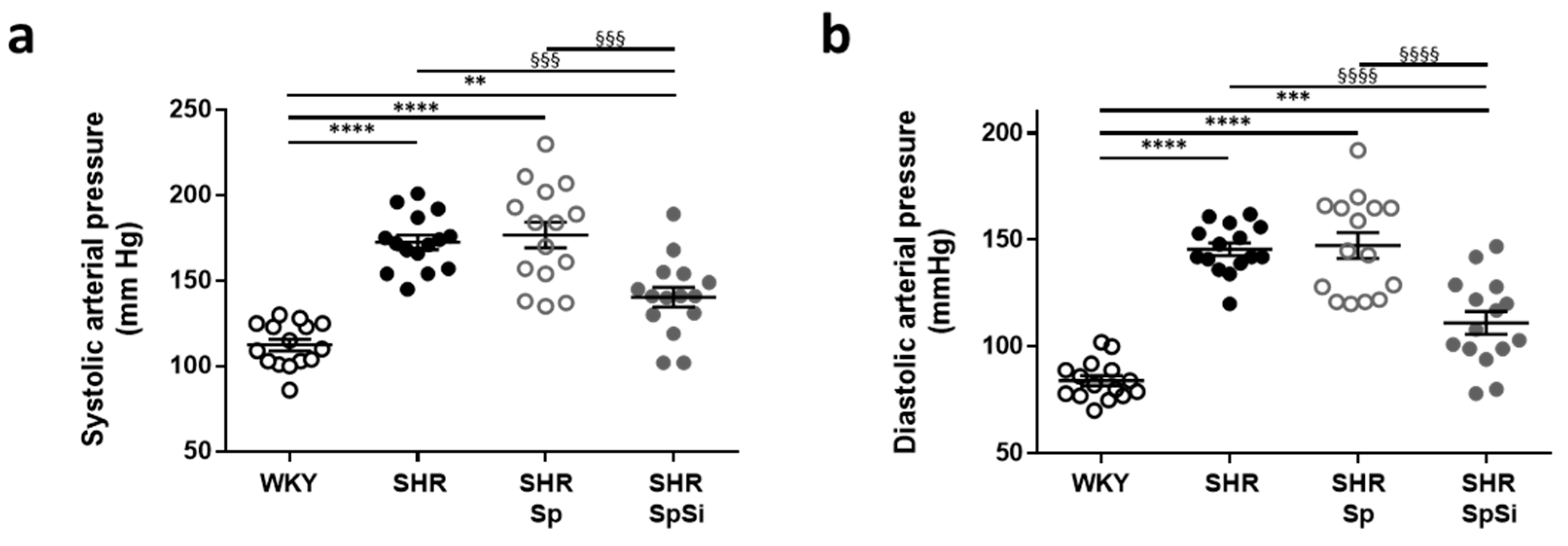
3.1. Effect of Supplementation on Blood Pressure
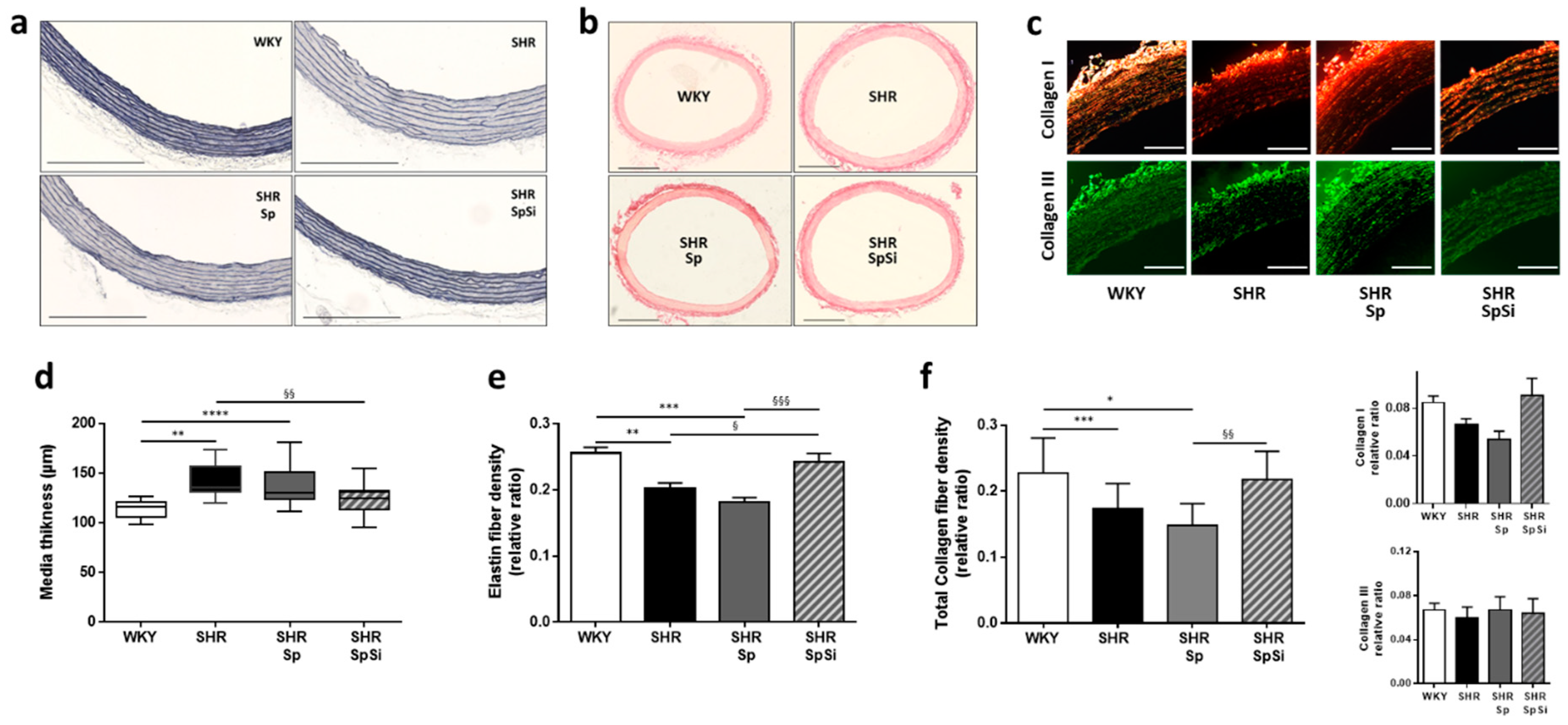
3.2. Morphometric and Ultrastructural Analysis of the Vascular Wall
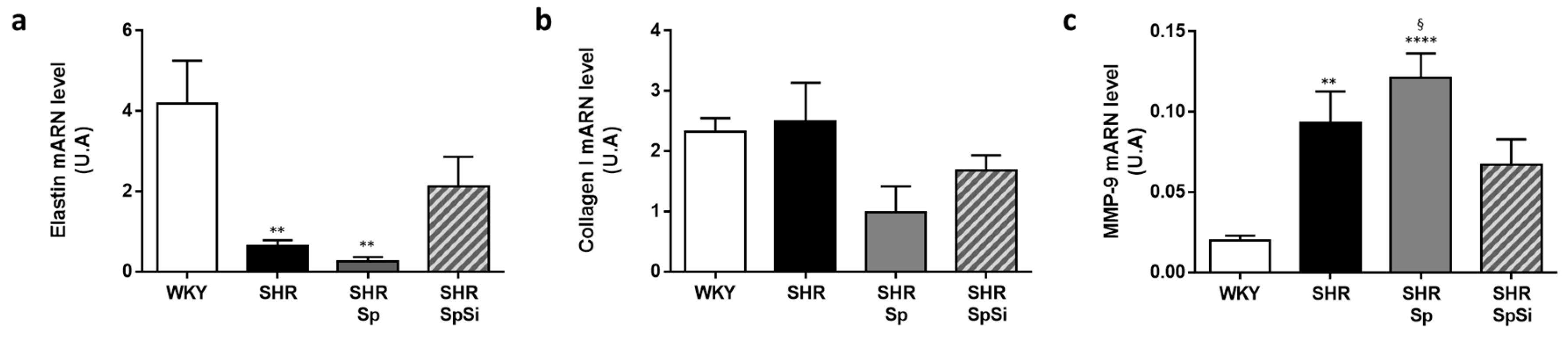
3.3. Analysis of Extracellular Matrix Components
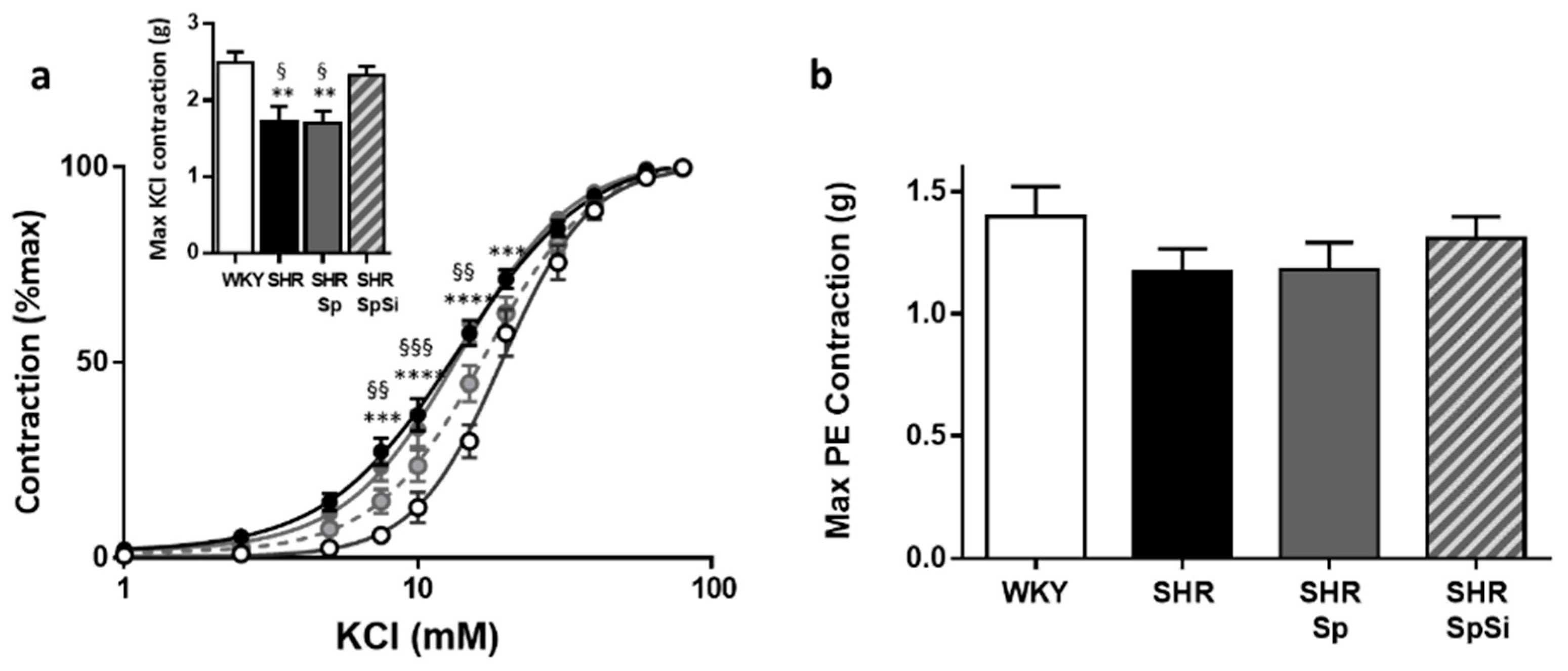
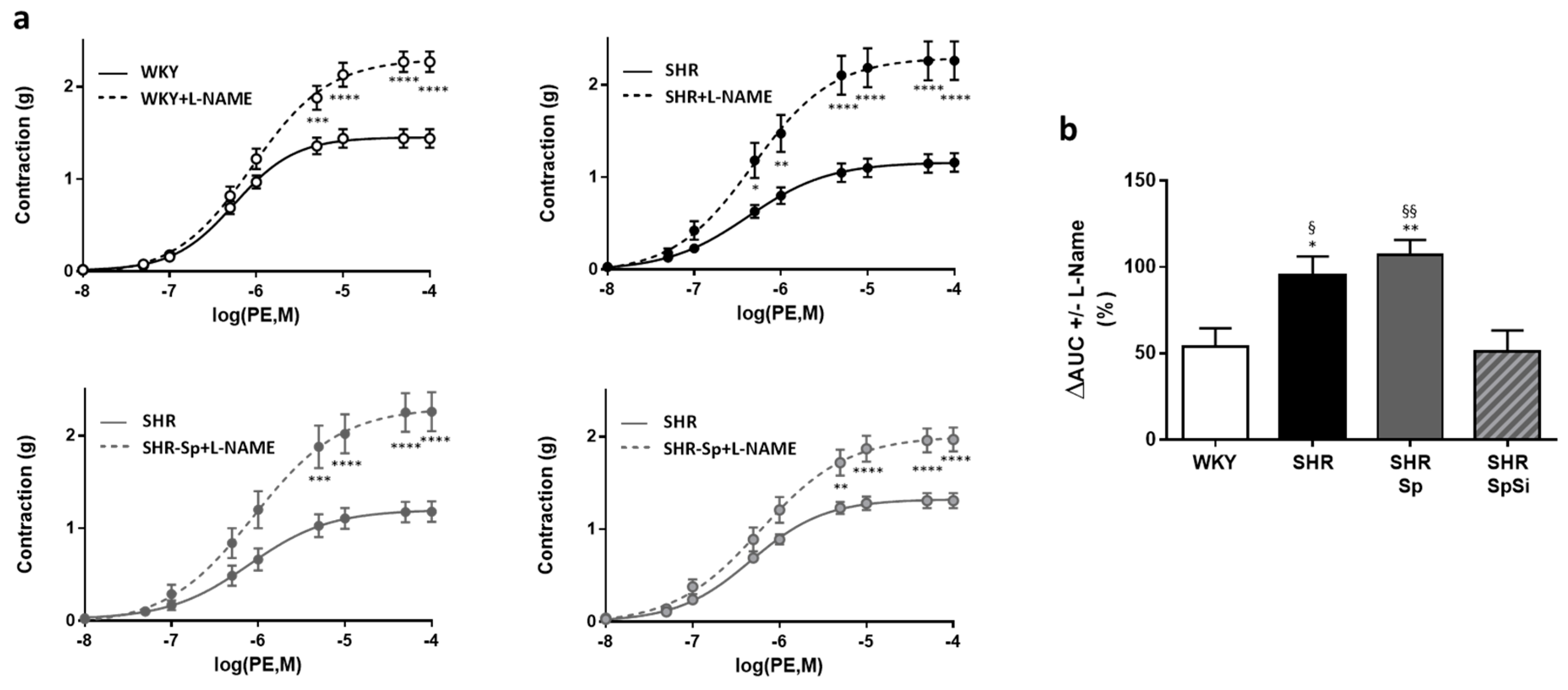
3.4. Evaluation of Vascular Contractile Properties of Aorta
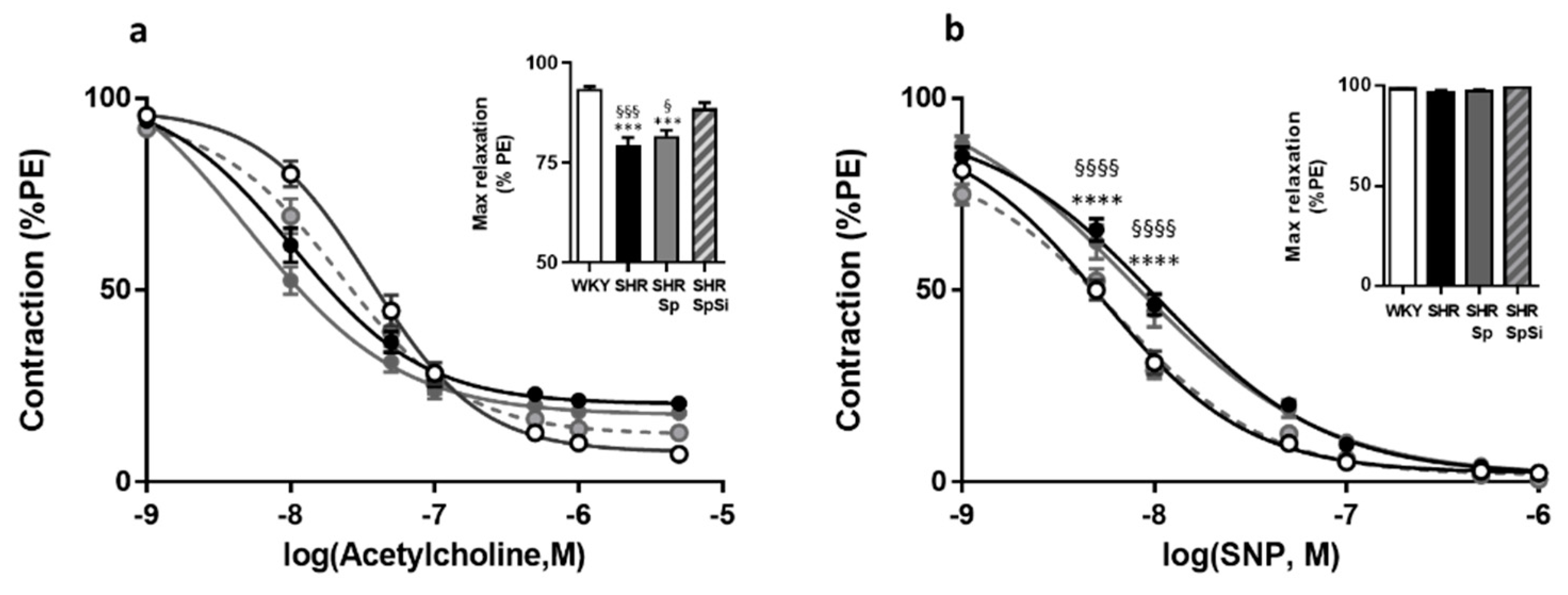
3.5. Evaluation of Vasorelaxant Capacity of the Aorta
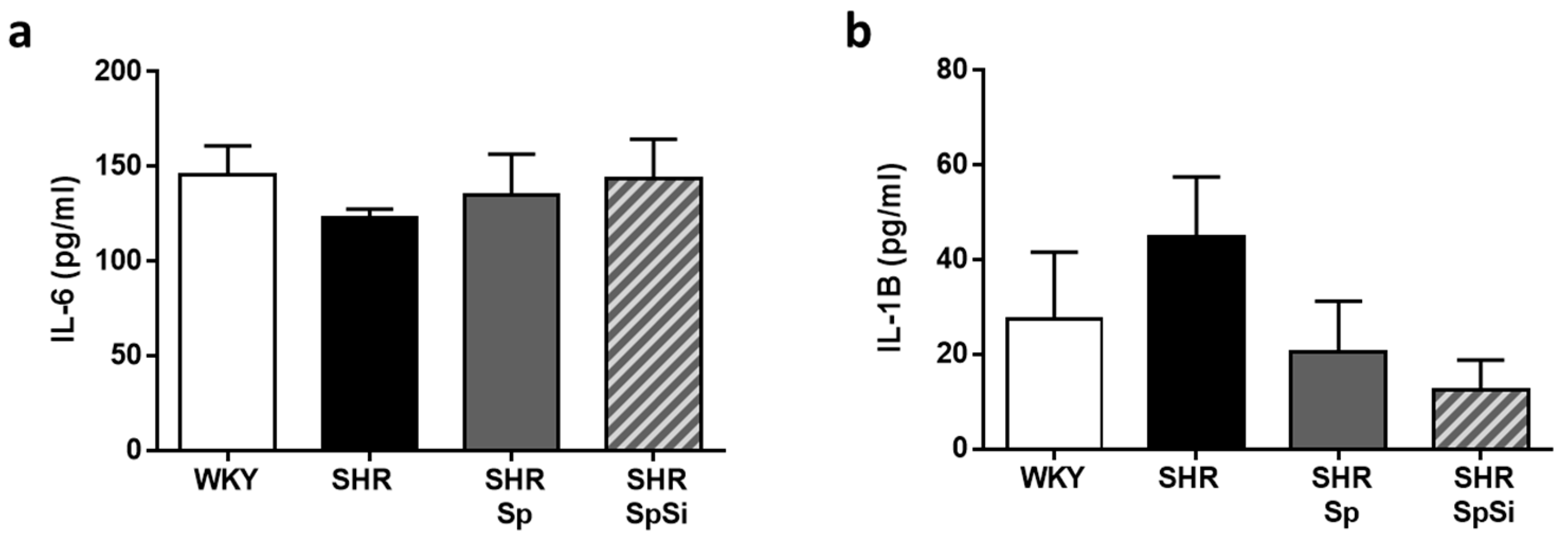
3.6. Analysis of IL-6 and IL-1B Inflammation Markers
3.7. Effect of Supplementation on Cardiac Morphology and Function
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Schwarz, K. Silicon, Fibre, and Atherosclerosis. Lancet 1977, 309, 454–457. [Google Scholar] [CrossRef]
- Nielsen, F.H. Update on the possible nutritional importance of silicon. J. Trace Elem. Med. Biol. 2014, 28, 379–382. [Google Scholar] [CrossRef] [PubMed]
- Carlisle, E.M. Silicon as a trace nutrient. Sci. Total Environ. 1988, 73, 95–106. [Google Scholar] [CrossRef]
- Jugdaohsingh, R.; Watson, A.I.E.; Pedro, L.D.; Powell, J.J. The decrease in silicon concentration of the connective tissues with age in rats is a marker of connective tissue turnover. Bone 2015, 75, 40–48. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Loeper, J.; Loeper, J.; Fragny, M. The Physiological Role of the Silicon and its AntiAtheromatous Action. In Biochemistry of Silicon and Related Problems; Bendz, G., Lindqvist, I., Runnström-Reio, V., Eds.; Nobel Foundation Symposia; Springer: Boston, MA, USA, 1978; pp. 281–296. [Google Scholar]
- Charnot, Y.; Peres, G. Change in the absorption and tissue metabolism of silicon in relation to age, sex and various endocrine glands. Lyon Med. 1971, 226, 85–88. [Google Scholar] [PubMed]
- Bissé, E.; Epting, T.; Beil, A.; Lindinger, G.; Lang, H.; Wieland, H. Reference values for serum silicon in adults. Anal. Biochem. 2005, 337, 130–135. [Google Scholar] [CrossRef]
- Kr, M. Dietary Silicon: Is Biofortification Essential? J. Nutr. Food Sci. Forecast 2018, 1, 1006. [Google Scholar]
- McCarty, M.F. Reported antiatherosclerotic activity of silicon may reflect increased endothelial synthesis of heparan sulfate proteoglycans. Med. Hypotheses 1997, 49, 175–176. [Google Scholar] [CrossRef]
- Carlisle, E.M. The nutritional essentiality of silicon. Nutr. Rev. 1982, 40, 193–198. [Google Scholar] [CrossRef]
- Schwarz, K.; Ricci, B.A.; Punsar, S.; Karvonen, M.J. Inverse relation of silicon in drinking water and atherosclerosis in Finland. Lancet Lond. Engl. 1977, 1, 538–539. [Google Scholar] [CrossRef]
- Jugdaohsingh, R.; Tucker, K.L.; Qiao, N.; Cupples, L.A.; Kiel, D.P.; Powell, J.J. Dietary silicon intake is positively associated with bone mineral density in men and premenopausal women of the Framingham Offspring cohort. J. Bone Miner. Res. 2004, 19, 297–307. [Google Scholar] [CrossRef] [PubMed]
- Pennington, J.A. Silicon in foods and diets. Food Addit. Contam. 1991, 8, 97–118. [Google Scholar] [CrossRef] [PubMed]
- Jugdaohsingh, R. Silicon and bone health. J. Nutr. Health Aging 2007, 11, 99–110. [Google Scholar] [PubMed]
- Jurkić, L.M.; Cepanec, I.; Pavelić, S.K.; Pavelić, K. Biological and therapeutic effects of ortho-silicic acid and some ortho-silicic acid-releasing compounds: New perspectives for therapy. Nutr. Metab. 2013, 10, 2. [Google Scholar] [CrossRef] [PubMed]
- Mazo, V.K.; Gmoshinski, I.V.; Zorin, S.N. New food sources of essential trace elements produced by biotechnology facilities. Biotechnol. J. 2007, 2, 1297–1305. [Google Scholar] [CrossRef]
- Borowitzka, M.A. Spirulina in human nutrition and health. J. Appl. Phycol. 2009, 21, 747. [Google Scholar] [CrossRef]
- Deng, R.; Chow, T.-J. Hypolipidemic, antioxidant, and antiinflammatory activities of microalgae Spirulina. Cardiovasc. Ther. 2010, 28, e33–e45. [Google Scholar] [CrossRef]
- Vidé, J.; Virsolvy, A.; Romain, C.; Ramos, J.; Jouy, N.; Richard, S.; Cristol, J.-P.; Gaillet, S.; Rouanet, J.-M. Dietary silicon-enriched spirulina improves early atherosclerosis markers in hamsters on a high-fat diet. Nutrition 2015, 31, 1148–1154. [Google Scholar] [CrossRef]
- Stehouwer, C.D.A.; Henry, R.M.A.; Ferreira, I. Arterial stiffness in diabetes and the metabolic syndrome: A pathway to cardiovascular disease. Diabetologia 2008, 51, 527. [Google Scholar] [CrossRef]
- Mulvany, M.J.; Baumbach, G.L.; Aalkjaer, C.; Heagerty, A.M.; Korsgaard, N.; Schiffrin, E.L.; Heistad, D.D. Vascular remodeling. Hypertension 1996, 28, 505–506. [Google Scholar]
- Tsamis, A.; Krawiec, J.T.; Vorp, D.A. Elastin and collagen fibre microstructure of the human aorta in ageing and disease: A review. J. R. Soc. Interface 2013, 10. [Google Scholar] [CrossRef] [PubMed]
- Vidé, J.; Bonafos, B.; Fouret, G.; Benlebna, M.; Poupon, J.; Jover, B.; Casas, F.; Jouy, N.; Feillet-Coudray, C.; Gaillet, S.; et al. Spirulina platensis and silicon-enriched spirulina equally improve glucose tolerance and decrease the enzymatic activity of hepatic NADPH oxidase in obesogenic diet-fed rats. Food Funct. 2018, 9, 6165–6178. [Google Scholar] [CrossRef] [PubMed]
- Rouhana, S.; Farah, C.; Roy, J.; Finan, A.; de Araujo, G.R.; Bideaux, P.; Scheuermann, V.; Saliba, Y.; Reboul, C.; Cazorla, O.; et al. Early calcium handling imbalance in pressure overload-induced heart failure with nearly normal left ventricular ejection fraction. Biochim. Biophys. Acta Mol. Basis Dis. 2019, 1865, 230–242. [Google Scholar] [CrossRef] [PubMed]
- Arganda-Carreras, I.; Fernández-González, R.; Muñoz-Barrutia, A.; Ortiz-De-Solorzano, C. 3D reconstruction of histological sections: Application to mammary gland tissue. Microsc. Res. Tech. 2010, 73, 1019–1029. [Google Scholar] [CrossRef] [PubMed]
- Duansak, N.; Schmid-Schönbein, G.W. The oxygen free radicals control MMP-9 and transcription factors expression in the spontaneously hypertensive rat. Microvasc. Res. 2013, 90, 154–161. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sprague, A.H.; Khalil, R.A. Inflammatory cytokines in vascular dysfunction and vascular disease. Biochem. Pharmacol. 2009, 78, 539–552. [Google Scholar] [CrossRef] [Green Version]
- Vidé, J.; Romain, C.; Feillet-Coudray, C.; Bonafos, B.; Cristol, J.P.; Fouret, G.; Rouanet, J.-M.; Gaillet, S. Assessment of potential toxicological aspects of dietary exposure to silicon-rich spirulina in rats. Food Chem. Toxicol. 2015, 80, 108–113. [Google Scholar] [CrossRef]
- Seravalle, G.; Grassi, G. Obesity and hypertension. Pharmacol. Res. 2017, 122, 1–7. [Google Scholar] [CrossRef]
- Harvey, A.; Montezano, A.C.; Lopes, R.A.; Rios, F.; Touyz, R.M. Vascular Fibrosis in Aging and Hypertension: Molecular Mechanisms and Clinical Implications. Can. J. Cardiol. 2016, 32, 659–668. [Google Scholar] [CrossRef] [Green Version]
- Van Gorp, A.W.; Schenau, D.S.; Hoeks, A.P.; Boudier, H.A.; de Mey, J.G.; Reneman, R.S. In spontaneously hypertensive rats alterations in aortic wall properties precede development of hypertension. Am. J. Physiol. Heart Circ. Physiol. 2000, 278, H1241–H1247. [Google Scholar] [CrossRef]
- Sehgel, N.L.; Sun, Z.; Hong, Z.; Hunter, W.C.; Hill, M.A.; Vatner, D.E.; Vatner, S.F.; Meininger, G.A. Augmented vascular smooth muscle cell stiffness and adhesion when hypertension is superimposed on aging. Hypertension 2015, 65, 370–377. [Google Scholar] [CrossRef] [PubMed]
- Martin, K.R. The chemistry of silica and its potential health benefits. J. Nutr. Health Aging 2007, 11, 94–97. [Google Scholar] [PubMed]
- Castro, M.M.; Rizzi, E.; Prado, C.M.; Rossi, M.A.; Tanus-Santos, J.E.; Gerlach, R.F. Imbalance between matrix metalloproteinases and tissue inhibitor of metalloproteinases in hypertensive vascular remodeling. Matrix Biol. J. Int. Soc. Matrix Biol. 2010, 29, 194–201. [Google Scholar] [CrossRef] [PubMed]
- Loeper, J.; Goy-Loeper, J.; Rozensztajn, L.; Fragny, M. The antiatheromatous action of silicon. Atherosclerosis 1979, 33, 397–408. [Google Scholar] [CrossRef]
- Gendre, P. Alterations of ultrastructure of the aortic wall of the rabbit after atherogenic treatment: Preventive action of a silicium derivative. C. R. Seances Soc. Biol. Fil. 1967, 161, 2177–2180. [Google Scholar]
- Maehira, F.; Motomura, K.; Ishimine, N.; Miyagi, I.; Eguchi, Y.; Teruya, S. Soluble silica and coral sand suppress high blood pressure and improve the related aortic gene expressions in spontaneously hypertensive rats. Nutr. Res. 2011, 31, 147–156. [Google Scholar] [CrossRef]
- Harvey, A.; Montezano, A.C.; Touyz, R.M. Vascular biology of ageing-Implications in hypertension. J. Mol. Cell. Cardiol. 2015, 83, 112–121. [Google Scholar] [CrossRef]
- Cox, R.H. Basis for the altered arterial wall mechanics in the spontaneously hypertensive rat. Hypertension 1981, 3, 485–495. [Google Scholar] [CrossRef]
- Mizutani, K.; Ikeda, K.; Kawai, Y.; Yamori, Y. Biomechanical properties and chemical composition of the aorta in genetic hypertensive rats. J. Hypertens. 1999, 17, 481–487. [Google Scholar] [CrossRef]
| WKY | SHR | SHR-Sp | SHR-SpSi | |
|---|---|---|---|---|
| Body weight (g) | 473.8 ± 6.8 | 441.1 ± 6.2 **, § | 464.6 ± 5.8 | 475.1 ± 10.3 |
| Body weight gain (g) | 137.2 ± 4.6 | 104.6 ± 6.2 * | 121.3 ± 8.4 | 113.2 ± 5.6 |
| Mean arterial pressure (mmHg) | 95.2 ± 2.5 | 157.1 ± 3.2 ****, §§§§ | 160.2 ±6.6 ****, §§§§ | 123.2 ±5.5 *** |
| Cardiac Frequency (bpm) | 348 ± 7 | 329 ± 7 | 347 ± 5 | 339 ± 7 |
| Ejection Fraction (%) | 68.8 ± 2.2 | 60.3 ± 1.6 ** | 60.8 ± 1.2 ** | 62.1 ±1.5 * |
| Left ventricular mass (mg) | 851 ± 45 | 1010 ± 43 * | 1041 ± 39 | 972 ± 43 |
| Post wall thickness, diastole (mm) | 1.65 ± 0.06 | 2.03 ± 0.07 *** | 2.19 ± 0.04 *** | 2.02 ± 0.06 *** |
| Post wall thickness, systole (mm) | 2.84 ± 0.11 | 3.17 ± 0.12 | 3.17 ± 0.07 | 3.13 ± 0.08 |
| KCl | PE | L-NAME/PE | Ach | SNP | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Emax (g) | EC50 (mM) | Emax (g) | EC50 (nM) | Emax (g) | EC50 (nM) | Emax (%PE) | EC50 (µM) | E10-8(%PE) | EC50 (nM) | |
| WHY | 2.49 ± 0.14 | 19.9 ± 1.4 | 1.4 ± 0.12 | 605 ± 81 | 2.27 ± 0.11 | 1790 ± 420 | 93.5 ± 0.6 | 36.1 ± 5.7 | 98.6 ± 0.3 | 5.7 ± 0.7 |
| SHR | 1.73 ± 0.19 **, § | 14.1 ± 0.6 **, § | 1.17 ± 0.1 | 550 ± 114 | 2.26 ± 0.21 | 1736 ± 677 | 79.5 ± 1.7 ****, §§§§ | 10.4 ± 2.1 | 97.1 ± 0.9 | 10.8 ± 1.5 ***, § |
| SHR-Sp | 1.69 ± 0.16 **, § | 13.9 ± 0.9 **, § | 1.18 ± 0.11 | 566 ± 89 | 2.26 ± 0.21 | 1827 ± 784 | 81.6 ± 1.5 ****,§ | 5.7 ± 2.5 | 97.3 ± 0.9 | 12.5 ± 0.3 ****, §§§ |
| SHR-SpSi | 2.33 ± 0.11 | 18.4 ± 1.04 | 1.31 ± 0.09 | 532 ± 62 | 1.97 ± 0.13 | 1340 ± 336 | 88.6 ± 1.4 | 22.1 ± 4.5 | 99.3 ± 0.3 | 6.9 ± 0.6 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arthur-Ataam, J.; Bideaux, P.; Charrabi, A.; Sicard, P.; Fromy, B.; Liu, K.; Eddahibi, S.; Pasqualin, C.; Jouy, N.; Richard, S.; et al. Dietary Supplementation with Silicon-Enriched Spirulina Improves Arterial Remodeling and Function in Hypertensive Rats. Nutrients 2019, 11, 2574. https://doi.org/10.3390/nu11112574
Arthur-Ataam J, Bideaux P, Charrabi A, Sicard P, Fromy B, Liu K, Eddahibi S, Pasqualin C, Jouy N, Richard S, et al. Dietary Supplementation with Silicon-Enriched Spirulina Improves Arterial Remodeling and Function in Hypertensive Rats. Nutrients. 2019; 11(11):2574. https://doi.org/10.3390/nu11112574
Chicago/Turabian StyleArthur-Ataam, Joanna, Patrice Bideaux, Azzouz Charrabi, Pierre Sicard, Bérengère Fromy, Kiaoling Liu, Saadia Eddahibi, Côme Pasqualin, Nicolas Jouy, Sylvain Richard, and et al. 2019. "Dietary Supplementation with Silicon-Enriched Spirulina Improves Arterial Remodeling and Function in Hypertensive Rats" Nutrients 11, no. 11: 2574. https://doi.org/10.3390/nu11112574
APA StyleArthur-Ataam, J., Bideaux, P., Charrabi, A., Sicard, P., Fromy, B., Liu, K., Eddahibi, S., Pasqualin, C., Jouy, N., Richard, S., & Virsolvy, A. (2019). Dietary Supplementation with Silicon-Enriched Spirulina Improves Arterial Remodeling and Function in Hypertensive Rats. Nutrients, 11(11), 2574. https://doi.org/10.3390/nu11112574