Effects of Nutrient Intake Timing on Post-Exercise Glycogen Accumulation and Its Related Signaling Pathways in Mouse Skeletal Muscle
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Experimental Procedures
2.2.1. Experiment 1
2.2.2. Experiment 2
2.3. Analytical Methods
2.3.1. Blood Analysis
2.3.2. Glycogen Levels in Skeletal Muscle
2.3.3. Muscle Homogenization
2.3.4. Western Blotting
2.4. Statistical Analysis
3. Results
3.1. Experiment 1
Blood Glucose and Plasma Insulin Levels after the Nutrient Intake
3.2. Experiment 2
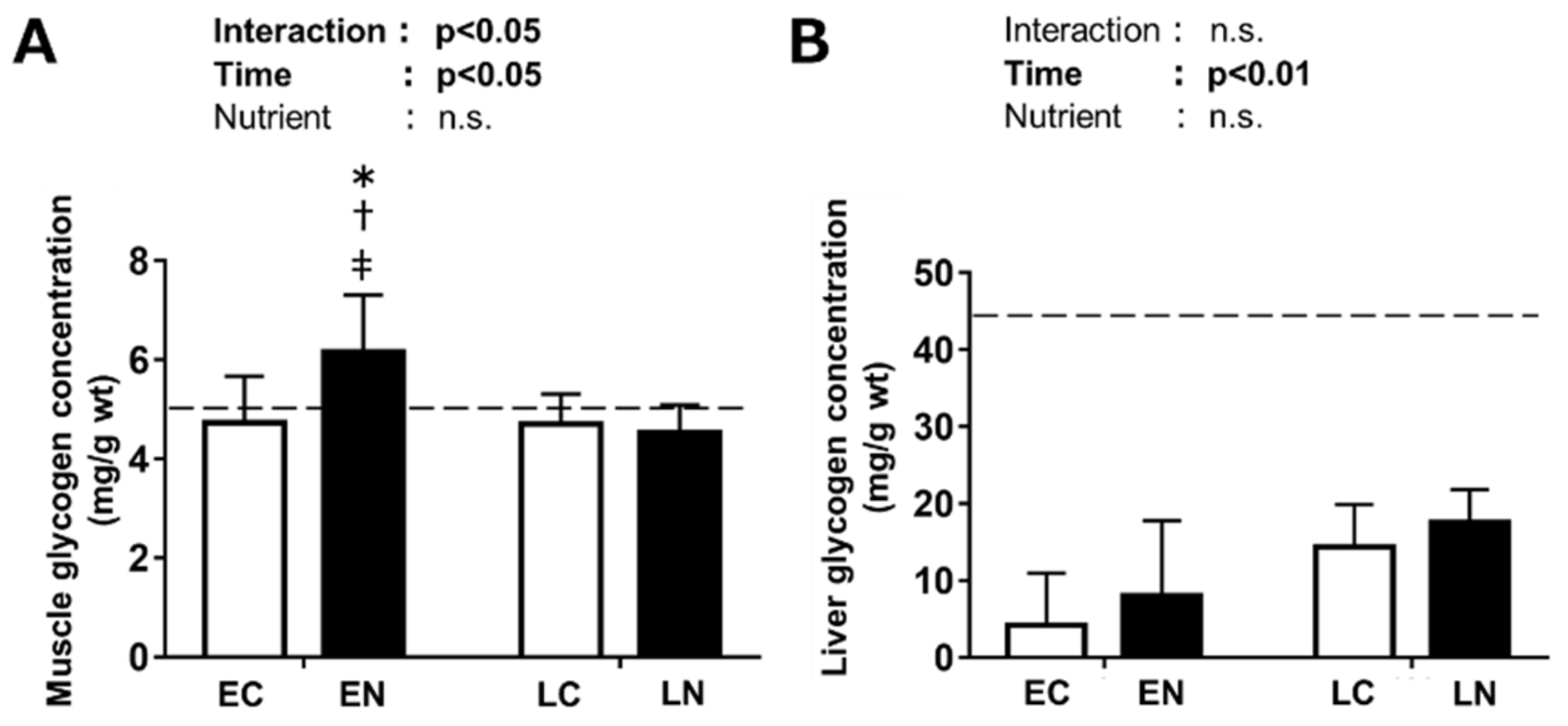
3.2.1. Glycogen Level in the Plantaris Muscle and Liver
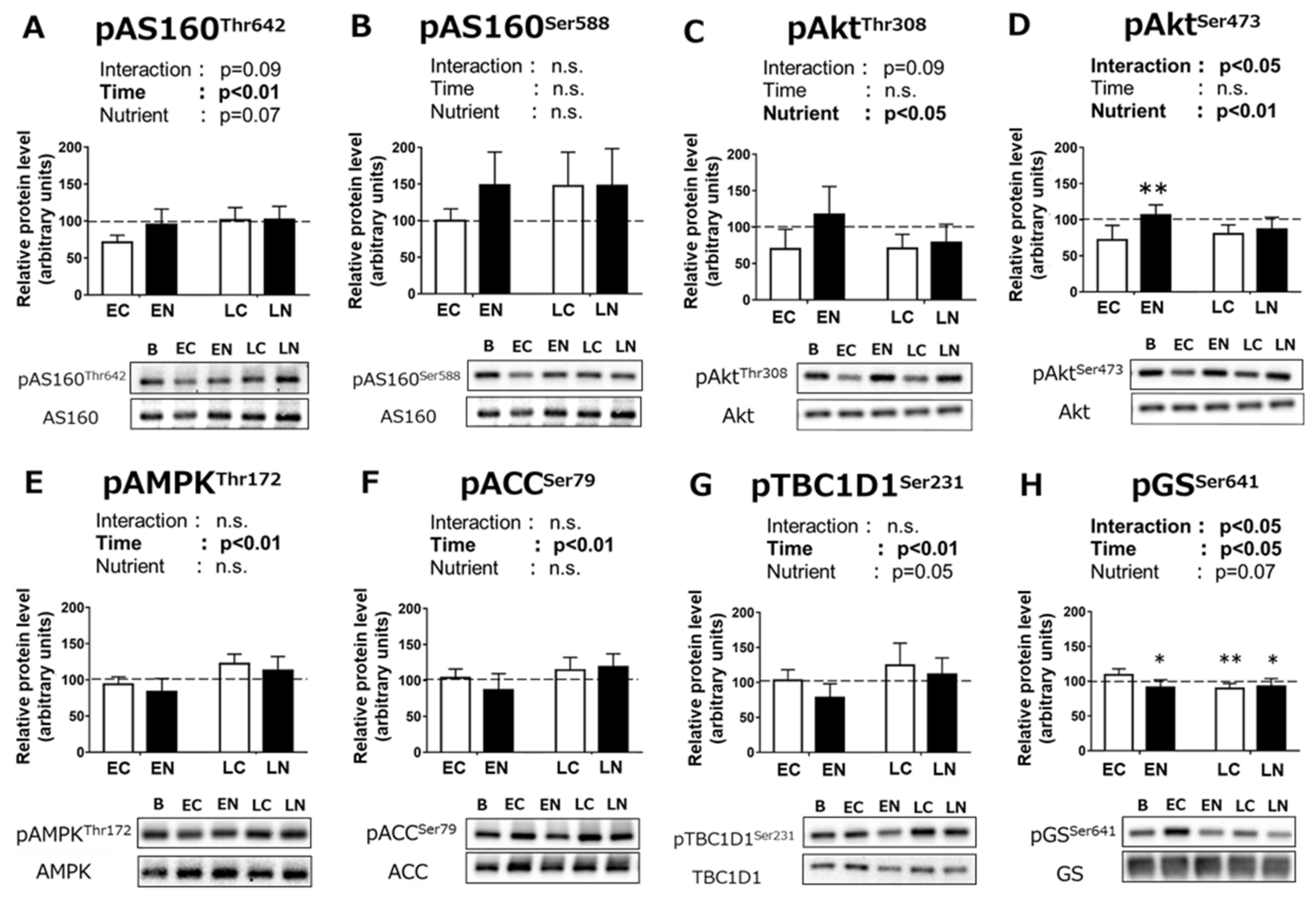
3.2.2. Phosphorylation Levels of Signaling Pathway Proteins Related to Carbohydrate Metabolism
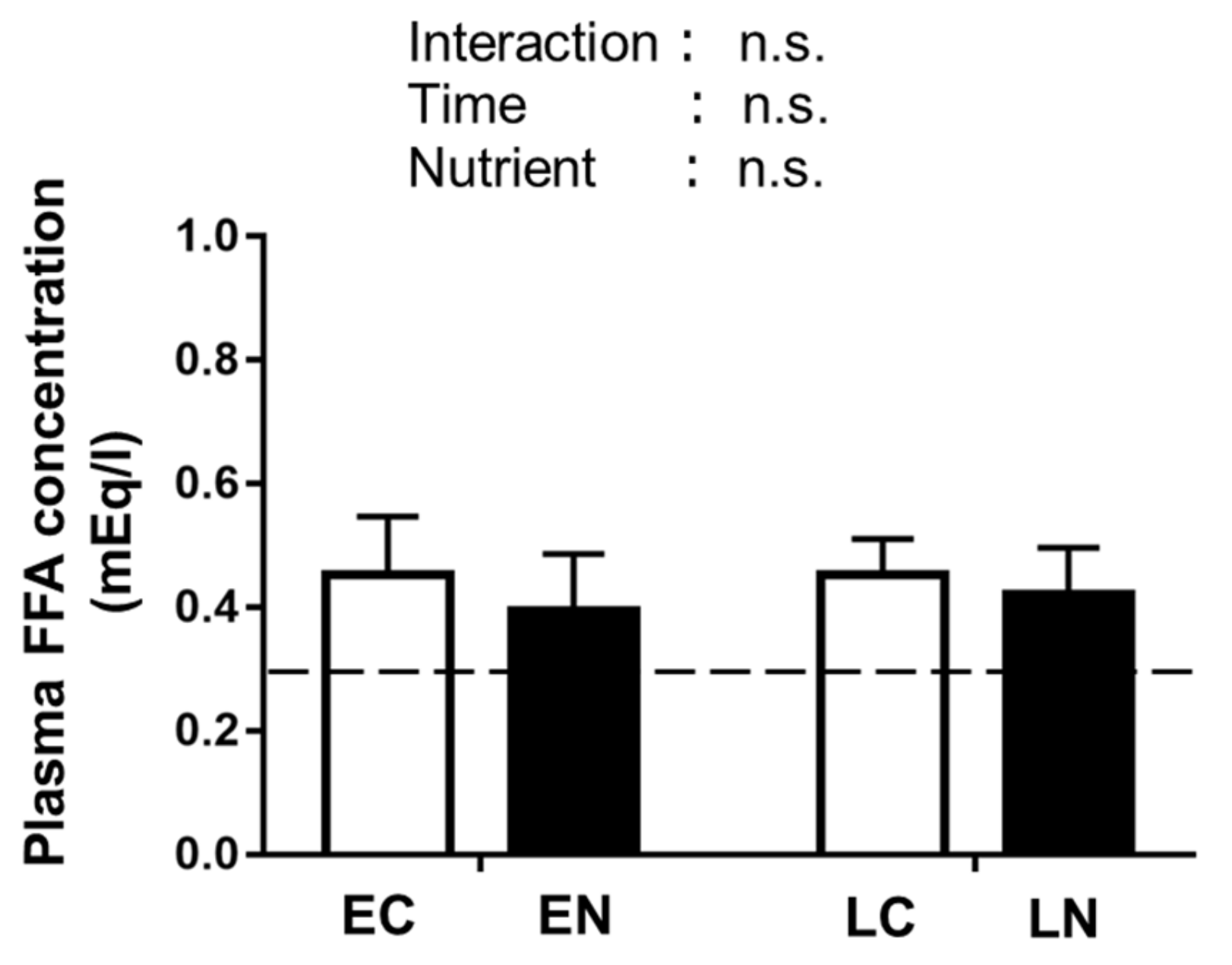
3.2.3. Plasma Free Fatty Acid (FFA) Level
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Romijn, J.A.; Coyle, E.F.; Sidossis, L.S.; Gastaldelli, A.; Horowitz, J.F.; Endert, E.; Wolfe, R.R. Regulation of endogenous fat and carbohydrate metabolism in relation to exercise intensity and duration. Am. J. Physiol. Endocrinol. Metab. 1993, 265, E380–E391. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- van Loon, L.J.; Greenhaff, P.L.; Constantin-Teodosiu, D.; Saris, W.H.; Wagenmakers, A.J. The effects of increasing exercise intensity on muscle fuel utilisation in humans. J. Physiol. 2001, 536, 295–304. [Google Scholar] [CrossRef] [PubMed]
- Hawley, J.A.; Leckey, J.J. Carbohydrate Dependence During Prolonged, Intense Endurance Exercise. Sports Med. 2015, 45 (Suppl. 1), S5–S12. [Google Scholar] [CrossRef]
- Ørtenblad, N.; Westerblad, H.; Nielsen, J. Muscle glycogen stores and fatigue. J. Physiol. 2013, 591, 4405–4413. [Google Scholar] [CrossRef] [PubMed]
- Burke, L.M.; van Loon, L.J.C.; Hawley, J.A. Postexercise muscle glycogen resynthesis in humans. J. Appl. Physiol. 2017, 122, 1055–1067. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alghannam, A.; Gonzalez, J.; Betts, J. Restoration of Muscle Glycogen and Functional Capacity: Role of Post-Exercise Carbohydrate and Protein Co-Ingestion. Nutrients 2018, 10, 253. [Google Scholar] [CrossRef]
- Murray, B.; Rosenbloom, C. Fundamentals of glycogen metabolism for coaches and athletes. Nutr. Rev. 2018, 76, 243–259. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ivy, J.L.; Katz, A.L.; Cutler, C.L.; Sherman, W.M.; Coyle, E.F. Muscle glycogen synthesis after exercise: Effect of time of carbohydrate ingestion. J. Appl. Physiol. 1988, 64, 1480–1485. [Google Scholar] [CrossRef]
- Levenhagen, D.K.; Gresham, J.D.; Carlson, M.G.; Maron, D.J.; Borel, M.J.; Flakoll, P.J. Postexercise nutrient intake timing in humans is critical to recovery of leg glucose and protein homeostasis. Am. J. Physiol. Endocrinol. Metab. 2001, 280, E982–E993. [Google Scholar] [CrossRef]
- Parkin, J.A.; Carey, M.F.; Martin, I.K.; Stojanovska, L.; Febbraio, M.A. Muscle glycogen storage following prolonged exercise: Effect of timing of ingestion of high glycemic index food. Med. Sci. Sports Exerc. 1997, 29, 220–224. [Google Scholar] [CrossRef]
- Costill, D.L.; Bowers, R.; Branam, G.; Sparks, K. Muscle glycogen utilization during prolonged exercise on successive days. J. Appl. Physiol. 1971, 31, 834–838. [Google Scholar] [CrossRef] [PubMed]
- Kirwan, J.P.; Costill, D.L.; Mitchell, J.B.; Houmard, J.A.; Flynn, M.G.; Fink, W.J.; Beltz, J.D. Carbohydrate balance in competitive runners during successive days of intense training. J. Appl. Physiol. 1988, 65, 2601–2606. [Google Scholar] [CrossRef] [PubMed]
- Fisher, J.S.; Nolte, L.A.; Kawanaka, K.; Han, D.-H.; Jones, T.E.; Holloszy, J.O. Glucose transport rate and glycogen synthase activity both limit skeletal muscle glycogen accumulation. Am. J. Physiol. Endocrinol. Metab. 2002, 282, E1214–E1221. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jentjens, R.; Jeukendrup, A.E. Determinants of Post-Exercise Glycogen Synthesis During Short-Term Recovery. Sport. Med. 2003, 33, 117–144. [Google Scholar] [CrossRef]
- Richter, E.A.; Garetto, L.P.; Goodman, M.N.; Ruderman, N.B. Muscle glucose metabolism following exercise in the rat: Increased sensitivity to insulin. J. Clin. Investig. 1982, 69, 785–793. [Google Scholar] [CrossRef]
- Garetto, L.P.; Richter, E.A.; Goodman, M.N.; Ruderman, N.B. Enhanced muscle glucose metabolism after exercise in the rat: The two phases. Am. J. Physiol. Endocrinol. Metab. 1984, 246, E471–E475. [Google Scholar] [CrossRef]
- Richter, E.A.; Garetto, L.P.; Goodman, M.N.; Ruderman, N.B. Enhanced muscle glucose metabolism after exercise: Modulation by local factors. Am. J. Physiol. Endocrinol. Metab. 1984, 246, E476–E482. [Google Scholar] [CrossRef]
- Goodyear, L.J.; Hirshman, M.F.; King, P.A.; Horton, E.D.; Thompson, C.M.; Horton, E.S. Skeletal muscle plasma membrane glucose transport and glucose transporters after exercise. J. Appl. Physiol. 1990, 68, 193–198. [Google Scholar] [CrossRef]
- Hansen, P.A.; Nolte, L.A.; Chen, M.M.; Holloszy, J.O. Increased GLUT-4 translocation mediates enhanced insulin sensitivity of muscle glucose transport after exercise. J. Appl. Physiol. 1998, 85, 1218–1222. [Google Scholar] [CrossRef]
- Villar-Palasí, C.; Guinovart, J.J. The role of glucose 6-phosphate in the control of glycogen synthase. FASEB J. 1997, 11, 544–558. [Google Scholar] [CrossRef]
- Jensen, J.; Lai, Y.-C. Regulation of muscle glycogen synthase phosphorylation and kinetic properties by insulin, exercise, adrenaline and role in insulin resistance. Arch. Physiol. Biochem. 2009, 115, 13–21. [Google Scholar] [CrossRef] [PubMed]
- Bouskila, M.; Hunter, R.W.; Ibrahim, A.F.M.; Delattre, L.; Peggie, M.; van Diepen, J.A.; Voshol, P.J.; Jensen, J.; Sakamoto, K. Allosteric Regulation of Glycogen Synthase Controls Glycogen Synthesis in Muscle. Cell Metab. 2010, 12, 456–466. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arias, E.B.; Kim, J.; Funai, K.; Cartee, G.D. Prior exercise increases phosphorylation of Akt substrate of 160 kDa (AS160) in rat skeletal muscle. Am. J. Physiol. Endocrinol. Metab. 2007, 292, E1191–E1200. [Google Scholar] [CrossRef] [PubMed]
- Funai, K.; Schweitzer, G.G.; Sharma, N.; Kanzaki, M.; Cartee, G.D. Increased AS160 phosphorylation, but not TBC1D1 phosphorylation, with increased postexercise insulin sensitivity in rat skeletal muscle. Am. J. Physiol. Endocrinol. Metab. 2009, 297, E242–E251. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schweitzer, G.G.; Arias, E.B.; Cartee, G.D. Sustained postexercise increases in AS160 Thr642 and Ser588 phosphorylation in skeletal muscle without sustained increases in kinase phosphorylation. J. Appl. Physiol. 2012, 113, 1852–1861. [Google Scholar] [CrossRef]
- Kramer, H.F.; Witczak, C.A.; Fujii, N.; Jessen, N.; Taylor, E.B.; Arnolds, D.E.; Sakamoto, K.; Hirshman, M.F.; Goodyear, L.J. Distinct Signals Regulate AS160 Phosphorylation in Response to Insulin, AICAR, and Contraction in Mouse Skeletal Muscle. Diabetes 2006, 55, 2067–2076. [Google Scholar] [CrossRef] [Green Version]
- Funai, K.; Schweitzer, G.G.; Castorena, C.M.; Kanzaki, M.; Cartee, G.D. In vivo exercise followed by in vitro contraction additively elevates subsequent insulin-stimulated glucose transport by rat skeletal muscle. Am. J. Physiol. Endocrinol. Metab. 2010, 298, E999–E1010. [Google Scholar] [CrossRef] [Green Version]
- Irimia, J.M.; Tagliabracci, V.S.; Meyer, C.M.; Segvich, D.M.; DePaoli-Roach, A.A.; Roach, P.J. Muscle Glycogen Remodeling and Glycogen Phosphate Metabolism following Exhaustive Exercise of Wild Type and Laforin Knockout Mice. J. Biol. Chem. 2015, 290, 22686–22698. [Google Scholar] [CrossRef] [Green Version]
- Matsunaga, Y.; Sakata, Y.; Yago, T.; Nakamura, H.; Shimizu, T.; Takeda, Y. Effects of Glucose with Casein Peptide Supplementation on Post-Exercise Muscle Glycogen Resynthesis in C57BL/6J Mice. Nutrients 2018, 10, 753. [Google Scholar] [CrossRef]
- Lo, S.; Russell, J.C.; Taylor, A.W. Determination of glycogen in small tissue samples. J. Appl. Physiol. 1970, 28, 234–236. [Google Scholar] [CrossRef]
- Takahashi, Y.; Terada, S.; Banjo, M.; Seike, K.; Nakano, S.; Hatta, H. Effects of β-hydroxybutyrate treatment on glycogen repletion and its related signaling cascades in epitrochlearis muscle during 120 min of post-exercise recovery. Appl. Physiol. Nutr. Metab. 2019. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, Y.; Hijikata, K.; Seike, K.; Nakano, S.; Banjo, M.; Sato, Y.; Takahashi, K.; Hatta, H. Effects of Royal Jelly Administration on Endurance Training-Induced Mitochondrial Adaptations in Skeletal Muscle. Nutrients 2018, 10, 1735. [Google Scholar] [CrossRef] [PubMed]
- Kelley, D.E.; Mokan, M.; Simoneau, J.A.; Mandarino, L.J. Interaction between glucose and free fatty acid metabolism in human skeletal muscle. J. Clin. Investig. 1993, 92, 91–98. [Google Scholar] [CrossRef] [PubMed]
- Boden, G.; Chen, X.; Ruiz, J.; White, J.V.; Rossetti, L. Mechanisms of fatty acid-induced inhibition of glucose uptake. J. Clin. Investig. 1994, 93, 2438–2446. [Google Scholar] [CrossRef] [PubMed]
- Chalkley, S.M.; Hettiarachchi, M.; Chisholm, D.J.; Kraegen, E.W. Five-hour fatty acid elevation increases muscle lipids and impairs glycogen synthesis in the rat. Metabolism 1998, 47, 1121–1126. [Google Scholar] [CrossRef]
- Tamura, Y.; Matsunaga, Y.; Masuda, H.; Takahashi, Y.; Takahashi, Y.; Terada, S.; Hoshino, D.; Hatta, H. Postexercise whole body heat stress additively enhances endurance training-induced mitochondrial adaptations in mouse skeletal muscle. Am. J. Physiol. Integr. Comp. Physiol. 2014, 307, R931–R943. [Google Scholar] [CrossRef] [Green Version]
- Pederson, B.A.; Cope, C.R.; Schroeder, J.M.; Smith, M.W.; Irimia, J.M.; Thurberg, B.L.; DePaoli-Roach, A.A.; Roach, P.J. Exercise Capacity of Mice Genetically Lacking Muscle Glycogen Synthase. J. Biol. Chem. 2005, 280, 17260–17265. [Google Scholar] [CrossRef] [Green Version]
- Xirouchaki, C.E.; Mangiafico, S.P.; Bate, K.; Ruan, Z.; Huang, A.M.; Tedjosiswoyo, B.W.; Lamont, B.; Pong, W.; Favaloro, J.; Blair, A.R.; et al. Impaired glucose metabolism and exercise capacity with muscle-specific glycogen synthase 1 (gys1) deletion in adult mice. Mol. Metab. 2016, 5, 221–232. [Google Scholar] [CrossRef]
- Aoi, W.; Naito, Y.; Takanami, Y.; Ishii, T.; Kawai, Y.; Akagiri, S.; Kato, Y.; Osawa, T.; Yoshikawa, T. Astaxanthin improves muscle lipid metabolism in exercise via inhibitory effect of oxidative CPT I modification. Biochem. Biophys. Res. Commun. 2008, 366, 892–897. [Google Scholar] [CrossRef]
- Uruno, A.; Yagishita, Y.; Katsuoka, F.; Kitajima, Y.; Nunomiya, A.; Nagatomi, R.; Pi, J.; Biswal, S.S.; Yamamoto, M. Nrf2-Mediated Regulation of Skeletal Muscle Glycogen Metabolism. Mol. Cell. Biol. 2016, 36, 1655–1672. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.; Arias, E.B.; Pataky, M.W.; Goodyear, L.J.; Cartee, G.D. Postexercise improvement in glucose uptake occurs concomitant with greater γ3-AMPK activation and AS160 phosphorylation in rat skeletal muscle. Am. J. Physiol. Endocrinol. Metab. 2018, 315, E859–E871. [Google Scholar] [CrossRef] [PubMed]
- Kjøbsted, R.; Munk-Hansen, N.; Birk, J.B.; Foretz, M.; Viollet, B.; Björnholm, M.; Zierath, J.R.; Treebak, J.T.; Wojtaszewski, J.F.P. Enhanced Muscle Insulin Sensitivity After Contraction/Exercise Is Mediated by AMPK. Diabetes 2017, 66, 598–612. [Google Scholar] [CrossRef]
- Hingst, J.R.; Bruhn, L.; Hansen, M.B.; Rosschou, M.F.; Birk, J.B.; Fentz, J.; Foretz, M.; Viollet, B.; Sakamoto, K.; Færgeman, N.J.; et al. Exercise-induced molecular mechanisms promoting glycogen supercompensation in human skeletal muscle. Mol. Metab. 2018, 16, 24–34. [Google Scholar] [CrossRef] [PubMed]
- Steenberg, D.E.; Jørgensen, N.B.; Birk, J.B.; Sjøberg, K.A.; Kiens, B.; Richter, E.A.; Wojtaszewski, J.F.P. Exercise training reduces the insulin-sensitizing effect of a single bout of exercise in human skeletal muscle. J. Physiol. 2019, 597, 89–103. [Google Scholar] [CrossRef] [PubMed]
- Leto, D.; Saltiel, A.R. Regulation of glucose transport by insulin: Traffic control of GLUT4. Nat. Rev. Mol. Cell Biol. 2012, 13, 383–396. [Google Scholar] [CrossRef]
- Cartee, G.D. Mechanisms for greater insulin-stimulated glucose uptake in normal and insulin-resistant skeletal muscle after acute exercise. Am. J. Physiol. Endocrinol. Metab. 2015, 309, E949–E959. [Google Scholar] [CrossRef] [Green Version]
- Scheid, M.P.; Woodgett, J.R. Unravelling the activation mechanisms of protein kinase B/Akt. FEBS Lett. 2003, 546, 108–112. [Google Scholar] [CrossRef] [Green Version]
- Gonzalez, E.; McGraw, T.E. Insulin-modulated Akt subcellular localization determines Akt isoform-specific signaling. Proc. Natl. Acad. Sci. USA 2009, 106, 7004–7009. [Google Scholar] [CrossRef] [Green Version]
- Kurth-Kraczek, E.J.; Hirshman, M.F.; Goodyear, L.J.; Winder, W.W. 5’ AMP-activated protein kinase activation causes GLUT4 translocation in skeletal muscle. Diabetes 1999, 48, 1667–1671. [Google Scholar] [CrossRef]
- Treebak, J.T.; Glund, S.; Deshmukh, A.; Klein, D.K.; Long, Y.C.; Jensen, T.E.; Jørgensen, S.B.; Viollet, B.; Andersson, L.; Neumann, D.; et al. AMPK-mediated AS160 phosphorylation in skeletal muscle is dependent on AMPK catalytic and regulatory subunits. Diabetes 2006, 55, 2051–2058. [Google Scholar] [CrossRef]
- Kjøbsted, R.; Treebak, J.T.; Fentz, J.; Lantier, L.; Viollet, B.; Birk, J.B.; Schjerling, P.; Björnholm, M.; Zierath, J.R.; Wojtaszewski, J.F.P. Prior AICAR Stimulation Increases Insulin Sensitivity in Mouse Skeletal Muscle in an AMPK-Dependent Manner. Diabetes 2015, 64, 2042–2055. [Google Scholar] [CrossRef] [PubMed]
- Pehmøller, C.; Treebak, J.T.; Birk, J.B.; Chen, S.; MacKintosh, C.; Hardie, D.G.; Richter, E.A.; Wojtaszewski, J.F.P. Genetic disruption of AMPK signaling abolishes both contraction- and insulin-stimulated TBC1D1 phosphorylation and 14-3-3 binding in mouse skeletal muscle. Am. J. Physiol. Endocrinol. Metab. 2009, 297, E665–E675. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vichaiwong, K.; Purohit, S.; An, D.; Toyoda, T.; Jessen, N.; Hirshman, M.F.; Goodyear, L.J. Contraction regulates site-specific phosphorylation of TBC1D1 in skeletal muscle. Biochem. J. 2010, 431, 311–320. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Whitfield, J.; Paglialunga, S.; Smith, B.K.; Miotto, P.M.; Simnett, G.; Robson, H.L.; Jain, S.S.; Herbst, E.A.F.; Desjardins, E.M.; Dyck, D.J.; et al. Ablating the protein TBC1D1 impairs contraction-induced sarcolemmal glucose transporter 4 redistribution but not insulin-mediated responses in rats. J. Biol. Chem. 2017, 292, 16653–16664. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miyamoto, L.; Toyoda, T.; Hayashi, T.; Yonemitsu, S.; Nakano, M.; Tanaka, S.; Ebihara, K.; Masuzaki, H.; Hosoda, K.; Ogawa, Y.; et al. Effect of acute activation of 5′-AMP-activated protein kinase on glycogen regulation in isolated rat skeletal muscle. J. Appl. Physiol. 2007, 102, 1007–1013. [Google Scholar] [CrossRef]
- Hunter, R.W.; Treebak, J.T.; Wojtaszewski, J.F.P.; Sakamoto, K. Molecular mechanism by which AMP-activated protein kinase activation promotes glycogen accumulation in muscle. Diabetes 2011, 60, 766–774. [Google Scholar] [CrossRef]
- Roach, P.J.; Takeda, Y.; Larner, J. Rabbit skeletal muscle glycogen synthase. I. Relationship between phosphorylation state and kinetic properties. J. Biol. Chem. 1976, 251, 1913–1919. [Google Scholar]
- Lai, Y.C.; Zarrinpashneh, E.; Jensen, J. Additive effect of contraction and insulin on glucose uptake and glycogen synthase in muscle with different glycogen contents. J. Appl. Physiol. 2010, 108, 1106–1115. [Google Scholar] [CrossRef] [Green Version]
- Maehlum, S.; Felig, P.; Wahren, J. Splanchnic glucose and muscle glycogen metabolism after glucose feeding during postexercise recovery. Am. J. Physiol. Endocrinol. Metab. 1978, 235, E255–E260. [Google Scholar] [CrossRef]
- Hamilton, K.S.; Gibbons, F.K.; Bracy, D.P.; Lacy, D.B.; Cherrington, A.D.; Wasserman, D.H. Effect of prior exercise on the partitioning of an intestinal glucose load between splanchnic bed and skeletal muscle. J. Clin. Investig. 1996, 98, 125–135. [Google Scholar] [CrossRef]
- Gunderson, H.; Wehmeyer, N.; Burnett, D.; Nauman, J.; Hartzell, C.; Savage, S. Exercise and exhaustion effects on glycogen synthesis pathways. J. Appl. Physiol. 1996, 81, 2020–2026. [Google Scholar] [CrossRef] [PubMed] [Green Version]


© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Takahashi, Y.; Matsunaga, Y.; Banjo, M.; Takahashi, K.; Sato, Y.; Seike, K.; Nakano, S.; Hatta, H. Effects of Nutrient Intake Timing on Post-Exercise Glycogen Accumulation and Its Related Signaling Pathways in Mouse Skeletal Muscle. Nutrients 2019, 11, 2555. https://doi.org/10.3390/nu11112555
Takahashi Y, Matsunaga Y, Banjo M, Takahashi K, Sato Y, Seike K, Nakano S, Hatta H. Effects of Nutrient Intake Timing on Post-Exercise Glycogen Accumulation and Its Related Signaling Pathways in Mouse Skeletal Muscle. Nutrients. 2019; 11(11):2555. https://doi.org/10.3390/nu11112555
Chicago/Turabian StyleTakahashi, Yumiko, Yutaka Matsunaga, Mai Banjo, Kenya Takahashi, Yosuke Sato, Kohei Seike, Suguru Nakano, and Hideo Hatta. 2019. "Effects of Nutrient Intake Timing on Post-Exercise Glycogen Accumulation and Its Related Signaling Pathways in Mouse Skeletal Muscle" Nutrients 11, no. 11: 2555. https://doi.org/10.3390/nu11112555




