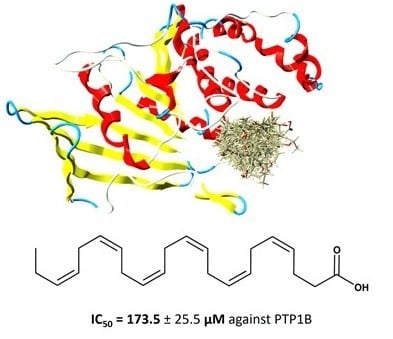
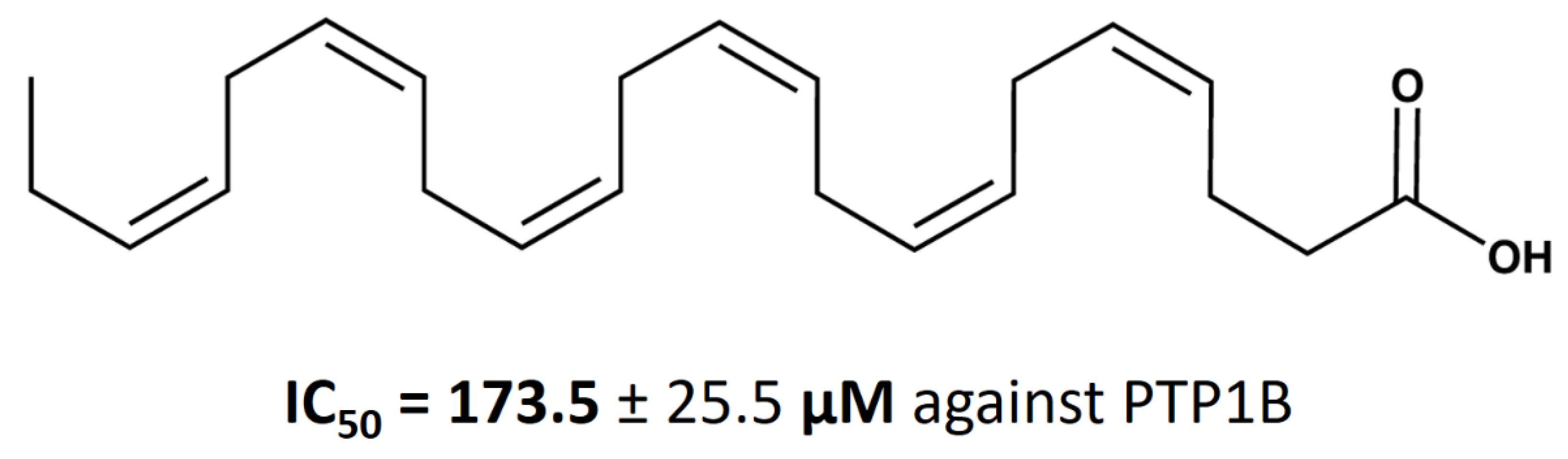
Docosahexaenoic Acid Inhibits PTP1B Phosphatase and the Viability of MCF-7 Breast Cancer Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Recombinant PTP1B Activity Assay
2.2. Cell Culture
2.3. Cell Viability Assay
2.4. Computational Analysis
2.5. Statistical Analysis
3. Results
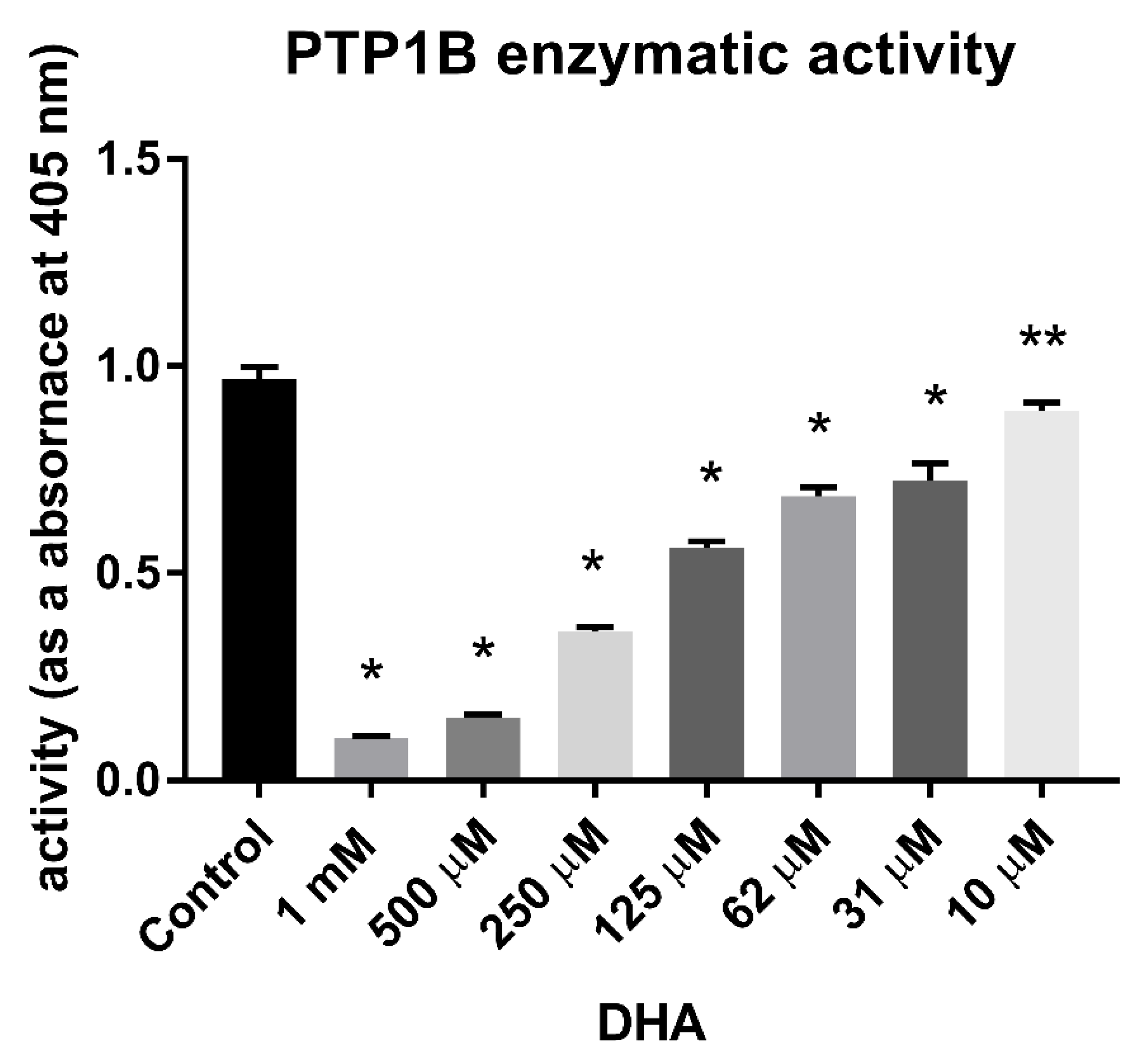
3.1. Inhibitory Effect of DHA on the Enzymatic Activity of PTP1B
3.2. Calculation of DHA IC50 Values for PTP1B
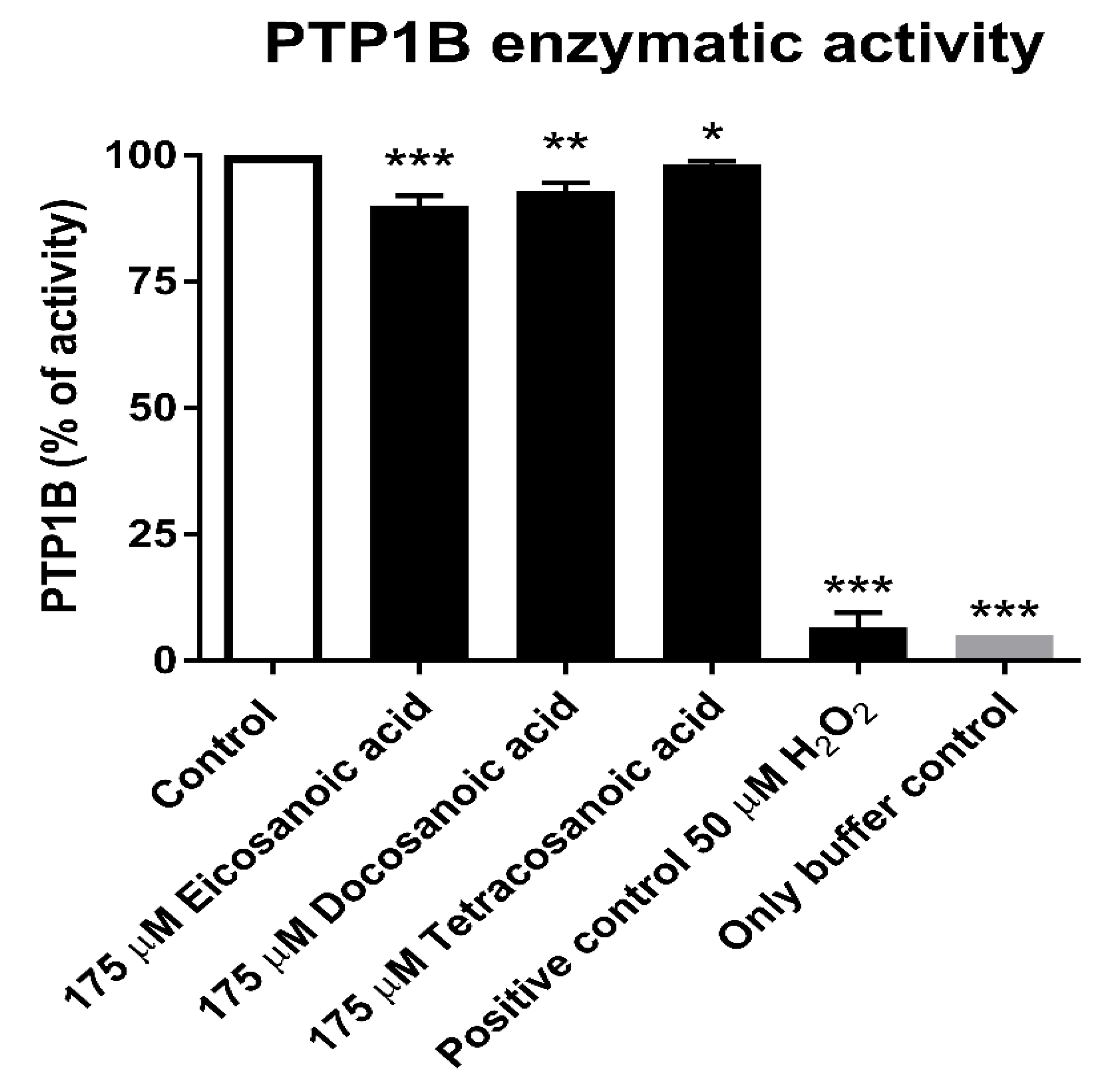
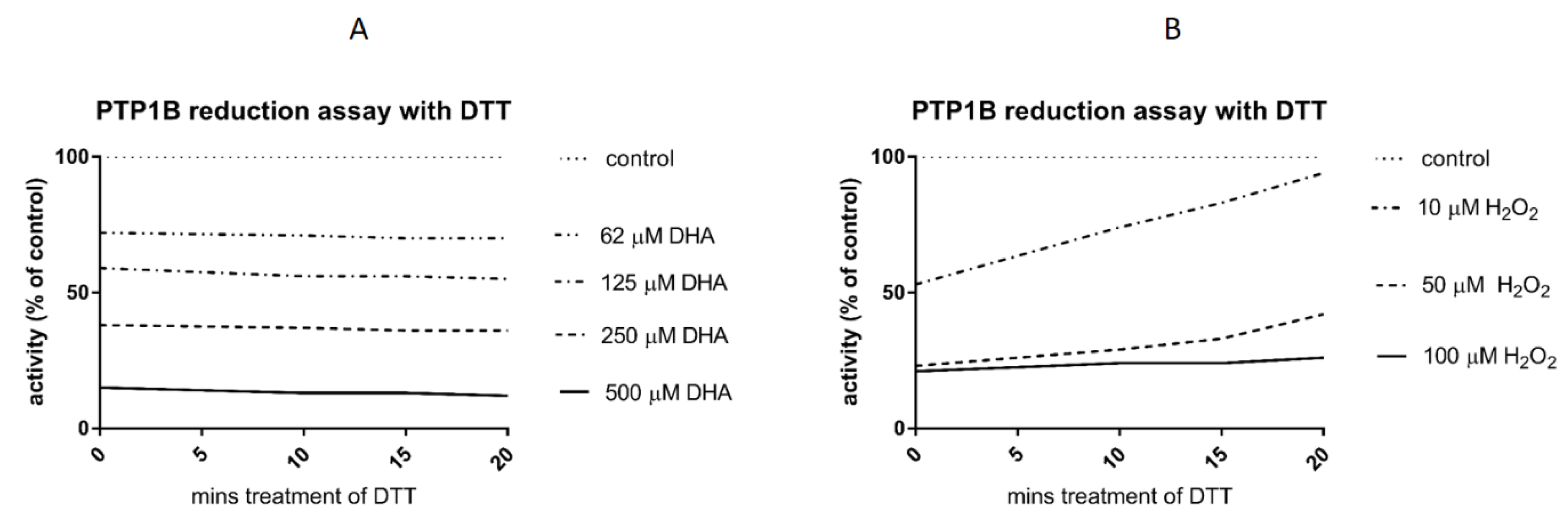
3.3. Reduction Assay
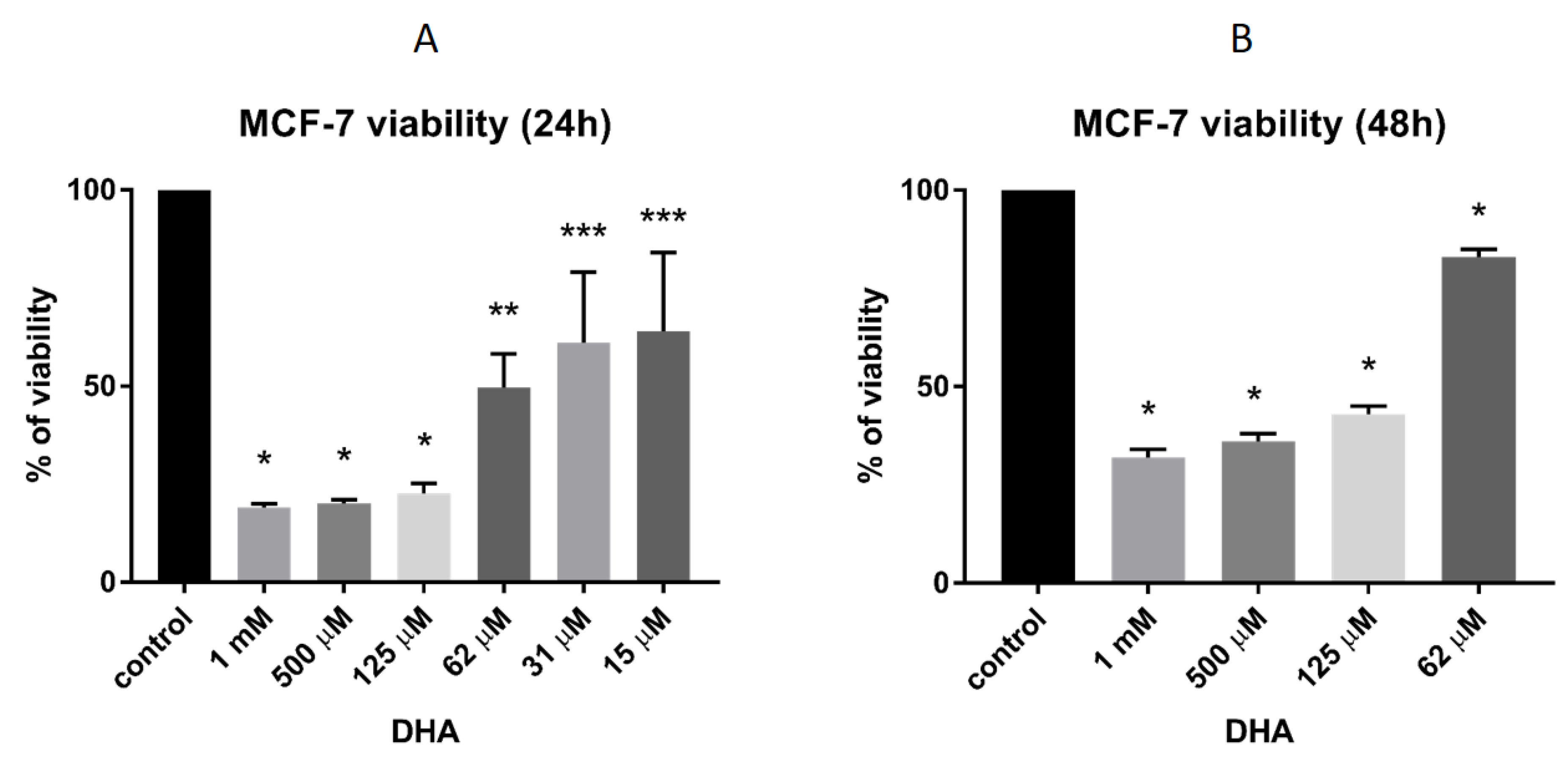
3.4. DHA Effect on the Viability of MCF-7 Breast Cancer Cells
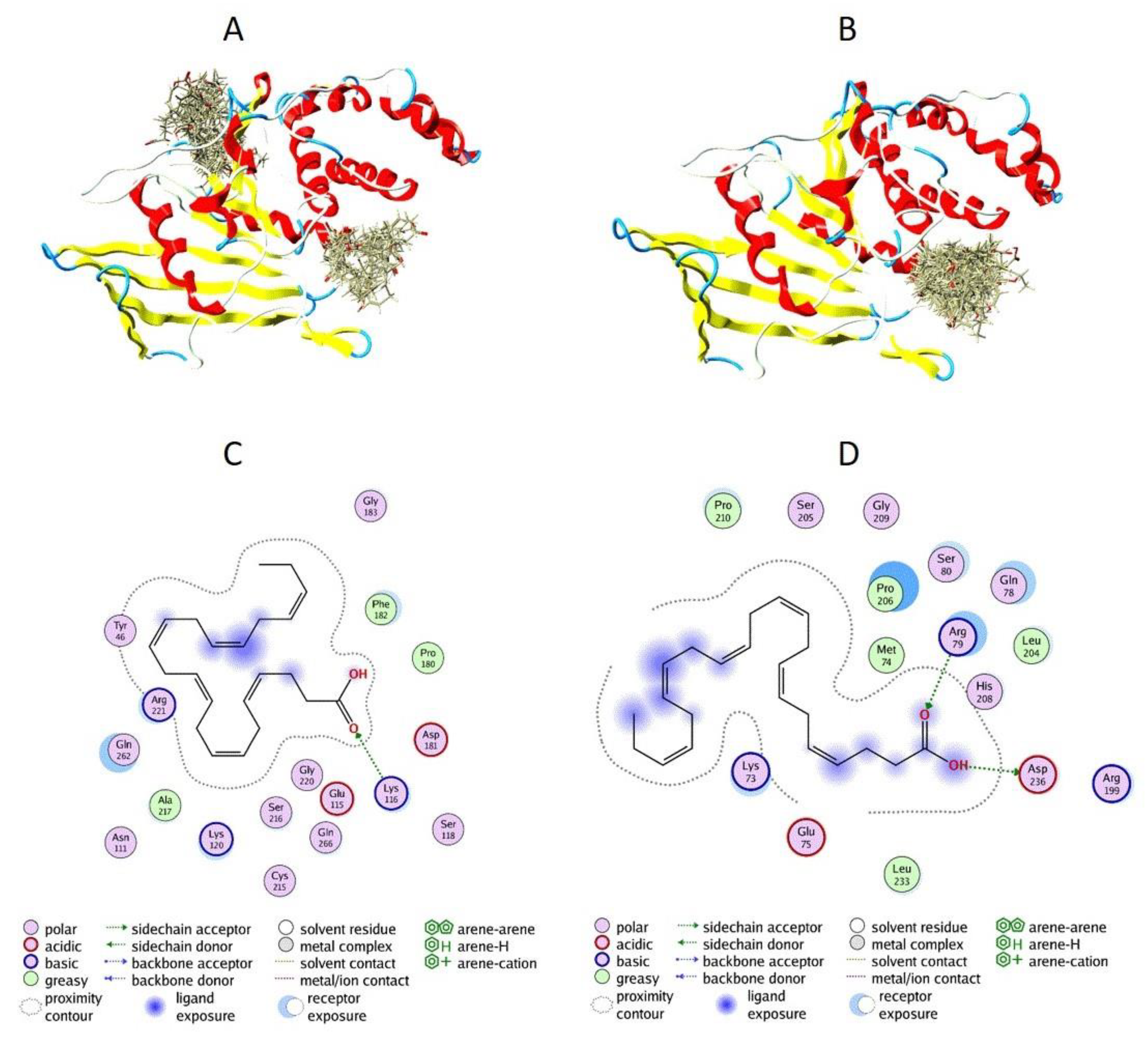
3.5. Docking of DHA to PTP1B
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Nunes-Xavier, C.E.; Martin-Perez, J.; Elson, A.; Pulido, R. Protein tyrosine phosphatases as novel targets in breast cancer therapy. Biochim. Biophys Acta 2013, 1836, 211–226. [Google Scholar] [CrossRef] [PubMed]
- Balavenkatraman, K.K.; Aceto, N.; Britschgi, A.; Mueller, U.; Bence, K.K.; Neel, B.G.; BentiresAlj, M. Epithelial protein-tyrosine phosphatase 1B contributes to the induction of mammary tumors by HER2/Neu but is not essential for tumor maintenance. Mol. Cancer Res. 2011, 9, 1377–1384. [Google Scholar] [CrossRef] [PubMed]
- Aceto, N.; Bentires-Alj, M. Targeting protein-tyrosine phosphatases in breast cancer. Oncotarget 2012, 3, 514–515. [Google Scholar] [CrossRef] [PubMed]
- Julien, S.G.; Dube, N.; Read, M.; Penney, J.; Paquet, M.; Han, Y.; Kennedy, B.P.; Muller, W.J.; Tremblay, M.L. Protein tyrosine phosphatase 1B deficiency or inhibition delays ErbB2-induced mammary tumorigenesis and protects from lung metastasis. Nat. Genet. 2007, 39, 338–346. [Google Scholar] [CrossRef] [PubMed]
- Gu, F.; Dube, N.; Kim, J.W.; Cheng, A.; Ibarra-Sanchez Mde, J.; Tremblay, M.L.; Boisclair, Y.R. Protein tyrosine phosphatase 1B attenuates growth hormone-mediated JAK2-STAT signaling. Mol. Cell. Biol. 2003, 23, 3753–3762. [Google Scholar] [CrossRef] [PubMed]
- Scott, L.M.; Lawrence, H.R.; Sebti, S.M.; Lawrence, N.J.; Wu, J. Targeting protein tyrosine phosphatases for anticancer drug discovery. Cur. Pharm. Des. 2010, 16, 1843–1862. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.S.; Wang, R.R.; Yue, H.; Zheng, Z.H.; Lu, X.H.; Wang, S.Q.; Dong, W.L.; Wang, R.L. Design, synthesis, biological evaluation and molecular dynamics studies of 4-thiazolinone derivatives as protein tyrosine phosphatase 1B (PTP1B) inhibitors. J. Biomol. Struct. Dyn. 2019, 19, 1–11. [Google Scholar] [CrossRef]
- Jin, J.; Ye, X.; Boateng, D.; Dai, K.; Ye, F.; Du, P.; Yu, H. Identification and characterization of potent and selective inhibitors targeting protein tyrosine phosphatase 1B (PTP1B). Bioorg. Med. Chem. Lett. 2019, 29, 2358–2363. [Google Scholar] [CrossRef]
- Hussain, H.; Green, I.R.; Abbas, G.; Adekenov, S.M.; Hussain, W.; Ali, I. Protein tyrosine phosphatase 1B (PTP1B) inhibitors as potential anti-diabetes agents: Patent review (2015–2018). Expert Opin. Ther. Pat. 2019, 29, 689–702. [Google Scholar] [CrossRef]
- Han, R.Y.; Ge, Y.; Zhang, L.; Wang, Q.M. Design and biological evaluation of novel imidazolyl flavonoids as potent and selective protein tyrosine phosphatase inhibitors. Med. Chem. 2019. [Google Scholar] [CrossRef]
- Przychodzen, P.; Kuban-Jankowska, A.; Wyszkowska, R.; Barone, G.; Bosco, G.L.; Celso, F.L.; Kamm, A.; Daca, A.; Kostrzewa, T.; Gorska-Ponikowska, M. PTP1B phosphatase as a novel target of oleuropein activity in MCF-7 breast cancer model. Toxicol. Vitr. 2019, 13, 104624. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Kong, G.; Chen, Q.C.; Wang, G.; Li, J.; Xu, Y.; Iin, T.; Tian, Y.; Zhang, X.; Yao, X.; et al. Fatty acids as natural specific inhibitors of the proto-oncogenic protein Shp2. Bioorg. Med. Chem. Lett. 2011, 21, 6833–6837. [Google Scholar] [CrossRef] [PubMed]
- Kuban-Jankowska, A.; Gorska, M.; Tuszynski, J.A.; Churchill, C.D.; Winter, P.; Klobukowski, M.; Wozniak, M. Inactivation of Protein Tyrosine Phosphatases by Peracids Correlates with the Hydrocarbon Chain Length. Cell. Phys. Biochem. 2015, 36, 1069–1083. [Google Scholar] [CrossRef] [PubMed]
- Sargent, J.R. Fish oils and human diet. Br. J. Nutr. 1997, 78, 5–13. [Google Scholar] [CrossRef]
- Gil, A.; Gil, F. Fish, a Mediterranean source of n-3 PUFA: Benefits do not justify limiting consumption. Br. J. Nutr. 2015, 113, 58–67. [Google Scholar] [CrossRef]
- Huang, X.M.; Yang, Z.J.; Xie, Q.; Zhang, Z.K.; Ma, J.Y. Natural products for treating colorectal cancer: A mechanistic review. Biomed. Pharmacother. 2019, 117, 109142. [Google Scholar] [CrossRef]
- Nauroth, J.M.; Liu, Y.C.; Van Elswyk, M.; Bell, R.; Hall, E.B.; Chung, G.; Arterburn, L.M. Docosahexaenoic acid (DHA) and docosapentaenoic acid (DPAn-6) algal oils reduce inflammatory mediators in human peripheral mononuclear cells in vitro and paw edema in vivo. Lipids 2010, 45, 375–384. [Google Scholar] [CrossRef]
- Hossain, Z.; Hosokawa, M.; Takahashi, K. Growth inhibition and induction of apoptosis of colon cancer cell lines by applying marine phospholipid. Nutr. Cancer. 2009, 61, 123–130. [Google Scholar] [CrossRef]
- Larsson, S.C.; Kumlin, M.; Ingelman-Sundberg, M.; Wolk, A. Dietary long-chain n-3 fatty acids for the prevention of cancer: A review of potential mechanisms. Am. J. Clin. Nutr. 2004, 79, 935–945. [Google Scholar] [CrossRef]
- Berquin, I.M.; Edwards, I.J.; Chen, Y.Q. Multi-targeted therapy of cancer by omega-3 fatty acids. Cancer Lett. 2008, 269, 363–377. [Google Scholar] [CrossRef]
- Berquin, I.M.; Min, Y.; Wu, R.; Wu, J.; Perry, D.; Cline, J.M.; Thomas, M.J.; Thornburg, T.; Kulik, G.; Smith, A.; et al. Modulation of prostate cancer genetic risk by omega-3 and omega-6 fatty acids. J. Clin. Invest. 2007, 117, 1866–1875. [Google Scholar] [CrossRef] [PubMed]
- Chapkin, R.S.; Seo, J.; McMurray, D.N.; Lupton, J.R. Mechanisms by which docosahexaenoic acid and related fatty acids reduce colon cancer risk and inflammatory disorders of the intestine. Chem. Phys. Lipids 2008, 153, 14–23. [Google Scholar] [CrossRef] [PubMed]
- Ding, W.Q.; Vaught, J.L.; Yamauchi, H.; Lind, S.E. Differential sensitivity of cancer cells to docosahexaenoic acid-induced cytotoxicity: The potential importance of down-regulation of superoxide dismutase 1 expression. Mol. Cancer Ther. 2004, 9, 1109–1117. [Google Scholar]
- Kang, K.S.; Wang, P.; Yamabe, N.; Fukui, M.; Jay, T.; Zhu, B.T. Docosahexaenoic acid induces apoptosis in MCF-7 cells in vitro and in vivo via reactive oxygen species formation and caspase 8 activation. PLoS ONE 2010, 5, e10296. [Google Scholar] [CrossRef] [PubMed]
- Abdolahi, M.; Shokri, F.; Hosseini, M.; Shadanian, M.; Saboor-Yaraghi, A.A. The combined effect of all-trans-retinoic acid and docosahexaenoic acid on the induction of apoptosis in human breast cancer MCF-7 cells. J. Cancer Res. Ther. 2016, 12, 204–208. [Google Scholar]
- Wei, X.Q.; Li, X.; Xin, X.J.; Tong, Z.S. Clinical features and survival analysis of very young (age <35) breast cancer patients. Asian Pac. J. Cancer Prev. 2013, 14, 5949–5952. [Google Scholar]
- Fabian, C.J.; Kimler, B.F.; Hursting, S.D. Omega-3 fatty acids for breast cancer prevention and survivorship. Breast Cancer Res. 2015, 17, 62. [Google Scholar] [CrossRef]
- Zheng, J.S.; Hu, X.J.; Zhao, Y.M.; Yang, J.; Li, D. Intake of fish and marine n-3 polyunsaturated fatty acids and risk of breast cancer: Meta-analysis of data from 21 independent prospective cohort studies. Br. Med. J. 2013, 346, 1–10. [Google Scholar] [CrossRef]
- Gago-Dominguez, M.; Yuan, J.M.; Sun, C.L.; Lee, H.P.; Yu, M.C. Opposing effects of dietary n-3 and n-6 fatty acids on mammary carcinogenesis: The Singapore Chinese Health Study. Br. J. Cancer 2003, 89, 1686–1692. [Google Scholar] [CrossRef]
- Wakai, K.; Tamakoshi, K.; Date, C.; Fukui, M.; Suzuki, S.; Lin, Y.; Niwa, Y.; Nishio, K.; Yatsuya, H.; Kondo, T.; et al. Dietary intakes of fat and fatty acids and risk of breast cancer: A prospective study in Japan. Cancer Sci. 2005, 96, 590–599. [Google Scholar] [CrossRef]
- D’Eliseo, D.; Velotti, F. Omega-3 Fatty Acids and Cancer Cell Cytotoxicity: Implications for Multi-Targeted Cancer Therapy. J. Clin. Med. 2016, 5, 15. [Google Scholar] [CrossRef] [PubMed]
- Mansara, P.P.; Deshpande, R.A.; Vaidya, M.M.; Kaul-Ghanekar, R. Differential ratios of omega fatty acids (AA/EPA+DHA) modulate growth, lipid peroxidation and expression of tumor MARBPs in breast cancer cell lines MCF7 and MDA-MB-231. PLoS ONE 2015, 10, e0136542. [Google Scholar] [CrossRef] [PubMed]
- Chamras, H.; Ardashian, A.; Heber, D.; Glaspy, J.A. Fatty acid modulation of MCF-7 human breast cancer cell proliferation, apoptosis and differentiation. J. Nutr. Biochem. 2002, 13, 711–716. [Google Scholar] [CrossRef]
- Guo, Y.; Zhu, S.L.; Wu, Y.K.; He, Z.; Chen, Y.Q. Omega-3 free fatty acids attenuate insulin-promoted breast cancer cell proliferation. Nutr. Res. 2017, 42, 43–50. [Google Scholar] [CrossRef] [PubMed]
- Newell, M.; Goruk, S.; Mazurak, V.; Postovit, L.; Field, C.J. Role of docosahexaenoic acid in enhancement of docetaxel action in patient-derived breast cancer xenografts. Breast Cancer Res. Treat. 2019, 177, 357–367. [Google Scholar] [CrossRef] [PubMed]
- Orchard, T.S.; Gaudier-Diaz, M.M.; Phuwamongkolwiwat-Chu, P.; Andridge, R.; Lustberg, M.B.; Bomser, J.; Cole, R.M.; Belury, M.A.; DeVries, A.C. Low sucrose, omega-3 enriched diet has region-specific effects on neuroinflammation and synaptic function markers in a mouse model of doxorubicin-based chemotherapy. Nutrients 2018, 10, 2004. [Google Scholar] [CrossRef]
- Liu, M.; Wang, J.; Huang, B.; Chen, A.; Li, X. Oleuropein inhibits the proliferation and invasion of glioma cells via suppression of the AKT signaling pathway. Oncol. Rep. 2016, 36, 2009–2016. [Google Scholar] [CrossRef]
- Yu, M.; Liu, Z.; Liu, Y.; Zhou, X.; Sun, F.; Liu, Y.; Li, L.; Hua, S.; Zhao, Y.; Gao, H.; et al. PTP1B markedly promotes breast cancer progression and is regulated by miR-193a-3p. FEBS J. 2019, 286, 1136–1153. [Google Scholar] [CrossRef]
- Ostman, A.; Frijhoff, J.; Sandin, A.; Bohmer, F. Regulation of protein tyrosine phosphatases by reversible oxidation. J. Biochem. 2011, 150, 345–356. [Google Scholar] [CrossRef]
- Ross, S.H.; Lindsay, Y.; Safrany, S.T.; Lorenzo, O.; Villa, F.; Toth, R.; Clague, M.J.; Downes, C.P.; Leslie, N.R. Differential redox regulation within the PTP superfamily. Cell. Signal. 2007, 19, 1521–1530. [Google Scholar] [CrossRef]
- Applegate, K.R.; Glomset, J.A. Computer-based modeling of the conformation and packing properties of docosahexaenoic acid. J. Lipid Res. 1986, 27, 658–680. [Google Scholar] [PubMed]
- Scapin, G.; Patel, S.; Patel, V.; Kennedy, B.; Asante-Appiah, E. The structure of apo protein-tyrosine phosphatase 1B C215S mutant: More than just an S → O change. Protein Sci. 2001, 10, 1596–1605. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Zhang, J.; Lu, S.; Huang, W.; Geng, L.; Shen, Q.; Zhang, J. The mechanism of allosteric inhibition of protein tyrosine phosphatase 1B. PLoS ONE 2014, 9, e97668. [Google Scholar] [CrossRef] [PubMed]
- Hoff, R.H.; Hengge, A.C.; Wu, L.; Keng, Y.F.; Zhang, Z.Y. Effects on general acid catalysis from mutations of the invariant tryptophan and arginine residues in the protein tyrosine phosphatase from Yersinia. Biochemistry 2000, 39, 46–54. [Google Scholar] [CrossRef] [PubMed]
- Bellomo, E.; Birla Singh, K.; Massarotti, A.; Hogstrand, C.; Maret, W. The metal face of protein tyrosine phosphatase 1B. Coord. Chem. Rev. 2016, 327–328, 70–83. [Google Scholar] [CrossRef] [PubMed]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kuban-Jankowska, A.; Gorska-Ponikowska, M.; Sahu, K.K.; Kostrzewa, T.; Wozniak, M.; Tuszynski, J. Docosahexaenoic Acid Inhibits PTP1B Phosphatase and the Viability of MCF-7 Breast Cancer Cells. Nutrients 2019, 11, 2554. https://doi.org/10.3390/nu11112554
Kuban-Jankowska A, Gorska-Ponikowska M, Sahu KK, Kostrzewa T, Wozniak M, Tuszynski J. Docosahexaenoic Acid Inhibits PTP1B Phosphatase and the Viability of MCF-7 Breast Cancer Cells. Nutrients. 2019; 11(11):2554. https://doi.org/10.3390/nu11112554
Chicago/Turabian StyleKuban-Jankowska, Alicja, Magdalena Gorska-Ponikowska, Kamlesh Kumar Sahu, Tomasz Kostrzewa, Michal Wozniak, and Jack Tuszynski. 2019. "Docosahexaenoic Acid Inhibits PTP1B Phosphatase and the Viability of MCF-7 Breast Cancer Cells" Nutrients 11, no. 11: 2554. https://doi.org/10.3390/nu11112554
APA StyleKuban-Jankowska, A., Gorska-Ponikowska, M., Sahu, K. K., Kostrzewa, T., Wozniak, M., & Tuszynski, J. (2019). Docosahexaenoic Acid Inhibits PTP1B Phosphatase and the Viability of MCF-7 Breast Cancer Cells. Nutrients, 11(11), 2554. https://doi.org/10.3390/nu11112554