Extra-Virgin Olive Oil Modifies the Changes Induced in Non-Nervous Organs and Tissues by Experimental Autoimmune Encephalomyelitis Models
Abstract
1. Introduction
2. Material and Methods
2.1. Animals
2.2. Experimental Protocol
2.3. Sample Preparation and Study
2.4. Clinical Score Evaluation
2.5. Statistics
3. Results
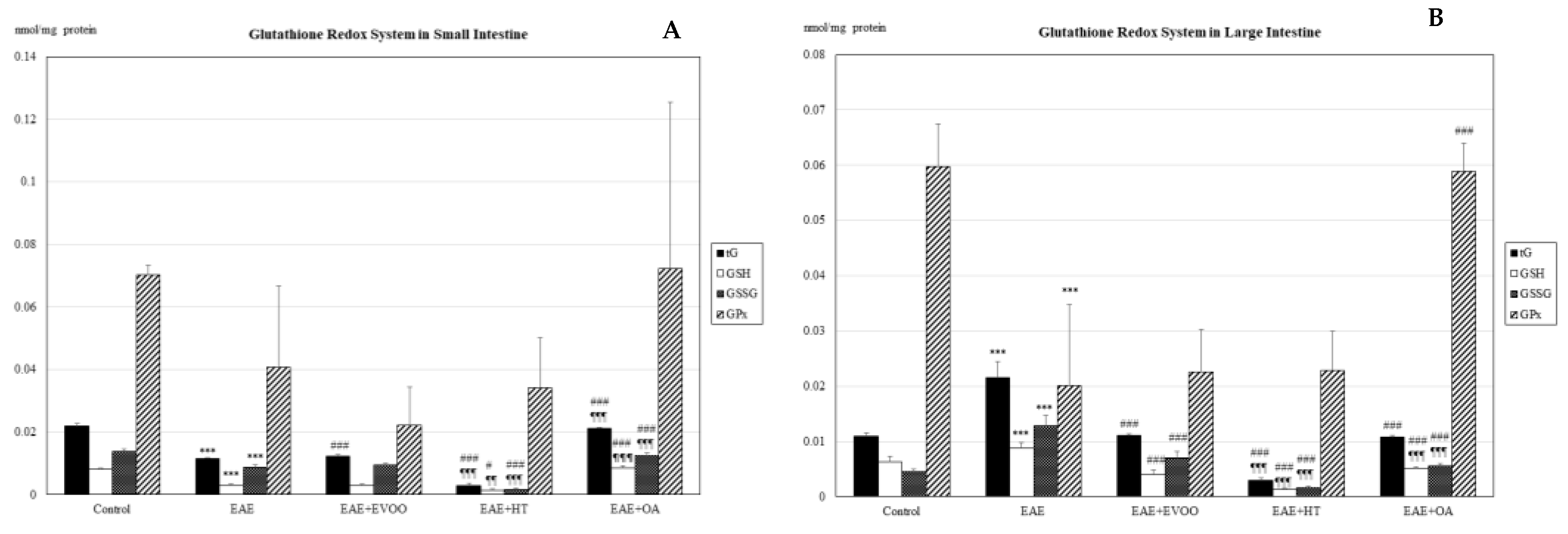
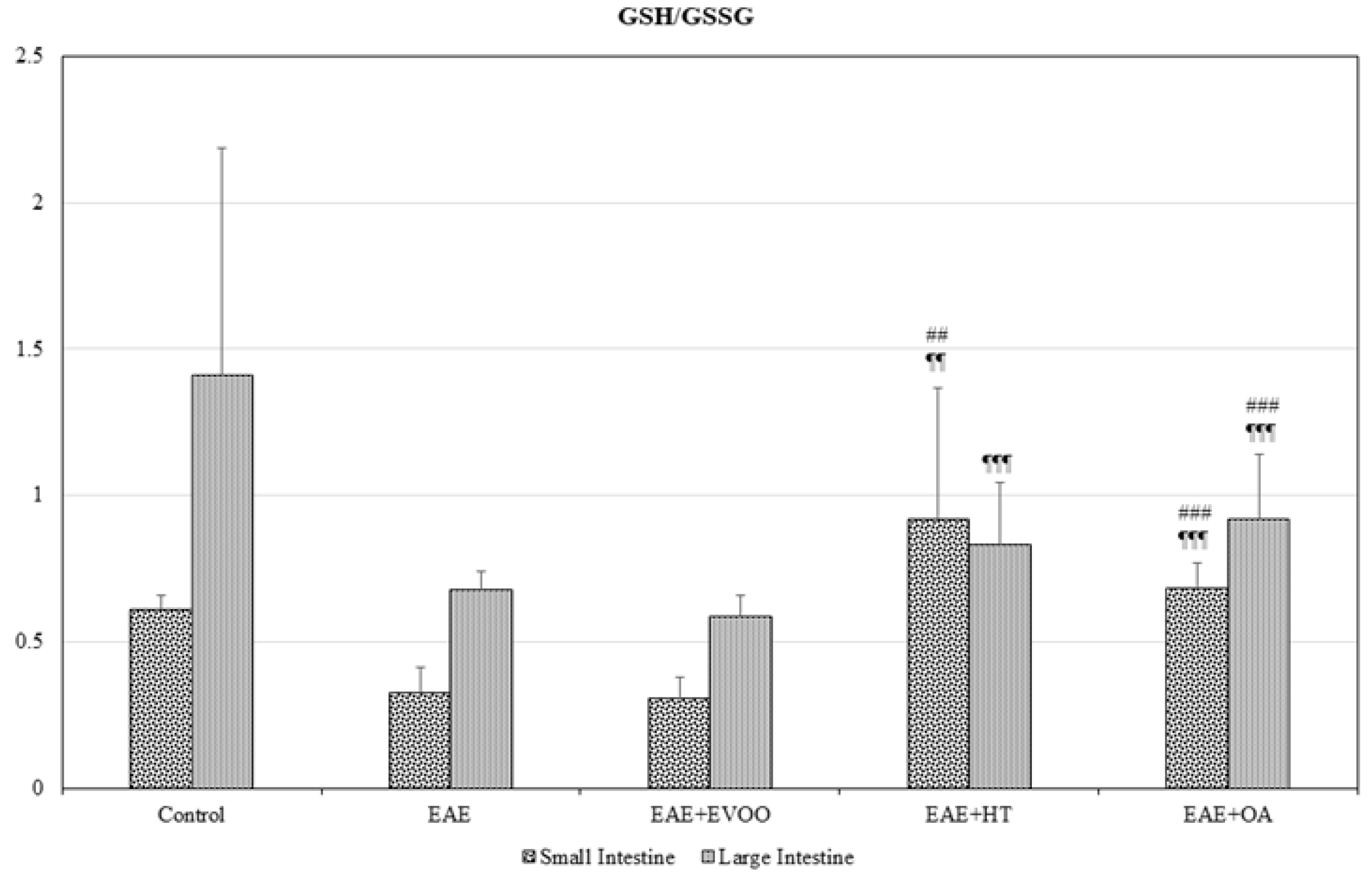
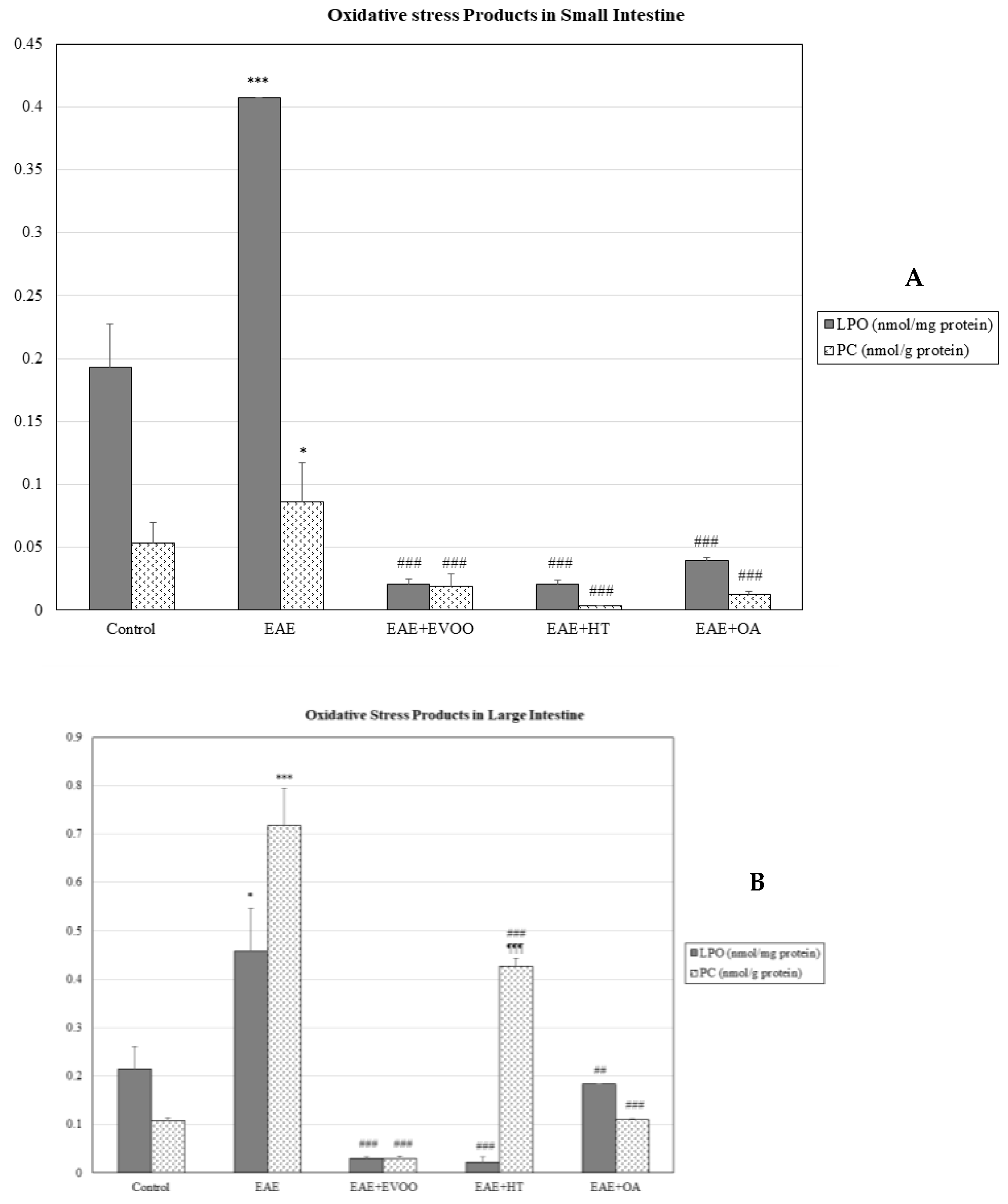
3.1. Oxidative Stress in Different Body Organs
3.2. EVOO, HT, and OA against the Oxidative Stress of EAE
3.3. Correlation of LPS and LBP with LPO and CP
3.4. EVOO, HT, and OA against the Microbiota
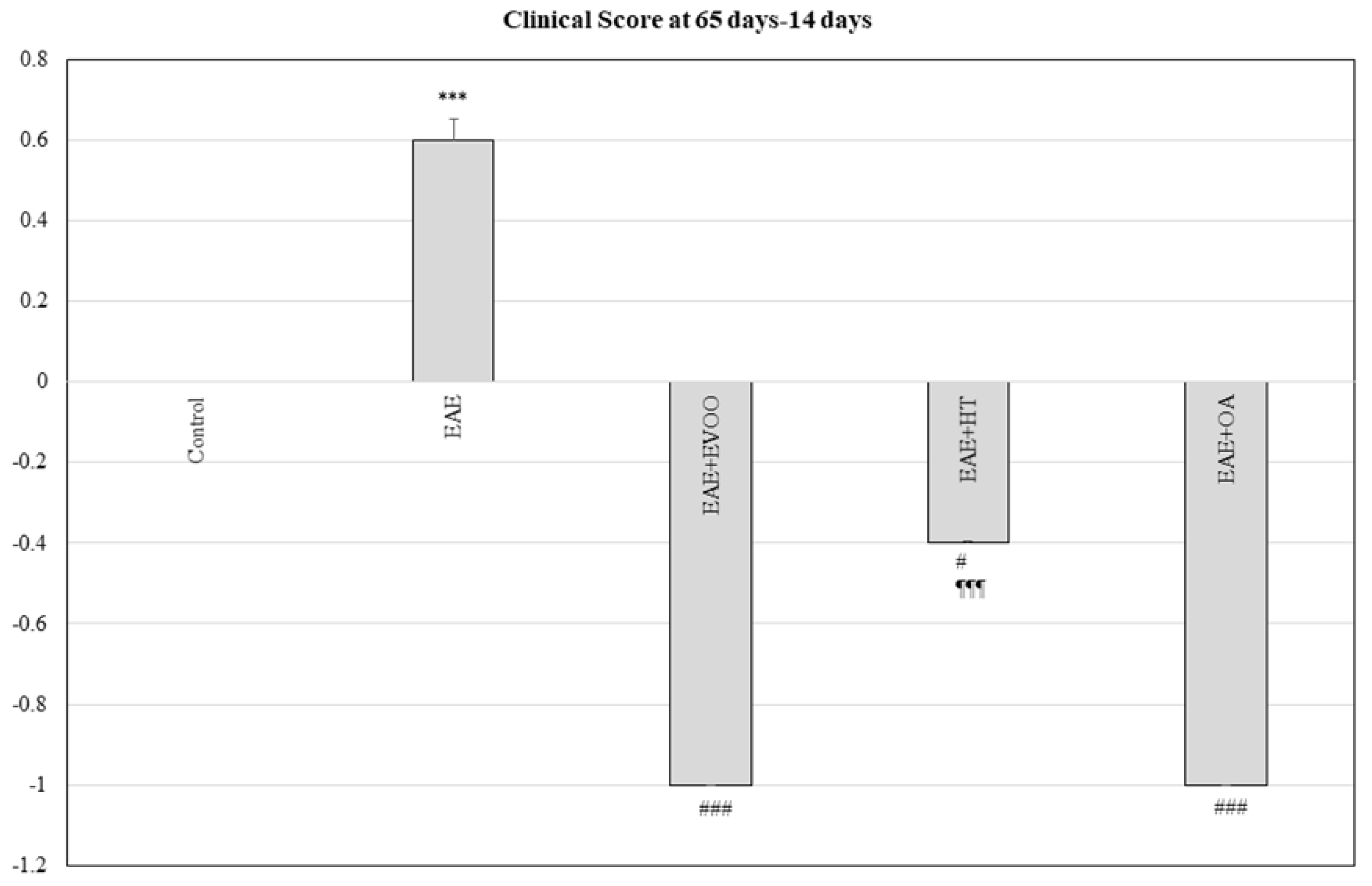
3.5. Clinical Score at 65 Days minus 14 Days and Correlation with LPS, LBP, LPO, and CP
4. Discussion
5. Conclusions
- (1)
- The oxidative damage of the EAE not only affects the CNS but also the principal body organs (small and large intestines, liver, kidney, and heart).
- (2)
- The bacterial microbiota endotoxin seems to be implicated in the production of inflammatory phenomena and subsequent oxidative stress in the intestinal tissue and in other organs.
- (3)
- Treatment with EVOO, HT, and OA reduces the bacterial endotoxin levels in the intestines at the same time as minimizing the oxidative damage in extra-nervous organs.
- (4)
- EVOO, HT, and OA improve the clinical score of the disease itself.
- (1)
- verifying the mechanism by which the intestinal microbiota is responsible for the inflammatory phenomena and the oxidative damage produced by the EAE and MS, not only in the CNS, but also in other organs;
- (2)
- finding out the molecular action mechanisms of LPS in the phenomena leading to the transendothelial migration of the immune system in the CNS and other organs affected by the EAE and MS; and
- (3)
- identifying the action mechanism/s of the EVOO in its protective effects in EAE and MS.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Compston, A.; Coles, A. Multiple sclerosis. Lancet 2008, 372, 1502–1517. [Google Scholar] [CrossRef]
- Ortiz, G.G.; Pacheco-Moisés, F.P.; Bitzer-Quintero, O.K.; Ramírez-Anguiano, A.C.; Flores-Alvarado, L.J.; Ramírez-Ramírez, V.; Macias-Islas, M.A.; Torres-Sánchez, E.D. Immunology and oxidative stress in multiple sclerosis: Clinical and basic approach. Clin. Dev. Immunol. 2013, 2013, 708659. [Google Scholar] [CrossRef] [PubMed]
- Hassanpour-Dehkordi, A.; Jivad, N. Comparison of regular aerobics and yoga on the quality of life in patients with multiple sclerosis. Med. J. Islam. Repub. Iran 2014, 6, 141. [Google Scholar]
- Escribano, B.M.; Aguilar-Luque, M.; Bahamonde, C.; Conde, C.; Lillo, R.; Sanchez-Lopez, F.; Giraldo, A.I.; Cruz, A.H.; Luque, E.; Gascon, F.; et al. Natalizumab Modifies Catecholamines Levels Present in Patients with Relapsing-Remitting Multiple Sclerosis. Curr. Pharm. Des. 2016, 22, 4876–4880. [Google Scholar] [CrossRef]
- Creanza, T.M.; Liguori, M.; Liuni, S.; Nuzziello, N.; Ancona, N. Meta-Analysis of Differential Connectivity in Gene Co-Expression Networks in Multiple Sclerosis. Int. J. Mol. Sci. 2016, 17, 936. [Google Scholar] [CrossRef]
- Cree, B.A.C. Multiple sclerosis. In Current Diagnosis and Treatment in Neurology; Brust, J.C.M., Ed.; Lange Medical Books/McGraw-Hill Medical: New York, NY, USA, 2007. [Google Scholar]
- Morandi, B.; Bramanti, P.; Bonaccorsi, I.; Montalto, E.; Oliveri, D.; Pezzino, G.; Navarra, M.; Ferlazzo, G. Role of natural killer cells in the pathogenesis and progression of multiple sclerosis. Pharmacol. Res. 2008, 57, 1–5. [Google Scholar] [CrossRef]
- Goldenberg, M. Multiple sclerosis review. P&T 2012, 37, 175–184. [Google Scholar]
- Spiro, D.B. Early onset multiple sclerosis: A review for nurse practioners. J. Pediatr. Health Care 2012, 26, 399–408. [Google Scholar] [CrossRef]
- Van Horssen, J.; Witte, M.E.; Schreibelt, G.; de Vries, H.E. Radical changes in multiple sclerosis pathogenesis. Biochim. Biophys. Acta 2011, 1812, 141–150. [Google Scholar] [CrossRef]
- Das, U.N. Is multiple sclerosis a proresolution deficiency disorder? Nutrition 2012, 28, 951–958. [Google Scholar] [CrossRef]
- Sorto-Gomez, T.E.; Ortiz, G.G.; Pacheco, F.P.; Torres-Sanchez, E.D.; Ramirez-Ramirez, V.; Macias-Islas, M.A.; de la Rosa, A.C.; Velázquez-Brizuela, I.E. Effect of fish oil on glutathione redox system in multiple sclerosis. Am. J. Neurodegener. Dis. 2016, 5, 145–151. [Google Scholar] [PubMed]
- Haider, L.; Fischer, M.T.; Frischer, J.M.; Bauer, J.; Höftberger, R.; Botond, G.; Esterbauer, H.; Binder, C.J.; Witztum, J.L.; Lassmann, H. Oxidative damage in multiple sclerosis lesions. Brain 2011, 134, 1914–1924. [Google Scholar] [CrossRef] [PubMed]
- Adamczyk-Sowa, M.; Galiniak, S.; Żyracka, E.; Grzesik, M.; Naparło, K.; Sowa, P.; Bartosz, G.; Sadowska-Bartosz, I. Oxidative Modification of Blood Serum Proteins in Multiple Sclerosis after Interferon Beta and Melatonin Treatment. Oxid. Med. Cell. Longev. 2017, 2017, 7905148. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, S.R.; Kallaur, A.P.; Simão, A.N.; Morimoto, H.K.; Lopes, J.; Panis, C.; Petenucci, D.L.; da Silva, E.; Cecchini, R.; Kaimen-Maciel, D.R.; et al. Oxidative stress in multiple sclerosis patients in clinical remission: Association with the expanded disability status scale. J. Neurol. Sci. 2012, 321, 49–53. [Google Scholar] [CrossRef] [PubMed]
- Fiorini, A.; Koudriavtseva, T.; Bucaj, E.; Coccia, R.; Foppoli, C.; Giorgi, A.; Schininà, M.E.; Di Domenico, F.; De Marco, F.; Perluigi, M. Involvement of oxidative stress in occurrence of relapses in multiple sclerosis: The spectrum of oxidatively modified serum proteins detected by proteomics and redox proteomics analysis. PLoS ONE 2013, 8, E65184. [Google Scholar]
- Mir, F.; Lee, D.; Ray, H.; Sadiq, S.A. CSF isoprostane levels are a biomarker of oxidative stress in multiple sclerosis. Neurol. Neuroimmunol. Neuroinflamm. 2014, 1, E21. [Google Scholar] [CrossRef]
- Wang, P.; Xie, K.; Wang, C.; Bi, J. Oxidative stress induced by lipid peroxidation is related with inflammation of demyelination and neurodegeneration in multiple sclerosis. Eur. Neurol. 2014, 72, 249–254. [Google Scholar] [CrossRef]
- Ochoa-Repáraz, J.; Mielcarz, D.W.; Ditrio, L.E.; Burroughs, A.R.; Foureau, D.M.; Haque-Begum, S.; Kasper, L.H. Role of gut comensal microflora in the development of experimental autoinmune encephalomyelitis. J. Immunol. 2009, 183, 6041–6050. [Google Scholar] [CrossRef]
- Berer, K.; Mues, M.; Koutrolos, M.; Rasbi, Z.A.; Boziki, M.; Johner, C.; Wekerle, H.; Krishnamoorthy, G. Commensal microbiota and myelin autoantigen cooperate to trigger autoimmune demyelination. Nature 2011, 479, 538–541. [Google Scholar] [CrossRef]
- Lee, Y.K.; Menezes, J.S.; Umesaki, Y.; Mazmanian, S.K. Proinflammatory T-cell responses to gut microbiota promote experimental autoinmune encephalomyelitis. Proc. Natl. Acad. Sci. USA 2011, 108, 4615–4622. [Google Scholar] [CrossRef]
- Buscarinu, M.C.; Romano, S.; Mechelli, R.; Pizzolato Umeton, R.; Ferraldeschi, M.; Fornasiero, A.; Reniè, R.; Cerasoli, B.; Morena, E.; Romano, C.; et al. Intestinal Permeability in Relapsing-Remitting Multiple Sclerosis. Neurotherapeutics 2018, 15, 68–74. [Google Scholar] [CrossRef] [PubMed]
- Escribano, B.M.; Medina-Fernández, F.J.; Aguilar-Luque, M.; Agüera, E.; Feijoo, M.; Garcia-Maceira, F.I.; Lillo, R.; Vieyra-Reyes, P.; Giraldo, A.I.; Luque, E.; et al. Lipopolysaccharide Binding Protein and Oxidative Stress in a Multiple Sclerosis Model. Neurotherapeutics 2017, 14, 199–211. [Google Scholar] [CrossRef] [PubMed]
- Escribano, B.M.; Luque, E.; Aguilar-Luque, M.; Feijóo, M.; Caballero-Villarraso, J.; Torres, L.A.; Ramirez, V.; García-Maceira, F.I.; Agüera, E.; Santamaria, A.; et al. Dose-dependent S-allyl cysteine ameliorates multiple sclerosis disease related pathology by reducing oxidative stress and biomarkers of dysbiosis in experimental autoimmune encephalomyelitis. Eur. J. Pharmacol. 2017, 815, 266–273. [Google Scholar] [CrossRef] [PubMed]
- Conde, C.; Escribano, B.M.; Luque, E.; Aguilar-Luque, M.; Feijóo, M.; Ochoa, J.J.; LaTorre, M.; Giraldo, A.I.; Lillo, R.; Agüera, E.; et al. The protective effect of extra-virgin olive oil inthe experimental model of multiple sclerosis in the rat. Nutr. Neurosci. 2018. [Google Scholar] [CrossRef]
- Farzi, A.; Fröhlich, E.E.; Holzer, P. Gut Microbiota and the Neuroendocrine System. Neurotherapeutics 2018, 15, 5–22. [Google Scholar] [CrossRef]
- Middleton, E., Jr. Effect of plant flavonoids on immune and inflammatory cell function. Adv. Exp. Med. Biol. 1998, 439, 175–182. [Google Scholar]
- Hollman, P.C.; Katan, M.B. Health effects and bioavailability of dietary flavonols. Free Radic. Res. 1999, 31, S75–S80. [Google Scholar] [CrossRef]
- Eastwood, M.A. Interaction of dietary antioxidants in vivo: How fruit and vegetables prevent disease? QJM Mon. J. Assoc. Phys. 1999, 92, 527–530. [Google Scholar] [CrossRef]
- Santangelo, C.; Vari, R.; Scazzocchio, B.; De Sanctis, P.; Giovannini, C.; D’Archivio, M.; Masella, R. Anti-inflammatory activity of extra virgin olive oil polyphenols: Which role in the prevention and treatment of immune-mediated inflammatory diseases? Endocr. Metab. Immune Disord. Drug Targets 2018, 18, 36–50. [Google Scholar] [CrossRef]
- Liuzzi, G.M.; Latronico, T.; Branà, M.T.; Gramegna, P.; Coniglio, M.G.; Rossano, R.; Larocca, M.; Riccio, P. Structure-dependent inhibition of gelatinases by dietary antioxidants in rat astrocytes and sera of multiple sclerosis patients. Neurochem. Res. 2011, 36, 518–527. [Google Scholar] [CrossRef]
- Angeloni, C.; Malaguti, M.; Barbalace, M.C.; Hrelia, S. Bioactivity of Olive Oil Phenols in Neuroprotection. Int. J. Mol. Sci. 2017, 18, E2230. [Google Scholar] [CrossRef] [PubMed]
- Rosenberg, G.A.; Dencoff, J.E.; Correa, N., Jr.; Reiners, M.; Ford, C.C. Effect of steroids on CSF matrix metalloproteinases in multiple sclerosis: Relation to blood-brain barrier injury. Neurology 1996, 46, 1626–1632. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, S.M.; Lau, L.; Yong, V.W. MMPs in the central nervous system: Where the good guys go bad. Semin. Cell. Dev. Biol. 2008, 19, 42–51. [Google Scholar] [CrossRef] [PubMed]
- Martín, R.; Carvalho-Tavares, J.; Hernández, M.; Arnés, M.; Ruiz-Gutiérrez, V.; Nieto, M.L. Beneficial actions of oleanolic acid in an experimental model of multiple sclerosis: A potential therapeutic role. Biochem. Pharmacol. 2010, 79, 198–208. [Google Scholar] [CrossRef]
- Martín, R.; Hernández, M.; Córdova, C.; Nieto, M.L. Natural triterpenes modulate immune-inflammatory markers of experimental autoimmune encephalomyelitis: Therapeutic implications for multiple sclerosis. Br. J. Pharmacol. 2012, 166, 1708–1723. [Google Scholar] [CrossRef]
- Reeves, P.G.; Nielsen, F.H.; Fahey, G.C., Jr. AIN-93 purified diets for laboratory rodents: Final report of the American Institute of Nutrition ad hoc writing committee on the reformulation of the AIN-76A rodent diet. J. Nutr. 1993, 123, 1939–1951. [Google Scholar] [CrossRef]
- Flohe, L.; Gunzler, W.A. Assays of glutathione peroxidase. Methods Enzymol. 1984, 105, 114–121. [Google Scholar]
- Levine, R.L.; Garland, D.; Oliver, C.N.; Amici, A.; Climent, I.; Lenz, A.G. Determination of carbonyl content in oxidatively modified proteins. Methods Enzymol. 1990, 186, 464–478. [Google Scholar]
- Perez-Nievas, B.G.; Garcia-Bueno, B.; Madrigal, J.L.; Leza, J.C. Chronic immobilisation stress ameliorates clinical score and neuroinflammation in a MOG-induced EAE in Dark Agouti rats: Mechanisms implicated. J. Neuroinflamm. 2010, 7, 60. [Google Scholar] [CrossRef]
- Yang, X.; Yan, J.; Feng, J. Surfactant protein A is expressed in the central nervous system of rats with experimental autoimmune encephalomyelitis, and suppresses inflammation in human astrocytes and microglia. Mol. Med. Rep. 2017, 15, 3555–3565. [Google Scholar] [CrossRef]
- Luo, J.M.; Wan, Y.S.; Liu, Z.Q.; Wang, G.R.; Floros, J.; Zhou, H.H. Regularity of distribution of immunoreactive pulmonary surfactant protein A in rat tissues. Int. J. Mol. Med. 2004, 14, 343–351. [Google Scholar] [CrossRef] [PubMed]
- Schob, S.; Schicht, M.; Sel, S.; Stiller, D.; Kekulé, A.S.; Paulsen, F.; Maronde, E.; Bräuer, L. The detection of surfactant proteins a, b, c and d in the human brain and their regulation in cerebral infarction, autoimmune conditions and infections of the CNS. PLoS ONE 2013, 8, E74412. [Google Scholar] [CrossRef]
- Bourbon, J.R.; Ducroc, R. Pulmonary surfactant protein A (SP-A) is expressed by epithelial cells of small and large intestine. J. Biol. Chem. 1995, 270, 12162–12169. [Google Scholar]
- Snyder, G.D.; Oberley-Deegan, R.E.; Goss, K.L.; Romig-Martin, S.A.; Stoll, L.L.; Snyder, J.M.; Weintraub, N.L. Surfactant protein D is expressed and modulates inflammatory responses in human coronary artery smooth muscle cells. Am. J. Physiol. Heart Circ. Physiol. 2008, 294, H2053–H2059. [Google Scholar] [CrossRef] [PubMed]
- Aparicio-Soto, M.; Sánchez-Hidalgo, M.; Rosillo, M.Á.; Castejón, M.L.; Alarcón-de-la-Lastra, C. Extra virgin olive oil: A key functional food for prevention of immune-inflammatory diseases. Food Funct. 2016, 7, 4492–4505. [Google Scholar] [CrossRef] [PubMed]
- Quiles, J.L.; Barja, G.; Battino, M.; Mataix, J.; Solfrizzi, V. Role of Olive Oil and Monounsaturated Fatty Acids in Mitochondrial Oxidative Stress and Aging. Nutr. Rev. 2006, 64, S31–S39. [Google Scholar] [CrossRef]
- Blekas, G.; Vassilakis, C.; Harizanis, C.; Tsimidou, M.; Boskou, D.G. Biophenols in table olives. J. Agric. Food Chem. 2002, 50, 3688–3692. [Google Scholar] [CrossRef]
- Granados-Principal, S.; Quiles, J.L.; Ramirez-Tortosa, C.L.; Sanchez-Rovira, P.; Ramirez-Tortosa, M.C. Hydroxytyrosol: From laboratory investigations to future clinical trials. Nutr. Rev. 2010, 68, 191–206. [Google Scholar] [CrossRef]
- Kitsati, N.; Mantzaris, M.D.; Galaris, D. Hydroxytyrosol inhibits hydrogen peroxide-induced apoptotic signaling via labile iron chelation. Redox Biol. 2016, 10, 233–242. [Google Scholar] [CrossRef]
- González-Correa, J.A.; Muñoz-Marín, J.; Arrebola, M.M.; Guerrero, A.; Narbona, F.; López-Villodres, J.A.; De La Cruz, J.P. Dietary virgin olive oil reduces oxidative stress and cellular damage in rat brain slices subjected to hypoxia-reoxygenation. Lipids 2007, 42, 921–929. [Google Scholar] [CrossRef]
- Pitozzi, V.; Jacomelli, M.; Catelan, D.; Servili, M.; Taticchi, A.; Biggeri, A.; Dolara, P.; Giovannelli, L. Long-term dietary extra-virgin olive oil rich in polyphenols reverses age-related dysfunctions in motor coordination and contextual memory in mice: Role of oxidative stress. Rejuvenation Res. 2012, 15, 601–612. [Google Scholar] [CrossRef] [PubMed]
- D’Angelo, S.; Manna, C.; Migliardi, V.; Mazzoni, O.; Morrica, P.; Capasso, G.; Pontoni, G.; Galletti, P.; Zappia, V. Pharmacokinetics and metabolism of hydroxytyrosol, a natural antioxidant from olive oil. Drug Metab. Dispos. 2001, 29, 1492–1498. [Google Scholar] [PubMed]
- Tremlett, H.; Waubant, E. The Gut Microbiota and Pediatric Multiple Sclerosis: Recent Findings. Neurotherapeutics 2018, 15, 102–108. [Google Scholar] [CrossRef] [PubMed]
- Dietrich, J.B. The adhesion molecule ICAM-1 and its regulation in relation with the blood-brain barrier. J. Neuroimmunol. 2002, 128, 58–68. [Google Scholar] [CrossRef]
- Lee, B.K.; Lee, W.J.; Jung, Y.-S. Chrysin Attenuates VCAM-1 Expression and Monocyte Adhesion in Lipopolysaccharide-Stimulated Brain Endothelial Cells by Preventing NF-κB Signaling. Int. J. Mol. Sci. 2017, 18, 1424. [Google Scholar] [CrossRef] [PubMed]
- Tasset, I.; Agüera, E.; Sánchez-López, F.; Feijóo, M.; Giraldo, A.I.; Cruz, A.H.; Gascón, F.; Túnez, I. Peripheral oxidative stress in relapsing–remitting multiple sclerosis. Clin. Biochem. 2012, 45, 440–444. [Google Scholar] [CrossRef]
- De Punder, K.; Pruimboom, L. Stress induces endotoxemia and lowgradeinflammation by increasing barrier permeability. Front. Immunol. 2015, 6, 223. [Google Scholar] [CrossRef] [PubMed]

| Glutathione Redox System | |||||
|---|---|---|---|---|---|
| Heart | |||||
| tG (nmol/mg protein) | GSH (nmol/mg protein) | GSSG (nmol/mg protein) | GSH/GSSG | GPx (nmol/mg protein) | |
| Control | 0.01086 ± 0.00019 | 0.00580 ± 0.00031 | 0.00506 ± 0.00031 | 1.152 ± 0.308 | 0.01326 ± 0.02017 |
| EAE | 0.01180 ± 0.00074 a | 0.00597 ± 0.00035 | 0.00583 ± 0.00105 | 1.062 ± 0.235 | 0.11050 ± 0.02344 a |
| EAE + EVOO | 0.01088 ± 0.00057 d | 0.00804 ± 0.00112 d | 0.00283 ± 0.00075 d | 3.069 ± 1.000 d | 0.03539 ± 0.02955 d |
| EAE + HT | 0.00377 ± 0.00025 d,g | 0.00166 ± 0.00005 d,g | 0.00211 ± 0.00029 d | 0.802 ± 0.386 g | 0.00616 ± 0.00126 d,g |
| EAE + OA | 0.02201 ± 0.00131 d,g | 0.00609 ± 0.00215 g | 0.01592 ± 0.00121 d,g | 0.391 ± 0.552 d,g | 0.01283 ± 0.00190 d,g |
| Kidney | |||||
| tG (nmol/mg protein) | GSH (nmol/mg protein) | GSSG (nmol/mg protein) | GSH/GSSG | GPx (nmol/mg protein) | |
| Control | 0.01827 ± 0.00015 | 0.00618 ± 0.00089 | 0.01209 ± 0.00095 | 0.518 ± 0.240 | 0.04728 ± 0.00149 |
| EAE | 0.01170 ± 0.00047 | 0.00473 ± 0.00128 a | 0.00698 ± 0.00152 a | 0.737 ± 0.137 | 0.04170 ± 0.01636 |
| EAE + EVOO | 0.01135 ± 0.00061 | 0.00866 ± 0.00057 d | 0.00270 ± 0.00097 d | 3.000 ± 1.000 d | 0.05123 ± 0.02927 |
| EAE + HT | 0.00305 ± 0.00059 | 0.00106 ± 0.00032 d,g | 0.00199 ± 0.00033 d | 0.533 ± 0.230 g | 0.05620 ± 0.02231 |
| EAE + OA | 0.01970 ± 0.00161 | 0.00749 ± 0.00073 d,g | 0.01221 ± 0.00136 d,g | 0.619 ± 0.087 g | 0.05642 ± 0.02262 |
| Liver | |||||
| tG (nmol/mg protein) | GSH (nmol/mg protein) | GSSG (nmol/mg protein) | GSH/GSSG | GPx (nmol/mg protein) | |
| Control | 0.01078 ± 0.00025 | 0.00773 ± 0.00128 | 0.00305 ± 0.00123 | 3.000 ± 2.000 | 0.09980 ± 0.00756 |
| EAE | 0.03050 ± 0.04167 | 0.00470 ± 0.00039 | 0.02580 ± 0.04174 | 0.868 ± 0.343 | 0.03595 ± 0.02465 |
| EAE + EVOO | 0.01121 ± 0.00031 | 0.03236 ± 0.04630 | 0.02116 ± 0.04628 | 5.000 ± 7.081 d | 0.08238 ± 0.01286 |
| EAE + HT | 0.00473 ± 0.00048 d | 0.00154 ± 0.00019 d,g | 0.00319 ± 0.00052 | 0.494 ± 0.240 g | 0.19775 ± 0.05116 |
| EAE + OA | 0.02200 ± 0.00049 | 0.00782 ± 0.00034 g | 0.01418 ± 0.00066 | 0.553 ± 0.047 g | 0.26600 ± 0.07108 |
| Oxidative Stress Products | ||
|---|---|---|
| Heart | ||
| LPO (nmol/mg protein) | CP (nmol/g protein) | |
| Control | 0.11158 ± 0.02087 | 0.03420 ± 0.00388 |
| EAE | 0.41875 ± 0.00000 a | 0.41875 ± 0.02344 a |
| EAE + EVOO | 0.09625 ± 0.02918 d | 0.02249 ± 0.00986 d |
| EAE + HT | 0.00769 ± 0.00065 d | 0.00616 ± 0.00126 d,g |
| EAE + OA | 0.02064 ± 0.00496 d,g | 0.01283 ± 0.00190 d |
| Kidney | ||
| LPO (nmol/mg protein) | CP (nmol/g protein) | |
| Control | 0.19280 ± 0.03422 | 0.00743 ± 0.00112 |
| EAE | 0.45750 ± 0.01063 a | 0.08493 ± 0.02702a |
| EAE + EVOO | 0.08875 ± 0.01001 d | 0.02920 ± 0.01707 d |
| EAE + HT | 0.00683 ± 0.00111 d,g | 0.00326 ± 0.00039 d |
| EAE + OA | 0.01867 ± 0.00159 d,g | 0.01989 ± 0.00181 d |
| Liver | ||
| LPO (nmol/mg protein) | CP (nmol/g protein) | |
| Control | 0.11140 ± 0.02013 | 0.01076 ± 0.00220 |
| EAE | 0.40250 ± 0.07214 a | 0.09568 ± 0.02642 a |
| EAE + EVOO | 0.10663 ± 0.00616 f | 0.03815 ± 0.01375 d |
| EAE + HT | 0.00826 ± 0.00189 d,g | 0.00660 ± 0.00083 d,h |
| EAE + OA | 0.02768 ± 0.00533 d,g | 0.03332 ± 0.00207 d |
| Pearson’s Correlation | ||
|---|---|---|
| Small Intestine | ||
| LPO | CP | |
| LPS | 0.636 (0.001) | 0.542 (0.008) |
| LBP | 0.816 (0.000) | 0.777 (0.000) |
| Large Intestine | ||
| LPO | CP | |
| LPS | 0.759 (0.000) | 0.581 (0.004) |
| LBP | 0.703 (0.000) | 0.747 (0.000) |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Conde, C.; Escribano, B.M.; Luque, E.; Feijóo, M.; Caballero-Villarraso, J.; Valdelvira, M.E.; Ochoa-Sepúlveda, J.J.; Lillo, R.; Paz, E.; Santamaría, A.; et al. Extra-Virgin Olive Oil Modifies the Changes Induced in Non-Nervous Organs and Tissues by Experimental Autoimmune Encephalomyelitis Models. Nutrients 2019, 11, 2448. https://doi.org/10.3390/nu11102448
Conde C, Escribano BM, Luque E, Feijóo M, Caballero-Villarraso J, Valdelvira ME, Ochoa-Sepúlveda JJ, Lillo R, Paz E, Santamaría A, et al. Extra-Virgin Olive Oil Modifies the Changes Induced in Non-Nervous Organs and Tissues by Experimental Autoimmune Encephalomyelitis Models. Nutrients. 2019; 11(10):2448. https://doi.org/10.3390/nu11102448
Chicago/Turabian StyleConde, Cristina, Begoña M. Escribano, Evelio Luque, Montserrat Feijóo, Javier Caballero-Villarraso, Manuel E. Valdelvira, Juan J. Ochoa-Sepúlveda, Rafael Lillo, Elier Paz, Abel Santamaría, and et al. 2019. "Extra-Virgin Olive Oil Modifies the Changes Induced in Non-Nervous Organs and Tissues by Experimental Autoimmune Encephalomyelitis Models" Nutrients 11, no. 10: 2448. https://doi.org/10.3390/nu11102448
APA StyleConde, C., Escribano, B. M., Luque, E., Feijóo, M., Caballero-Villarraso, J., Valdelvira, M. E., Ochoa-Sepúlveda, J. J., Lillo, R., Paz, E., Santamaría, A., Agüera, E., & Túnez, I. (2019). Extra-Virgin Olive Oil Modifies the Changes Induced in Non-Nervous Organs and Tissues by Experimental Autoimmune Encephalomyelitis Models. Nutrients, 11(10), 2448. https://doi.org/10.3390/nu11102448





