The Effectiveness of Different Doses of Iron Supplementation and the Prenatal Determinants of Maternal Iron Status in Pregnant Spanish Women: ECLIPSES Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Data Collection
2.2.1. Baseline Data (on Week 12 of Gestation)
2.2.2. Data Recorded during Scheduled Study Visits
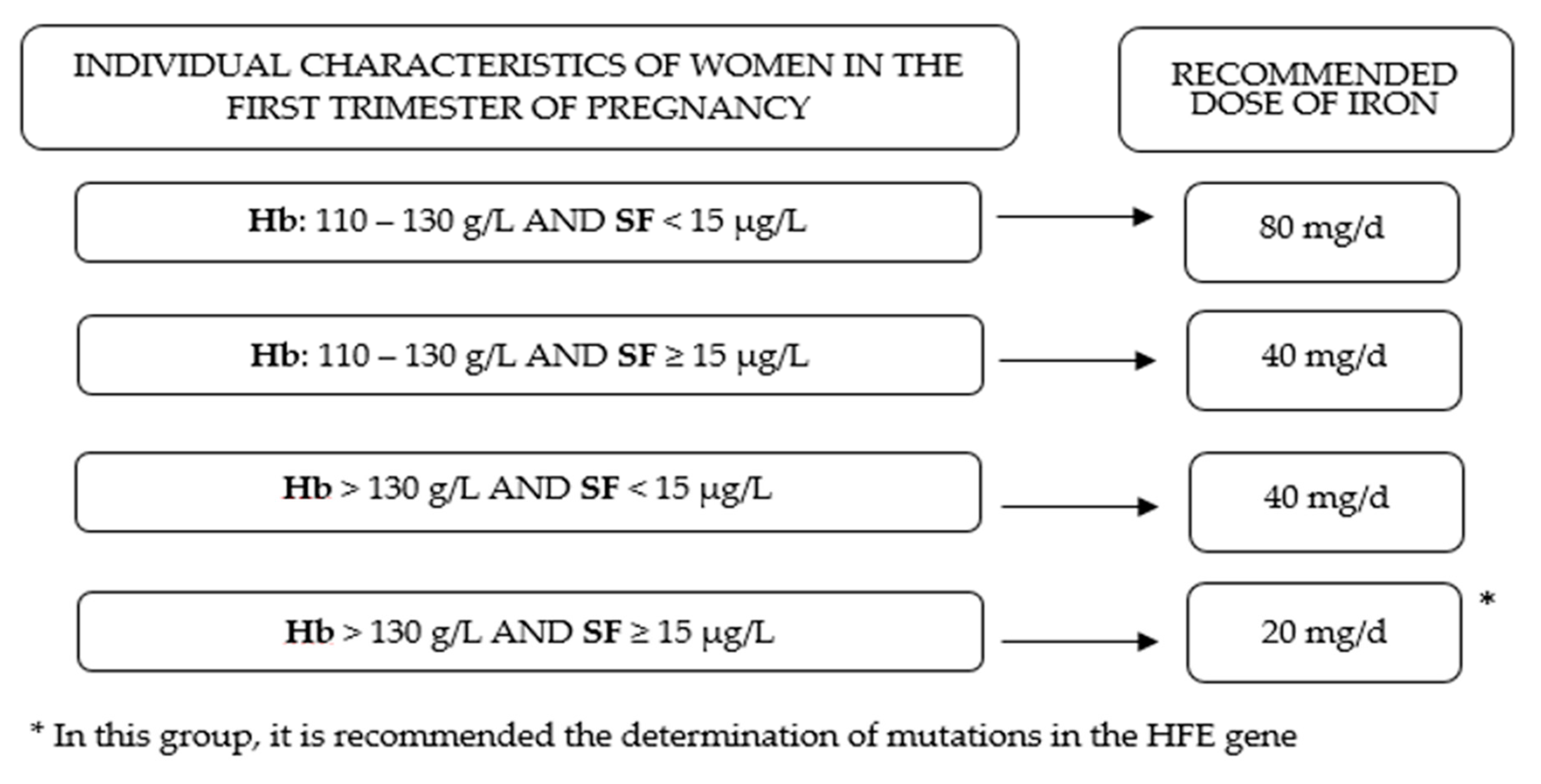
2.2.3. Definition of Iron Status
2.3. Statistical Analysis
3. Results
4. Discussion
Strengths and Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- De Benoist, B.; McLean, E.; Egli, I.; Cogswell, M. Worldwide Prevalence of Anaemia 1993–2005: WHO Global Database on Anaemia; WHO: Geneva, Switzerland, 2008. [Google Scholar]
- World Health Organization. The Global Prevalence of Anaemia in 2011; WHO: Geneva, Switzerland, 2015. [Google Scholar]
- Milman, N.; Taylor, C.L.; Merkel, J.; Brannon, P.M. Iron status in pregnant women and women of reproductive age in Europe. Am. J. Clin. Nutr. 2017, 106, 1655S–1662S. [Google Scholar] [CrossRef] [PubMed]
- The Global Library of Women’s Medicine. Available online: https://www.glowm.com/Critical_current_issue/page/25 (accessed on 22 May 2019).
- Radlowski, E.C.; Johnson, R.W. Perinatal iron deficiency and neurocognitive development. Front. Hum. Neurosci. 2013, 7, 585. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Martínez, C.; Canals, J.; Aranda, N.; Ribot, B.; Escribano, J.; Arija, V. Effects of iron deficiency on neonatal behavior at different stages of pregnancy. Early Hum. Dev. 2011, 87, 165–169. [Google Scholar] [CrossRef] [PubMed]
- Rahmati, S.; Azami, M.; Badfar, G.; Parizad, N.; Sayehmiri, K. The relationship between maternal anemia during pregnancy with preterm birth: A systematic review and meta–analysis. J. Matern. Fetal Neonatal Med. 2018, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Figueiredo, A.C.M.G.; Gomes-Filho, I.S.; Silva, R.B.; Pereira, P.P.S.; Mata, F.A.F.D.; Lyrio, A.O.; Souza, E.S.; Cruz, S.S.; Pereira, M.G. Maternal anemia and low birth weight: A systematic review and meta–analysis. Nutrients 2018, 10, 601. [Google Scholar] [CrossRef] [PubMed]
- Ribot, B.; Aranda, N.; Viteri, F.E.; Hernández-Martínez, C.; Canals, J.; Arija, V. Depleted iron stores without anaemia early in pregnancy carries increased risk of lower birthweight even when supplemented daily with moderate iron. Hum. Reprod. 2012, 27, 1260–1266. [Google Scholar] [CrossRef] [PubMed]
- Vallée, L. Fer et neurodéveloppement. Arch. Pediatr. 2017, 24, 5S18–5S22. [Google Scholar] [CrossRef]
- Todorich, B.; Pasquini, J.M.; Garcia, C.I.; Paez, P.M.; Connor, J.R. Oligodendrocytes and myelination: The role of iron. Glia 2009, 57, 467–478. [Google Scholar] [CrossRef] [PubMed]
- Beard, J. Iron Deficiency alters brain development and functioning. J. Nutr. 2003, 133, 1468S–1472S. [Google Scholar] [CrossRef] [PubMed]
- Abbaspour, N.; Hurrell, R.; Kelishadi, R. Review on iron and its importance for human health. J. Res. Med. Sci. 2014, 19, 164–174. [Google Scholar] [PubMed]
- Gupta, C.P. Role of Iron (Fe) in Body. IOSR J. Appl. Chem. 2014, 7, 38–46. [Google Scholar] [CrossRef]
- Marangoni, F.; Cetin, I.; Verduci, E.; Canzone, G.; Giovannini, M.; Scollo, P.; Corsello, G.; Poli, A. Maternal diet and nutrient requirements in pregnancy and breastfeeding. An Italian Consensus Document. Nutrients 2016, 8, 629. [Google Scholar] [CrossRef] [PubMed]
- Bothwell, T. Iron requirements in pregnancy and strategies to meet them. Am. J. Clin. Nutr. 2000, 72, 257S–264S. [Google Scholar] [CrossRef] [PubMed]
- Kominiarek, M.A.; Rajan, P. Nutrition recommendations in pregnancy and lactation. Med. Clin. N. Am. 2016, 100, 1199–1215. [Google Scholar] [CrossRef] [PubMed]
- Piñero, D.J.; Connor, J.R. Iron in the brain: An important contributor in normal and diseased states. Neuroscientist 2000, 6, 435–453. [Google Scholar] [CrossRef]
- Stevens, G.A.; Finucane, M.M.; De-Regil, L.M.; Paciorek, C.J.; Flaxman, S.R.; Branca, F.; Peña-Rosas, J.P.; Bhutta, Z.A.; Ezzati, M. Global, regional, and national trends in haemoglobin concentration and prevalence of total and severe anaemia in children and pregnant and non-pregnant women for 1995–2011: A systematic analysis of population–Representative data. Lancet Glob. Heal. 2013, 1, 16–25. [Google Scholar] [CrossRef]
- Allen, L.H. Anemia and iron deficiency: Effects on pregnancy outcome. Am. J. Clin. Nutr. 2000, 71, 1280S–1284S. [Google Scholar] [CrossRef]
- Ghio, A.J.; Hilborn, E.D.; Stonehuerner, J.G.; Dailey, L.A.; Carter, J.D.; Richards, J.H.; Crissman, K.M.; Foronjy, R.F.; Uyeminami, D.L.; Pinkerton, K.E. Particulate matter in cigarette smoke alters iron homeostasis to produce a biological effect. Am. J. Respir. Crit. Care Med. 2008, 178, 1130–1138. [Google Scholar] [CrossRef]
- Miller, E.M. The reproductive ecology of iron in women. Am. J. Phys. Anthropol. 2016, 159, S172–S195. [Google Scholar] [CrossRef]
- Balarajan, Y.; Ramakrishnan, U.; Özaltin, E.; Shankar, A.H.; Subramanian, S.V. Anaemia in low-income and middle-income countries. Lancet 2011, 378, 2123–2135. [Google Scholar] [CrossRef]
- Beutler, E.; Felitti, V.; Gelbart, T.; Waalen, J. Haematological effects of the C282Y HFE mutation in homozygous and heterozygous states among subjects of northern and southern European ancestry. Br. J. Haematol. 2003, 120, 887–893. [Google Scholar] [CrossRef]
- Gordeuk, V.R.; Brannon, P.M. Ethnic and genetic factors of iron status in women of reproductive age. Am. J. Clin. Nutr. 2017, 106, S1594–S1599. [Google Scholar] [CrossRef] [PubMed]
- Peña-Rosas, J.P.; Viteri, F.E. Effects and safety of preventive oral iron or iron+folic acid supplementation for women during pregnancy. Cochrane Database Syst. Rev. 2009, CD004736. [Google Scholar] [CrossRef]
- Arija, V.; Ribot, B.; Aranda, N. Prevalence of iron deficiency states and risk of haemoconcentration during pregnancy according to initial iron stores and iron supplementation. Public Health Nutr. 2013, 16, 1371–1378. [Google Scholar] [CrossRef] [PubMed]
- Rayman, M.P.; Barlis, J.; Evans, R.W.; Redman, C.W.G.; King, L.J. Abnormal iron parameters in the pregnancy syndrome preeclampsia. Am. J. Obstet. Gynecol. 2002, 187, 412–418. [Google Scholar] [CrossRef] [PubMed]
- Gaillard, R.; Eilers, P.H.C.; Yassine, S.; Hofman, A.; Steegers, E.A.P.; Jaddoe, V.W.V. Risk factors and consequences of maternal anaemia and elevated haemoglobin levels during pregnancy: A population-based prospective cohort study. Paediatr. Perinat. Epidemiol. 2014, 28, 213–226. [Google Scholar] [CrossRef]
- Aranda, N.; Hernández-Martínez, C.; Arija, V.; Ribot, B.; Canals, J. Haemoconcentration risk at the end of pregnancy: Effects on neonatal behaviour. Public Health Nutr. 2017, 20, 1405–1413. [Google Scholar] [CrossRef] [PubMed]
- Aranda, N.; Ribot, B.; Viteri, F.E.; Cavallé, P.; Arija, V. Predictors of haemoconcentration at delivery: Association with low birth weight. Eur. J. Nutr. 2013, 52, 1631–1639. [Google Scholar] [CrossRef] [PubMed]
- Arija, V.; Fargas, F.; March, G.; Abajo, S.; Basora, J.; Canals, J.; Ribot, B.; Aparicio, E.; Serrat, N.; Hernández-Martínez, C.; et al. Adapting iron dose supplementation in pregnancy for greater effectiveness on mother and child health: Protocol of the ECLIPSES randomized clinical trial. BMC Pregnancy Childbirth 2014, 14, 33. [Google Scholar] [CrossRef]
- Aranda, N.; Ribot, B.; Garcia, E.; Viteri, F.E.; Arija, V. Pre-pregnancy iron reserves, iron supplementation during pregnancy, and birth weight. Early Hum. Dev. 2011, 87, 791–797. [Google Scholar] [CrossRef]
- Trinidad Rodríguez, I.; Fernández Ballart, J.; Cucó Pastor, G.; Biarnés Jordà, E.; Arija Val, V. Validation of a short questionnaire on frequency of dietary intake: Reproducibility and validity. Nutr. Hosp. 2008, 23, 242–252. [Google Scholar]
- Trichopoulou, A.; Costacou, T.; Bamia, C.; Trichopoulos, D. Adherence to a Mediterranean diet and survival in a Greek population. N. Engl. J. Med. 2003, 348, 2599–2608. [Google Scholar] [CrossRef] [PubMed]
- Fagerström, K.O. Measuring degree of physical dependence to tobacco smoking with reference to individualization of treatment. Addict. Behav. 1978, 3, 235–241. [Google Scholar] [CrossRef]
- Craig, C.L.; Marshall, A.L.; Sjöström, M.; Bauman, A.E.; Booth, M.L.; Ainsworth, B.E.; Pratt, M.; Ekelund, U.; Yngve, A.; Sallis, J.F.; et al. International physical activity questionnaire: 12-country reliability and validity. Med. Sci. Sports Exerc. 2003, 35, 1381–1395. [Google Scholar] [CrossRef] [PubMed]
- Classificació catalana d’ocupacions 2011 (CCO–2011). Adaptació de la CNO–2011. Available online: https://www.idescat.cat/serveis/biblioteca/docs/cat/cco2011.pdf (accessed on 22 May 2019).
- Brannon, P.M.; Taylor, C.L. Iron supplementation during pregnancy and infancy: Uncertainties and implications for research and policy. Nutrients 2017, 9, 1327. [Google Scholar] [CrossRef] [PubMed]
- Aranda, N.; Viteri, F.E.; Montserrat, C.; Arija, V. Effects of C282Y, H63D, and S65C HFE gene mutations, diet, and life-style factors on iron status in a general Mediterranean population from Tarragona, Spain. Ann. Hematol. 2010, 89, 767–773. [Google Scholar] [CrossRef] [PubMed]
- Philpott, C.C. Molecular aspects of iron absorption: Insights into the role of HFE in hemochromatosis. Hepatology. 2002, 35, 993–1001. [Google Scholar] [CrossRef]
- Fisher, A.L.; Nemeth, E. Iron homeostasis during pregnancy. Am. J. Clin. Nutr. 2017, 106, S1567–S1574. [Google Scholar] [CrossRef]
- Anderson, G.J.; Frazer, D.M. Current understanding of iron homeostasis. Am. J. Clin. Nutr. 2017, 106, S1559–S1566. [Google Scholar] [CrossRef]
- Milman, N.; Bergholt, T.; Eriksen, L.; Byg, K.E.; Graudal, N.; Pedersen, P.; Hertz, J. Iron prophylaxis during pregnancy—How much iron is needed? A randomized dose-response study of 20–80 mg ferrous iron daily in pregnant women. Acta Obstet. Gynecol. Scand. 2005, 84, 238–247. [Google Scholar] [CrossRef]
- Milman, N.; Byg, K.E.; Bergholt, T.; Eriksen, L.; Hvas, A.M. Body iron and individual iron prophylaxis in pregnancy—Should the iron dose be adjusted according to serum ferritin? Ann. Hematol. 2006, 85, 567–573. [Google Scholar] [CrossRef] [PubMed]
- Milman, N. Oral iron prophylaxis in pregnancy: Not too little and not too much! J. Pregnancy 2012, 2012, 514345. [Google Scholar] [CrossRef]
- Casanueva, E.; Viteri, F.E.; Mares-Galindo, M.; Meza-Camacho, C.; Loría, A.; Schnaas, L.; Valdés-Ramos, R. Weekly iron as a safe alternative to daily supplementation for nonanemic pregnant women. Arch. Med. Res. 2006, 37, 674–682. [Google Scholar] [CrossRef] [PubMed]
- ACC/SCN. Fourth Report on the World Nutrition Situation: Nutrition Throughout the Life Cycle; ACC/SCN in collaboration with IFPRI: Geneva, Switzerland, 2000. [Google Scholar]
- Alwan, N.A.; Hamamy, H. Maternal iron status in pregnancy and long-term health outcomes in the offspring. J. Pediatr. Genet. 2015, 4, 111–123. [Google Scholar] [PubMed]
- Lindquist, A.; Kurinczuk, J.; Redshaw, M.; Knight, M. Experiences, utilisation and outcomes of maternity care in England among women from different socio-economic groups: Findings from the 2010 National Maternity Survey. BJOG Int. J. Obstet. Gynecol. 2015, 122, 1610–1617. [Google Scholar] [CrossRef] [PubMed]
- Larson, C.P. Poverty during pregnancy: Its effects on child health outcomes. Paediatr. Child. Health 2007, 12, 673. [Google Scholar] [CrossRef] [PubMed]
- Benyamin, B.; Esko, T.; Ried, J.S.; Radhakrishnan, A.; Vermeulen, S.H.; Traglia, M.; Gögele, M.; Anderson, D.; Broer, L.; Podmore, C.; et al. Novel loci affecting iron homeostasis and their effects in individuals at risk for hemochromatosis. Nat. Commun. 2014, 5, 4926. [Google Scholar] [CrossRef] [PubMed]
- Adams, P.C.; Reboussin, D.M.; Barton, J.C.; McLaren, C.E.; Eckfeldt, J.H.; McLaren, G.D.; Dawkins, F.W.; Acton, R.T.; Harris, E.L.; Gordeuk, V.R.; et al. Hemochromatosis and iron-overload screening in a racially diverse population. N. Engl. J. Med. 2005, 352, 1769–1778. [Google Scholar] [CrossRef] [PubMed]
- Hollerer, I.; Bachmann, A.; Muckenthaler, M.U. Pathophysiological consequences and benefits of HFE mutations: 20 years of research. Haematologica 2017, 102, 809–817. [Google Scholar] [CrossRef] [PubMed]
- Nemeth, E.; Ganz, T. Regulation of iron metabolism by hepcidin. Annu. Rev. Nutr. 2006, 26, 323–342. [Google Scholar] [CrossRef]
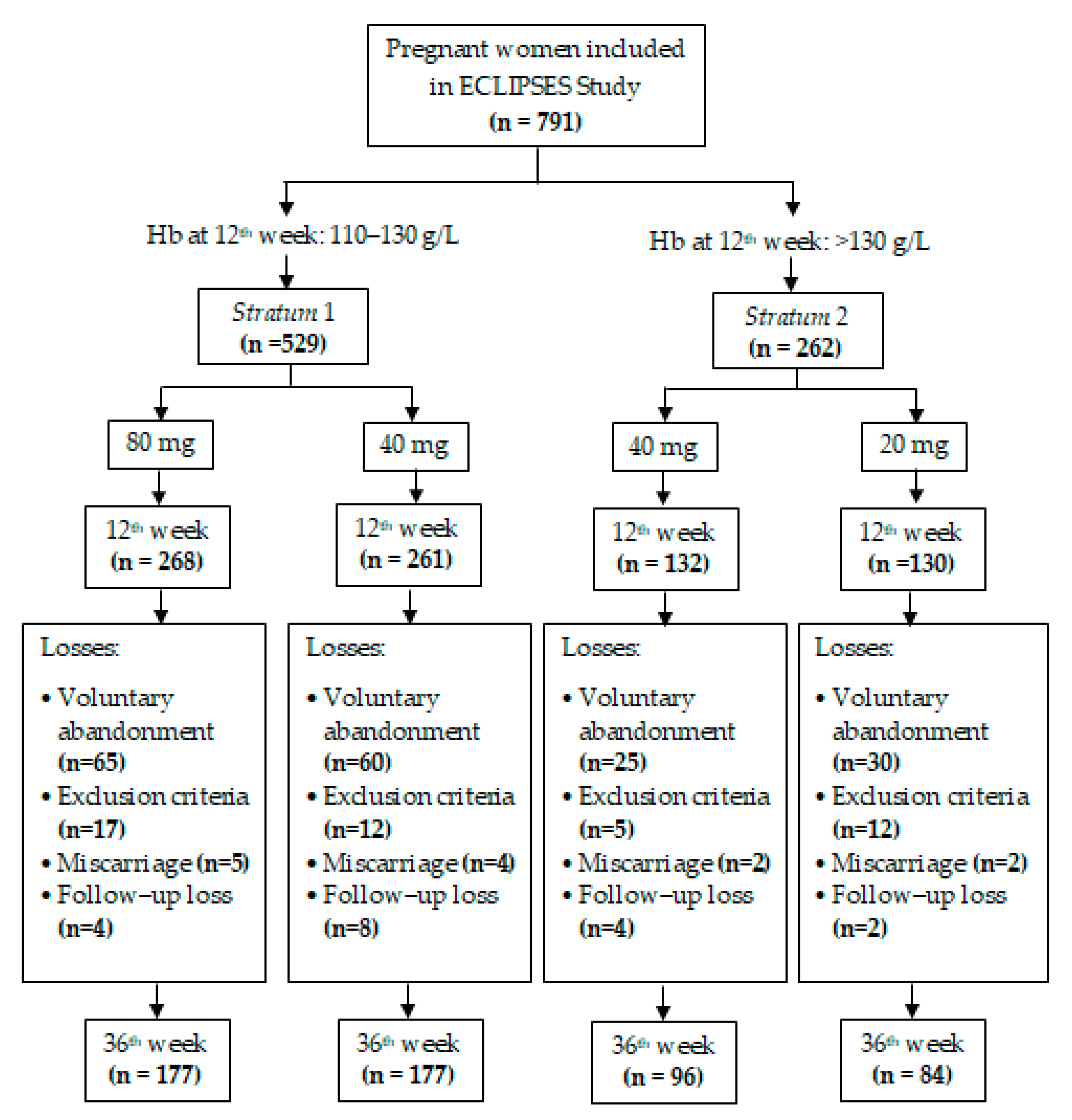
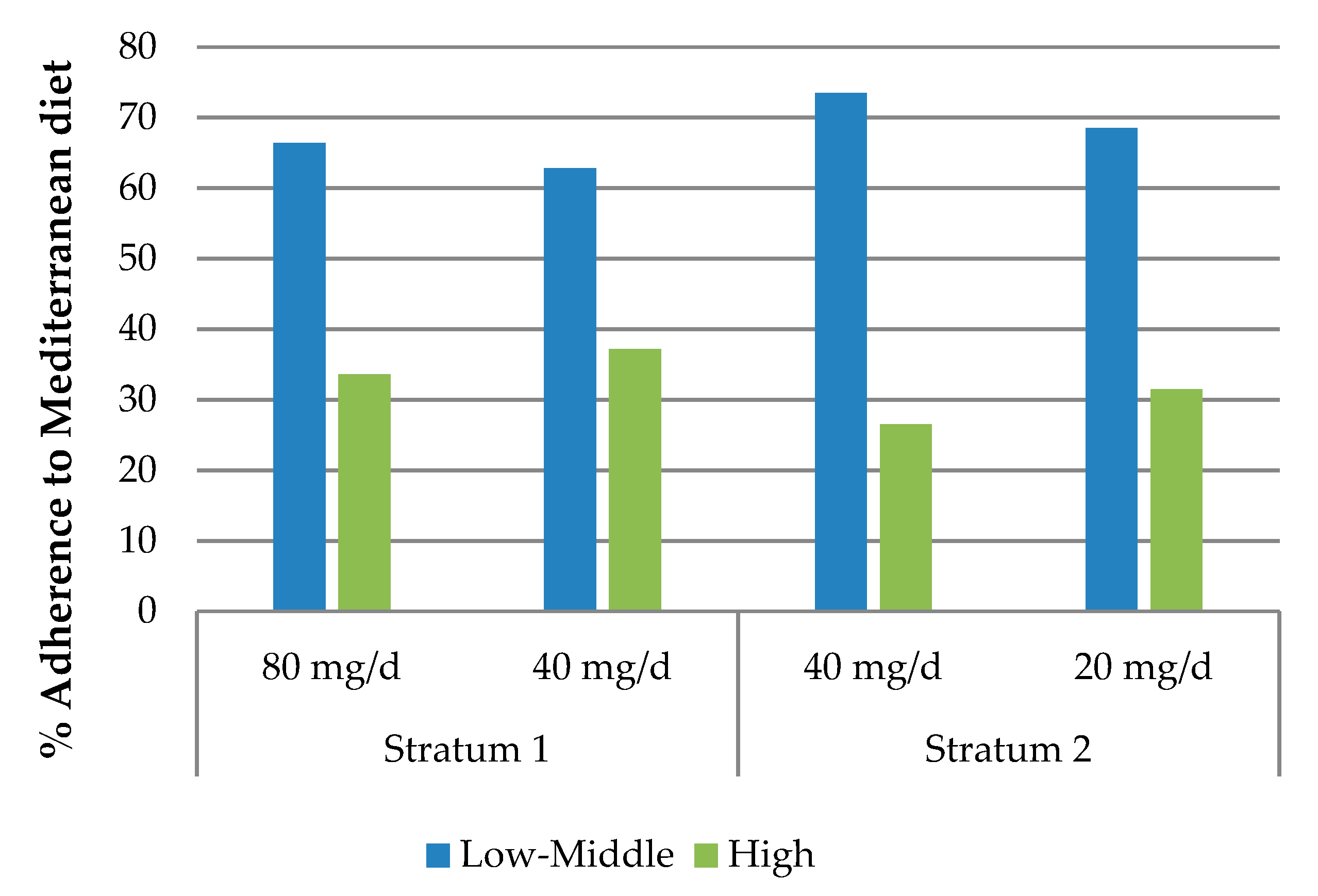
| Stratum 1 (n = 529) | Stratum 2 (n = 262) | p | |||
|---|---|---|---|---|---|
| Mean | SD | Mean | SD | ||
| Age, years | 30.40 | 5.07 | 30.21 | 5.28 | 0.613 |
| Weight, Kg | 64.83 | 11.31 | 67.17 | 13.60 | 0.017 |
| Pre–pregnancy BMI, Kg/m2 | 24.66 | 4.13 | 25.82 | 5.08 | 0.001 |
| Gestational weight gain, Kg | 11.11 | 8.17 | 9.69 | 9.47 | 0.030 |
| % (n) | % (n) | ||||
| Smoking | 16.6 (88) | 20.2 (53) | 0.214 | ||
| Parity | 62.3 (329) | 55.7 (146) | 0.075 | ||
| Planned pregnancy | 79.8 (422) | 80.5 (211) | 0.801 | ||
| Use of hormonal contraception | 18.3 (97) | 18.1 (47) | 0.929 | ||
| Pre–pregnancy BMI | |||||
| Underweight | 1.3 (7) | 2.3 (6) | 0.314 | ||
| Normal weight | 60.9 (322) | 51.5 (135) | 0.012 | ||
| Overweight | 25.7 (136) | 27.9 (73) | 0.518 | ||
| Obesity | 12.1 (64) | 18.3 (48) | 0.018 | ||
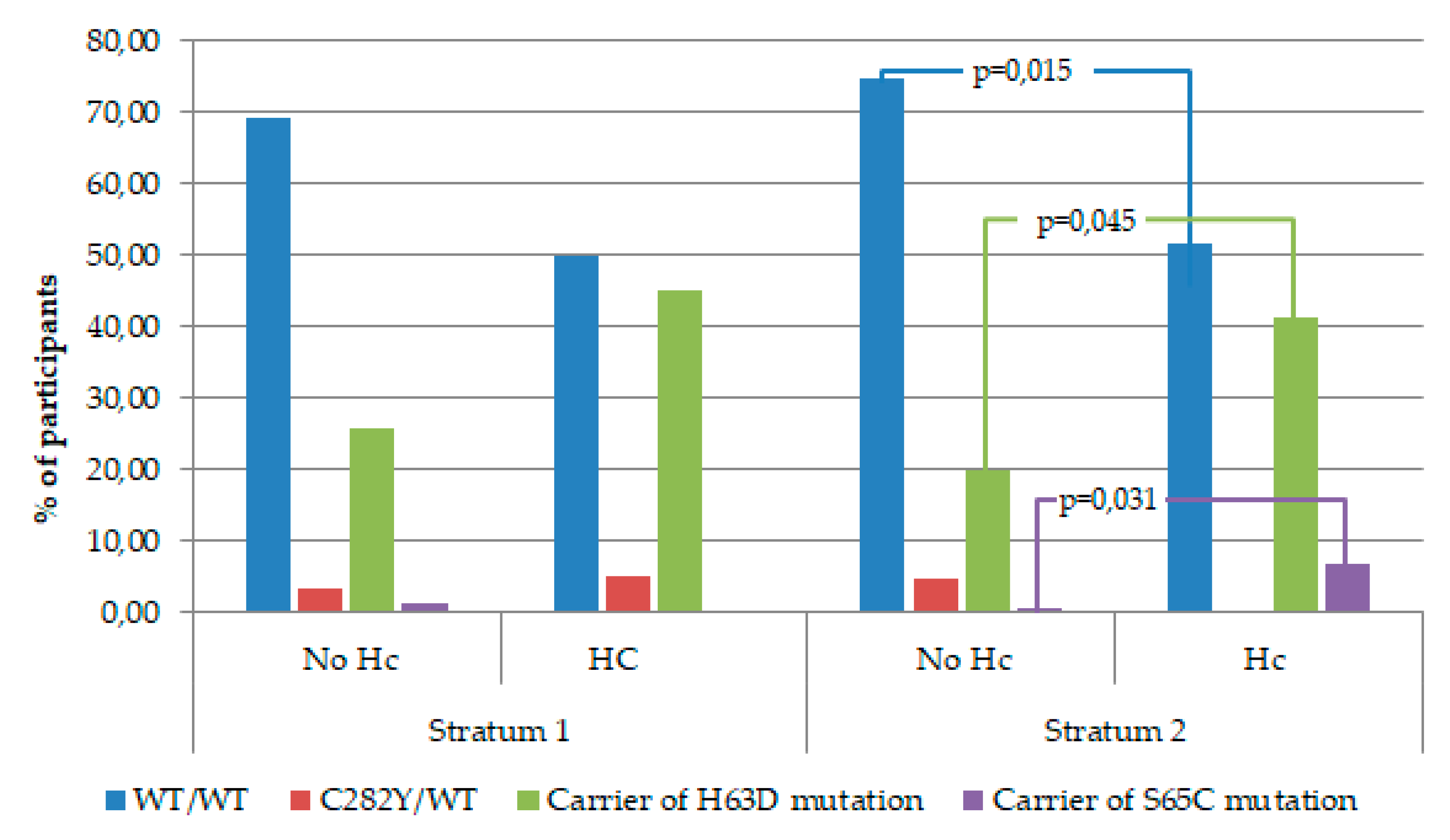
| HFE gene mutation | 31.7 (130) | 32.53 (68) | 0.834 | ||
| HFE genotype | |||||
| WT/WT | 67.3 (280) | 66.2 (141) | 0.779 | ||
| C282Y/WT | 3.8 (16) | 2.8 (6) | 0.506 | ||
| Carrier of H63D mutation | 27.4 (114) | 29.1 (62) | 0.652 | ||
| Carrier of S65C mutation | 1.4 (6) | 1.9 (4) | 0.679 | ||
| Family socioeconomic status | |||||
| Low | 16.4 (87) | 15.6 (41) | 0.774 | ||
| Middle | 66.7 (353) | 67.6 (177) | 0.816 | ||
| High | 16.8 (89) | 16.8 (44) | 0.991 | ||
| Maternal ethnic origin | |||||
| Caucasian | 82.8 (405) | 82.9 (203) | 0.991 | ||
| Asian | 0.2 (1) | 0.8 (2) | 0.221 | ||
| Arab | 7.8 (38) | 8.2 (20) | 0.853 | ||
| Black | 1.8 (9) | 0.4 (1) | 0.114 | ||
| Latin American | 7.4 (36) | 7.7 (19) | 0.849 | ||
| Adherence to Mediterranean diet | |||||
| Low–Middle | 64.7 (342) | 71.0 (186) | 0.075 | ||
| High | 35.3 (187) | 29.0 (76) | 0.075 | ||
| Stratum 1 | Stratum 2 | |||||
|---|---|---|---|---|---|---|
| 80 g/d | 40 g/d | p | 40 g/d | 20 g/d | p | |
| 12th week | ||||||
| Hemoglobin (g/L) | 123.26 (5.32) | 123.44 (4.77) | 0.689 | 135.68 (4.59) | 136.61 (4.44) | 0.098 |
| Serum ferritin (µg/L) | 38.95 (26.10) | 38.20 (25.05) | 0.740 | 38.50 (28.98) | 40.75 (30.00) | 0.965 |
| Mean corpuscular volume (fL) | 87.08 (6.36) | 87.30 (6.63) | 0.696 | 88.53 (3.43) | 88.54 (3.74) | 0.980 |
| C–reactive protein (mg/L) | 0.73 (0.62) | 0.74 (0.72) | 0.815 | 0.72 (0.54) | 0.70 (0.53) | 0.779 |
| Iron deficiency (%) | 14.2 (38) | 14.2 (37) | 0.999 | 14.4 (19) | 12.3 (16) | 0.620 |
| 36th week | ||||||
| Hemoglobin (g/L) | 117.63 (7.55) | 117.21 (8.35) | 0.622 | 123.07 (10.19) | 121.04 (8.85) | 0.157 |
| Serum ferritin (µg/L) | 17.19 (11.53) | 14.70 (9.38) | 0.042 | 11.10 (8.10) | 11.00 (6.80) | 0.798 |
| Mean corpuscular volume (fL) | 89.31 (6.87) | 88.19 (12.06) | 0.261 | 90.23 (4.19) | 89.61 (4.11) | 0.299 |
| C–reactive protein (mg/L) | 0.76 (0.74) | 0.71 (0.64) | 0.470 | 0.70 (0.69) | 0.75 (0.56) | 0.593 |
| Iron deficiency (%) | 38.2 (71) | 51 (98) | 0.012 | 66 (68) | 69.7 (62) | 0.590 |
| Iron deficiency anemia (%) | 8.5 (15) | 9.6 (17) | 0.711 | 7.3 (7) | 11.9 (10) | 0.291 |
| Anemia (%) | 11.9 (21) | 13 (23) | 0.747 | 8.3 (8) | 11.9 (10) | 0.426 |
| Hemoconcentration (%) | 6.8 (12) | 7.9 (14) | 0.684 | 24 (23) | 13.1 (11) | 0.063 |
| Hemoglobin levels | ||||
| Independent variables | β | SE | p | Model |
| a Intervention (0:80 mg/d, 1:40 mg/d) | −0.42 | 0.85 | 0.622 | R2 = − 0.002 p = 0.622 |
| b Intervention (0:80 mg/d, 1:40 mg/d) | 0.33 | 0.92 | 0.718 | R2 = 0.031 p = 0.050 |
| Hemoglobin on week 12 of pregnancy | 0.25 | 0.10 | 0.015 | |
| Serum ferritin on week 12 of pregnancy | 1.70 | 0.66 | 0.010 | |
| Serum ferritin levels | ||||
| Independent variables | β | SE | p | Model |
| a Intervention (0:80 mg/d, 1:40 mg/d) | −0.10 | 0.06 | 0.085 | R2 = 0.004 p = 0.085 |
| c Intervention (0:80 mg/d, 1:40 mg/d) | −0.12 | 0.05 | 0.026 | R2 = 0.436 p< 0.001 |
| Serum ferritin on week 12 of pregnancy | 0.60 | 0.04 | <0.001 | |
| Maternal age (0:25–34 years, 1:<25 years) | 0.05 | 0.08 | 0.559 | |
| Maternal age (0:25–34 years, 1:≥35 years) | 0.21 | 0.07 | 0.002 | |
| Iron deficiency (0:no, 1:yes) | ||||
| Independent variables | OR | 95% CI | p | Model |
| a Intervention (0:80 mg/d, 1:40 mg/d) | 1.69 | 1.12–2.54 | 0.012 | R2 Nagelkerke = 0.022 p = 0.012 |
| c Intervention (0:80 mg/d, 1:40 mg/d) | 1.82 | 1.09–3.03 | 0.022 | R2 Nagelkerke = 0.241 p < 0.001 |
| Serum ferritin on week 12 of pregnancy | 0.29 | 0.19–0.45 | <0.001 | |
| Anemia (0:no, 1:yes) | ||||
| Independent variables | OR | 95% CI | p | Model |
| a Intervention (0:80 mg/d, 1:40 mg/d) | 1.11 | 0.59–2.09 | 0.747 | R2 Nagelkerke = 0.001 p = 0.747 |
| b Intervention (0:80 mg/d, 1:40 mg/d) | 1.70 | 0.80–3.61 | 0.166 | R2 Nagelkerke = 0.146 p = 0.027 |
| Planned pregnancy (0:no, 1:yes) | 3.57 | 1.00–12.80 | 0.050 | |
| Serum ferritin on week 12 of pregnancy | 0.54 | 0.32–0.90 | 0.018 | |
| Iron–deficiency anemia (0:no, 1:yes) | ||||
| Independent variables | OR | 95% CI | p | Model |
| a Intervention (0:80 mg/d, 1:40 mg/d) | 1.15 | 0.55–2.38 | 0.711 | R2 Nagelkerke = 0.001 p = 0.711 |
| b Intervention (0:80 mg/d, 1:40 mg/d) | 1.58 | 0.67–3.71 | 0.299 | R2 Nagelkerke = 0.19 p = 0.004 |
| Serum ferritin on week 12 of pregnancy | 0.32 | 0.17–0.59 | <0.001 | |
| Hemoconcentration (0:no, 1:yes) | ||||
| Independent variables | OR | 95% CI | p | Model |
| a Intervention (0:80 mg/d, 1:40 mg/d) | 1.18 | 0.53–2.63 | 0.684 | R2 Nagelkerke = 0.001 p = 0.684 |
| b Intervention (0:80 mg/d, 1:40 mg/d) | 1.44 | 0.52–3.97 | 0.481 | R2 Nagelkerke = 0.166 p = 0.071 |
| Genotype HFE (0:WT/WT, 1: carrier of H63D) | 3.28 | 0.09–6.68 | 0.026 | |
| Genotype HFE (0:WT/WT, 1: C282Y/WT) | 1.93 | 0.21–18.02 | 0.566 | |
| Parity (0:no, 1:yes) | 0.26 | 0.09–0.76 | 0.014 |
| SF < 15 µg/L | ||||
| Hemoglobin levels | β | SE | p | Model |
| Crude model | – 7.06 | 2.43 | 0.006 | R2 = 0.129 p = 0.006 |
| a Adjusted model | – 8.81 | 2.40 | 0.001 | R2 = 0.32 p = 0.003 |
| Iron deficiency (0:no, 1:yes) | OR | 95% CI | p | Model |
| Crude model | 3.10 | 0.93–10.39 | 0.066 | R2 Nagelkerke = 0.091 p = 0.060 |
| b Adjusted model | 4.51 | 0.78–26.08 | 0.092 | R2 Nagelkerke = 0.429 p = 0.013 |
| Anemia (0:no, 1:yes) | OR | 95% CI | p | Model |
| Crude model | 5.50 | 1.05–28.75 | 0.043 | R2 Nagelkerke = 0.145 p = 0.025 |
| a Adjusted model | 29.14 | 1.67–508.56 | 0.021 | R2 Nagelkerke = 0.596 p = 0.020 |
| Iron–deficiency anemia (0:no, 1:yes) | OR | 95% CI | p | Model |
| Crude model | 5.50 | 1.05–28.75 | 0.043 | R2 Nagelkerke = 0.145 p = 0.025 |
| a Adjusted model | 29.14 | 1.67–508.56 | 0.021 | R2 Nagelkerke = 0.596 p = 0.020 |
| SF≥15 µg/L | ||||
| Hemoglobin levels | β | SE | p | Model |
| Crude model | 0.75 | 0.88 | 0.395 | R2 = −0.001 p = 0.395 |
| a Adjusted model | 0.42 | 0.96 | 0.664 | R2 = 0.035 p = 0.031 |
| Hemoglobin levels | ||||
| Independent variables | β | SE | p | Model |
| a Intervention (0:40 mg/d, 1:20 mg/d) | −1.91 | 1.44 | 0.188 | R2 = 0.004 p = 0.188 |
| b Intervention (0:40 mg/d, 1:20 mg/d) | −2.50 | 1.47 | 0.092 | R2 = 0.116 p = 0.003 |
| Genotype HFE (0:WT/WT, 1: carrier of H63D) | 3.93 | 1.74 | 0.025 | |
| Genotype HFE (0:WT/WT, 1: C282Y/WT) | 1.34 | 3.70 | 0.718 | |
| Hemoglobin on week 12 of pregnancy | 0.72 | 0.16 | <0.001 | |
| Serum ferritin levels | ||||
| Independent variables | β | SE | p | Model |
| a Intervention (0:40 mg/d, 1:20 mg/d) | 0.02 | 0.07 | 0.734 | R2 = −0.003 p = 0.734 |
| c Intervention (0:40 mg/d, 1:20 mg/d) | 0.01 | 0.08 | 0.954 | R2 = 0.218 p < 0.001 |
| Maternal age (0:25–34 years, 1:<25 years) | −0.28 | 0.11 | 0.013 | |
| Maternal age (0:25–34 years, 1:≥35 years) | 0.12 | 0.10 | 0.221 | |
| Serum ferritin on week 12 of pregnancy | 0.42 | 0.06 | <0.001 | |
| Low iron stores (0:no, 1:yes) | ||||
| Independent variables | OR | 95% CI | p | Model |
| a Intervention (0:40 mg/d, 1:20 mg/d) | 1.18 | 0.64–2.17 | 0.590 | R2 Nagelkerke = 0.002 p = 0.590 |
| c Intervention (0:40 mg/d, 1:20 mg/d) | 1.45 | 0.69–3.02 | 0.326 | R2 Nagelkerke = 0.229 p = 0.003 |
| Maternal age (0:25–34 years, 1:<25 years) | 3.07 | 0.78–12.12 | 0.109 | |
| Maternal age (0:25–34 years, 1:≥35 years) | 0.37 | 0.16–0.91 | 0.029 | |
| Serum ferritin on week 12 of pregnancy | 0.36 | 0.19–0.68 | 0.002 | |
| Anemia (0:no, 1:yes) | ||||
| Independent variables | OR | 95% CI | p | Model |
| a Intervention (0:40 mg/d, 1:20 mg/d) | 1.48 | 0.56–3.96 | 0.428 | R2 Nagelkerke = 0.007 p = 0.426 |
| b Intervention (0:40 mg/d, 1:20 mg/d) | 2.01 | 0.44–9.09 | 0.364 | R2 Nagelkerke = 0.468 p = 0.002 |
| SES (0:low; 1:middle + high) | 0.06 | 0.01–0.40 | 0.003 | |
| Serum ferritin on week 12 of pregnancy | 0.26 | 0.08–0.66 | 0.023 | |
| Iron–deficiency anemia (0:no, 1:yes) | ||||
| Independent variables | OR | 95% CI | p | Model |
| a Intervention (0:40 mg/d, 1:20 mg/d) | 1.78 | 0.62–4.74 | 0.295 | R2 Nagelkerke = 0.013 p = 0.291 |
| b Intervention (0:40 mg/d, 1:20 mg/d) | 2.01 | 0.44–9.09 | 0.364 | R2 Nagelkerke = 0.468 p = 0.002 |
| SES (0:low; 1:middle + high) | 0.06 | 0.01–0.40 | 0.003 | |
| Serum ferritin on week 12 of pregnancy | 0.26 | 0.08–0.66 | 0.023 | |
| Hemoconcentration (0:no, 1:yes) | ||||
| Independent variables | OR | 95% CI | p | Model |
| a Intervention (0:40 mg/d, 1:20 mg/d) | 0.48 | 0.22–1.05 | 0.067 | R2 Nagelkerke = 0.031 p = 0.060 |
| b Intervention (0:40 mg/d, 1:20 mg/d) | 0.31 | 0.11–0.92 | 0.035 | R2 Nagelkerke = 0.282 p = 0.004 |
| Hemoglobin on week 12 of pregnancy | 1.20 | 1.08–1.33 | 0.001 | |
| Genotype HFE (0:WT/WT, 1: carrier of H63D) | 3.09 | 1.10–8.71 | 0.033 | |
| Genotype HFE (0:WT/WT, 1: C282Y/WT) | 0.00 | . | 0.999 |
| SF<15 µg/L | ||||
| Serum ferritin levels | β | SE | p | Model |
| Crude model | −0.24 | 0.14 | 0.083 | R2 = 0.061 p = 0.083 |
| b Adjusted model | −0.39 | 0.15 | 0.014 | R2 = 0.344 p = 0.021 |
| Iron deficiency (0:no, 1:yes) | OR | 95% CI | p | Model |
| Crude model | 6.11 | 0.60–62.23 | 0.126 | R2 Nagelkerke = 0.163 p = 0.085 |
| b Adjusted model | 42.09 | 0.00–50.00 | 0.614 | R2 Nagelkerke = 0.924 p = 0.001 |
| SF≥15 µg/L | ||||
| Hemoglobin levels | β | SE | p | Model |
| Crude model | –1.75 | 1.54 | 0.260 | R2 = 0.002 p = 0.260 |
| a Adjusted model | –2.00 | 1.56 | 0.200 | R2 = 0.184 p < 0.001 |
| Serum ferritin levels | β | SE | p | Model |
| Crude model | 0.06 | 0.08 | 0.455 | R2 = −0.002 p = 0.455 |
| b Adjusted model | 0.08 | 0.09 | 0.368 | R2 = 0.077 p = 0.008 |
| Anemia (0:no, 1:yes) | OR | 95% CI | p | Model |
| Crude model | 1.34 | 0.43–4.20 | 0.611 | R2 Nagelkerke = 0.004 p = 0.611 |
| a Adjusted model | 1.02 | 0.20–5.10 | 0.984 | R2 Nagelkerke = 0.383 p = 0.007 |
| Iron–deficiency anemia (0:no, 1:yes) | OR | 95% CI | p | Model |
| Crude model | 1.63 | 0.50–5.39 | 0.421 | R2 Nagelkerke = 0.010 p = 0.417 |
| a Adjusted model | 1.02 | 0.20–5.10 | 0.984 | R2 Nagelkerke = 0.383 p = 0.007 |
| Hemoconcentration (0:no, 1:yes) | OR | 95% CI | p | Model |
| Crude model | 0.46 | 0.20–1.06 | 0.068 | R2 Nagelkerke = 0.035 p = 0.061 |
| a Adjusted model | 0.25 | 0.07–0.85 | 0.027 | R2 Nagelkerke = 0.261 p = 0.001 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Iglesias Vázquez, L.; Arija, V.; Aranda, N.; Aparicio, E.; Serrat, N.; Fargas, F.; Ruiz, F.; Pallejà, M.; Coronel, P.; Gimeno, M.; et al. The Effectiveness of Different Doses of Iron Supplementation and the Prenatal Determinants of Maternal Iron Status in Pregnant Spanish Women: ECLIPSES Study. Nutrients 2019, 11, 2418. https://doi.org/10.3390/nu11102418
Iglesias Vázquez L, Arija V, Aranda N, Aparicio E, Serrat N, Fargas F, Ruiz F, Pallejà M, Coronel P, Gimeno M, et al. The Effectiveness of Different Doses of Iron Supplementation and the Prenatal Determinants of Maternal Iron Status in Pregnant Spanish Women: ECLIPSES Study. Nutrients. 2019; 11(10):2418. https://doi.org/10.3390/nu11102418
Chicago/Turabian StyleIglesias Vázquez, Lucía, Victoria Arija, Núria Aranda, Estefanía Aparicio, Núria Serrat, Francesc Fargas, Francisca Ruiz, Meritxell Pallejà, Pilar Coronel, Mercedes Gimeno, and et al. 2019. "The Effectiveness of Different Doses of Iron Supplementation and the Prenatal Determinants of Maternal Iron Status in Pregnant Spanish Women: ECLIPSES Study" Nutrients 11, no. 10: 2418. https://doi.org/10.3390/nu11102418
APA StyleIglesias Vázquez, L., Arija, V., Aranda, N., Aparicio, E., Serrat, N., Fargas, F., Ruiz, F., Pallejà, M., Coronel, P., Gimeno, M., & Basora, J. (2019). The Effectiveness of Different Doses of Iron Supplementation and the Prenatal Determinants of Maternal Iron Status in Pregnant Spanish Women: ECLIPSES Study. Nutrients, 11(10), 2418. https://doi.org/10.3390/nu11102418







