Fermented Goat Milk Consumption Enhances Brain Molecular Functions during Iron Deficiency Anemia Recovery
Abstract
1. Introduction
2. Materials and Methods
2.1. Fermentation and Dehydration of the Milks
2.2. Animals
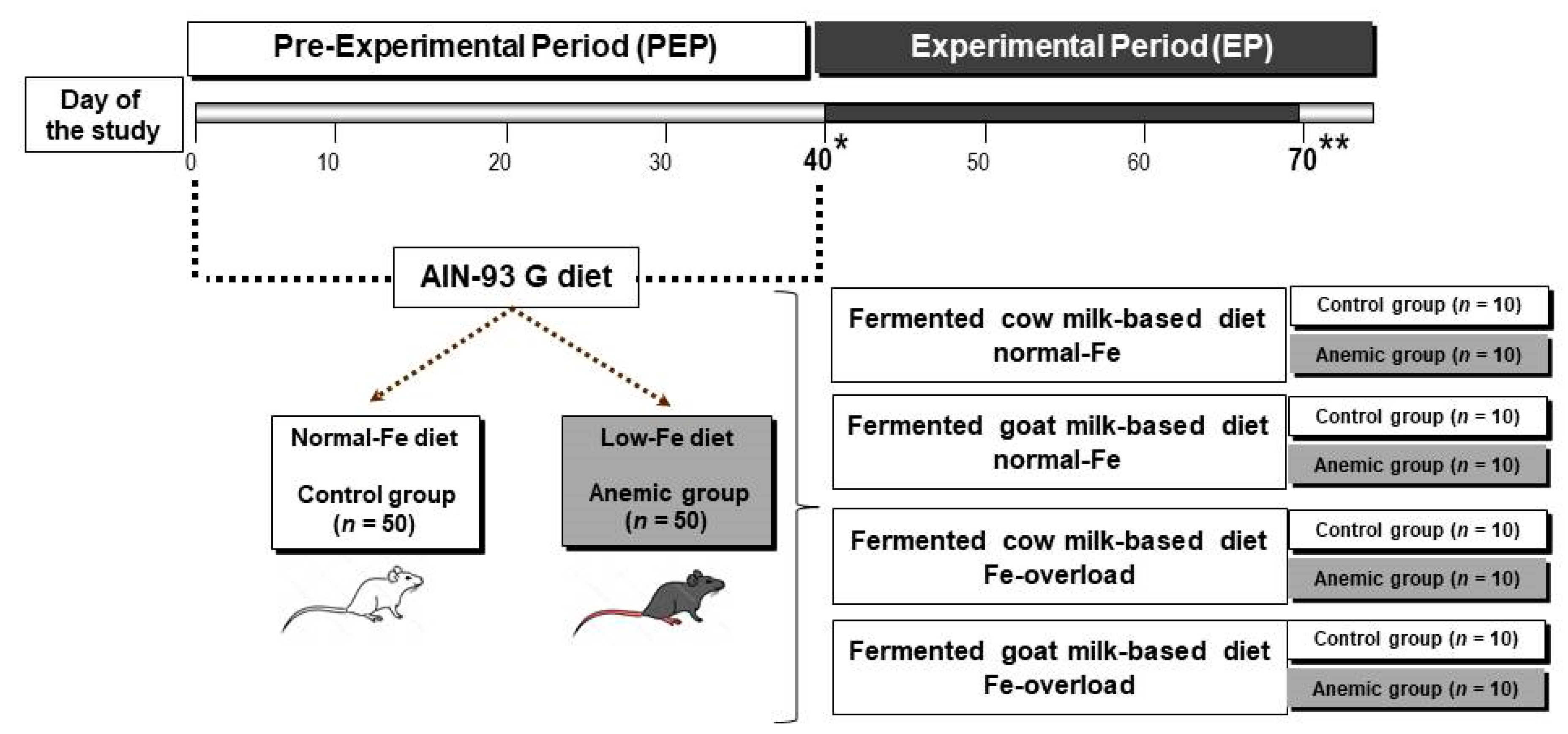
2.3. Experimental Design
2.4. Hematological Test
2.5. Iron Assessments
2.6. Dopamine
2.7. Serotonin
2.8. MAO-A and MAO-B
2.9. Irisin
2.10. Synaptophysin
2.11. Neuropeptides Assessment
2.12. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kyu, H.H.; Pinho, C.; Wagner, J.A.; Brown, J.C.; Bertozzi-Villa, A.; Charlson, F.J.; Coffeng, L.E.; Dandona, L.; Erskine, H.E.; Ferrari, A.J.; et al. Global and National Burden of Diseases and Injuries Among Children and Adolescents Between 1990 and 2013: Findings From the Global Burden of Disease 2013 Study. JAMA Pediatrics 2016, 170, 267–287. [Google Scholar] [CrossRef]
- Beard, J.L.; Connor, J.R. Iron status and neural functioning. Ann. Rev. Nutr. 2003, 23, 41–58. [Google Scholar] [CrossRef] [PubMed]
- Cusick, S.E.; Georgieff, M.K.; Rao, R. Approaches for Reducing the Risk of Early-Life Iron Deficiency-Induced Brain Dysfunction in Children. Nutrients 2018, 10, 227. [Google Scholar] [CrossRef] [PubMed]
- Murray-Kolb, L.E.; Wenger, M.J.; Scott, S.P.; Rhoten, S.E.; Lung’aho, M.G.; Haas, J.D. Consumption of Iron-Biofortified Beans Positively Affects Cognitive Performance in 18- to 27-Year-Old Rwandan Female College Students in an 18-Week Randomized Controlled Efficacy Trial. J. Nutr. 2017, 147, 2109–2117. [Google Scholar] [CrossRef] [PubMed]
- Wenger, M.J.; DellaValle, D.M. Effect of iron deficiency on simultaneous measures of behavior, brain activity, and energy expenditure in the performance of a cognitive task. Nutr. Neurosci. 2019, 22, 196–206. [Google Scholar] [CrossRef] [PubMed]
- Murray-Kolb, L.E. Iron and brain functions. Curr. Opin. Clin. Nutr. Metab. Care 2013, 16, 703–707. [Google Scholar] [CrossRef] [PubMed]
- Pickett, J.L.; Theberge, D.C.; Brown, W.S.; Schweitzer, S.U.; Nissenson, A.R. Normalizing hematocrit in dialysis patients improves brain function. Am. J. Kidney Dis. Off. J. Natl. Kidney Found. 1999, 33, 1122–1130. [Google Scholar] [CrossRef]
- Nissenson, A.R. Epoetin and cognitive function. Am. J. Kidney Dis. Off. J. Natl. Kidney Found. 1992, 20, 21–24. [Google Scholar] [PubMed]
- Moreno, F.; Sanz-Guajardo, D.; Lopez-Gomez, J.M.; Jofre, R.; Valderrabano, F. Increasing the Hematocrit Has a Beneficial Effect on Quality of Life and Is Safe in Selected Hemodialysis Patients. J. Am. Soc. Nephrol. 2000, 11, 335. [Google Scholar]
- Falkingham, M.; Abdelhamid, A.; Curtis, P.; Fairweather-Tait, S.; Dye, L.; Hooper, L. The effects of oral iron supplementation on cognition in older children and adults: A systematic review and meta-analysis. Nutr. J. 2010, 9, 4. [Google Scholar] [CrossRef]
- Bahrami, A.; Khorasanchi, Z. Anemia is associated with cognitive impairment in adolescent girls: A cross-sectional survey. Appl. Neuropsychol. Child 2019, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Camfield, D.A.; Owen, L.; Scholey, A.B.; Pipingas, A.; Stough, C. Dairy constituents and neurocognitive health in ageing. Br. J. Nutr. 2011, 106, 159–174. [Google Scholar] [CrossRef] [PubMed]
- Crichton, G.E.; Murphy, K.J.; Bryan, J. Dairy intake and cognitive health in middle-aged South Australians. Asia Pac. J. Clin. Nutr. 2010, 19, 161–171. [Google Scholar] [PubMed]
- Moreno-Fernandez, J.; Diaz-Castro, J.; Pulido-Moran, M.; Alferez, M.J.; Boesch, C.; Sanchez-Alcover, A.; Lopez-Aliaga, I. Fermented Goat’s Milk Consumption Improves Duodenal Expression of Iron Homeostasis Genes during Anemia Recovery. J. Agric. food Chem. 2016, 64, 2560–2568. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Fernandez, J.; Diaz-Castro, J.; Alferez, M.J.; Hijano, S.; Nestares, T.; Lopez-Aliaga, I. Production and chemical composition of two dehydrated fermented dairy products based on cow or goat milk. J. Dairy Res. 2016, 83, 81–88. [Google Scholar] [CrossRef] [PubMed]
- Reeves, P.G.; Nielsen, F.H.; Fahey, G.C., Jr. AIN-93 purified diets for laboratory rodents: Final report of the American Institute of Nutrition ad hoc writing committee on the reformulation of the AIN-76A rodent diet. J. Nutr. 1993, 123, 1939–1951. [Google Scholar] [CrossRef]
- Pallares, I.; Lisbona, F.; Aliaga, I.L.; Barrionuevo, M.; Alferez, M.J.; Campos, M.S. Effect of iron deficiency on the digestive utilization of iron, phosphorus, calcium and magnesium in rats. Br. J. Nutr. 1993, 70, 609–620. [Google Scholar] [CrossRef] [PubMed]
- Raja, K.B.; Simpson, R.J.; Peters, T.J. Intestinal iron absorption studies in mouse models of iron-overload. Br. J. Haematol. 1994, 86, 156–162. [Google Scholar] [CrossRef] [PubMed]
- Lozoff, B.; Beard, J.; Connor, J.; Barbara, F.; Georgieff, M.; Schallert, T. Long-lasting neural and behavioral effects of iron deficiency in infancy. Nutr. Rev. 2006, 64, S34–S43. [Google Scholar] [CrossRef]
- Zlokovic, B.V. Neurovascular pathways to neurodegeneration in Alzheimer’s disease and other disorders. Nat. Rev. Neurosci. 2011, 12, 723. [Google Scholar] [CrossRef]
- Hasselblatt, M.; Ehrenreich, H.; Siren, A.L. The brain erythropoietin system and its potential for therapeutic exploitation in brain disease. J. Neurosurg. Anesthesiol. 2006, 18, 132–138. [Google Scholar] [CrossRef] [PubMed]
- Munoz, P.; Humeres, A. Iron deficiency on neuronal function. Biometals Int. J. Role Met. Ions Biol. Biochem. Med. 2012, 25, 825–835. [Google Scholar] [CrossRef] [PubMed]
- Nieoullon, A. Dopamine and the regulation of cognition and attention. Prog. Neurobiol. 2002, 67, 53–83. [Google Scholar] [CrossRef]
- Unger, E.L.; Wiesinger, J.A.; Hao, L.; Beard, J.L. Dopamine D2 receptor expression is altered by changes in cellular iron levels in PC12 cells and rat brain tissue. J. Nutr. 2008, 138, 2487–2494. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Fernandez, J.; Diaz-Castro, J. Iron Deficiency and Neuroendocrine Regulators of Basal Metabolism, Body Composition and Energy Expenditure in Rats. Nutrients 2019, 11, 631. [Google Scholar] [CrossRef] [PubMed]
- McEwen, B.S.; Sapolsky, R.M. Stress and cognitive function. Curr. Opin. Neurobiol. 1995, 5, 205–216. [Google Scholar] [CrossRef]
- Youdim, M.B.; Ben-Shachar, D.; Yehuda, S. Putative biological mechanisms of the effect of iron deficiency on brain biochemistry and behavior. Am. J. Clin. Nutr. 1989, 50, 607–617. [Google Scholar] [CrossRef] [PubMed]
- Shukla, A.; Agarwal, K.N.; Chansuria, J.P.; Taneja, V. Effect of latent iron deficiency on 5-hydroxytryptamine metabolism in rat brain. J. Neurochem. 1989, 52, 730–735. [Google Scholar] [CrossRef]
- Finch, C.A.; Miller, L.R.; Inamdar, A.R.; Person, R.; Seiler, K.; Mackler, B. Iron deficiency in the rat. Physiological and biochemical studies of muscle dysfunction. J. Clin. Investig. 1976, 58, 447–453. [Google Scholar] [CrossRef]
- Morse, A.C.; Beard, J.L.; Azar, M.R.; Jones, B.C. Sex and Genetics are Important Cofactors in Assessing the Impact of Iron Deficiency on the Developing Mouse Brain. Nutr. Neurosci. 1999, 2, 323–335. [Google Scholar] [CrossRef]
- Kaladhar, M.; Rao, B.S. Effect of maternal iron deficiency in rat on serotonin uptake in vitro by brain synaptic vesicles in the offspring. J. Neurochem. 1983, 40, 1768–1770. [Google Scholar] [CrossRef] [PubMed]
- Youdim, M.B.; Ben-Shachar, D. Minimal brain damage induced by early iron deficiency: Modified dopaminergic neurotransmission. Isr. J. Med. Sci. 1987, 23, 19–25. [Google Scholar] [PubMed]
- Li, Y.; Kim, J.; Buckett, P.D.; Böhlke, M.; Maher, T.J.; Wessling-Resnick, M. Severe postnatal iron deficiency alters emotional behavior and dopamine levels in the prefrontal cortex of young male rats. J. Nutr. 2011, 141, 2133–2138. [Google Scholar] [CrossRef] [PubMed]
- Wrann, C.D. FNDC5/irisin—Their role in the nervous system and as a mediator for beneficial effects of exercise on the brain. Brain Plast. 2015, 1, 55–61. [Google Scholar] [CrossRef] [PubMed]
- Golub, M.S.; Hogrefe, C.E. Fetal iron deficiency and genotype influence emotionality in infant rhesus monkeys. J. Nutr. 2015, 145, 647–653. [Google Scholar] [CrossRef] [PubMed]
- Kostoglou-Athanassiou, I.; Forsling, M.L.; Navarra, P.; Grossman, A.B. Oxytocin release is inhibited by the generation of carbon monoxide from the rat hypothalamus-further evidence for carbon monoxide as a neuromodulator. Brain Res. Mol. Brain Res. 1996, 42, 301–306. [Google Scholar] [CrossRef]
- Singh, M.; Mukhopadhyay, K. Alpha-melanocyte stimulating hormone: An emerging anti-inflammatory antimicrobial peptide. BioMed Res. Int. 2014, 2014, 874610. [Google Scholar] [CrossRef]
- Veening, J.G.; Barendregt, H.P. The effects of beta-endorphin: State change modification. Fluids Barriers CNS 2015, 12, 3. [Google Scholar] [CrossRef]
- Gordon, S.L.; Harper, C.B.; Smillie, K.J.; Cousin, M.A. A Fine Balance of Synaptophysin Levels Underlies Efficient Retrieval of Synaptobrevin II to Synaptic Vesicles. PLoS ONE 2016, 11, e0149457. [Google Scholar] [CrossRef]
- Alladi, P.A.; Wadhwa, S.; Singh, N. Effect of prenatal auditory enrichment on developmental expression of synaptophysin and syntaxin 1 in chick brainstem auditory nuclei. Neuroscience 2002, 114, 577–590. [Google Scholar] [CrossRef]
- Joca, S.R.; Guimaraes, F.S.; Del-Bel, E. Inhibition of nitric oxide synthase increases synaptophysin mRNA expression in the hippocampal formation of rats. Neurosci. Lett. 2007, 421, 72–76. [Google Scholar] [CrossRef] [PubMed]
- Ano, Y.; Ozawa, M.; Kutsukake, T.; Sugiyama, S.; Uchida, K.; Yoshida, A.; Nakayama, H. Preventive effects of a fermented dairy product against Alzheimer’s disease and identification of a novel oleamide with enhanced microglial phagocytosis and anti-inflammatory activity. PLoS ONE 2015, 10, e0118512. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Aliaga, I.; Garcia-Pedro, J.D.; Moreno-Fernandez, J.; Alferez, M.J.M.; Lopez-Frias, M.; Diaz-Castro, J. Fermented goat milk consumption improves iron status and evokes inflammatory signalling during anemia recovery. Food Funct. 2018, 9, 3195–3201. [Google Scholar] [CrossRef] [PubMed]
- Lourenco, M.V.; Ledo, J.H. Targeting Alzheimer’s pathology through PPARgamma signaling: Modulation of microglial function. J. Neurosci. Off. J. Soc. Neurosci. 2013, 33, 5083–5084. [Google Scholar] [CrossRef] [PubMed]
- Xia, M.Q.; Qin, S.X.; Wu, L.J.; Mackay, C.R.; Hyman, B.T. Immunohistochemical study of the beta-chemokine receptors CCR3 and CCR5 and their ligands in normal and Alzheimer’s disease brains. Am. J. Pathol. 1998, 153, 31–37. [Google Scholar] [CrossRef]
- Mrak, R.E.; Griffin, W.S. Potential inflammatory biomarkers in Alzheimer’s disease. J. Alzheimer’s Dis. JAD 2005, 8, 369–375. [Google Scholar] [CrossRef]
- Cheignon, C.; Tomas, M.; Bonnefont-Rousselot, D.; Faller, P.; Hureau, C.; Collin, F. Oxidative stress and the amyloid beta peptide in Alzheimer’s disease. Redox Biol. 2018, 14, 450–464. [Google Scholar] [CrossRef]
- Chi, H.; Chang, H.-Y.; Sang, T.-K. Neuronal cell death mechanisms in major neurodegenerative diseases. Int. J. Mol. Sci. 2018, 19, 3082. [Google Scholar] [CrossRef]
- Moreno-Fernandez, J.; Diaz-Castro, J.; Alferez, M.J.; Nestares, T.; Ochoa, J.J.; Sanchez-Alcover, A.; Lopez-Aliaga, I. Fermented goat milk consumption improves melatonin levels and influences positively the antioxidant status during nutritional ferropenic anemia recovery. Food Funct. 2016, 7, 834–842. [Google Scholar] [CrossRef]
- Moreno-Fernandez, J.; Diaz-Castro, J.; Alferez, M.J.; Boesch, C.; Nestares, T.; Lopez-Aliaga, I. Fermented goat milk improves antioxidant status and protects from oxidative damage to biomolecules during anemia recovery. J. Sci. Food Agric. 2017, 97, 1433–1442. [Google Scholar] [CrossRef]
- Hare, D.J.; Double, K.L. Iron and dopamine: A toxic couple. Brain J. Neurol. 2016, 139, 1026–1035. [Google Scholar] [CrossRef] [PubMed]
- Naoi, M.; Riederer, P.; Maruyama, W. Modulation of monoamine oxidase (MAO) expression in neuropsychiatric disorders: Genetic and environmental factors involved in type A MAO expression. J. Neural Transm. 2016, 123, 91–106. [Google Scholar] [CrossRef] [PubMed]
- Shrihari, T.G. BETA—Endorphins—A Novel Natural Holistic Healer. J. Microb. Biochem. Technol. 2018, 10, 25–26. [Google Scholar]
- Miwa, C.P.; de Lima, M.N.; Scalco, F.; Vedana, G.; Mattos, R.; Fernandez, L.L.; Hilbig, A.; Schroder, N.; Vianna, M.R. Neonatal iron treatment increases apoptotic markers in hippocampal and cortical areas of adult rats. Neurotox. Res. 2011, 19, 527–535. [Google Scholar] [CrossRef]
- Ano, Y.; Ayabe, T.; Kutsukake, T.; Ohya, R.; Takaichi, Y.; Uchida, S.; Yamada, K.; Uchida, K.; Takashima, A.; Nakayama, H. Novel lactopeptides in fermented dairy products improve memory function and cognitive decline. Neurobiol. Aging 2018, 72, 23–31. [Google Scholar] [CrossRef]
- Nagatsu, T.; Sawada, M. Molecular mechanism of the relation of monoamine oxidase B and its inhibitors to Parkinson’s disease: Possible implications of glial cells. J. Neural Transm. Suppl. 2006, 71, 53–65. [Google Scholar]
- Son, B.; Jun, S.Y.; Seo, H.; Youn, H.; Yang, H.J.; Kim, W.; Kim, H.K.; Kang, C.; Youn, B. Inhibitory effect of traditional oriental medicine-derived monoamine oxidase B inhibitor on radioresistance of non-small cell lung cancer. Sci. Rep. 2016, 6, 21986. [Google Scholar] [CrossRef]
- Luger, T.A.; Scholzen, T.E.; Brzoska, T.; Bohm, M. New insights into the functions of alpha-MSH and related peptides in the immune system. Ann. N. Y. Acad. Sci. 2003, 994, 133–140. [Google Scholar] [CrossRef]
| Normal-Fe Control Group (n = 50) | Low-Fe Anemic Group (n = 50) | |
|---|---|---|
| Hemoglobin concentration (g·L−1) | 130.62 ± 2.77 | 60.46 ± 2.77 * |
| Red blood cells (1012·L−1) | 7.11 ± 0.22 | 3.19 ± 0.30 * |
| Hematocrit (%) | 41.13 ± 1.03 | 13.12 ± 1.33 * |
| Mean corpuscular volume (fL) | 54.92 ± 0.52 | 37.21 ± 0.37 * |
| Mean corpuscular hemoglobin (pg) | 20.01 ± 0.15 | 14.22 ± 0.53 * |
| Mean corpuscular hemoglobin concentration (g·dL−1) | 36.12 ± 0.37 | 30.56 ± 0.73 * |
| Red cell distribution width (%) | 16.33 ± 0.45 | 19.67 ± 0.35 * |
| Platelets (109·L−1) | 731 ± 70.11 | 2123 ± 112 * |
| White blood cells (109·L−1) | 8.32 ± 0.41 | 8.45 ± 0.59 |
| Lymphocytes (106·mL−1) | 7.76 ± 0.51 | 5.62 ± 0.77 * |
| Fe (µg·L−1) | 1342 ± 97.31 | 607 ± 55.22 * |
| Total iron-binding capacity (µg·L−1) | 2623 ± 179 | 18002 ± 539 * |
| Transferrin saturation (%) | 49.22 ± 5.86 | 4.01 ± 0.45 * |
| Ferritin (µg·L−1) | 79.54 ± 2.23 | 49.12 ± 1.58 * |
| Hepcidin (ng·mL−1) | 13.25 ± 0.32 | 15.42 ± 0.68 * |
| Fe Content | Fermented Cow Milk Diet | Fermented Goat Milk Diet | Two-Way ANOVA | |||||
|---|---|---|---|---|---|---|---|---|
| Control Group | Anemic Group | Control Group | Anemic Group | Diet | Anemia | Fe Content | ||
| Hemoglobin concentration (g·L−1) | Normal | 127.73 ± 2.35 | 128.42 ± 2.43 | 131.91 ± 2.44 | 130.01 ± 2.39 | NS 1 | NS | <0.001 |
| Overload | 141.97 ± 2.53 C | 140.18 ± 2.87 C | 141.43 ± 2.77 C | 145.62 ± 2.92 BC | <0.05 | NS | ||
| Red blood cells (1012·L−1) | Normal | 7.21 ± 0.18 | 7.16 ± 0.20 | 7.35 ± 0.22 | 7.32 ± 0.19 | NS | NS | <0.05 |
| Overload | 7.01 ± 0.19 | 7.19 ± 0.19 | 8.12 ± 0.28 AC | 7.21 ± 0.22 | <0.01 | NS | ||
| Hematocrit (%) | Normal | 40.22 ± 1.12 | 39.22 ± 1.01 | 41.87 ± 1.41 A | 43.05 ± 1.11 B | <0.01 | NS | <0.01 |
| Overload | 39.87 ± 1.25 | 45.11 ± 2.34 C | 44.75 ± 1.51 AC | 44.91 ± 1.41 C | <0.05 | NS | ||
| Mean corpuscular volume (fL) | Normal | 57.28 ± 0.54 | 55.28 ± 0.53 | 57.41 ± 0.57 | 55.22 ± 0.54 | NS | NS | NS |
| Overload | 56.90 ± 0.60 | 53.39 ± 0.54 | 56.62 ± 0.54 | 56.33 ± 0.51 B | <0.05 | NS | ||
| Platelets (109·L−1) | Normal | 935.62 ± 72.11 | 961.53 ± 67.33 | 929.21 ± 78.11 | 937.32 ± 68.53 | NS | NS | NS |
| Overload | 939.22 ± 71.24 | 963.29 ± 70.21 | 936.12 ± 79.76 | 942.12 ± 70.25 | NS | NS | ||
| Serum Fe (µg·L−1) | Normal | 1323 ± 81.88 | 1347 ± 85.33 | 1362 ± 88.22 | 1332 ± 91.13 | NS | NS | <0.01 |
| Overload | 1576 ± 98.56 C | 1592 ± 96.25 C | 1552 ± 97.89 C | 1569 ± 95.76 C | NS | NS | ||
| Total iron-binding capacity (µg·L−1) | Normal | 2782 ± 153 | 2788 ± 142 | 2791 ± 143 | 2786 ± 152 | NS | NS | <0.01 |
| Overload | 3151 ± 167 C | 3234 ± 171 C | 3241 ± 166 C | 3188 ± 169 C | NS | NS | ||
| Transferrin saturation (%) | Normal | 46.22 ± 0.91 | 45.50 ± 0.87 | 46.79 ± 0.78 | 46.89 ± 0.91 | NS | NS | <0.01 |
| Overload | 47.85 ± 1.21 C | 47.62 ± 1.12 C | 48.96 ± 1.11 C | 48.79 ± 1.07 C | NS | NS | ||
| Serum ferritin (µg·L−1) | Normal | 83.11 ± 1.56 | 83.77 ± 1.29 | 84.31 ± 1.65 | 82.24 ± 1.82 | NS | NS | <0.01 |
| Overload | 86.95 ± 1.88 C | 85.98 ± 1.76 C | 86.96 ± 1.83 C | 87.03 ± 1.79 C | NS | NS | ||
| Serum hepcidin (ng·mL−1) | Normal | 16.21 ± 0.61 | 16.37 ± 0.53 | 14.11 ± 0.58 A | 14.33 ± 0.61 B | <0.01 | NS | NS |
| Overload | 16.42 ± 0.58 | 16.39 ± 0.51 | 15.07 ± 0.58A | 14.22± 0.57B | <0.01 | NS | ||
| Normal-Fe Control Group (n = 10) | Low-Fe Anemic Group (n = 10) | |
|---|---|---|
| Dopamine | 1970.00 ± 180.21 | 1560.01 ± 90.05 * |
| Serotonin | 9179.0 ± 174.9 | 9409.0 ± 1813.5 |
| MAO-A | 7856.3 ± 225.2 | 6622.3 ± 212.7 ** |
| MAO-B | 267.96 ± 25.85 | 219.85 ± 10.81 |
| Neurotensin | 513.40 ± 48.18 | 442.61 ± 37.63 |
| Oxytocin | 249.71 ± 21.22 | 181.85 ± 17.86 ** |
| Irisin | 21.17 ± 1.19 | 16.74 ± 0.90 ** |
| Synaptophysin | 771.79 ± 36.97 | 1121.37 ± 28.62 ** |
| α-MSH | 616.34 ± 20.52 | 200.58 ± 44.29 ** |
| β-Endorphin | 2431.5 ± 126.0 | 1308.3 ± 186.6 ** |
| Fe Content | Fermented Cow Milk Diet | Fermented Goat Milk Diet | Two-Way ANOVA | |||||
|---|---|---|---|---|---|---|---|---|
| Control Group | Anemic Group | Control Group | Anemic Group | Diet | Anemia | Fe Content | ||
| Dopamine | Normal | 1210.00 ± 40.01 | 980.14 ± 30.07 | 1710.10 ± 16.07 A | 1320.11 ± 11.23 B | <0.01 | NS | <0.05 |
| Overload | 3130.02 ± 22.14 D | 1440.21 ± 30.12 CD | 1400.02 ± 60.04 A | 1260 ± 40.33 C | <0.01 | < 0.01 | ||
| Serotonin | Normal | 33651.9 ± 3334.5 | 61341.9 ± 5820.1 C | 39278.9 ± 4571.7 | 82050.5 ± 2995.1 BC | <0.05 | <0.001 | <0.001 |
| Overload | 26346.4 ± 2943.3 D | 29564.3 ± 2806.3 D | 20981.4 ± 852.7 D | 23688.0 ± 962.7 D | NS | NS | ||
| MAO-A | Normal | 8220.1 ± 261.5 | 7279.3 ± 298.6 C | 7390.3 ± 211.9 A | 6716.7 ± 483.3 B | <0.05 | <0.05 | <0.01 |
| Overload | 13935.1 ± 282.3 D | 7152.6 ± 498.0 C | 8403.7 ± 353.4 AD | 4001.8 ± 168.3 BCD | <0.001 | <0.001 | ||
| MAO-B | Normal | 260.96 ± 10.81 | 237.89 ± 11.57 C | 215.13 ± 10.10 A | 208.93 ± 10.07 B | <0.001 | <0.05 | <0.01 |
| Overload | 327.70 ± 9.56 D | 259.96 ± 6.68 C | 305.24 ± 9.01 D | 267.52 ± 7.90 CD | NS | <0.01 | ||
| Neurotensin | Normal | 317.98 ± 11.94 | 405.37 ± 18.23 C | 336.36 ± 21.37 | 373.21 ± 10.15 | NS | <0.05 | <0.05 |
| Overload | 360.77 ± 12.67 D | 345.61 ± 12.80 D | 355.79 ± 10.39 | 341.56 ± 9.98 | NS | NS | ||
| Oxytocin | Normal | 145.57 ± 9.27 | 173.42 ± 33.90 C | 182.41 ± 7.27 A | 177.74 ± 1.87 | <0.05 | <0.05 | <0.001 |
| Overload | 217.27 ± 3.58 D | 86.95 ± 0.13 CD | 225.52 ± 3.94 D | 89.85 ± 1.57 CD | NS | <0.01 | ||
| Irisin | Normal | 16.75 ± 0.70 | 15.96 ± 0.35 | 19.18 ± 1.26 | 17.24 ± 1.03 | NS | NS | NS |
| Overload | 19.32 ± 0.60 | 16.66 ± 0.85 | 17.58 ± 0.79 | 15.64 ± 0.70 | NS | NS | ||
| Synaptophysin | Normal | 836.97 ± 31.40 | 907.33 ± 27.57 C | 1091.02 ± 26.92 A | 730.09 ± 20.44 BC | <0.001 | <0.01 | <0.001 |
| Overload | 552.01 ± 40.56 D | 789.02 ± 49.59 CD | 1290.56 ± 51.89 AD | 883.40 ± 24.74 BCD | <0.001 | <0.001 | ||
| α-MSH | Normal | 310.87 ± 14.95 | 86.28 ± 1.38 C | 1162.05 ± 9.07 A | 494.28 ± 22.64 BC | <0.001 | <0.001 | <0.001 |
| Overload | 669.74 ± 33.48 D | 251.69 ± 18.04 CD | 726.85 ± 21.86 A D | 312.54 ± 9.40 BC D | <0.01 | <0.001 | ||
| β-Endorphin | Normal | 2426.5 ± 79.4 | 744.3 ± 39.9 C | 2551.9 ± 131.7 | 671.3 ± 36.4 C | NS | <0.01 | <0.05 |
| Overload | 741.6 ± 41.3 D | 688.6 ± 40.9 | 1344.2 ± 71.5 AD | 640.1 ± 34.0 C | <0.05 | NS | ||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moreno-Fernández, J.; López-Aliaga, I.; García-Burgos, M.; J.M. Alférez, M.; Díaz-Castro, J. Fermented Goat Milk Consumption Enhances Brain Molecular Functions during Iron Deficiency Anemia Recovery. Nutrients 2019, 11, 2394. https://doi.org/10.3390/nu11102394
Moreno-Fernández J, López-Aliaga I, García-Burgos M, J.M. Alférez M, Díaz-Castro J. Fermented Goat Milk Consumption Enhances Brain Molecular Functions during Iron Deficiency Anemia Recovery. Nutrients. 2019; 11(10):2394. https://doi.org/10.3390/nu11102394
Chicago/Turabian StyleMoreno-Fernández, Jorge, Inmaculada López-Aliaga, María García-Burgos, María J.M. Alférez, and Javier Díaz-Castro. 2019. "Fermented Goat Milk Consumption Enhances Brain Molecular Functions during Iron Deficiency Anemia Recovery" Nutrients 11, no. 10: 2394. https://doi.org/10.3390/nu11102394
APA StyleMoreno-Fernández, J., López-Aliaga, I., García-Burgos, M., J.M. Alférez, M., & Díaz-Castro, J. (2019). Fermented Goat Milk Consumption Enhances Brain Molecular Functions during Iron Deficiency Anemia Recovery. Nutrients, 11(10), 2394. https://doi.org/10.3390/nu11102394







