Dietary Compounds for Targeting Prostate Cancer
Abstract
1. Introduction
2. Results
2.1. Apoptosis and Dietary Compounds
2.2. Anti-Angiogenesis and Dietary Compounds
2.3. Anti-Metastasis and Dietary Compounds
2.4. MiRNA Regulation and Dietary Compounds
2.5. Multi-Drug Resistance (MDR) and Dietary Compounds
3. Discussion
4. Methods
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| miRNA | microRNA |
| MDR | multi-drug-resistance |
| PARP | poly adenosine diphosphate ribose polymerase |
| PTEN | phosphatase and tensin homolog |
| Akt | protein kinase B |
| SHH | sonic hedgehog |
| FLIP | cellular FLICE (FADD-like IL-1β-converting enzyme)-inhibitory protein |
| VEGF | vascular endothelial growth factor |
| GM-CSF | granulocyte-macrophage colony-stimulating factor |
| IL-12 | interleukin 12; KAI1, Kangai 1 |
| OPG | osteoprotegerin; DNA, deoxyribonucleic Acid |
| BAX | BCL2 Associated X |
| LNCaP | lymph node carcinoma of the prostate |
| ROS | reactive oxygen species |
| NF-kB1 | nuclear factor kappa-light-chain-enhancer of activated B cells 1 |
| HDAC3 | histone deacetylase 3 |
| Puma | p53 upregulated modulator of apoptosis |
| NOXA | NADPH oxidase activator |
| JNK | Janus kinase |
| MMP | mitochondrial membrane potential |
| RIPK1 | receptor-interacting serine/threonine-protein kinase 1 |
| Bcl-2 | B-cell lymphoma 2 |
| Bim | Bcl-2-like protein 11 |
| FADD | Fas-associated protein with death domain |
| TRAIL | TNF-related apoptosis inducing ligand |
| BITC | 3-butenyl isothiocyanate |
| IC50 | the half maximal inhibitory concentration |
| NK-kB | nuclear factor kappa light chain enhancer of activated B cells |
| MTA1 | metastasis-associated protein 1 |
| STAT3 IHC | signal transducer and activator of transcription 3 Immunohistochemistry |
| LLDT-288 | (14S)-14β-(1-(2-morpholinoethyl)-1H-indazol-5-ylamino)mthylepitriptolide |
| ECM | extra-cellular matrix; EMT, epithelial-to-mesenchymal transition |
| TRAMP | transgenic adenocarcinoma of mouse prostate |
| NE-Ca | N-ethylcarboxamideadenosine |
| PLA2 | phospholipases A2; COX, cyclooxygenase |
| AA | acetic acid |
| CXCR4 | C-X-C chemokine receptor type 4 |
| PI3K | phosphoinositide 3-kinases |
| CXCL12, | C-X-C motif chemokine ligand 12 |
| MRP1 | multidrug resistance-associated protein 1 |
| GSTπ | glutathione S-transferase Pi |
| GST | glutathione S-transferase |
| PPV | personalized peptide vaccination |
| Mo-MDSC | monocytic myeloid-derived suppressor cells |
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: Globocan estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA 2018, 68, 394–424. [Google Scholar] [PubMed]
- Hauner, K.; Maisch, P.; Retz, M. Side effects of chemotherapy. Der Urologe. Ausg. A 2017, 56, 472–479. [Google Scholar] [CrossRef] [PubMed]
- American Cancer Society. Key Statistics for Prostate Cancer. Available online: https://www.cancer.org/cancer/prostate-cancer/about/key-statistics.html#references (accessed on 1 August 2019).
- Dogan, S.E.; Mizrak, D.; Alkan, A.; Demirkazik, A. Docetaxel-induced pericardial effusion. J. Oncol. Pharm. Pract. 2017, 23, 389–391. [Google Scholar] [CrossRef] [PubMed]
- Hughes, C.L.; Dhiman, T.R. Dietary compounds in relation to dietary diversity and human health. J. Med. Food 2002, 5, 51–68. [Google Scholar] [CrossRef] [PubMed]
- Sung, S.; Kwon, D.; Um, E.; Kim, B. Could polyphenols help in the control of rheumatoid arthritis? Molecules 2019, 24, 1589. [Google Scholar] [CrossRef]
- Direito, R.; Rocha, J. Anti-inflammatory effects of persimmon (diospyros kaki l.) in experimental rodent rheumatoid arthritis. J. Diet. Suppl. 2019, 1–21. [Google Scholar] [CrossRef]
- Nwanna, E.E.; Ibukun, E.O.; Oboh, G. Eggplant (solanum spp) supplemented fruits diet modulated the activities of ectonucleoside triphosphate diphosphohydrolase (entpdase), monoamine oxidase (mao), and cholinesterases (ache/bche) in the brain of diabetic wistar male rats. J. Food Biochem. 2019, 43, e12910. [Google Scholar] [CrossRef]
- Lee, J.E.; Song, H.S.; Park, M.N.; Kim, S.H. Ethanol extract of oldenlandia diffusa herba attenuates scopolamine-induced cognitive impairments in mice via activation of bdnf, p-creb and inhibition of acetylcholinesterase. Int. J. Mol. Sci. 2018, 19, 363. [Google Scholar] [CrossRef]
- Hwang, D.; Kim, M.; Park, H.; Jeong, M.I.; Jung, W.; Kim, B. Natural products and acute myeloid leukemia: A review highlighting mechanisms of action. Nutrients 2019, 11, 1010. [Google Scholar] [CrossRef]
- Abutaha, N.; Nasr, F.A.; Al-Zharani, M.; Alqahtani, A.S. Effects of hexane root extract of ferula hermonis boiss. On human breast and colon cancer cells: An in vitro and in vivo study. BioMed Res. Int. 2019, 2019, 3079895. [Google Scholar] [CrossRef]
- Noman, O.M.; Mubarak, M.; Abdelhabib, S.; Wadaan, M.A.; Shen, H.; Qu, Z.; Harata-Lee, Y.; Aung, T.N.; Cui, J.; Wang, W.; et al. Understanding the mechanistic contribution of herbal extracts in compound kushen injection with transcriptome analysis. BioMed Res. Int. 2019, 9, 632. [Google Scholar]
- Kim, C.; Kim, B. Anti-cancer natural products and their bioactive compounds inducing er stress-mediated apoptosis: A review. Nutrients 2018, 10, 1021. [Google Scholar] [CrossRef] [PubMed]
- Asif, M. Health effects of omega-3,6,9 fatty acids: Perilla frutescens is a good example of plant oils. Orient. Pharm. Exp. Med. 2011, 11, 51–59. [Google Scholar] [CrossRef] [PubMed]
- Nobili, S.; Lippi, D.; Witort, E.; Donnini, M.; Bausi, L.; Mini, E.; Capaccioli, S. Natural compounds for cancer treatment and prevention. Pharmacol. Res. 2009, 59, 365–378. [Google Scholar] [CrossRef] [PubMed]
- Banikazemi, Z.; Haji, H.A.; Mohammadi, M.; Taheripak, G.; Iranifar, E.; Poursadeghiyan, M.; Moridikia, A.; Rashidi, B.; Taghizadeh, M.; Mirzaei, H. Diet and cancer prevention: Dietary compounds, dietary micrornas, and dietary exosomes. J. Cell Biochem. 2018, 119, 185–196. [Google Scholar] [CrossRef] [PubMed]
- Qiang, Z.; Meng, L.; Yi, C.; Yu, L.; Chen, W.; Sha, W. Curcumin regulates the mir-21/pten/akt pathway and acts in synergy with pd98059 to induce apoptosis of human gastric cancer mgc-803 cells. J. Int. Med. Res. 2019, 47, 1288–1297. [Google Scholar] [CrossRef]
- Feng, T.; Wei, Y.; Lee, R.J.; Zhao, L. Liposomal curcumin and its application in cancer. Int. J. Nanomed. 2017, 12, 6027–6044. [Google Scholar] [CrossRef]
- Fu, J.; Shrivastava, A.; Shrivastava, S.K.; Srivastava, R.K.; Shankar, S. Triacetyl resveratrol upregulates mirna200 and suppresses the shh pathway in pancreatic cancer: A potential therapeutic agent. Int. J. Oncol. 2019, 54, 1306–1316. [Google Scholar]
- Ko, J.-H.; Sethi, G.; Um, J.-Y.; Shanmugam, M.K.; Arfuso, F.; Kumar, A.P.; Bishayee, A.; Ahn, K.S. The role of resveratrol in cancer therapy. Int. J. Mol. Sci. 2017, 18, 2589. [Google Scholar] [CrossRef]
- Gao, Z.; Gao, W.; Zeng, S.-L.; Li, P.; Liu, E.H. Chemical Structures, Bioactivities and Molecular Mechanisms of Citrus Polymethoxyflavones. J. Funct. Foods. 2018, 40, 498–509. [Google Scholar] [CrossRef]
- Hassan, M.; Watari, H.; AbuAlmaaty, A.; Ohba, Y.; Sakuragi, N. Apoptosis and molecular targeting therapy in cancer. BioMed Res. Int. 2014, 2014, 150845. [Google Scholar] [CrossRef] [PubMed]
- Prager, G.W.; Poettler, M.; Unseld, M.; Zielinski, C.C. Angiogenesis in cancer: Anti-vegf escape mechanisms. Transl. Lung Cancer Res. 2012, 1, 14–25. [Google Scholar] [PubMed]
- Park, G.; Choi, K.-C. Advanced New Strategies for Metastatic Cancer Treatment by Therapeutic Stem Cells and Oncolytic Virotherapy. Oncotarget 2016, 7, 58684–58695. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Cornelison, R.; Llaneza, C.D.; Landen, N.C. Emerging therapeutics to overcome chemoresistance in epithelial ovarian cancer: A mini-review. Int. J. Mol. Sci. 2017, 18, 2171. [Google Scholar] [CrossRef] [PubMed]
- Adiwidjaja, J.; McLachlan, A.J.; Boddy, A.V. Curcumin as a clinically-promising anti-cancer agent: Pharmacokinetics and drug interactions. Expert Opin. Drug Metab. Toxicol. 2017, 13, 953–972. [Google Scholar] [CrossRef] [PubMed]
- Cho, H.D.; Lee, J.H.; Moon, K.D.; Park, K.H.; Lee, M.K.; Seo, K.I. Auriculasin-induced ros causes prostate cancer cell death via induction of apoptosis. Food Chem. Toxicol. 2018, 111, 660–669. [Google Scholar] [CrossRef] [PubMed]
- Pascoal, A.C.; Ehrenfried, C.A.; Lopez, B.G.; de Araujo, T.M.; Pascoal, V.D.; Gilioli, R.; Anhe, G.F.; Ruiz, A.L.; Carvalho, J.E.; Stefanello, M.E.; et al. Antiproliferative activity and induction of apoptosis in pc-3 cells by the chalcone cardamonin from campomanesia adamantium (myrtaceae) in a bioactivity-guided study. Molecules 2014, 19, 1843–1855. [Google Scholar] [CrossRef]
- Deb Majumdar, I.; Devanabanda, A.; Fox, B.; Schwartzman, J.; Cong, H.; Porco, J.A., Jr.; Weber, H.C. Synthetic cyclohexenyl chalcone natural products possess cytotoxic activities against prostate cancer cells and inhibit cysteine cathepsins in vitro. Biochem. Biophys. Res. Commun. 2011, 416, 397–402. [Google Scholar] [CrossRef]
- Jeong, M.H.; Ko, H.; Jeon, H.; Sung, G.J.; Park, S.Y.; Jun, W.J.; Lee, Y.H.; Lee, J.; Lee, S.W.; Yoon, H.G.; et al. Delphinidin induces apoptosis via cleaved hdac3-mediated p53 acetylation and oligomerization in prostate cancer cells. Oncotarget 2016, 7, 56767–56780. [Google Scholar] [CrossRef]
- Park, K.H.; Yin, J.; Yoon, K.H.; Hwang, Y.J.; Lee, M.W. Antiproliferative effects of new dimeric ellagitannin from cornus alba in prostate cancer cells including apoptosis-related s-phase arrest. Molecules 2016, 21, 137. [Google Scholar] [CrossRef]
- Puente, J.; Grande, E.; Medina, A.; Maroto, P.; Lainez, N.; Arranz, J.A. Docetaxel in prostate cancer: A familiar face as the new standard in a hormone-sensitive setting. Ther. Adv. Med. Oncol. 2017, 9, 307–318. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; He, W.Y.; Zeng, Y.Z.; Hossain, A.; Gou, X. Inhibiting autophagy overcomes docetaxel resistance in castration-resistant prostate cancer cells. Int. Urol. Nephrol. 2018, 50, 675–686. [Google Scholar] [CrossRef] [PubMed]
- Zheng, L.; Chen, S.; Cao, Y.; Zhao, L.; Gao, Y.; Ding, X.; Wang, X.; Gu, Y.; Wang, S.; Zhu, Z.; et al. Combination of comprehensive two-dimensional prostate cancer cell membrane chromatographic system and network pharmacology for characterizing membrane binding active components from radix et rhizoma rhei and their targets. J. Chromatogr. A 2018, 1564, 145–154. [Google Scholar] [CrossRef]
- Hanafi, M.M.M.; Afzan, A.; Yaakob, H.; Aziz, R.; Sarmidi, M.R.; Wolfender, J.L.; Prieto, J.M. In vitro pro-apoptotic and anti-migratory effects of ficus deltoidea l. Plant extracts on the human prostate cancer cell lines pc3. Front. Pharmacol. 2017, 8, 895. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Ren, K.; Dong, H.; Song, F.; Chen, J.; Guo, Y.; Liu, Y.; Tao, W.; Zhang, Y. Flavonoids from persimmon (diospyros kaki l.) leaves inhibit proliferation and induce apoptosis in pc-3 cells by activation of oxidative stress and mitochondrial apoptosis. Chem. Biol. Interact. 2017, 275, 210–217. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Lao, Y.; Zhang, H.; Wang, X.; Tan, H.; Lin, Z.; Xu, H. The natural compound guttiferone f sensitizes prostate cancer to starvation induced apoptosis via calcium and jnk elevation. BMC Cancer 2015, 15, 254. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.X.; Lin, Z.M.; Wang, M.J.; Dong, Y.W.; Niu, H.M.; Young, C.Y.; Lou, H.X.; Yuan, H.Q. Jungermannenone a and b induce ros- and cell cycle-dependent apoptosis in prostate cancer cells in vitro. Acta Pharmacol. Sin. 2016, 37, 814–824. [Google Scholar] [CrossRef]
- Zhao, Y.; Chen, R.; Wang, Y.; Qing, C.; Wang, W.; Yang, Y. In vitro and in vivo efficacy studies of lavender angustifolia essential oil and its active constituents on the proliferation of human prostate cancer. Integr. Cancer Ther. 2017, 16, 215–226. [Google Scholar] [CrossRef]
- Wang, Q.; Xu, J.; Li, X.; Zhang, D.; Han, Y.; Zhang, X. Comprehensive two-dimensional pc-3 prostate cancer cell membrane chromatography for screening anti-tumor components from radix sophorae flavescentis. J. Sep. Sci. 2017, 40, 2688–2693. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, L.; Wang, J.; Cheng, W.; Zhang, J.; Li, X.; Zhang, Z.; Gong, J.; Ghosh, R.; Kumar, A.P.; et al. Combination of nexrutine and docetaxel suppresses nfkappab-mediated activation of c-flip. Mol. Carcinog. 2017, 56, 2200–2209. [Google Scholar] [CrossRef]
- Lu, Z.; Wang, H.; Zhu, M.; Song, W.; Wang, J.; Wu, C.; Kong, Y.; Guo, J.; Li, N.; Liu, J.; et al. Ophiopogonin D′, a natural product from radix ophiopogonis, induces in vitro and in vivo ripk1-dependent and caspase-independent apoptotic death in androgen-independent human prostate cancer cells. Front. Pharmacol. 2018, 9, 432. [Google Scholar] [CrossRef] [PubMed]
- Yun, J.M.; Kweon, M.H.; Kwon, H.; Hwang, J.K.; Mukhtar, H. Induction of apoptosis and cell cycle arrest by a chalcone panduratin a isolated from kaempferia pandurata in androgen-independent human prostate cancer cells pc3 and du145. Carcinogenesis 2006, 27, 1454–1464. [Google Scholar] [CrossRef] [PubMed]
- Adaramoye, O.; Erguen, B.; Nitzsche, B.; Hopfner, M.; Jung, K.; Rabien, A. Punicalagin, a polyphenol from pomegranate fruit, induces growth inhibition and apoptosis in human pc-3 and lncap cells. Chem. Biol. Interact. 2017, 274, 100–106. [Google Scholar] [CrossRef] [PubMed]
- Han, H.; Qiu, L.; Wang, X.; Qiu, F.; Wong, Y.; Yao, X. Physalins a and b inhibit androgen-independent prostate cancer cell growth through activation of cell apoptosis and downregulation of androgen receptor expression. Biol. Pharm. Bull. 2011, 34, 1584–1588. [Google Scholar] [CrossRef] [PubMed]
- Amujuri, D.; Siva, B.; Poornima, B.; Sirisha, K.; Sarma, A.V.S.; Lakshma Nayak, V.; Tiwari, A.K.; Purushotham, U.; Suresh Babu, K. Synthesis and biological evaluation of schizandrin derivatives as potential anti-cancer agents. Eur. J. Med. Chem. 2018, 149, 182–192. [Google Scholar] [CrossRef] [PubMed]
- Gao, C.; Zhou, Y.; Jiang, Z.; Zhao, Y.; Zhang, D.; Cong, X.; Cao, R.; Li, H.; Tian, W. Cytotoxic and chemosensitization effects of scutellarin from traditional chinese herb scutellaria altissima l. In human prostate cancer cells. Oncol. Rep. 2017, 38, 1491–1499. [Google Scholar] [CrossRef] [PubMed]
- Pan, B.; Zhong, W.; Deng, Z.; Lai, C.; Chu, J.; Jiao, G.; Liu, J.; Zhou, Q. Inhibition of prostate cancer growth by solanine requires the suppression of cell cycle proteins and the activation of ros/p38 signaling pathway. Cancer Med. 2016, 5, 3214–3222. [Google Scholar] [CrossRef]
- Wei, S.; Fukuhara, H.; Chen, G.; Kawada, C.; Kurabayashi, A.; Furihata, M.; Inoue, K.; Shuin, T. Terrestrosin d, a steroidal saponin from tribulus terrestris l., inhibits growth and angiogenesis of human prostate cancer in vitro and in vivo. Pathobiology 2014, 81, 123–132. [Google Scholar] [CrossRef]
- Liu, W.; Kou, B.; Ma, Z.K.; Tang, X.S.; Lv, C.; Ye, M.; Chen, J.Q.; Li, L.; Wang, X.Y.; He, D.L. Tetrandrine suppresses proliferation, induces apoptosis, and inhibits migration and invasion in human prostate cancer cells. Asian J. Androl. 2015, 17, 850–853. [Google Scholar]
- Levrier, C.; Rockstroh, A.; Gabrielli, B.; Kavallaris, M.; Lehman, M.; Davis, R.A.; Sadowski, M.C.; Nelson, C.C. Discovery of thalicthuberine as a novel antimitotic agent from nature that disrupts microtubule dynamics and induces apoptosis in prostate cancer cells. Cell Cycle 2018, 17, 652–668. [Google Scholar] [CrossRef]
- Akhtar, N.; Syed, D.N.; Khan, M.I.; Adhami, V.M.; Mirza, B.; Mukhtar, H. The pentacyclic triterpenoid, plectranthoic acid, a novel activator of ampk induces apoptotic death in prostate cancer cells. Oncotarget 2016, 7, 3819–3831. [Google Scholar] [CrossRef]
- Klosek, M.; Mertas, A.; Krol, W.; Jaworska, D.; Szymszal, J.; Szliszka, E. Tumor necrosis factor-related apoptosis-inducing ligand-induced apoptosis in prostate cancer cells after treatment with xanthohumol-a natural compound present in humulus lupulus l. Int. J. Mol. Sci. 2016, 17, 837. [Google Scholar] [CrossRef]
- Arora, R.; Kumar, R.; Mahajan, J.; Vig, A.P.; Singh, B.; Singh, B.; Arora, S. 3-butenyl isothiocyanate: A hydrolytic product of glucosinolate as a potential cytotoxic agent against human cancer cell lines. J. Food Sci. Technol. 2016, 53, 3437–3445. [Google Scholar] [CrossRef]
- Levrier, C.; Sadowski, M.C.; Rockstroh, A.; Gabrielli, B.; Kavallaris, M.; Lehman, M.; Davis, R.A.; Nelson, C.C. 6alpha-acetoxyanopterine: A novel structure class of mitotic inhibitor disrupting microtubule dynamics in prostate cancer cells. Mol. Cancer Ther. 2017, 16, 3–15. [Google Scholar] [CrossRef]
- Endo, S.; Hoshi, M.; Matsunaga, T.; Inoue, T.; Ichihara, K.; Ikari, A. Autophagy inhibition enhances anticancer efficacy of artepillin c, a cinnamic acid derivative in brazilian green propolis. Biochem. Biophys. Res. Commun. 2018, 497, 437–443. [Google Scholar] [CrossRef]
- Kim, Y.J.; Park, H.B.; Yoo, J.H.; Kwon, H.C.; Kim, J.; Yang, H.O. Glionitrin a, a new diketopiperazine disulfide, activates atm-atr-chk1/2 via 53bp1 phosphorylation in du145 cells and shows antitumor effect in xenograft model. Biol. Pharm. Bull. 2014, 37, 378–386. [Google Scholar] [CrossRef][Green Version]
- Mukhopadhyay, A.; Hanold, L.E.; Thayele Purayil, H.; Gisemba, S.A.; Senadheera, S.N.; Aldrich, J.V. Macrocyclic peptides decrease c-myc protein levels and reduce prostate cancer cell growth. Cancer Biol. Ther. 2017, 18, 571–583. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, M.; Wang, D.; Li, X.; Wang, W.; Lou, H.; Yuan, H. Malformin a1 promotes cell death through induction of apoptosis, necrosis and autophagy in prostate cancer cells. Cancer Chemother. Pharmacol. 2016, 77, 63–75. [Google Scholar] [CrossRef]
- Kundu, S.; Kim, T.H.; Yoon, J.H.; Shin, H.S.; Lee, J.; Jung, J.H.; Kim, H.S. Viriditoxin regulates apoptosis and autophagy via mitotic catastrophe and microtubule formation in human prostate cancer cells. Int. J. Oncol. 2014, 45, 2331–2340. [Google Scholar] [CrossRef]
- Wu, S.Y.; Sung, P.J.; Chang, Y.L.; Pan, S.L.; Teng, C.M. Heteronemin, a spongean sesterterpene, induces cell apoptosis and autophagy in human renal carcinoma cells. BioMed Res. Int. 2015, 2015, 738241. [Google Scholar] [CrossRef]
- Lee, M.G.; Liu, Y.C.; Lee, Y.L.; El-Shazly, M.; Lai, K.H.; Shih, S.P.; Ke, S.C.; Hong, M.C.; Du, Y.C.; Yang, J.C.; et al. Heteronemin, a marine sesterterpenoid-type metabolite, induces apoptosis in prostate lncap cells via oxidative and er stress combined with the inhibition of topoisomerase ii and hsp90. Mar. Drugs 2018, 16, 204. [Google Scholar] [CrossRef]
- Guedes, J.P.; Pereira, C.S.; Rodrigues, L.R.; Corte-Real, M. Bovine milk lactoferrin selectively kills highly metastatic prostate cancer pc-3 and osteosarcoma mg-63 cells in vitro. Front. Oncol. 2018, 8, 200. [Google Scholar] [CrossRef]
- Sato, C.; Kaneko, S.; Sato, A.; Virgona, N.; Namiki, K.; Yano, T. Combination effect of delta-tocotrienol and gamma-tocopherol on prostate cancer cell growth. J. Nutr. Sci. Vitaminol. 2017, 63, 349–354. [Google Scholar] [CrossRef]
- Tao, X.; Xu, L.; Yin, L.; Han, X.; Qi, Y.; Xu, Y.; Song, S.; Zhao, Y.; Peng, J. Dioscin induces prostate cancer cell apoptosis through activation of estrogen receptor-beta. Cell Death Dis. 2017, 8, e2989. [Google Scholar] [CrossRef]
- Xu, H.; Fan, X.; Zhang, G.; Liu, X.; Li, Z.; Li, Y.; Jiang, B. Lldt-288, a novel triptolide analogue exhibits potent antitumor activity in vitro and in vivo. Biomed. Pharmacother. Biomed. Pharmacother. 2017, 93, 1004–1009. [Google Scholar] [CrossRef]
- Dhar, S.; Kumar, A.; Zhang, L.; Rimando, A.M.; Lage, J.M.; Lewin, J.R.; Atfi, A.; Zhang, X.; Levenson, A.S. Dietary pterostilbene is a novel mta1-targeted chemopreventive and therapeutic agent in prostate cancer. Oncotarget 2016, 7, 18469–18484. [Google Scholar] [CrossRef]
- Rui, X.; Pan, H.F.; Shao, S.L.; Xu, X.M. Anti-tumor and anti-angiogenic effects of fucoidan on prostate cancer: Possible jak-stat3 pathway. BMC Complement Alt. Med. 2017, 17, 378. [Google Scholar] [CrossRef]
- Li, X.; Fan, S.; Pan, X.; Xiaokaiti, Y.; Duan, J.; Shi, Y.; Pan, Y.; Tie, L.; Wang, X.; Li, Y. Nordihydroguaiaretic acid impairs prostate cancer cell migration and tumor metastasis by suppressing neuropilin 1. Oncotarget 2016, 7, 86225–86238. [Google Scholar] [CrossRef]
- Li, K.; Dias, S.J.; Rimando, A.M.; Dhar, S.; Mizuno, C.S.; Penman, A.D.; Lewin, J.R.; Levenson, A.S. Pterostilbene acts through metastasis-associated protein 1 to inhibit tumor growth, progression and metastasis in prostate cancer. PLoS ONE 2013, 8, e57542. [Google Scholar] [CrossRef]
- Ryu, N.H.; Park, K.R.; Kim, S.M.; Yun, H.M.; Nam, D.; Lee, S.G.; Jang, H.J.; Ahn, K.S.; Kim, S.H.; Shim, B.S.; et al. A hexane fraction of guava leaves (psidium guajava l.) induces anticancer activity by suppressing akt/mammalian target of rapamycin/ribosomal p70 s6 kinase in human prostate cancer cells. J. Med. Food 2012, 15, 231–241. [Google Scholar] [CrossRef]
- Lee, S.C.; Chan, W.K.; Lee, T.W.; Lam, W.H.; Wang, X.; Chan, T.H.; Wong, Y.C. Effect of a prodrug of the green tea polyphenol (-)-epigallocatechin-3-gallate on the growth of androgen-independent prostate cancer in vivo. Nutr. Cancer 2008, 60, 483–491. [Google Scholar] [CrossRef] [PubMed]
- Chintharlapalli, S.; Papineni, S.; Lei, P.; Pathi, S.; Safe, S. Betulinic acid inhibits colon cancer cell and tumor growth and induces proteasome-dependent and -independent downregulation of specificity proteins (sp) transcription factors. BMC Cancer 2011, 11, 371. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Yang, D.; Wang, S.; Tang, W.; Liu, M.; Davis, M.; Chen, J.; Rae, J.M.; Lawrence, T.; Lippman, M.E. (-)-gossypol enhances response to radiation therapy and results in tumor regression of human prostate cancer. Mol. Cancer Ther. 2005, 4, 197–205. [Google Scholar] [PubMed]
- Harikumar, K.B.; Sung, B.; Tharakan, S.T.; Pandey, M.K.; Joy, B.; Guha, S.; Krishnan, S.; Aggarwal, B.B. Sesamin manifests chemopreventive effects through the suppression of nf-kappa b-regulated cell survival, proliferation, invasion, and angiogenic gene products. Mol. Cancer Res. MCR 2010, 8, 751–761. [Google Scholar] [CrossRef] [PubMed]
- Tang, S.N.; Zhang, J.; Wu, W.; Jiang, P.; Puppala, M.; Zhang, Y.; Xing, C.; Kim, S.H.; Jiang, C.; Lü, J. Chemopreventive effects of korean angelica versus its major pyranocoumarins on two lineages of transgenic adenocarcinoma of mouse prostate carcinogenesis. Cancer Prev. Res. 2015, 8, 835–844. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Li, W.; Lin, M.; Garcia, M.; Mulholland, D.; Lilly, M.; Martins-Green, M. Luteolin, ellagic acid and punicic acid are natural products that inhibit prostate cancer metastasis. Carcinogenesis 2014, 35, 2321–2330. [Google Scholar] [CrossRef] [PubMed]
- Mehta, R.G.; Murillo, G.; Naithani, R.; Peng, X. Cancer chemoprevention by natural products: How far have we come? Pharm. Res. 2010, 27, 950–961. [Google Scholar] [CrossRef]
- Wang, L.; Ho, J.; Glackin, C.; Martins-Green, M. Specific pomegranate juice components as potential inhibitors of prostate cancer metastasis. Transl. Oncol. 2012, 5, 344–355. [Google Scholar] [CrossRef]
- Siddiqui, I.A.; Asim, M.; Hafeez, B.B.; Adhami, V.M.; Tarapore, R.S.; Mukhtar, H. Green tea polyphenol egcg blunts androgen receptor function in prostate cancer. FASEB J. 2011, 25, 1198–1207. [Google Scholar] [CrossRef]
- Yang, K.; Gao, Z.Y.; Li, T.Q.; Song, W.; Xiao, W.; Zheng, J.; Chen, H.; Chen, G.H.; Zou, H.Y. Anti-tumor activity and the mechanism of a green tea (camellia sinensis) polysaccharide on prostate cancer. Int. J. Biol. Macromol. 2019, 122, 95–103. [Google Scholar] [CrossRef]
- Dhar, S.; Hicks, C.; Levenson, A.S. Resveratrol and prostate cancer: Promising role for micrornas. Mol. Nutr. Food Res. 2011, 55, 1219–1229. [Google Scholar] [CrossRef] [PubMed]
- Hirata, H.; Hinoda, Y.; Shahryari, V.; Deng, G.; Tanaka, Y.; Tabatabai, Z.L.; Dahiya, R. Genistein downregulates onco-mir-1260b and upregulates sfrp1 and smad4 via demethylation and histone modification in prostate cancer cells. Br. J. Cancer 2014, 110, 1645–1654. [Google Scholar] [CrossRef] [PubMed]
- Khor, T.O.; Huang, Y.; Wu, T.Y.; Shu, L.; Lee, J.; Kong, A.N. Pharmacodynamics of curcumin as DNA hypomethylation agent in restoring the expression of nrf2 via promoter cpgs demethylation. Biochem. Pharmacol. 2011, 82, 1073–1078. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.H.; Yue, J.; Sims, M.; Pfeffer, L.M. The curcumin analog ef24 targets nf-κb and mirna-21, and has potent anticancer activity in vitro and in vivo. PLoS ONE 2013, 8, e71130. [Google Scholar] [CrossRef] [PubMed]
- Vardi, A.; Bosviel, R.; Rabiau, N.; Adjakly, M.; Satih, S.; Dechelotte, P.; Boiteux, J.P.; Fontana, L.; Bignon, Y.J.; Guy, L.; et al. Soy phytoestrogens modify DNA methylation of gstp1, rassf1a, eph2 and brca1 promoter in prostate cancer cells. In Vivo 2010, 24, 393–400. [Google Scholar] [PubMed]
- Sakurai, M.A.; Ozaki, Y.; Okuzaki, D.; Naito, Y.; Sasakura, T.; Okamoto, A.; Tabara, H.; Inoue, T.; Hagiyama, M.; Ito, A.; et al. Gefitinib and luteolin cause growth arrest of human prostate cancer pc-3 cells via inhibition of cyclin g-associated kinase and induction of mir-630. PLoS ONE 2014, 9, e100124. [Google Scholar] [CrossRef] [PubMed]
- Morad, S.A.; Schmidt, C.; Buchele, B.; Schneider, B.; Wenzler, M.; Syrovets, T.; Simmet, T. (8r)-3beta,8-dihydroxypolypoda-13e,17e,21-triene induces cell cycle arrest and apoptosis in treatment-resistant prostate cancer cells. J. Nat. Prod. 2011, 74, 1731–1736. [Google Scholar] [CrossRef] [PubMed]
- Lyn-Cook, B.D.; Rogers, T.; Yan, Y.; Blann, E.B.; Kadlubar, F.F.; Hammons, G.J. Chemopreventive effects of tea extracts and various components on human pancreatic and prostate tumor cells in vitro. Nutr. Cancer 1999, 35, 80–86. [Google Scholar] [CrossRef]
- Steentjes, L.; Siesling, S.; Drummond, F.J.; van Manen, J.G.; Sharp, L.; Gavin, A. Factors associated with current and severe physical side-effects after prostate cancer treatment: What men report. Eur. J. Cancer Care 2018, 27. [Google Scholar] [CrossRef]
- Wang, Y.; Shi, J.; Chai, K.; Ying, X.; Zhou, B.P. The role of snail in emt and tumorigenesis. Curr. Cancer Drug Targ. 2013, 13, 963–972. [Google Scholar] [CrossRef]
- Geiger, T.R.; Peeper, D.S. Metastasis mechanisms. Biochim. Biophys. Acta 2009, 1796, 293–308. [Google Scholar] [CrossRef] [PubMed]
- Jain, C.K.; Gupta, A.; Dogra, N.; Kumar, V.S.; Wadhwa, G.; Sharma, S.K. Microrna therapeutics: The emerging anticancer strategies. Recent Pat. Anticancer Drug Discov. 2014, 9, 286–296. [Google Scholar] [CrossRef] [PubMed]
- Brase, J.C.; Johannes, M.; Schlomm, T.; Fälth, M.; Haese, A.; Steuber, T.; Beissbarth, T.; Kuner, R.; Sültmann, H. Circulating mirnas are correlated with tumor progression in prostate cancer. Int. J. Cancer 2011, 128, 608–616. [Google Scholar] [CrossRef] [PubMed]
- Porkka, K.P.; Pfeiffer, M.J.; Waltering, K.K.; Vessella, R.L.; Tammela, T.L.J.; Visakorpi, T. Microrna expression profiling in prostate cancer. Cancer Res. 2007, 67, 6130–6135. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.; Croce, C.M. The role of micrornas in human cancer. Signal Transduct. Targ. Ther. 2016, 1, 15004. [Google Scholar] [CrossRef] [PubMed]
- Krasnov, G.S.; Dmitriev, A.A.; Sadritdinova, A.F.; Volchenko, N.N.; Slavnova, E.N.; Danilova, T.V.; Snezhkina, A.V.; Melnikova, N.V.; Fedorova, M.S.; Lakunina, V.A.; et al. Molecular genetic mechanisms of drug resistance in prostate cancer. Mol. Biol. 2015, 49, 638–648. [Google Scholar] [CrossRef]
- Kumar, A.; Jaitak, V. Natural products as multidrug resistance modulators in cancer. Eur. J. Med. Chem. 2019, 176, 268–291. [Google Scholar] [CrossRef] [PubMed]
- Guo, Q.; Cao, H.; Qi, X.; Li, H.; Ye, P.; Wang, Z.; Wang, D.; Sun, M. Research progress in reversal of tumor multi-drug resistance via natural products. Anticancer Agents Med. Chem. 2017, 17, 1466–1476. [Google Scholar] [CrossRef] [PubMed]
- Baguley, B.C. Multiple drug resistance mechanisms in cancer. Mol. Biotechnol. 2010, 46, 308–316. [Google Scholar] [CrossRef]
- Langer, R.; Ott, K.; Feith, M.; Lordick, F.; Specht, K.; Becker, K.; Hofler, H. High pretherapeutic thymidylate synthetase and mrp-1 protein levels are associated with nonresponse to neoadjuvant chemotherapy in oesophageal adenocarcinoma patients. J. Surg. Oncol. 2010, 102, 503–508. [Google Scholar] [CrossRef]
- Shi, H.; Lu, D.; Shu, Y.; Shi, W.; Lu, S.; Wang, K. Expression of multidrug resistance-related proteins p-glycoprotein, glutathione-s-transferases, topoisomerase-ii and lung resistance protein in primary gastric cardiac adenocarcinoma. Hepatogastroenterology 2008, 55, 1530–1536. [Google Scholar] [PubMed]
- Deb, G.; Shankar, E.; Thakur, V.S.; Ponsky, L.E.; Bodner, D.R.; Fu, P.; Gupta, S. Green tea-induced epigenetic reactivation of tissue inhibitor of matrix metalloproteinase-3 suppresses prostate cancer progression through histone-modifying enzymes. Mol. Carcinog. 2019, 58, 1194–1207. [Google Scholar] [CrossRef] [PubMed]
- Shankar, E.; Kanwal, R.; Candamo, M.; Gupta, S. Dietary phytochemicals as epigenetic modifiers in cancer: Promise and challenges. Semin. Cancer Biol. 2016, 40–41, 82–99. [Google Scholar] [CrossRef] [PubMed]
- Gathirua-Mwangi, W.G.; Zhang, J. Dietary factors and risk for advanced prostate cancer. Eur. J. Cancer Prev. 2014, 23, 96–109. [Google Scholar] [CrossRef] [PubMed]
- Canene-Adams, K.; Lindshield, B.L.; Wang, S.; Jeffery, E.H.; Clinton, S.K.; Erdman, J.W., Jr. Combinations of tomato and broccoli enhance antitumor activity in dunning r3327-h prostate adenocarcinomas. Cancer Res. 2007, 67, 836–843. [Google Scholar] [CrossRef] [PubMed]
- Taylor, W.F.; Moghadam, S.E.; Moridi Farimani, M.; Ebrahimi, S.N.; Tabefam, M.; Jabbarzadeh, E. A multi-targeting natural compound with growth inhibitory and anti-angiogenic properties re-sensitizes chemotherapy resistant cancer. PLoS ONE 2019, 14, e0218125. [Google Scholar] [CrossRef] [PubMed]
- Imran, M.; Rauf, A.; Khan, I.A.; Shahbaz, M.; Qaisrani, T.B.; Fatmawati, S.; Abu-Izneid, T.; Imran, A.; Rahman, K.U.; Gondal, T.A. Thymoquinone: A novel strategy to combat cancer: A review. Biomed. Pharmacother. Biomed. Pharmacother. 2018, 106, 390–402. [Google Scholar] [CrossRef] [PubMed]
- Dybkowska, E.; Sadowska, A.; Swiderski, F.; Rakowska, R.; Wysocka, K. The occurrence of resveratrol in foodstuffs and its potential for supporting cancer prevention and treatment. A review. Roczniki Panstwowego Zakladu Higieny 2018, 69, 5–14. [Google Scholar]
- Shokoohinia, Y.; Jafari, F.; Mohammadi, Z.; Bazvandi, L.; Hosseinzadeh, L.; Chow, N.; Bhattacharyya, P.; Farzaei, M.H.; Farooqi, A.A.; Nabavi, S.M.; et al. Potential anticancer properties of osthol: A comprehensive mechanistic review. Nutrients 2018, 10, 36. [Google Scholar] [CrossRef]
- Dahut, W.L.; Gulley, J.L.; Arlen, P.M.; Liu, Y.; Fedenko, K.M.; Steinberg, S.M.; Wright, J.J.; Parnes, H.; Chen, C.C.; Jones, E.; et al. Randomized phase ii trial of docetaxel plus thalidomide in androgen-independent prostate cancer. J. Clin. Oncol. 2004, 22, 2532–2539. [Google Scholar] [CrossRef]
- Koga, N.; Moriya, F.; Waki, K.; Yamada, A.; Itoh, K.; Noguchi, M. Immunological efficacy of herbal medicines in prostate cancer patients treated by personalized peptide vaccine. Cancer Sci. 2017, 108, 2326–2332. [Google Scholar] [CrossRef] [PubMed]
- Paller, C.J.; Denmeade, S.R.; Carducci, M.A. Challenges of conducting clinical trials of natural products to combat cancer. Clin. Adv. Hematol. Oncol. H&O 2016, 14, 447–455. [Google Scholar]
- Shankar, E.; Goel, A.; Gupta, K.; Gupta, S. Plant flavone apigenin: An emerging anticancer agent. Curr. Pharmacol. Rep. 2017, 3, 423–446. [Google Scholar] [CrossRef] [PubMed]
- Baker, J.; Ajani, J.; Scotté, F.; Winther, D.; Martin, M.; Aapro, M.S.; von Minckwitz, G. Docetaxel-related side effects and their management. Eur. J. Oncol. Nurs. 2009, 13, 49–59. [Google Scholar] [CrossRef] [PubMed]
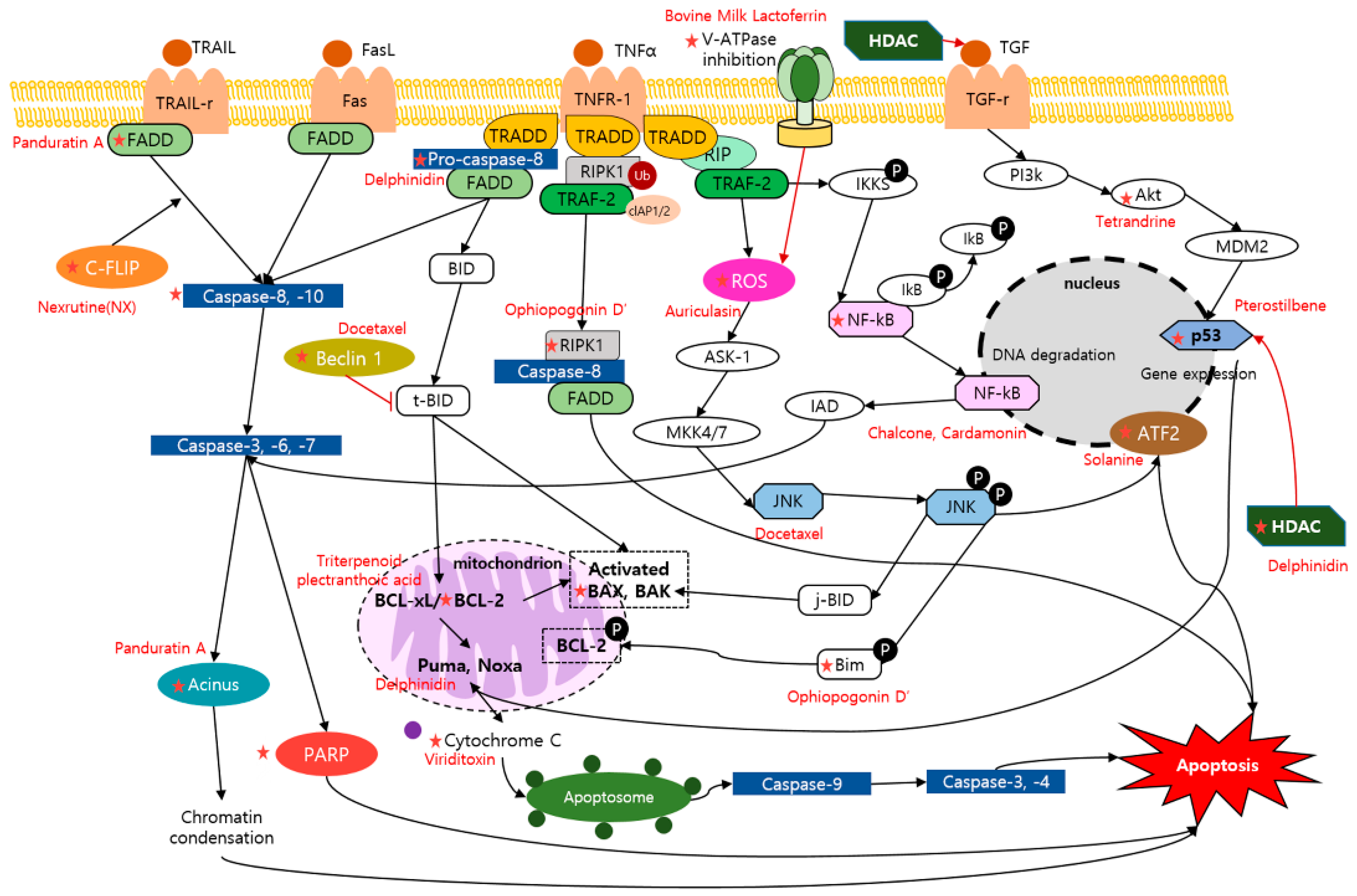

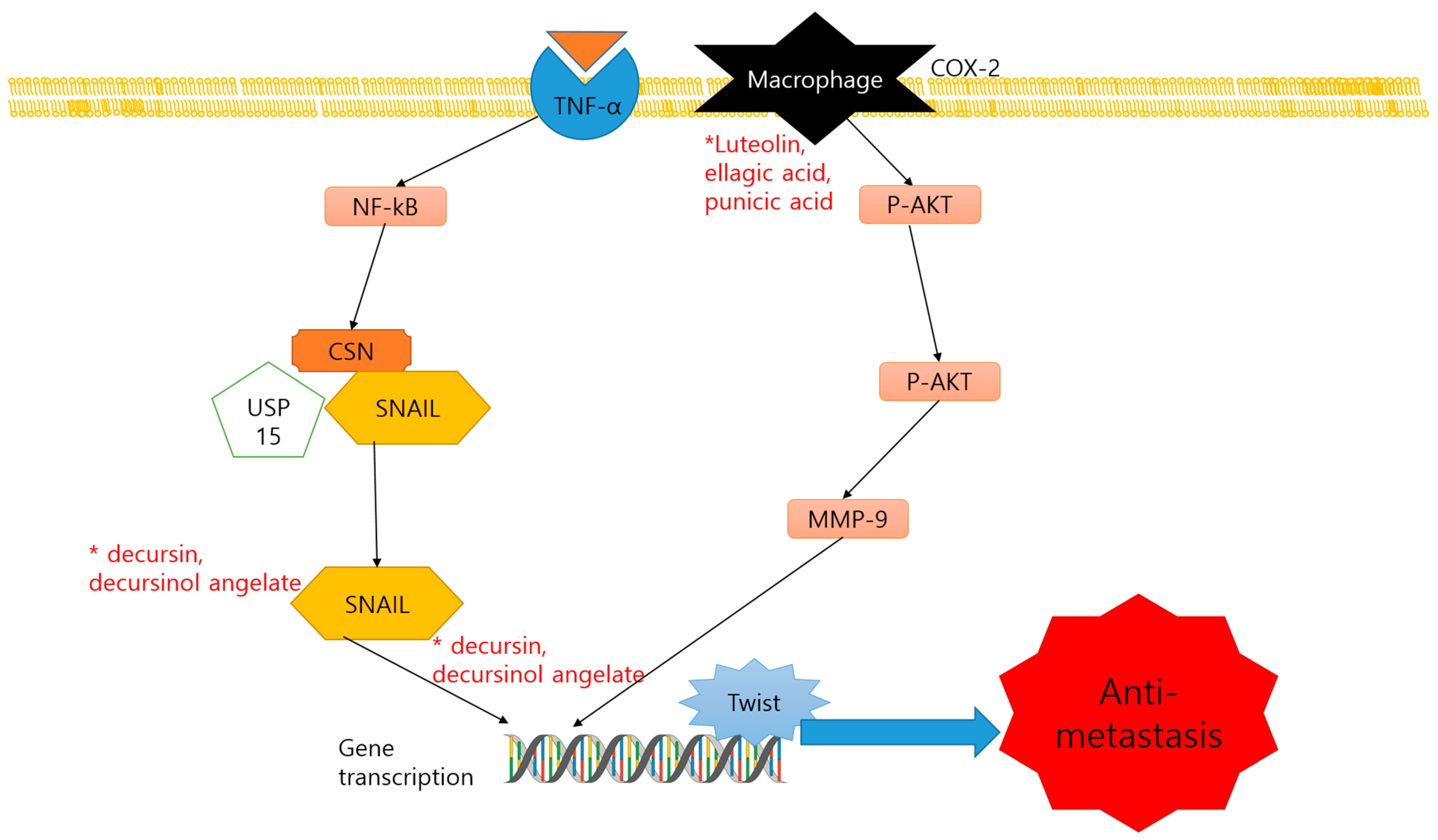
| Compound | Classification | Source | Organism | Cell Line/Animal Model | Dose; Duration | Mechanism | Reference |
|---|---|---|---|---|---|---|---|
| Auriculasin | Isoflavonoids | Flemingia philippinensis Merr. and Rolfe | Plant | LNCaP | 5 μM/24 h | Bax, c-PARP, ROS↑ | [27] |
| Bcl-2↓ | |||||||
| Chalcone Cardamonin | Flavonoids | Campomanesia adamantium Myrtaceae | Plant | PC3 | 11.35 μg/mL/12, 24, 48 h | NF-kB ↓ | [28] |
| Cyclohexenyl chalcone panduratin A (PA) and nicolaioidesin C (NC) | Flavones | Boesenbergia pandurate Roxb | Plant | PC3, DU145 | 5, 10, 20 μM/2 days | [29] | |
| Delphinidin | Anthocianidin | Pomegranates, berries, grapes, beets, and eggplants | Plant | (1) LNCaP | 50, 100, 150 μM/24 h | (1) c-caspase-3, -7, c- PARP, c-HDAC3, Puma, Bax, Noxa ↑ | [30] |
| (2) DU145 | Pro-caspase-8, HDAC3 ↓ | ||||||
| (3) PC3 | (3) HDAC3↓ | ||||||
| Dimeric ellagitannins (cornusiin A, camptothin B, C75H56O48 (amorphous)) | Tannins | Cornus alba Linne. | Plant | (1) DU145 | (1) 20, 50, 100 μM/48 h | [31] | |
| (2) LNCaP | (2) 5, 10, 20 μM/48 h | ||||||
| Docetaxel | Flavones | Taxus baccata L. | Plant | PC3, DU145 | 100 ng/mL/12 h, 24 h | c-PARP, p-Bcl-2, Beclin1, p-JNK↑ | [33] |
| (semisynthetic) | |||||||
| Emodin, rhapontigrnin | Anthraquinones | Rheum palmatum Linne. | Plant | DU145 | 50 μM/48 h | [34] | |
| FD1a, FD1c, FD1h, FD2a, FD2c, FD2h | Flavonoids | Ficus deltoidea Linne. | Plant | (1) PC3 | (1) 19 mg/mL/72, 96 h | (1) c-caspase 3, 7, Bax, Bcl-2 ↑ | [35] |
| (2) LNCaP | (2) 23 mg/mL/72 h | (2) c-caspase-3, -7 ↑ | |||||
| Flavonoid | Flavonoids | Diospyros kaki Linne. | Plant | PC3 | 12.5, 25 μg/mL/24 h | Bax, c-caspase-3↑ | [36] |
| Bcl-2↓ | |||||||
| Guttiferone F | Benzophenones | Allanblackia stuhlmannii Engl. | Plant | (1) PC3 | 10 μM/6, 12, 24 h | (1) & (2) c-caspase-9, −7, −3, c-PARP ↑ | [37] |
| (2) LNCaP | caspase-9, -7, -3, t-PARP ↓ | ||||||
| (2) Bcl-2, Bcl-2/Bax ↓ | |||||||
| Jungermannenone A and B | ent-kaurane diterpenoids | Jungermannia fauriana Beauverd. | Plant | PC3 | JA: 1.5 μM/L/12, 24, 48 h | c-caspase-3, c-PARP ↑ | [38] |
| JB: 5 μM/L/12, 24, 48 h | |||||||
| Linalool, Linalyl acetate | Flavonoids | Lavandula angustifolia Mill. | Plant | (1) PC3, DU145 | (1) 2.5 µM/24 h | [39] | |
| (2) Nude mice PC-3 prostate cancer cell xenograft model | (2) 10, 25, 50, 100, 200 mg/kg/7 days | ||||||
| Matrine, Oxymatrine, Sophocarpine, Xanthohumol | Alkaloids | Sophorae flavescens Aiton | Plant | PC3 | 0.1–2 mg/mL/72 h | [40] | |
| Nexrutine (NX) | Alkaloids, Phenolic compounds, flavone glycosides | Phellodendron amurense Rupr. | Plant | PC3 | 10 μM/72 h | c-caspase-3 ↑ | [41] |
| c-FLIP ↓ | |||||||
| Ophiopogonin D | Steroidal Glycoside | Ophiopogon japonicus Linne. f. | Plant | (1) PC3, DU145 | (1) 2.5, 5.0 μM/6h | (1) RIPK1, Bim ↑ | [42] |
| (2) 2.5 or 5.0 mg/kg; 24 days (5 days a week) | c-RIPK1, c-caspase-8, -10, Bid↓ | ||||||
| (2) BALB/c nude xenograft mice | |||||||
| Panduratin A | Flavones | Kaempferia pandurata Roxb. | Plant | (1) PC3, DU145 | (1) 20 μM/24 h | (1) Bax, FADD, TRAIL, c-PARP ↑ | [43] |
| Bcl-2, Bid, acinus, t-PARP, pro-caspase -3, -6, -8, -9 ↓ | |||||||
| Punicalagin | Ellagitanin | Punica granatum Linne. | Plant | (1) PC3 | (1), (2) 100 μM/24 h, 12 h | (1) c-caspase-3, -8 ↑ | [44] |
| (2) LNCaP | |||||||
| Physalin A, B | Steroids | Physalis alkekengi Linne. | Plant | C42B, CWR22Rv1 | 10 μM/12, 24, 48 h, or 5, 10, 16 μM/24 h | t-PARP, pro-caspase 3 ↓ | [45] |
| Schizandrin (1) derivatives compound 5 | N/A | Schisandra grandiflora Wall. | Plant | DU145 | 1, 2 μM/48 h | [46] | |
| Scutellarin | Flavone | Scutellaria altissima Linne. | Plant | PC3 | 200, 400, 600 μM/24 h | c-caspase-3, -9, Bax↑ | [47] |
| MMP, Bcl-2, ↓ | |||||||
| Solanine | Glycoalkaloids | Solanum nigrum Linne. | Plant | (1) DU145 | (1) 10–160 μM/L/1 h | (1) P38 pathway, p-ATF2, Bax↑ | [48] |
| (2) Six-to eight-week- old male nude mice with subcutaneous DU145 prostate cancer cell xenografts | (2) 5 mg/kg/4 weeks | Bcl-2 ↓ | |||||
| Terrestrosin D | Steroids | Tribulus terrestris Linne. | Plant | (1) PC3 | (1) 2, 5 μM/24 h | (1) MMP ↑ | [49] |
| (2) Male nude mice (5 weeks of age, BALB/c) | (2) 25, 50 mg/kg/4wks (3 times a week) | ||||||
| Tetrandrine | Alkaloids | Stephania tetrandra S. Moore | Plant | DU145, PC3 | 2.5, 5.0 10.0 μM/48 h | c-caspase-3, c-PARP, Bax↑ | [50] |
| Akt, Bcl-2 ↓ | |||||||
| Thalicthuberine | Alkaloids | Hernandia albiflora Kubitzki | Plant | (1) LNCaP, | (1) 2.5 μM/48h | (1) c-caspase-3, -7 ↑ | [51] |
| (2) CEM | (2) 5, 10 μM/24 h | (2) caspase-3, -7 ↑ | |||||
| (3) VCR-R | (3) 60 μM/24h | (3) caspase-3, -7 ↑ | |||||
| Triterpenoid plectranthoic acid (PA) | Hopanoids | Ficus microcarpa Linne.f. | Plant | DU145, PC3, NB26 | 20, 40 μM/24 h | Bax, c-PARP, c-caspase3 vinculin ↑ | [52] |
| Bcl-xl, Bcl-2 ↓ | |||||||
| Xanthohumol | Prenylated Chalconoid | Humulus lupulus Linne. | Plant | LNCaP | 50 μM/2, 8 h | c-caspase-8, -9, -3, Bax↑ | [53] |
| (Combination of TRAIL) | |||||||
| 3-butenyl isothiocyanate | Isothiocyanate | Brassica juncea Linne. Czen | Plant | PC3 | 0.041 μL/mL, 0.060 μL/mL/12-14 h | c-caspase-3 ↑ | [54] |
| MMP ↓ | |||||||
| 6α-acetoxyanopterine | Alkaloids | Anopterus macleayanus F.Muell. | Plant | (1) LNCaP | 1.25, 2.5, 5 nM/24 h | (1) c-PARP↑ | [55] |
| (2) PC3 |
| Compound | Classification | Source | Organism | Cell Line/Animal Model | Dose; Duration | Mechanism | Reference |
|---|---|---|---|---|---|---|---|
| Artepillin C | Phenolic acid | Propolis | Fungus | CRPC CWR22Rv1 | 50 μM/24 h | c-PARP, c-caspase-3 ↑ | [56] |
| Diketopiperazine disulfide glionitrin A | Diketopiperazine metabolite | Aspergillus fumigatus Fresen. Sphingomonas sp. (KMK-001) | Fungus | (1) DU145 | (1) 0.75, 1.5, 3 μM/24 h | (1) c-caspase-8, -9, -3, c-PARP, Bax ↑ | [57] |
| (2) Five weeks-old male BALB/c-nu mice (17–22 g) b earing xenografts of DU145 c ells | (2) 5, 10 mg/kg/27 d | Bid, PARP ↓ | |||||
| D-Trp isomerized CJ-15, 208 | Macrocyclic Peptide | Fungus | Fungus | PC3 | 10 μM/24, 48 h | [58] | |
| (cyclo [Phe-D-Pro-Phe-Trp]) | |||||||
| Malformin A1 | Quassinoids | Aspergillus niger Tiegh. | Fungus | PC3, LNCaP | 150 nM/6, 12, 24 h | c-caspase-3, c-PARP ↑ | [59] |
| Bcl-2 ↓ | |||||||
| Viriditoxin | N/A | Paecilomyces variotii (Bissochlamys spectabilis Udagawa and Shoji Suzuki) | Fungus | LNCaP | 0.1, 0.5, 1 μM/48 h | c-PARP, Bax and cytochrome c, c-caspase-3 ↑ | [60] |
| Bcl-2 ↓ |
| Compound | Classification | Source | Organism | Cell Line/Animal Model | Dose; Duration | Mechanism | Reference |
|---|---|---|---|---|---|---|---|
| Heteronemin | Sesterpenoid | Hyrtios sp. | Metazoa | (1) LNCaP, PC3 (2) Male immunodeficient athymic mice | (1) 2.56, 5.12 μM/24 h (2) 1 mg/kg/29 days | GAPDH, c-caspase -3, c-PARP↑ | [62] |
| Bovine Milk Lactoferrin | Glycoprotein | Milk | Animal | PC3 | 175 μM/48 h or 72 h | V-ATPase↑ | [63] |
| δTocotrienol, γ tocopherol (each and combined) | Tocotrienol (vitamin E) | Vitamin E | N/A | LNCaP | 10 μM/48 h | [64] | |
| Dioscin | Spirostanyl glycoside, Hexacyclic triterpenoid | N/A | N/A | (1) PC3 (2) PC3 cell tumor xenograft model | (1) 5.6, 10 μM/24 h (2) 80 mg/kg/28 days | (1) c-PARP, c-caspase-3, Bax ↑ Bcl-2 ↓ | [65] |
| LLDT-288 | Triptolide analogue | N/A | N/A | (1) PC3 (2) Human prostate (PC-3) xenograft mice model | (1) 1, 10 μM/36 h (2) 5, 10, 20mg/kg, PO, BID/21 days | (1) c-PARP, c-caspase-3, Bax ↑ NF-kB, caspase-3, -9, Bcl-2 ↓ | [66] |
| Pterostilbene | Stilbenoid | N/A | N/A | (2) Ptenf/f mice Pten+/f mice | (2) 10 mg/kg/3–30 weeks | (2) C-caspase3, p21, p27, Ac-p53/p53 ↑ | [67] |
| Mechanism | Compounds |
|---|---|
| PARP | Artepillin C |
| Auriculasin | |
| Delphinidin | |
| Dioscin | |
| Diketopiperazine disulfide glionitrin A | |
| Docetaxel | |
| Jungermannenone A and B | |
| LLDT-288 | |
| Malformin A1 | |
| Panduratin A | |
| Physlain A, B | |
| Tetrandrine | |
| Triterpenoid plectranthoic acid | |
| Viriditoxin | |
| Xanthohumol | |
| 6α-acetoxyanopterine | |
| Caspase | Artepillin C |
| Delphinidin | |
| Dioscin | |
| Diketopiperazine disulfide glionitrin A | |
| FD1a, FD1c, FD1h, FD2a, FD2c, FD2h | |
| Flavonoid | |
| Guttiferone F | |
| Heteronemin | |
| Scutellarin | |
| Tetrandrine | |
| Thalicthuberine | |
| Triterpenoid plectranthoic acid | |
| Xanthohumol | |
| 3-butenyl isothiocyanate | |
| C-FLIP | Nexrutine |
| FADD | Panduratin A |
| RIPK1 | Ophiopogonin D’ |
| ROS | Auriculasin |
| HDAC | Delphinidin |
| ATF2 | Solanine |
| Caspase-8 | Delphinidin |
| p53 | Pterostilbene |
| Beclin 1 | Docetaxel |
| JNK | Docetaxel |
| Cytochrome C | Viriditoxin |
| Puma, Noxa | Delphinidin |
| V-ATPase inhibition | Bovine Milk Lactoferrin |
| Acinus | Panduratin A |
| Bcl-xL | Triterpenoid plectranthoic acid |
| Bim | Ophiopogonin D’ |
| Akt | Tetrandrine |
| NF-kB | Chalcone |
| Cardamonin | |
| Bid | Diketopiperazine disulfide glionitrin A |
| Ophiopogonin D’ | |
| Panduratin A | |
| Ophiopogonin D’ | |
| Bcl-2 | Auriculasin |
| Dioscin | |
| Flavonoid | |
| Guttiferone F | |
| LLDT-288 | |
| Malformin A1 | |
| Panduratin A | |
| Scutellarin | |
| Solanine | |
| Tetrandrine | |
| Triterpenoid plectranthoic acid | |
| Viriditoxin | |
| Bax | Auriculasin |
| Delphinidin | |
| Dioscin | |
| Diketopiperazine disulfide glionitrin A | |
| FD1a, FD1c, FD1h, FD2a, FD2c, FD2h | |
| Flavonoid | |
| Guttiferone F | |
| LLDT-288 |
| Compound | Classification | Source | Organism | Cell Line/Animal Model | Dose; Duration | Mechanism | Reference |
|---|---|---|---|---|---|---|---|
| Fucoidan | Polysaccharide | Algae | Protist | DU-145 xenografts | 20 mg/kg/28 days | VEGF, Cyclin D1, Bcl-xL ↓ | [68] |
| Nordihydroguaiaretic acid | Tetrol | Larrea tridentate | Plant | PC3 | 20 μM/0, 0.5, 1, 2, 3 h | NRP1 ↓ | [69] |
| Terrestrosin D | Steroids | Tribulus terrestris L. | Plant | PC3, HUVEC | 50 mg/kg/28 days | [49] | |
| Resveratrol, Pterostilbene | Polyphenol, | Red wine | Plant | LNCaP, Du145 PC3 xenografts | 5–100 µM/24 h | MTA 1↓ | [70] |
| Stilbenol | 50 mg/kg/day/8 days | ||||||
| Sesamin | Lignans | Sesamum indicum | Plant | DU145 | 100 μM/12 h | ICAM-1, MMP-9, VEGF↓ | [75] |
| (1) Epigallocatechin-3-gallate | Polyphenol, Flavan | Green tea | Plant | CWR22R | (1) 50 mg/kg/20 days | (1), (2) CD31-positive endothelial cells ↓ | [72] |
| (2) peracetate of EGCG | (2) 86.7 mg/kg/20 days | ||||||
| Betulinic acid | Triterpenoid, | Birch bark | Plant | LNCaP/LNCaP xenografts | 5, 10, 15, 20 μmol/L/24 h | VEGF, Sp proteins↓ | [73] |
| Hydroxy- monocarboxylic acid | 10, 20 mg/kg/day/7 days | ||||||
| (−)-Gossypol | N/A | Cottonseed | Plant | NCr-nu/nu nude mice injected with PC-3 cells | 10 mg/kg × 5/day/4 weeks | Bcl-2 ↓ | [74] |
| Compound | Classification | Source | Organism | Cell Line/Animal Model | Dose; Duration | Mechanism | Reference |
|---|---|---|---|---|---|---|---|
| Decursin, decursinol angelate | Coumarins | Angelica gigas Nakai | Plant | (1) C57BL/6 (2) TRAMP mice | (1) 5 mg/mouse/8, 16, 28 weeks (2) 3 mg/mouse/8, 16, 28 weeks | (1) Snail2, Twist, Notch1, TGFBR2, E-cadherin ↓ (2) Snail2 ↓ | [76] |
| Luteolin, ellagic acid and punicic acid | Flavones, | Pomegranate | Plant | PCa xenograft SCID mice | 64 µg/5 days/8 weeks | PLA2, COX, CXCR4, Gα13, PI3K, p-AKT ↓ | [77] |
| Polyphenol |
| Compound | Classification | Source | Organism | Cell Line/Animal Model | Dose; Duration | Mechanism | Reference |
|---|---|---|---|---|---|---|---|
| Luteolin, Ellagic acid, Punicic acid | Flavones, polyphenols | Pomegranate juice | Plant | PC3 | 8 µg/mL/12 h | miR144, miR-133b, miR-1, miR-122, miR-34c, miR-200c, miR-127, miR-335, miR-124, miR-181a, miR-7, miR-215, miR-15a, Let-7d, miR-20a, miR-21, miR-9, miR-29b, miR-181b↓ | [79] |
| Epigallocatechin-3-gallate | Polyphenols, Flavan | Green tea | Plant | PCa xenografts | 3 mg/6 weeks | miRNA-330↑ miRNA-21↓ | [80] |
| Green tea polysaccharide | Polysaccharide | Camellia sinensis L. | Plant | PC3 | 25, 50, 100 μg/mL/48 h | miR-93↓ | [81] |
| Resveratrol | Stilbenoid | N/A | Plant | DU145, LNCaP | 50 mM/24 h | miR-17, miR-20a, miR-20b, miR-106a, miR-106b↑ | [82] |
| Genistein | Isoflavone | N/A | Plant | PC3, DU145 | 25μM/4 days | sFRP1, Smad4↑ miR-1260b↓ | [83] |
| Curcumin | Polyphenol | Curcoma longa | Plant | TRAMP C1 | 2.5 μM; 5 days | Nrf 2, NQO-1↑ | [84] |
| EF24 | Polyphenol | Curcoma longa | Plant | DU145 | 5 µM; 24 h | miR-345, miR-409, miR-10a miR-206↑ miR-21, miR-26a, miR-24, miR-30b miR-29a↓ | [85] |
| Genistein, Daidzein | Isoflavones | Soy | Plant | PC-3, DU-145, LNCaP | 40 μM, 110 μM; 48 h | GSTP1, EPHB2↑ | [86] |
| Gefitinib, Luteolin | Anilinoquinazoline, Flavones | Reseda luteola | Plant | PC-3, U2OS | 60 μM, 60 μM; 24 h, 48 h, 72 h | miR-630, miR-5703↑ | [87] |
| Compound | Classification | Source | Organism | Cell Line/Animal Model | Dose; Duration | Mechanism | Reference |
|---|---|---|---|---|---|---|---|
| 6-gingerol, 10-gingerol, 6-shogaol, and 10-shogaol | Ketone, guaiacol | Ginger | Plant | PC3R | 100 μM/24 h | MRP1, GSTπ↓ | [33] |
| Scutellarin | Glycosyloxyflavone | Scutellaria altissima L. | Plant | PC3 | 200 µM/48 h | γ-H2AX foci ↑ | [47] |
| (Flavone) | |||||||
| (8R)-3β,8-dihydroxypolypoda-13E,17E,21-triene | Bicyclic triterpenoid | Pistacia lentiscus | Plant | Drug-resistant PC-3 PC-3 tumor xenografts grown on the CAM | 30 μM/48, 72 h | Z-VAD-FMK ↑ | [88] |
| 10, 30, 100 μM/4 days | |||||||
| (1) Theaflavins, (2) epicatechin-3-gallate, (3) epigallocatechin-3-gallate | Polyphenols | Black and green tea | Plant | LNCaP | (1) 100 μg/mL/24 h | MDR-1 ↓ | [89] |
| (2), (3) 1, 10 μM/24 h |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Noh, S.; Choi, E.; Hwang, C.-H.; Jung, J.H.; Kim, S.-H.; Kim, B. Dietary Compounds for Targeting Prostate Cancer. Nutrients 2019, 11, 2401. https://doi.org/10.3390/nu11102401
Noh S, Choi E, Hwang C-H, Jung JH, Kim S-H, Kim B. Dietary Compounds for Targeting Prostate Cancer. Nutrients. 2019; 11(10):2401. https://doi.org/10.3390/nu11102401
Chicago/Turabian StyleNoh, Seungjin, Eunseok Choi, Cho-Hyun Hwang, Ji Hoon Jung, Sung-Hoon Kim, and Bonglee Kim. 2019. "Dietary Compounds for Targeting Prostate Cancer" Nutrients 11, no. 10: 2401. https://doi.org/10.3390/nu11102401
APA StyleNoh, S., Choi, E., Hwang, C.-H., Jung, J. H., Kim, S.-H., & Kim, B. (2019). Dietary Compounds for Targeting Prostate Cancer. Nutrients, 11(10), 2401. https://doi.org/10.3390/nu11102401








