Effect of Vasicinone against Paraquat-Induced MAPK/p53-Mediated Apoptosis via the IGF-1R/PI3K/AKT Pathway in a Parkinson’s Disease-Associated SH-SY5Y Cell Model
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Cell Culture and Treatments
2.3. MTT Assay
2.4. Annexin-V Staining Assay
2.5. Measurement of Intracellular ROS Generation
2.6. TUNEL Staining
2.7. Western Blotting Analysis
2.8. Statistical Analysis
3. Results
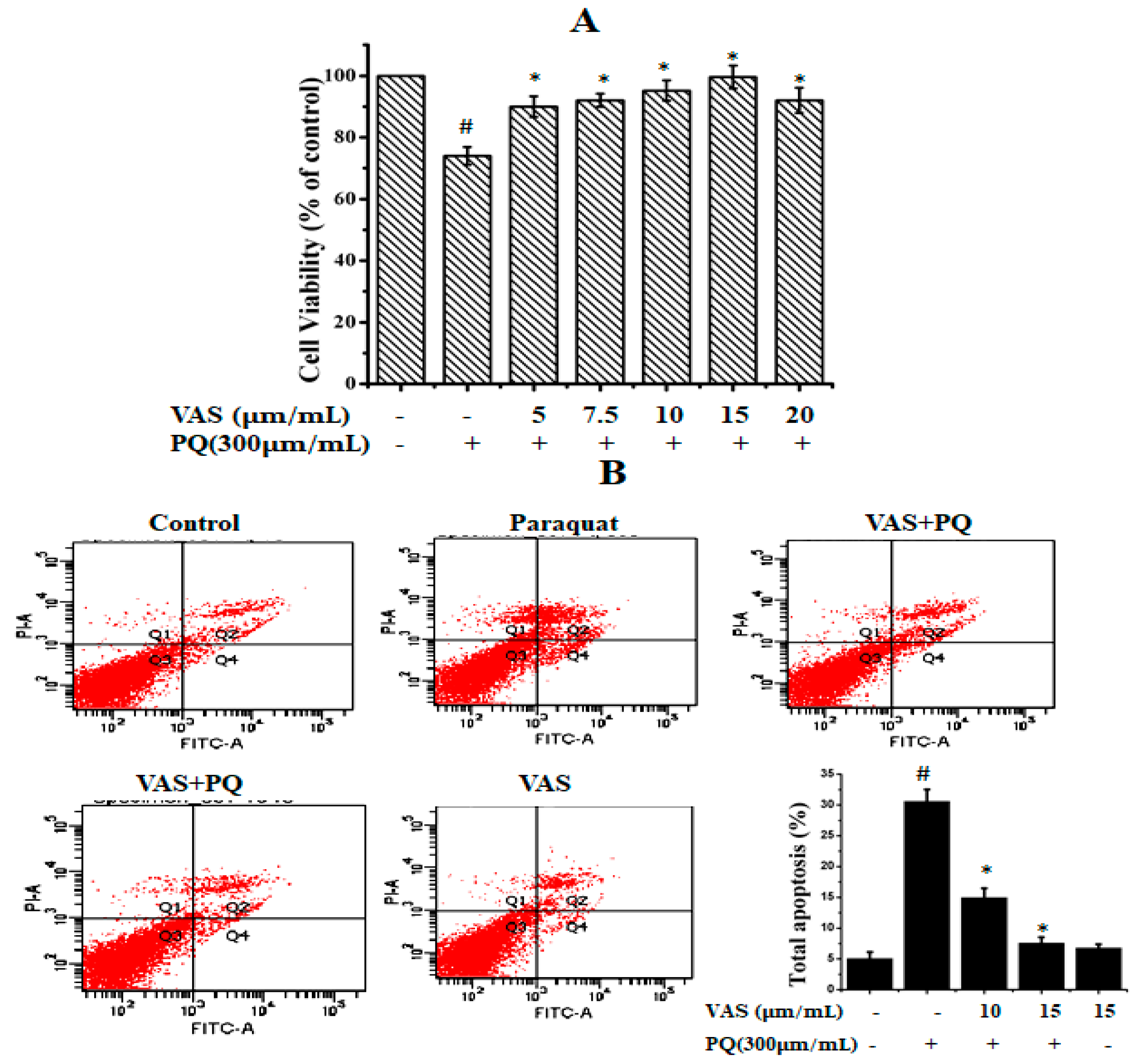
3.1. Vasicinone Inhibited Paraquat-Induced Cytotoxicity in SH-SY5Y Cells
3.2. Vasicinone Prevented the Effect of Paraquat on ROS Generation in SH-SY5Y Cells
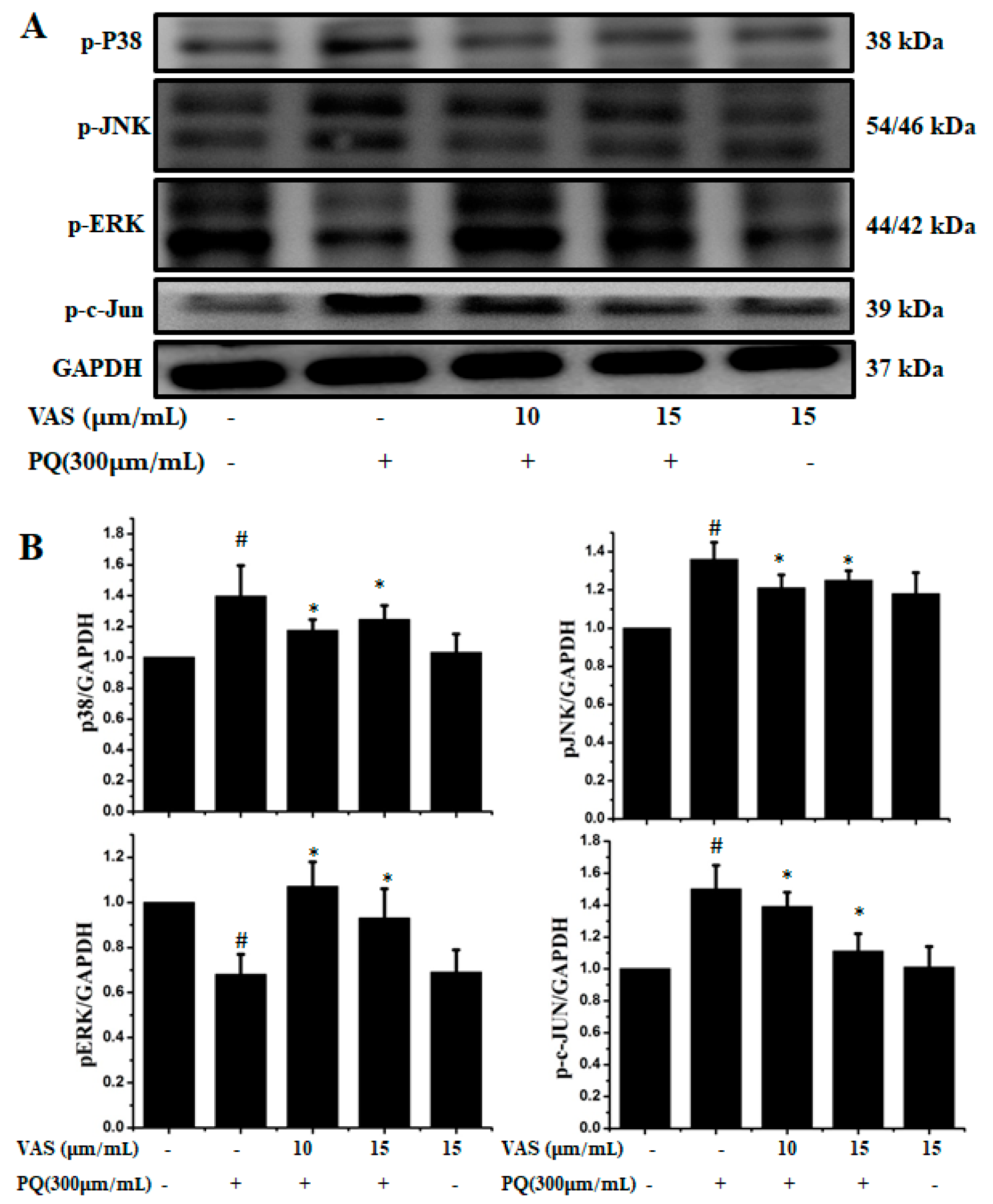
3.3. Vasicinone Inhibited the Paraquat-Induced Activation of the JNK and p38 MAPKs in SH-SY5Y Cells
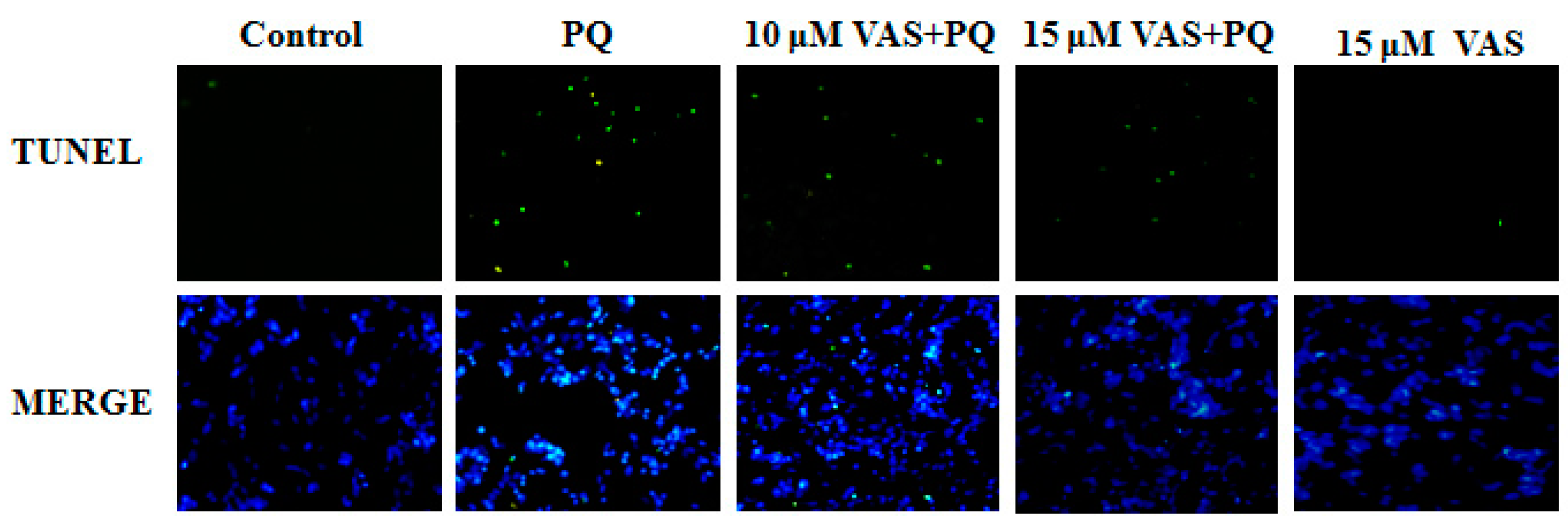
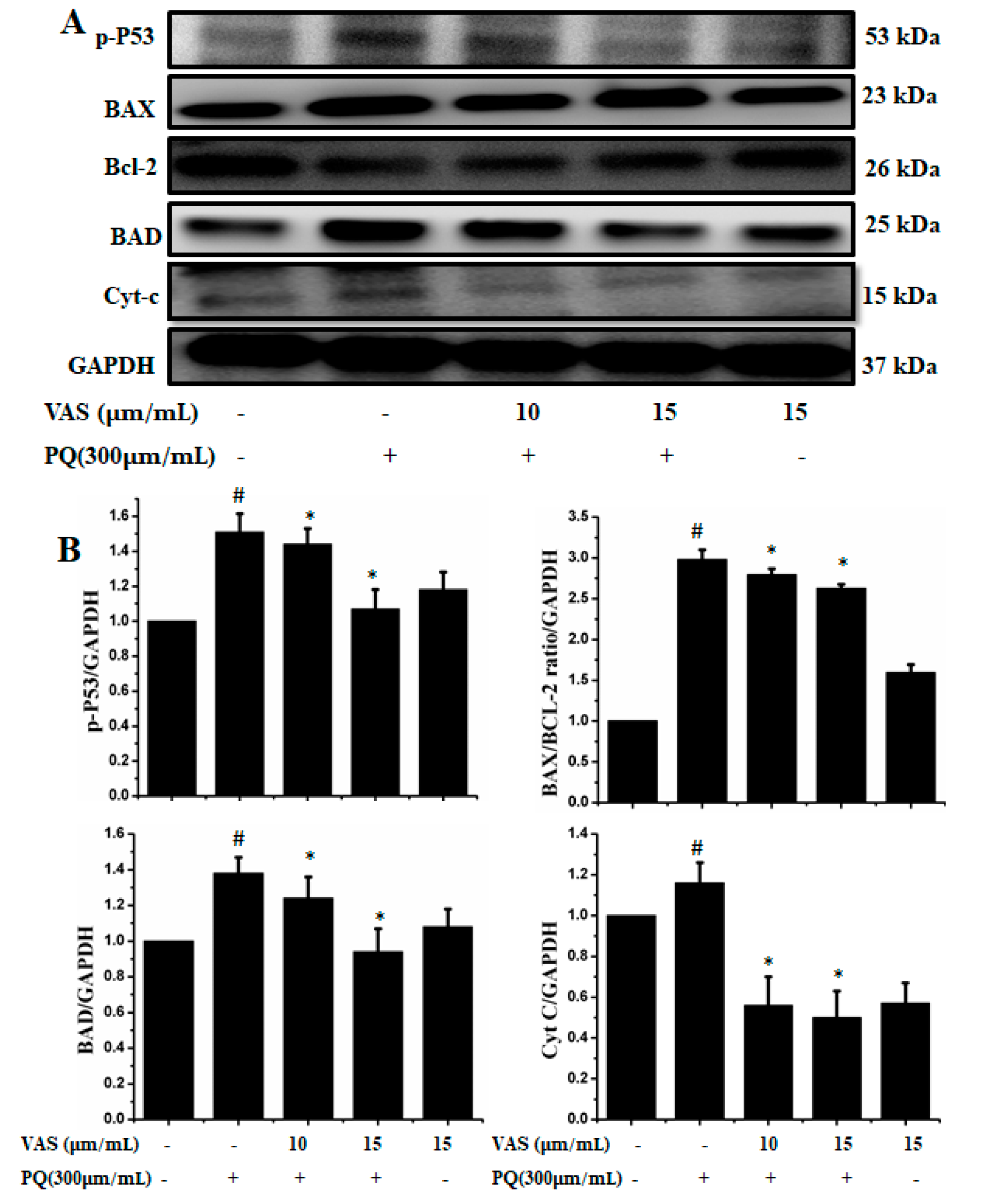
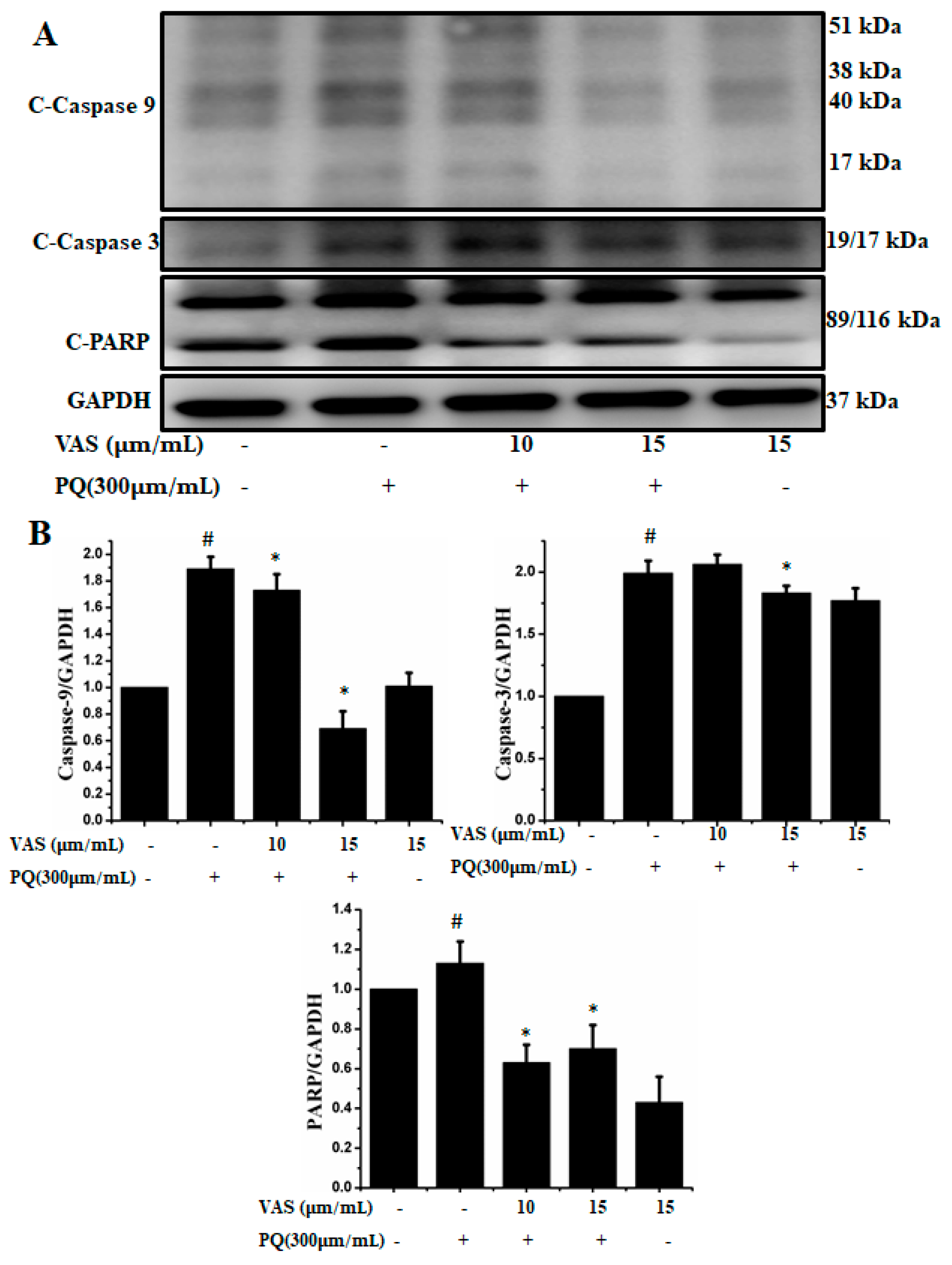
3.4. Vasicinone Inhibited Paraquat-Induced Apoptosis in SH-SY5Y Cells
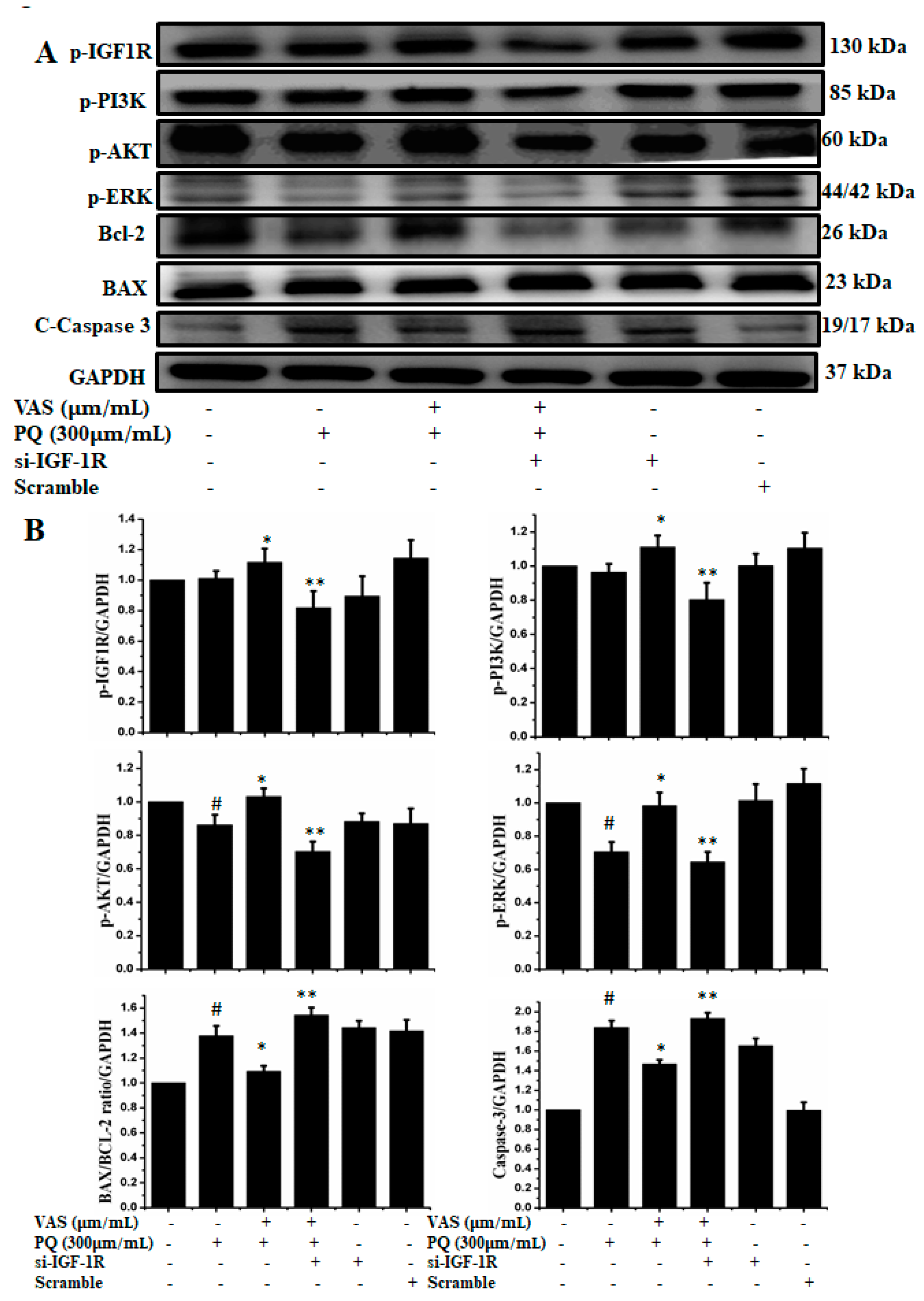
3.5. Vasicinone Regulated IGF-1R/PI3K/AKT and ERK Signaling Pathways in SH-SY5Y Cells
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Blesa, J.; Trigo-Damas, I.; Dileone, M.; Del Rey, N.L.; Hernandez, L.F.; Obeso, J.A. Compensatory mechanisms in Parkinson’s disease: Circuits adaptations and role in disease modification. Exp. Neurol. 2017, 298, 148–161. [Google Scholar] [CrossRef] [PubMed]
- Ortiz, G.G.; González-Usigli, H.; Pacheco-Moisés, F.P.; Mireles-Ramírez, M.A.; Sánchez-López, A.L.; Torres-Sánchez, E.D.; González-Renovato, E.D.; Flores-Alvarado, L.J.; Macías-Isla, M.A.; Rivero-Moragrega, P.; et al. Physiology and Pathology of Neuroimmunology: Role of Inflammation in Parkinson’s Disease. In Physiology and Pathology of Immunology; IntechOpen: London, UK, 2017; pp. 173–197. [Google Scholar]
- Blum, D.; Torch, S.; Lambeng, N.; Nissou, M.; Benabid, A.L.; Sadoul, R.; Verna, J.M. Molecular pathways involved in the neurotoxicity of 6-OHDA, dopamine and MPTP: Contribution to the apoptotic theory in Parkinson’s disease. Prog. Neurobiol. 2001, 65, 135–172. [Google Scholar] [CrossRef]
- Gong, P.; Deng, F.; Zhang, W.; Ji, J.; Liu, J.; Sun, Y.; Hu, J. Tectorigenin attenuates the MPP (+)-induced SH-SY5Y cell damage, indicating a potential beneficial role in Parkinson’s disease by oxidative stress inhibition. Exp. Ther. Med. 2017, 14, 4431–4437. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Moon, H.E.; Paek, S.H. Mitochondrial Dysfunction in Parkinson’s disease. Exp. Neurobiol. 2015, 24, 103–116. [Google Scholar] [CrossRef] [PubMed]
- Blesa, J.; Trigo-Damas, I.; Quiroga-Varela, A.; Jackson-Lewis, V.R. Oxidative stress and Parkinson’s disease. Front. Neuroanat 2015, 9, 91. [Google Scholar] [CrossRef] [PubMed]
- Hu, G.; Bidel, S.; Jousilahti, P.; Antikainen, R.; Tuomilehto, J. Coffee and tea consumption and the risk of Parkinson’s disease. Mov. Disord. 2007, 22, 2242–2248. [Google Scholar] [CrossRef]
- Ritz, B.; Ascherio, A.; Checkoway, H.; Marder, K.S.; Nelson, L.M.; Rocca, W.A.; Ross, G.W.; Strickland, D.; Van Den Eeden, S.K.; Gorell, J. Pooled analysis of tobacco use and risk of Parkinson disease. Arch. Neurol. 2007, 64, 990–997. [Google Scholar] [CrossRef]
- Vaccari, C.; El Dib, R.; De Camargo, J.L.V. Paraquat and Parkinson’s disease: A systematic review protocol according to the OHAT approach for hazard identification. Syst. Rev. 2017, 6, 98. [Google Scholar] [CrossRef]
- Jenner, P.; Morris, H.R.; Robbins, T.W.; Goedert, M.; Hardy, J.; Ben-Shlomo, Y.; Bolam, P.; Burn, D.; Hindle, J.V.; Brooks, D. Parkinson’s disease—The debate on the clinical phenomenology, aetiology, pathology and pathogenesis. J. Parkinsons Dis. 2013, 3, 1–11. [Google Scholar] [CrossRef]
- Goldman, S.M. Environmental toxins and Parkinson’s disease. Annu. Rev. Pharmacol. Toxicol. 2014, 54, 141–164. [Google Scholar] [CrossRef]
- Blesa, J.; Phani, S.; Jackson-Lewis, V.; Przedborski, S. Classic and new animal models of Parkinson’s disease. J. Biomed. Biotechnol. 2012, 2012, 845618. [Google Scholar] [CrossRef] [PubMed]
- Li, X.Z.; Zhang, S.N.; Liu, S.M.; Lu, F. Recent advances in herbal medicines treating Parkinson’s disease. Fitoterapia 2013, 84, 273–285. [Google Scholar] [CrossRef] [PubMed]
- Fu, W.; Zhuang, W.; Zhou, S.; Wang, V.X. Plant-derived neuroprotective agents in Parkinson’s disease. Am. J. Transl. Res. 2015, 7, 1189–1202. [Google Scholar] [PubMed]
- Bhattacharyya, D.; Pandit, S.; Jana, U.; Sen, S.; Sur, T.K. Hepatoprotective activity of Adhatoda vasica aqueous leaf extract on D-galactosamine-induced liver damage in rats. Fitoterapia 2005, 76, 223–225. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Wang, Y.; He, D.D.; Li, S.P.; Zhu, Y.D.; Jiang, B.; Cheng, X.M.; Wang, Z.; Wang, C.H. Antitussive, expectorant, and bronchodilating effects of quinazoline alkaloids (+/-)-vasicine, deoxyvasicine, and (+/-)-vasicinone from aerial parts of Peganum harmala L. Phytomedicine 2015, 22, 1088–1095. [Google Scholar] [CrossRef] [PubMed]
- Amin, A.H.; Mehta, D.R. A bronchodilator alkaloid (vasicinone) from Adhatoda vasica Nees. Nature 1959, 184, 1317. [Google Scholar] [CrossRef] [PubMed]
- Singh, B.; Sharma, R.A. Anti-inflammatory and antimicrobial properties of pyrroloquinazoline alkaloids from Adhatoda vasica Nees. Phytomedicine 2013, 20, 441–445. [Google Scholar] [CrossRef]
- Kulkarni, A.A. Ray of hope for cancer patients. In Proceedings of the International Seminar on Holistic Management of Cancer, Jerusalem, Geneva, 2 July 2019; Ayurvedic Education: Albuquerque, NM, USA, 1998; Volume 67, pp. 5–11. [Google Scholar]
- Roja, G.; Vikrant, B.H.; Sandur, S.K.; Sharma, A.; Pushpa, K.K. Accumulation of vasicine and vasicinone in tissue cultures of Adhatoda vasica and evaluation of the free radical-scavenging activities of the various crude extracts. Food Chem. 2011, 126, 1033–1038. [Google Scholar] [CrossRef]
- Jha, S.K.; Jha, N.K.; Kar, R.; Ambasta, R.K.; Kumar, P. P38 MAPK and PI3K/AKT Signalling Cascades in Parkinson’s Disease. Int. J. Mol. Cell Med. 2015, 4, 67–86. [Google Scholar]
- Lee, P.C.; Rhodes, S.L.; Sinsheimer, J.S.; Bronstein, J.; Ritz, B. Functional paraoxonase 1 variants modify the risk of Parkinson’s disease due to organophosphate exposure. Environ. Int. 2013, 56, 42–47. [Google Scholar] [CrossRef]
- Mata, I.F.; Leverenz, J.B.; Weintraub, D.; Trojanowski, J.Q.; Hurtig, H.I.; Van Deerlin, V.M.; Ritz, B.; Rausch, R.; Rhodes, S.L.; Factor, S.A.; et al. APOE, MAPT, and SNCA genes and cognitive performance in Parkinson disease. JAMA Neurol. 2014, 71, 1405–1412. [Google Scholar] [CrossRef] [PubMed]
- Hwang, O. Role of oxidative stress in Parkinson’s disease. Exp. Neurobiol. 2013, 22, 11–17. [Google Scholar] [CrossRef] [PubMed]
- Solhaug, A.; Refsnes, M.; Lag, M.; Schwarze, P.E.; Husoy, T.; Holme, J.A. Polycyclic aromatic hydrocarbons induce both apoptotic and anti-apoptotic signals in Hepa1c1c7 cells. Carcinogenesis 2004, 25, 809–819. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Ji, L.D.; Xu, L.H. Lead-induced apoptosis in PC 12 cells: Involvement of p53, Bcl-2 family and caspase-3. Toxicol. Lett. 2006, 166, 160–167. [Google Scholar] [CrossRef] [PubMed]
- Nair, V.D.; McNaught, K.S.; Gonzalez-Maeso, J.; Sealfo, SC.; Olanow, C.W. p53 mediates nontranscriptional cell death in dopaminergic cells in response to proteasome inhibition. J. Biol. Chem. 2006, 281, 39550–39560. [Google Scholar] [CrossRef]
- Yang, W.; Tiffany-Castiglioni, E. Paraquat-induced apoptosis in human neuroblastoma SH-SY5Y cells: Involvement of p53 and mitochondria. J. Toxicol. Environ. Health 2008, 71, 289–299. [Google Scholar] [CrossRef] [PubMed]
- Tsujimoto, Y. Bcl-2 family of proteins: Life-or-death switch in mitochondria. Biosci. Rep. 2002, 22, 47–58. [Google Scholar] [CrossRef]
- Cai, J.; Yang, J.; Jones, D.P. Mitochondrial control of apoptosis: The role of cytochrome c. Biochim. Biophys. Acta 1998, 1366, 139–149. [Google Scholar] [CrossRef]
- Mosley, R.L.; Benner, E.J.; Kadiu, I.; Thomas, M.; Boska, M.D.; Hasan, K.; Laurie, C.; Gendelman, H.E. Neuroinflammation, Oxidative Stress and the Pathogenesis of Parkinson’s disease. Clin. Neurosci. Res. 2006, 6, 261–281. [Google Scholar] [CrossRef]
- Li, P.; Nijhawan, D.; Budihardjo, I.; Srinivasula, SM.; Ahmad, M.; Alnemri, E.S.; Wang, X. Cytochrome C and dATP dependent formation of Apaf-1/caspase-9 complex initiates an apoptotic protease cascade. Cell 2004, 91, 479–489. [Google Scholar] [CrossRef]
- Kim, H.J.; Song, J.Y.; Park, H.J.; Park, H.K.; Yun, D.H.; Chung, J.H. Naringin Protects against Rotenone-induced Apoptosis in Human Neuroblastoma SH-SY5Y Cells. Korean J. Physiol. Pharmacol. 2009, 13, 281–285. [Google Scholar] [CrossRef]
- Niso-Santano, M.; Moran, J.M.; Garcia-Rubio, L.; Gomez-Martin, A.; Gonzalez-Polo, R.A.; Soler, G.; Fuentes, J.M. Low concentrations of paraquat induce early activation of extracellular signal-regulated kinase 1/2, protein kinase B, and c-Jun N-terminal kinase 1/2 pathways: Role of c-Jun N-terminal kinase in paraquat-induced cell death. Toxicol. Sci. 2006, 92, 507–515. [Google Scholar] [CrossRef]
- Zeng, Z.; Wang, D.; Gaur, U.; Rifang, L.; Wang, H.; Zheng, W. H2O2 attenuates IGF-1R tyrosine phosphorylation and its survival signaling properties in neuronal cells via NR2B containing NMDA receptor. Oncotarget 2017, 8, 65313–65328. [Google Scholar] [CrossRef][Green Version]
- Kim, C.; Park, S. IGF-1 protects SH-SY5Y cells against MPP (+)-induced apoptosis via PI3K/PDK-1/Akt pathway. Endocr. Connect. 2018, 7, 443–455. [Google Scholar] [CrossRef]


© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ju, D.-T.; Sivalingam, K.; Kuo, W.-W.; Ho, T.-J.; Chang, R.-L.; Chung, L.-C.; Day, C.H.; Viswanadha, V.P.; Liao, P.-H.; Huang, C.-Y. Effect of Vasicinone against Paraquat-Induced MAPK/p53-Mediated Apoptosis via the IGF-1R/PI3K/AKT Pathway in a Parkinson’s Disease-Associated SH-SY5Y Cell Model. Nutrients 2019, 11, 1655. https://doi.org/10.3390/nu11071655
Ju D-T, Sivalingam K, Kuo W-W, Ho T-J, Chang R-L, Chung L-C, Day CH, Viswanadha VP, Liao P-H, Huang C-Y. Effect of Vasicinone against Paraquat-Induced MAPK/p53-Mediated Apoptosis via the IGF-1R/PI3K/AKT Pathway in a Parkinson’s Disease-Associated SH-SY5Y Cell Model. Nutrients. 2019; 11(7):1655. https://doi.org/10.3390/nu11071655
Chicago/Turabian StyleJu, Da-Tong, Kalaiselvi Sivalingam, Wei-Wen Kuo, Tsung-Jung Ho, Ruey-Lin Chang, Li-Chin Chung, Cecilia Hsuan Day, Vijaya Padma Viswanadha, Po-Hsiang Liao, and Chih-Yang Huang. 2019. "Effect of Vasicinone against Paraquat-Induced MAPK/p53-Mediated Apoptosis via the IGF-1R/PI3K/AKT Pathway in a Parkinson’s Disease-Associated SH-SY5Y Cell Model" Nutrients 11, no. 7: 1655. https://doi.org/10.3390/nu11071655
APA StyleJu, D.-T., Sivalingam, K., Kuo, W.-W., Ho, T.-J., Chang, R.-L., Chung, L.-C., Day, C. H., Viswanadha, V. P., Liao, P.-H., & Huang, C.-Y. (2019). Effect of Vasicinone against Paraquat-Induced MAPK/p53-Mediated Apoptosis via the IGF-1R/PI3K/AKT Pathway in a Parkinson’s Disease-Associated SH-SY5Y Cell Model. Nutrients, 11(7), 1655. https://doi.org/10.3390/nu11071655




