Heart Histopathology and Mitochondrial Ultrastructure in Aged Rats Fed for 24 Months on Different Unsaturated Fats (Virgin Olive Oil, Sunflower Oil or Fish Oil) and Affected by Different Longevity
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Diets
2.2. Histopathological Analysis of Heart
2.3. Electron Microscopy Analysis of Heart
2.4. Heart Mitochondria Isolation
2.5. Fatty Acids Analysis of Dietary Fats and of Heart Mitochondrial Membranes
2.6. Mitochondrial Hydrogen Peroxide Content
2.7. Heart Mitochondrial Antioxidants Enzymes Analysis
2.8. α-Tocopherol and Coenzyme Q (CoQ) Determinations in Mitochondrial Membranes
2.9. Statistical Analysis
3. Results
3.1. Heart Mitochondrial Membrane Fatty Acids
3.2. Body and Heart Weight
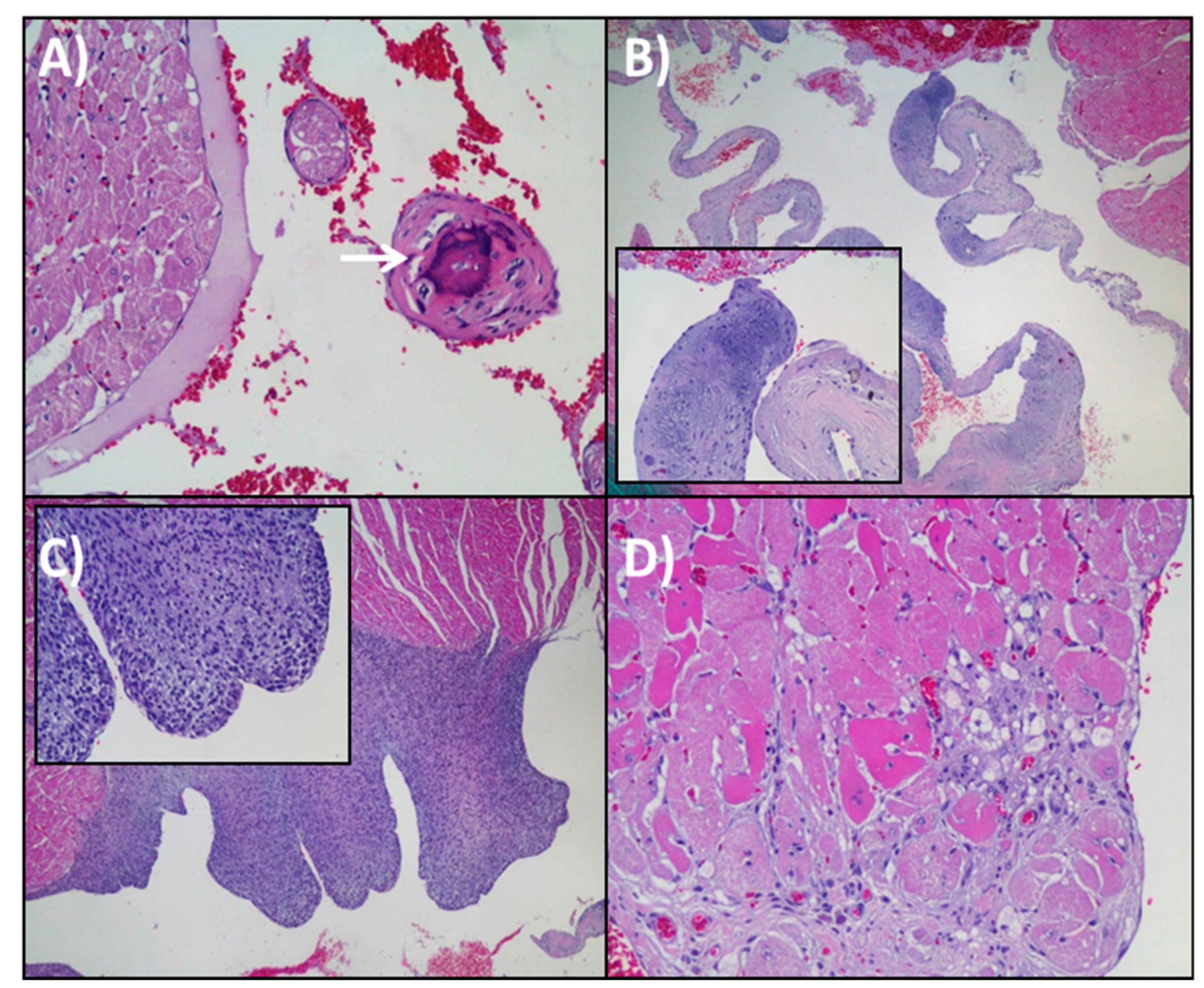
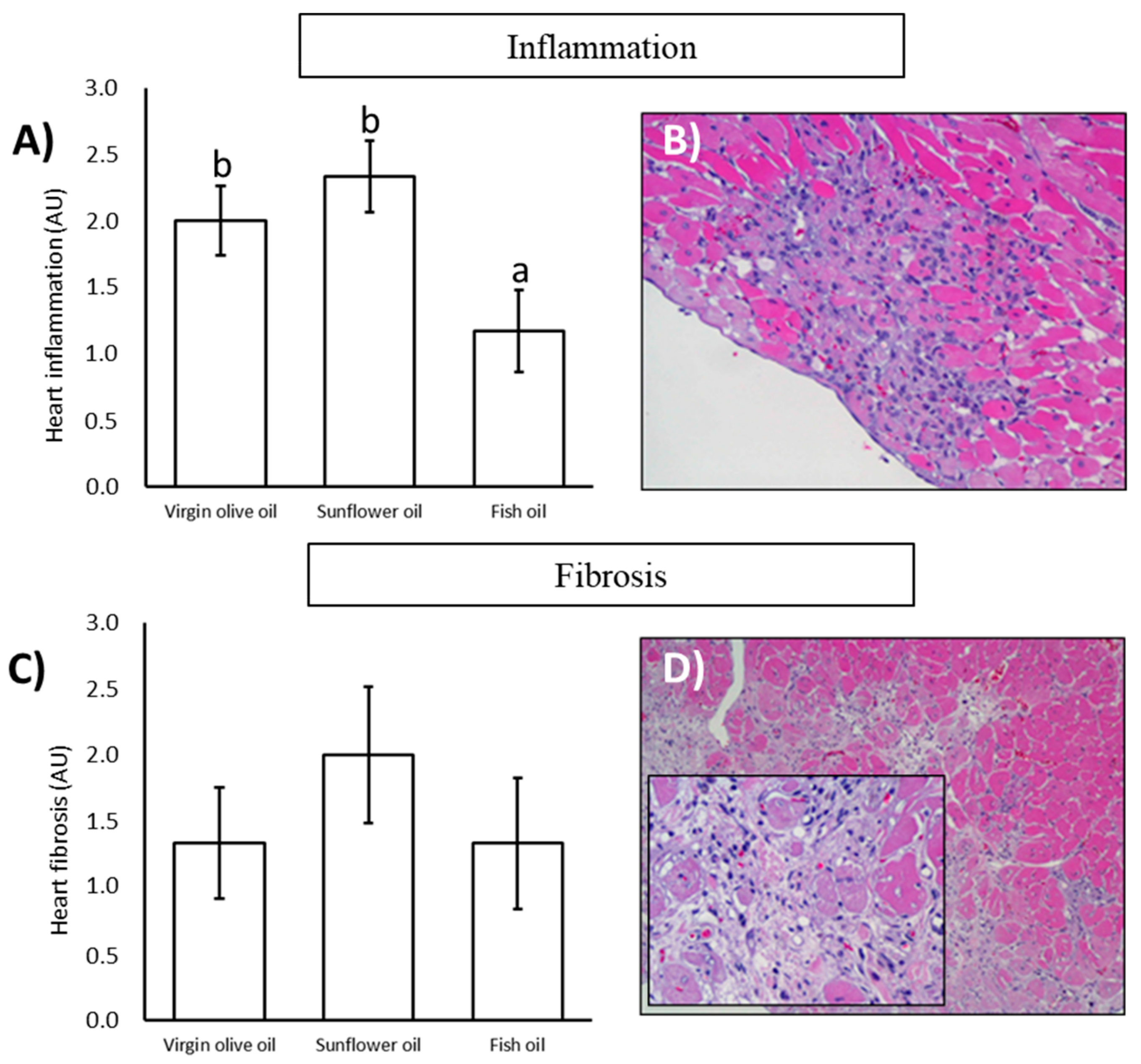
3.3. Histopathological Analysis
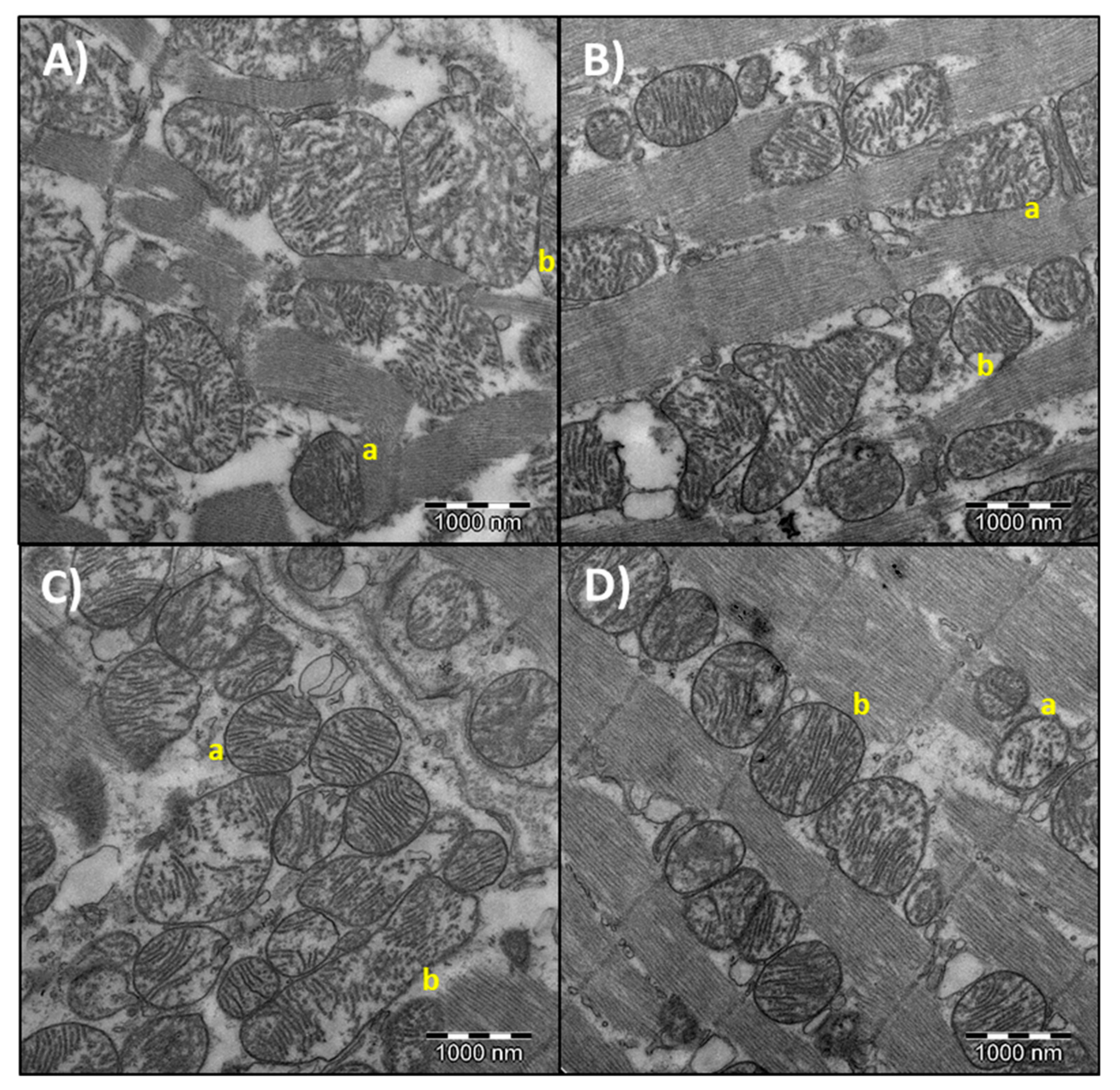
3.4. Heart Mitochondrial Morphometry
3.5. Oxidative Stress and Antioxidants Markers
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Niccoli, T.; Partridge, L. Ageing as a risk factor for disease. Curr. Biol. CB 2012, 22, R741–R752. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Cardiovascular Diseases (CVDs). Available online: https://www.who.int/news-room/fact-sheets/detail/cardiovascular-diseases-(cvds) (accessed on 29 June 2019).
- Quiles, J.L.; Ochoa, J.J.; Ramirez-Tortosa, M.C.; Huertas, J.R.; Mataix, J. Age-related mitochondrial DNA deletion in rat liver depends on dietary fat unsaturation. J. Gerontol. A Biol. Sci. Med. Sci. 2006, 61, 107–114. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Li, Y.; He, C.; Gou, B.; Song, M. Mitochondrial regulation of cardiac aging. Biochim. Biophys. Acta BBA Mol. Basis Dis. 2019, 1865, 1853–1864. [Google Scholar] [CrossRef]
- Harman, D. The free radical theory of aging. Antioxid. Redox Signal. 2003, 5, 557–561. [Google Scholar] [CrossRef] [PubMed]
- Kurz, F.T.; Aon, M.A.; O’Rourke, B.; Armoundas, A.A. Functional implications of cardiac mitochondria clustering. In Mitochondrial Dynamics in Cardiovascular Medicine; Santulli, G., Ed.; Advances in Experimental Medicine and Biology; Springer International Publishing: Cham, Switzerland, 2017; pp. 1–24. ISBN 978-3-319-55330-6. [Google Scholar]
- El’darov, C.M.; Vays, V.B.; Vangeli, I.M.; Kolosova, N.G.; Bakeeva, L.E. Morphometric examination of mitochondrial ultrastructure in aging cardiomyocytes. Biochem. Biokhimiia 2015, 80, 604–609. [Google Scholar] [CrossRef]
- Sastre, J.; Pallardó, F.V.; Viña, J. The role of mitochondrial oxidative stress in aging. Free Radic. Biol. Med. 2003, 35, 1–8. [Google Scholar] [CrossRef]
- Naderi-boldaji, V.; Joukar, S.; Noorafshan, A.; Raji-amirhasani, A.; Naderi-boldaji, S.; Bejeshk, M. The effect of blood flow restriction along with low-intensity exercise on cardiac structure and function in aging rat: Role of angiogenesis. Life Sci. 2018, 209, 202–209. [Google Scholar] [CrossRef]
- Olgar, Y.; Degirmenci, S.; Durak, A.; Billur, D.; Can, B.; Kayki-Mutlu, G.; Arioglu-Inan, E.; Turan, B. Aging related functional and structural changes in the heart and aorta: MitoTEMPO improves aged-cardiovascular performance. Exp. Gerontol. 2018, 110, 172–181. [Google Scholar] [CrossRef]
- Jahangir, A.; Sagar, S.; Terzic, A. Aging and cardioprotection. J. Appl. Physiol. 2007, 103, 2120–2128. [Google Scholar] [CrossRef]
- World Health Organization. Diet, Nutrition and the Prevention of Chronic Diseases: Report of a Joint WHO/FAO Expert Consultation. Available online: http://www.fao.org/3/AC911E/ac911e00.htm (accessed on 5 July 2019).
- Desnoyers, M.; Gilbert, K.; Madingou, N.; Gagné, M.-A.; Daneault, C.; Des Rosiers, C.; Rousseau, G. A high omega-3 fatty acid diet rapidly changes the lipid composition of cardiac tissue and results in cardioprotection. Can. J. Physiol. Pharmacol. 2018, 96, 916–921. [Google Scholar] [CrossRef]
- Orsavova, J.; Misurcova, L.; Ambrozova, J.V.; Vicha, R.; Mlcek, J. Fatty Acids composition of vegetable oils and its contribution to dietary energy intake and dependence of cardiovascular mortality on dietary intake of fatty Acids. Int. J. Mol. Sci. 2015, 16, 12871–12890. [Google Scholar] [CrossRef] [PubMed]
- Quiles, J.L.; Pamplona, R.; Ramirez-Tortosa, M.C.; Naudí, A.; Portero-Otin, M.; Araujo-Nepomuceno, E.; López-Frías, M.; Battino, M.; Ochoa, J.J. Coenzyme Q addition to an n-6 PUFA-rich diet resembles benefits on age-related mitochondrial DNA deletion and oxidative stress of a MUFA-rich diet in rat heart. Mech. Ageing Dev. 2010, 131, 38–47. [Google Scholar] [CrossRef] [PubMed]
- Venø, S.K.; Schmidt, E.B.; Bork, C.S. Polyunsaturated fatty acids and risk of ischemic stroke. Nutrients 2019, 11, 1467. [Google Scholar] [CrossRef]
- Ochoa, J.J.; Quiles, J.L.; Ibáñez, S.; Martínez, E.; López-Frías, M.; Huertas, J.R.; Mataix, J. Aging-related oxidative stress depends on dietary lipid source in rat postmitotic tissues. J. Bioenerg. Biomembr. 2003, 35, 267–275. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.D.; Li, Y.; Chiuve, S.E.; Stampfer, M.J.; Manson, J.E.; Rimm, E.B.; Willett, W.C.; Hu, F.B. Association of specific dietary fats with total and cause-specific mortality. JAMA Intern. Med. 2016, 176, 1134–1145. [Google Scholar] [CrossRef] [PubMed]
- Eilat-Adar, S.; Sinai, T.; Yosefy, C.; Henkin, Y. Nutritional recommendations for cardiovascular disease prevention. Nutrients 2013, 5, 3646–3683. [Google Scholar] [CrossRef]
- Ramirez-Tortosa, C.L.; Varela-López, A.; Navarro-Hortal, M.D.; Ramos-Pleguezuelos, F.M.; Márquez-Lobo, B.; Ramirez-Tortosa, M.C.; Ochoa, J.J.; Battino, M.; Quiles, J.L. Longevity and cause of death in male Wistar rats fed lifelong diets based on virgin olive oil, sunflower oil or fish oil. J. Gerontol. A Biol. Sci. Med. Sci. 2019. [Google Scholar] [CrossRef] [PubMed]
- Kain, V.; Ingle, K.A.; Kachman, M.; Baum, H.; Shanmugam, G.; Rajasekaran, N.S.; Young, M.E.; Halade, G.V. Excess ω-6 fatty acids influx in aging drives metabolic dysregulation, electrocardiographic alterations, and low-grade chronic inflammation. Am. J. Physiol. Heart Circ. Physiol. 2018, 314, H160–H169. [Google Scholar] [CrossRef] [PubMed]
- Reeves, P.G. Components of the AIN-93 diets as improvements in the AIN-76A diet. J. Nutr. 1997, 127, 838S–841S. [Google Scholar] [CrossRef]
- Rasband, W.S. ImageJ Software; National Institute Health: Bethesda, MD, USA, 1997.
- Fleischer, S.; McIntyre, J.O.; Vidal, J.C. Large-scale preparation of rat liver mitochondria in high yield. Methods Enzymol. 1979, 55, 32–39. [Google Scholar]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [PubMed]
- Lepage, G.; Roy, C.C. Direct transesterification of all classes of lipids in a one-step reaction. J. Lipid Res. 1986, 27, 114–120. [Google Scholar] [PubMed]
- LeBel, C.P.; Ischiropoulos, H.; Bondy, S.C. Evaluation of the probe 2′,7′-dichlorofluorescin as an indicator of reactive oxygen species formation and oxidative stress. Chem. Res. Toxicol. 1992, 5, 227–231. [Google Scholar] [CrossRef] [PubMed]
- Aebi, H. Catalase in vitro. Methods Enzymol. 1984, 105, 121–126. [Google Scholar] [PubMed]
- Flohé, L.; Günzler, W.A. Assays of glutathione peroxidase. Methods Enzymol. 1984, 105, 114–121. [Google Scholar]
- Quiles, J.L.; Ochoa, J.J.; Ramirez-Tortosa, M.C.; Linde, J.; Bompadre, S.; Battino, M.; Narbona, E.; Maldonado, J.; Mataix, J. Coenzyme Q concentration and total antioxidant capacity of human milk at different stages of lactation in mothers of preterm and full-term infants. Free Radic. Res. 2006, 40, 199–206. [Google Scholar] [CrossRef] [PubMed]
- Alvarado García, A.M.; Salazar Maya, Á.M. Análisis del concepto de envejecimiento. Gerokomos 2014, 25, 57–62. [Google Scholar] [CrossRef]
- Al-Okbi, S.Y.; El-qousy, S.M.; El-Ghlban, S.; Moawad, H.F. Role of borage seed oil and fish oil with or without turmeric and alpha- tocopherol in prevention of cardiovascular disease and fatty liver in rats. J. Oleo Sci. 2018, 67, 1551–1562. [Google Scholar] [CrossRef]
- Quiles, J.L.; Ochoa, J.J.; Ramirez-Tortosa, C.; Battino, M.; Huertas, J.R.; Martín, Y.; Mataix, J. Dietary fat type (virgin olive vs. sunflower oils) affects age-related changes in DNA double-strand-breaks, antioxidant capacity and blood lipids in rats. Exp. Gerontol. 2004, 39, 1189–1198. [Google Scholar] [CrossRef]
- Varela-Lopez, A.; Pérez-López, M.P.; Ramirez-Tortosa, C.L.; Battino, M.; Granados-Principal, S.; Ramirez-Tortosa, M.D.C.; Ochoa, J.J.; Vera-Ramirez, L.; Giampieri, F.; Quiles, J.L. Gene pathways associated with mitochondrial function, oxidative stress and telomere length are differentially expressed in the liver of rats fed lifelong on virgin olive, sunflower or fish oils. J. Nutr. Biochem. 2018, 52, 36–44. [Google Scholar] [CrossRef]
- Ochoa, J.J.; Pamplona, R.; Ramirez-Tortosa, M.C.; Granados-Principal, S.; Perez-Lopez, P.; Naudí, A.; Portero-Otin, M.; López-Frías, M.; Battino, M.; Quiles, J.L. Age-related changes in brain mitochondrial DNA deletion and oxidative stress are differentially modulated by dietary fat type and coenzyme Q10. Free Radic. Biol. Med. 2011, 50, 1053–1064. [Google Scholar] [CrossRef] [PubMed]
- Ochoa, J.J.; Quiles, J.L.; López-Frías, M.; Huertas, J.R.; Mataix, J. Effect of lifelong coenzyme Q10 supplementation on age-related oxidative stress and mitochondrial function in liver and skeletal muscle of rats fed on a polyunsaturated fatty acid (PUFA)-rich diet. J. Gerontol. A Biol. Sci. Med. Sci. 2007, 62, 1211–1218. [Google Scholar] [CrossRef] [PubMed]
- Quiles, J.L.; Huertas, J.R.; Mañas, M.; Ochoa, J.J.; Battino, M.; Mataix, J. Dietary fat type and regular exercise affect mitochondrial composition and function depending on specific tissue in the rat. J. Bioenerg. Biomembr. 2001, 33, 127–134. [Google Scholar] [CrossRef] [PubMed]
- Strong, R.; Miller, R.A.; Antebi, A.; Astle, C.M.; Bogue, M.; Denzel, M.S.; Fernandez, E.; Flurkey, K.; Hamilton, K.L.; Lamming, D.W.; et al. Longer lifespan in male mice treated with a weakly estrogenic agonist, an antioxidant, an α-glucosidase inhibitor or a Nrf2-inducer. Aging Cell 2016, 15, 872–884. [Google Scholar] [CrossRef]
- Roche, E.; Ramírez-Tortosa, C.L.; Arribas, M.I.; Ochoa, J.J.; Sirvent-Belando, J.E.; Battino, M.; Ramírez-Tortosa, M.C.; González-Alonso, A.; Pérez-López, M.P.; Quiles, J.L. Comparative analysis of pancreatic changes in aged rats fed life long with sunflower, fish, or olive oils. J. Gerontol. A Biol. Sci. Med. Sci. 2014, 69, 934–944. [Google Scholar] [CrossRef] [PubMed]
- Raclot, T. Selective mobilization of fatty acids from adipose tissue triacylglycerols. Prog. Lipid Res. 2003, 42, 257–288. [Google Scholar] [CrossRef]
- Lewis, D.J. Nonneoplastic lesions in the cardiovascular system. In Pathobiology of the Aging Rat; Mohr, U., Dungworth, D.L., Capen, C.C., Eds.; ILSI Press: Washington, DC, USA, 1992; Volume 1. [Google Scholar]
- Kim, E.J.; Song, B.G.; Sohn, H.R.; Hong, S.-M.; Park, D.W.; Heo, S.H.; Kim, K.Y.; Cho, W.-H.; Choi, S.-K. Senile cardiac calcification syndrome: A rare case of extensive calcification of left ventricular papillary muscle. Cardiol. Res. 2011, 2, 127–129. [Google Scholar] [CrossRef] [PubMed]
- Schwender, F.T. Papillary muscle calcification after inferoposterior myocardial infarction. Heart 2001, 86, e8. [Google Scholar] [CrossRef][Green Version]
- Aboulhoda, B.E. Age-related remodeling of the JAK/STAT/SOCS signaling pathway and associated myocardial changes: From histological to molecular level. Ann. Anat. Anat. Anz. Off. Organ Anat. Ges. 2017, 214, 21–30. [Google Scholar] [CrossRef]
- Beliveau, P.; Cheriet, F.; Anderson, S.A.; Taylor, J.L.; Arai, A.E.; Hsu, L.-Y. Quantitative assessment of myocardial fibrosis in an age-related rat model by ex vivo late gadolinium enhancement magnetic resonance imaging with histopathological correlation. Comput. Biol. Med. 2015, 65, 103–113. [Google Scholar] [CrossRef]
- Huet, E.; Gabison, E.; Vallee, B.; Mougenot, N.; Linguet, G.; Riou, B.; Jarosz, C.; Menashi, S.; Besse, S. Deletion of extracellular matrix metalloproteinase inducer/CD147 induces altered cardiac extracellular matrix remodeling in aging mice. J. Physiol. Pharmacol. Off. J. Pol. Physiol. Soc. 2015, 66, 355–366. [Google Scholar]
- O’Connell, T.D.; Block, R.C.; Huang, S.P.; Shearer, G.C. ω3-Polyunsaturated fatty acids for heart failure: Effects of dose on efficacy and novel signaling through free fatty acid receptor 4. J. Mol. Cell. Cardiol. 2017, 103, 74–92. [Google Scholar] [CrossRef] [PubMed]
- Halade, G.V.; Williams, P.J.; Lindsey, M.L.; Fernandes, G. Fish oil decreases inflammation and reduces cardiac remodeling in rosiglitazone treated aging mice. Pharmacol. Res. 2011, 63, 300–307. [Google Scholar] [CrossRef] [PubMed]
- Mayyas, F.; Jaradat, R.; Alzoubi, K.H. Cardiac effects of fish oil in a rat model of streptozotocin-induced diabetes. Nutr. Metab. Cardiovasc. Dis. NMCD 2018, 28, 592–599. [Google Scholar] [CrossRef] [PubMed]
- Richard, D.; Bausero, P.; Schneider, C.; Visioli, F. Polyunsaturated fatty acids and cardiovascular disease. Cell. Mol. Life Sci. 2009, 66, 3277. [Google Scholar] [CrossRef] [PubMed]
- Simopoulos, A.P. The importance of the omega-6/omega-3 fatty acid ratio in cardiovascular disease and other chronic diseases. Exp. Biol. Med. Maywood NJ 2008, 233, 674–688. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.X.; Weylandt, K.H. Modulation of inflammatory cytokines by omega-3 fatty acids. In Lipids in Health and Diseases; Subcellular Biochemistry; Springer: Dordrecht, The Netherlands, 2008; Volume 49, pp. 133–143. [Google Scholar]
- Chan, D.C. Mitochondria: Dynamic organelles in disease, aging, and development. Cell 2006, 125, 1241–1252. [Google Scholar] [CrossRef] [PubMed]
- Leduc-Gaudet, J.-P.; Picard, M.; St-Jean Pelletier, F.; Sgarioto, N.; Auger, M.-J.; Vallée, J.; Robitaille, R.; St-Pierre, D.H.; Gouspillou, G. Mitochondrial morphology is altered in atrophied skeletal muscle of aged mice. Oncotarget 2015, 6, 17923–17937. [Google Scholar] [CrossRef] [PubMed]
- Chalmers, S.; Saunter, C.D.; Girkin, J.M.; McCarron, J.G. Age decreases mitochondrial motility and increases mitochondrial size in vascular smooth muscle. J. Physiol. 2016, 594, 4283–4295. [Google Scholar] [CrossRef]
- Linton, P.-J.; Gurney, M.; Sengstock, D.; Mentzer, R.M.; Gottlieb, R.A. This old heart: Cardiac aging and autophagy. J. Mol. Cell. Cardiol. 2015, 83, 44–54. [Google Scholar] [CrossRef]
- Westrate, L.M.; Drocco, J.A.; Martin, K.R.; Hlavacek, W.S.; MacKeigan, J.P. Mitochondrial morphological features are associated with fission and fusion events. PLoS ONE 2014, 9, e95265. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.-Y.; Jin, J.; Li, S.-L.; Yan, J.; Zhen, C.-L.; Gao, J.-L.; Zhang, Y.-H.; Zhang, Y.-Q.; Shen, X.; Zhang, L.-S.; et al. Mitochondrial fission of smooth muscle cells is involved in artery constriction. Hypertens. Dallas Tex 1979 2016, 68, 1245–1254. [Google Scholar] [CrossRef] [PubMed]
- Frenzel, H.; Feimann, J. Age-dependent structural changes in the myocardium of rats. A quantitative light- and electron-microscopic study on the right and left chamber wall. Mech. Ageing Dev. 1984, 27, 29–41. [Google Scholar] [CrossRef]
- Rincón-Cervera, M.A.; Valenzuela, R.; Hernandez-Rodas, M.C.; Marambio, M.; Espinosa, A.; Mayer, S.; Romero, N.; Barrera, M.S.C.; Valenzuela, A.; Videla, L.A. Supplementation with antioxidant-rich extra virgin olive oil prevents hepatic oxidative stress and reduction of desaturation capacity in mice fed a high-fat diet: Effects on fatty acid composition in liver and extrahepatic tissues. Nutrition 2016, 32, 1254–1267. [Google Scholar] [CrossRef] [PubMed]
- Da Silva Pedroza, A.A.; Lopes, A.; Mendes da Silva, R.F.; Braz, G.R.; Nascimento, L.P.; Ferreira, D.S.; dos Santos, Â.A.; Batista-de-Oliveira-Hornsby, M.; Lagranha, C.J. Can fish oil supplementation and physical training improve oxidative metabolism in aged rat hearts? Life Sci. 2015, 137, 133–141. [Google Scholar] [CrossRef]
- Ochoa, J.J.; Quiles, J.L.; Huertas, J.R.; Mataix, J. Coenzyme Q10 protects from aging-related oxidative stress and improves mitochondrial function in heart of rats fed a polyunsaturated fatty acid (PUFA)-rich diet. J. Gerontol. Ser. A 2005, 60, 970–975. [Google Scholar] [CrossRef]
| Virgin Olive Oil | Sunflower Oil | Fish Oil | ||||
|---|---|---|---|---|---|---|
| 6 Months | 24 Months | 6 Months | 24 Months | 6 Months | 24 Months | |
| C14 | 201.7 ± 23.7 | 206.8 ± 72.2 | 191.3 ± 28.1 | 203.3 ± 30.0 | 151 ± 25.4 | 155.4 ± 49.8 |
| C16 | 112.4 ± 25.1 | 126.0 ± 44.7 | 115.6 ± 21.9 | 126.1 ± 36.7 | 79.8 ± 18.4 | 75.4 ± 22.2 |
| C16:1n9 | 3.61 ± 0.2 | 3.3 ± 0.4 | 2.5 ± 0.7 | 2.9 ± 0.8 | 3.2 ± 0.5 | 3.5 ± 0.8 |
| C18 | 18.8 ± 3.25 A,B | 21.3 ± 4.6 a,b | 23.1 ± 1.1 B | 24.2 ± 1.2 b | 15.6 ± 2.5 A | 14.8 ± 2.9 a |
| C18:1n9 | 21.9 ± 1.7 B | 21.2 ± 3.4 b | 16.8 ± 0.7 A | 15.7 ± 0.5 a | 10.1 ± 2.0 A | 12.2 ± 2.2 a |
| C18:2n6 | 16.9 ± 1.8 B | 16.0 ± 2.0 b | 23.3 ± ±1.7 C | 24.4 ± 3.4 c | 5.5 ± 0.7 A | 5.9 ± 1.0 a |
| C20:3n6 | 0.3 ± 0.1 | 0.4 ± 0.1 | 0.5 ± 0.2 | 0.5 ± 0.1 | 0.8 ± 0.2 | 0.8 ± 0.5 |
| C20:4n6 | 11.7 ± 1.5 B | 10.7 ± 2.4 b | 15.4 ± 0.4 C | 16.0 ± 1.8 c | 7.1 ± 0.9 A | 6.1 ± 1.3 a |
| C20:5n3 | 0.7 ± 0.2 A | 0.7 ± 0.3 a | 0.9 ± 0.5 A | 1.1 ± 0.7 a | 3.3 ± 0.6 B | 3.8 ± 1.5 b |
| C22:6n3 | 6.8 ± 1.9 A | 7.2 ± 2.3 a | 7.1 ± 2.5 A | 8.1 ± 3.9 a | 15.9 ± 2.6 B | 17.3 ± 4.4 b |
| C24:0 | 0.5 ± 0.4 | 0.3 ± 0.3 | 0.7 ± 0.3 | 0.9 ± 0.5 | 0.3 ± 0.2 | 0.4 ± 0.4 |
| C24:1n9 | 1.9 ± 0.3 B | 1.3 ± 0.4 b | 2.7 ± 0.9 B | 3.2 ± 1.3 b | 0.3 ± 0.1 A | 0.3 ± 0.2 a |
| Virgin Olive Oil | Sunflower Oil | Fish Oil | ||||
|---|---|---|---|---|---|---|
| 6 Months | 24 Months | 6 Months | 24 Months | 6 Months | 24 Months | |
| Body weight (g) | 309.2 ± 9.2 * | 534.5 ± 51.8 a,b | 301.5 ± 10.9 * | 508.3 ± 19.5 a | 315.3 ± 13.1 * | 604.0 ± 23.3 b |
| Heart weight (g) | 0.9 ± 0.1 * | 1.3 ± 0.1 | 0.9 ± 0.1 * | 1.3 ± 0.1 | 0.9 ± 0.0 * | 1.4 ± 0.1 |
| Heart/Body ratio | 0.0029 ± 0.0002 * | 0.0024 ± 0.0001 | 0.0030 ± 0.0001 * | 0.0026 ± 0.0000 | 0.0029 ± 0.0002 * | 0.0023 ± 0.0001 |
| Virgin Olive Oil | Sunflower Oil | Fish Oil | |
|---|---|---|---|
| Inflammation | |||
| Grade 0 | 0 (00.00%) | 0 (00.00%) | 1 (16.67%) |
| Grade I | 1 (16.67%) | 2 (33.33%) | 3 (50.00%) |
| Grade II | 4 (66.67%) | 1 (16.67%) | 2 (33.33%) |
| Grade III | 1 (16.67%) | 2 (33.33%) | 0 (00.00%) |
| Grade IV | 0 (00.00%) | 1 (16.67%) | 0 (00.00%) |
| Fibrosis | |||
| Grade 0 | 1 (16.67%) | 0 (00.00%) | 2 (33.33%) |
| Grade I | 3 (50.00%) | 3 (50.00%) | 1 (16.67%) |
| Grade II | 1 (16.67%) | 1 (16.67%) | 2 (33.33%) |
| Grade III | 1 (16.67%) | 1 (16.67%) | 1 (16.67%) |
| Grade IV | 0 (00.00%) | 1 (16.67%) | 0 (00.00%) |
| Lipofuscin depots | |||
| Grade 0 | 0 (00.00%) | 1 (16.67%) | 1 (16.67%) |
| Grade I | 1 (16.67%) | 1 (16.67%) | 0 (00.00%) |
| Grade II | 4 (66.67%) | 3 (50.00%) | 5 (83.33%) |
| Grade III | 1 (16.67%) | 1 (16.67%) | 0 (00.00%) |
| Coronary hyalinosis | |||
| Absence | 4 (66.67%) | 3 (50.00%) | 3 (50.00%) |
| Presence | 2 (33.33%) | 3 (50.00%) | 3 (50.00%) |
| Vacuolization | |||
| Grade 0 | 1 (16.67%) | 1 (16.67%) | 1 (16.67%) |
| Grade I | 5 (83.33%) | 3 (50.00%) | 5 (83.33%) |
| Grade II | 0 (00.00%) | 2 (33.33%) | 0 (00.00%) |
| Virgin Olive Oil | Sunflower Oil | Fish Oil | ||||
|---|---|---|---|---|---|---|
| 6 Months | 24 Months | 6 Months | 24 Months | 6 Months | 24 Months | |
| Area (µm2) | 0.41 ± 0.01 * | 0.72 ± 0.07 a,b | 0.38 ± 0.01 * | 0.59 ± 0.04 a | 0.37 ± 0.01 * | 0.97 ± 0.14 b |
| Perimeter (µm) | 2.65 ± 0.05 * | 3.22 ± 0.17 a,b | 2.54 ± 0.05 * | 2.93 ± 0.09 a | 2.58 ± 0.06 * | 3.69 ± 0.22 b |
| Circularity (AU) | 0.90 ± 0.01 * | 0.81 ± 0.01 | 0.88 ± 0.01 * | 0.83 ± 0.01 | 0.90 ± 0.01 * | 0.82 ± 0.03 |
| Aspect ratio (AU) | 1.23 ± 0.02 * | 1.57 ± 0.07 a,b | 1.31 ± 0.02 * | 1.56 ± 0.06 a | 1.28 ± 0.02 * | 1.62 ± 0.19 b |
| Solidity (AU) | 0.97 ± 0.00 * | 0.96 ± 0.00 | 0.97 ± 0.0 * | 0.96 ± 0.00 | 0.97 ± 0.00 * | 0.96 ± 0.00 |
| Virgin Olive Oil | Sunflower Oil | Fish Oil | ||||
|---|---|---|---|---|---|---|
| 6 Months | 24 Months | 6 Months | 24 Months | 6 Months | 24 Months | |
| DCFDA (nM/mg) | 2.79 ± 0.37 * | 9.05 ± 1.52 | 3.59 ± 0.74 * | 9.44 ± 1.27 | 1.66 ± 0.66 * | 11.04 ± 1.78 |
| α-tocopherol (µg/mg) | 1.49 ± 0.18 * | 3.99 ± 0.48 | 1.45 ± 0.10 * | 3.94 ± 0.30 | 1.04 ± 0.12 * | 4.15 ± 0.38 |
| CoQ9 (µg/mg) | 0.34 ± 0.08 * | 3.18 ± 0.15 b | 0.29 ± 0.03 * | 2.60 ± 0.18 a | 0.21 ± 002 * | 4.18 ± 0.62 c |
| CoQ10 (µg/mg) | 0.05 ± 0.01 * | 0.46 ± 0.06 b | 0.05 ± 0.02 * | 0.33 ± 0.04 a | 0.04 ± 0.01 * | 0.73 ± 0.09 c |
| Se-GPX (U/mg) | 435.94 ± 13.17 * | 571.58 ± 9.62 b | 443.64 ± 15.66 * | 520.27 ± 9.08 a | 500.89 ± 26.70 | 526.45 ± 19.30 a |
| Catalase (U/mg) | 36.61 ± 1.77 * | 61.63 ± 1.40 b | 45.76 ± 5.89 | 53.13 ± 1.72 a | 47.82 ± 3.98 * | 65.51 ± 3.14 b |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Navarro-Hortal, M.D.; Ramírez-Tortosa, C.L.; Varela-López, A.; Romero-Márquez, J.M.; Ochoa, J.J.; Ramírez-Tortosa, M.; Forbes-Hernández, T.Y.; Granados-Principal, S.; Battino, M.; Quiles, J.L. Heart Histopathology and Mitochondrial Ultrastructure in Aged Rats Fed for 24 Months on Different Unsaturated Fats (Virgin Olive Oil, Sunflower Oil or Fish Oil) and Affected by Different Longevity. Nutrients 2019, 11, 2390. https://doi.org/10.3390/nu11102390
Navarro-Hortal MD, Ramírez-Tortosa CL, Varela-López A, Romero-Márquez JM, Ochoa JJ, Ramírez-Tortosa M, Forbes-Hernández TY, Granados-Principal S, Battino M, Quiles JL. Heart Histopathology and Mitochondrial Ultrastructure in Aged Rats Fed for 24 Months on Different Unsaturated Fats (Virgin Olive Oil, Sunflower Oil or Fish Oil) and Affected by Different Longevity. Nutrients. 2019; 11(10):2390. https://doi.org/10.3390/nu11102390
Chicago/Turabian StyleNavarro-Hortal, María D., César L. Ramírez-Tortosa, Alfonso Varela-López, José M. Romero-Márquez, Julio J. Ochoa, MCarmen Ramírez-Tortosa, Tamara Y. Forbes-Hernández, Sergio Granados-Principal, Maurizio Battino, and José L. Quiles. 2019. "Heart Histopathology and Mitochondrial Ultrastructure in Aged Rats Fed for 24 Months on Different Unsaturated Fats (Virgin Olive Oil, Sunflower Oil or Fish Oil) and Affected by Different Longevity" Nutrients 11, no. 10: 2390. https://doi.org/10.3390/nu11102390
APA StyleNavarro-Hortal, M. D., Ramírez-Tortosa, C. L., Varela-López, A., Romero-Márquez, J. M., Ochoa, J. J., Ramírez-Tortosa, M., Forbes-Hernández, T. Y., Granados-Principal, S., Battino, M., & Quiles, J. L. (2019). Heart Histopathology and Mitochondrial Ultrastructure in Aged Rats Fed for 24 Months on Different Unsaturated Fats (Virgin Olive Oil, Sunflower Oil or Fish Oil) and Affected by Different Longevity. Nutrients, 11(10), 2390. https://doi.org/10.3390/nu11102390










