Abstract
It is unclear whether patients with non-celiac gluten sensitivity (NCGS) can tolerate gluten. We have evaluated the changes of both gastrointestinal symptoms and quality of life for NCGS patients after the re-introduction of dietary gluten. Twenty-two NCGS patients reporting functional gastroenterological symptoms and on gluten-free diet (GFD) for the previous three weeks were exposed to incremental gluten-containing diets. Three groups were compared at baseline (immediately after 3-weeks on GFD) and immediately after the return of symptomatology: (i) a group tolerating a low-gluten diet (3.5 g gluten/day, week 1, n = 8), (ii) a group tolerating a mid-gluten diet (8 g gluten/day, week 2, n = 6), and (iii) a group tolerating a high-gluten diet (13 g gluten/day, week 3, n = 8). Their gastrointestinal symptoms and quality of life were assessed at baseline and post-intervention. The most common symptoms were: constipation (46%), abdominal pain (50%) and dyspepsia (38%). A decrease in several short form health survey (SF-36) sub-scores (all p < 0.03) after gluten re-introduction was only observed in the group tolerating the low-gluten diet; the same group showed a lower post-intervention role-emotional SF-36 score (p = 0.01). Most gastrointestinal symptoms remained similar after gluten re-introduction. However, a decrease in the general perception of well-being was only found after gluten re-introduction in the group tolerating a low-gluten diet (p = 0.01); the same was true when comparing the post-intervention general well-being perception among the three groups (p = 0.050). In conclusion, dissimilar responses from patients with NCGS were observed after the re-introduction of gluten, with gluten at a low dosage affecting the quality of life and general well-being of a group of patients, whereas others tolerate even higher doses of dietary gluten.
1. Introduction
Non-celiac gluten sensitivity (NCGS) is characterized by intestinal and extra-intestinal symptoms related to the ingestion of gluten-containing foods, in subjects that are not affected by either celiac disease (CD) or wheat allergy [1,2,3]. The symptomatology commonly found in NCGS comprises: bloating, abdominal pain, diarrhea, epigastric pain, nausea, aerophagia, lack of well-being, tiredness, headache, foggy mind, and anxiety among other symptoms [4]. Symptoms disappear after starting on a gluten-free diet (GFD) and appear again after a gluten challenge within a few hours or a couple of days [5,6]. However, this latter finding can be attributed to a placebo/nocebo effect [7,8]. Several studies have evaluated the effect of a gluten re-challenge in NCGS patients after GFD (a summary of studies is shown in Table 1). According to a recent meta-analysis, there is a wide range of patients relapsing after a gluten challenge (between 7% and 77%) and no effect of a gluten challenge was found on the risk of relapse [9]. These results are in line with another review of studies on patients with suspected NCGS, indicating that only 16% of them show clear gluten-specific symptoms [10]. These studies highlight the fact that further methodological considerations are required in studies evaluating the gluten challenge.

Table 1.
Summary of clinical trials evaluating a gluten re-challenge in NCGS patients.
The current clinical consensus is that the diagnostic criteria on NCGS should include self-reported gluten intolerance, negative serology for CD (including immunoglobulin A (IgA) endomysial antibodies, IgA tissue transglutaminase antibodies, and IgG de-amidated gliadin peptide antibodies) and the absence of villous atrophy at duodenal histology (whilst on a gluten-containing diet) [1,3,11].
Similarly to CD and wheat allergy, the cornerstone of NCGS treatment is the withdrawal of gluten-containing foods. Although considered safe and effective, the lifelong elimination of gluten from the diet carries psychological and social implications. Patients with CD report about concerns related to the management of their social relationships and life routine [12]. Support and education are important to enable patients to adapt to their new diet [13]. However, given the uncertainty on the pathogenesis and trigger(s) of NCGS, it is not clear how strict such a new diet needs to be, how long its implementation and how to monitor the efficacy of the treatment other than by clinical response. Clinical experience suggests that patients affected by NCGS range from those who need to adhere to a strict GFD to those who can tolerate potential cross-contamination without any clinical consequences [14].
NCGS is a disorder treated with a GFD. There is currently discussion whether the symptoms described in NCGS are exclusively due to the ingestion of gluten proteins rather than other components included in wheat [15]. Wheat has some components that are different from gluten proteins and can be harmful to patients suffering from NCGS, including wheat germ agglutinins (WGA), amylase inhibitors/trypsin (ATI), and fermentable oligo/di/monosaccharides and polyols (FODMAP) [16,17,18,19]. ATIs are a family of structurally similar proteins, which serve as protective proteins in wheat and other cereals, by inhibiting the enzymes (trypsin and trypsin-like activities) of wheat and some parasites [20] ATIs have been described as triggers of the activation of innate immunity in intestinal cells [18]. WGAs [19], similar to ATIs, serve as protective proteins as they are resistant to heat and proteolysis. WGAs have shown to promote the production of pro-inflammatory cytokines, which affect the integrity of the intestinal epithelium [21]. Finally, FODMAP-containing foods include such components as oligosaccharides, disaccharides, monosaccharides, and sugar alcohols. They are resistant to digestion and can ferment completely or partially in the large intestine. Their efficacy in the treatment of gastrointestinal symptoms related to IBS has been described, and their function is being evaluated in various pathologies affecting the intestine [22,23].
There is currently no data that can support any recommendations on the need for, or frequency of, repeated follow-up visits in these patients. It is considered good clinical care to study these patients at regular intervals in order to ensure they remain healthy and to involve a nutritionist to make sure they are not at risk of nutrient deficiencies. It is also advisable that the continued need for “strict” avoidance of all gluten-related products be regularly reviewed following recovery because some patients can possibly follow a less restrictive diet with no recurrence of symptoms. A lifelong strict GFD (as in CD) vs. an “on-demand” approach is the main question. Many experts recommend that patients should undergo periodic re-evaluation with the re-introduction of gluten (e.g., every 6–12 months) [8].
A GFD leads to the complete disappearance of symptoms in most patients with NCGS but in some cases the level of improvement after gluten withdrawal is only partial. However, it should be mentioned that the level of tolerance varies among individuals and there are patients with NCGS who do not tolerate even very small amounts of gluten. As it is presently unclear whether gluten sensitivity is a permanent or transient condition, the re-introduction of gluten after 1–2 years on a GFD is potentially advisable [33]. Thus, the aim of the present study was to evaluate the changes in gastrointestinal symptoms and quality of life for NCGS patients after exposure to different amounts of dietary gluten.
2. Materials and Methods
Between 2013 and 2014, patients reporting functional gastroenterological symptoms according to the Rome III criteria [34] were invited to participate in this study. All were recruited from the gastroenterological outpatient clinic at the Center for Prevention and Diagnosis of Celiac Disease, Gastroenterology and Endoscopy Unit, Fondazione IRCCS Ca’ Granda Ospedale Maggiore Policlinico in Milan (Italy). The patients who agreed to participate gave their written informed consent and were enrolled in the study. The local Ethics Committee for Human Research of the City of Milan approved the study protocol. The trial was registered in ClinicalTrial.gov (NCT01864993).
The inclusion criteria were: adult age (>18 years old), being on a gluten-containing diet, with negative anti-tissue transglutaminase IgA, normal IgA dosage, negative IgE-mediated wheat allergy as verified by skin prick test and serological IgE dosage. The exclusion criteria were: diagnosis of CD, wheat allergy, inflammatory bowel disease, psychiatric disorders, major abdominal surgery (in particular, intestinal resections), diabetes mellitus, systemic autoimmune diseases, previous anaphylactic episodes, any systemic disorders, patients already following or having followed a GFD regimen in the previous six months, pregnant or breastfeeding women, and patients already on pharmacological therapy. The patients were evaluated by a gastroenterologist and a qualified nutritionist. The diagnosis of NCGS was made in accordance with the latest NCGS consensus [4]. After recruitment, patients were requested to follow a GFD plan for 3-weeks before the start of the dietary intervention (i.e., the low/mid/high-gluten diet). Their overall health, gastrointestinal symptoms, and quality of life were assessed by medical examination. Their adherence to the GFD was evaluated according to the celiac dietary adherence test (CDAT) [35]. The CDAT is a clinically relevant, easily administrated 7-item instrument which allows the standardized evaluation of GFD adherence. It is a sensitive tool developed using standard psychometric techniques. Only those patients with excellent or very good GFD adherence were included in the study. CDAT is based on a score ranging from 7 to 35 against seven questions, each on a 5-point scale, with higher scores denoting worse GFD adherence [35].
2.1. Intervention
Twenty-four patients were recruited (Figure 1). As mentioned above, all the recruited patients were instructed to follow a strict GFD for 3 weeks. After that time, the intervention period started. A qualified nutritionist designed a personalized GFD adjusted to match daily requirements for energy, macronutrients and micronutrients. A structured 3-week dietary plan was indicated and explained to every patient, to cover structured meals, foods/beverages and alternatives for food purchase. The patients were also encouraged to immediately contact the nutritionist by phone in case of any doubt related to the diet. After the three weeks on the GFD, the intervention period started. The patients started the study with a low-gluten diet during the first week (3.5–4 g gluten/day, week 1, n = 24). Two patients dropped out of the study during the first week because they did not want to continue the diet. Afterwards, the patients who did not report adverse symptoms were administered a mid-gluten diet in the second week (6.7–8 g gluten/day, week 2, n = 14). Then, the patients who passed the second week were started on a high-gluten diet for the following week (10–13 g gluten/day, week 3, n = 8). Each patient had been instructed to immediately contact the research team at the end of each week should any of the previously reported symptoms at the beginning of the study return. A clinical evaluation was then arranged and the patient was to stop their gluten-containing diet and return to the GFD (i.e., for patients reporting adverse symptomatology at the end of the first week) or to the previous gluten-containing diet (i.e., for patients reporting adverse symptomatology at the end of the second and third week). In such cases, the nutritionist would also reinforce the instructions and food education on the practice of the GFD. A flow-chart of patients is shown in Figure 1. The patients with symptomatic relapse at the end of week 1 returned to the GFD (as indicated in the previous three weeks after recruitment). The patients who experienced a symptomatic relapse at the end of week 2 returned to a low-gluten diet (3.5–4 g/day) and stayed on that dietary treatment until the end of week 3. Finally, none of the patients who had undergone the high-gluten diet (n = 8) reported any worsening of gastrointestinal symptoms and at the end of week 3, they were instructed to return to their regular dietary pattern.
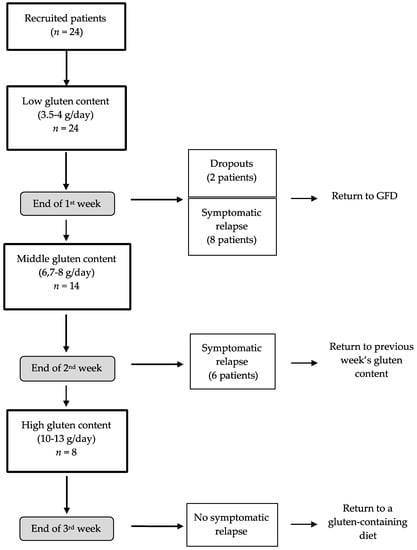
Figure 1.
Flow-chart of the patients’ activity in the study. Two patients (drop-outs) decided to abandon the diet during the first week of intervention; GFD: gluten-free diet.
2.2. Diets
The nutritional evaluation aimed to assess anthropometrical parameters, nutritional status, and usual dietary patterns. At the beginning of the study, after clinical evaluation, a structured 7-day dietary plan was generated for each patient, adjusted to his/her daily nutritional requirements for energy, macronutrients and micronutrients. For each week, according to the low/mid/high-gluten amount contained, meals were listed (breakfast, morning snack, lunch, afternoon snack, dinner and other snacks during the day) with specific foods/beverages (see examples in Table 2). For week 1 the source of gluten was only wheat pasta (50 g, about 3.5–4 g of gluten) administered during dinner. In week 2 the sources of gluten were wheat pasta (50 g, about 3.5–4 g of gluten) during dinner and wheat bread (50 g, about 3.2–4 g gluten) during the daytime. For week 3 the sources of gluten were wheat pasta (60 g at lunch and 60 g at dinner, ~8.4–9.6 g gluten) and wheat bread (30 g during the day, 1.9–3 g gluten). The gluten content of each of the three diets was calculated referring to Schalk et al. [36]. In that study, the gluten content was determined through a comprehensive strategy to isolate gluten protein fractions and gluten protein types (GPT) from wheat, rye, barley, and oat flours. All of the isolated GPTs were fully characterized by means of analytical reversed-phase high-performance liquid chromatography (RP-HPLC), sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), N-terminal sequencing, electrospray-ionization quadrupole time-of-flight mass spectrometry (LC-ESI-QTOF-MS) and untargeted LC-MS/MS of chymotryptic hydrolysates of the single GPT. Successively, all of the GPTs were reproducibly isolated in high purity from the flours and were made suitable to be used as a reference material, i.e., for calibration of liquid chromatography tandem mass spectrometry methods or enzyme-linked immunosorbent assays (ELISAs) [36].

Table 2.
Example of the three dietary plans used and differing in the amount of gluten contained 1.
2.3. Gastrointestinal Symptoms and Quality of Life
The gastrointestinal symptoms and quality of life of each patient were assessed at the beginning of the study and soon after the return of symptoms after administering one of the gluten-containing diets (i.e., after gluten exposure). A visual analogue scale (VAS) was used to assess the patient’s gastrointestinal symptoms and general perception of well-being as previously described by our group [23]. This instrument recorded the severity of specific symptoms: abdominal pain, bloating, postprandial fullness, early satiety, epigastric pain, non-specific functional gastrointestinal symptoms, and satisfaction with stool consistency. For each question, each patient was asked to put a mark along a 10-cm long line with one end 0 meaning “absence of symptom” and the other end 10 “severe symptomatology”. A further VAS evaluated the satisfaction about the current level of general well-being, with 0 meaning “completely unsatisfied” and 10 “absolutely satisfied”.
The patient’s quality of life was evaluated through the short form health survey (SF-36) questionnaire. This instrument comprises 36 questions that conceptually refer to eight health domains [37]. The patients were asked to answer each question and then domain-specific scores ranging between 0 and 100 were calculated, where 100 represented the best possible perception of quality of life.
2.4. Statistical Analysis
The data are provided as mean ± SEM or median (interquartile range) unless indicated otherwise. Twenty-two patients were included for analysis. Comparisons were made according to the group of patients that reported the return of symptomatology after gluten exposure, that is 3 groups: low-gluten (patients that reported adverse symptomatology after 1 week on a gluten-containing diet, n = 8), mid-gluten (patients that reported adverse symptomatology after 2 weeks on a gluten-containing diet, n = 6), and high-gluten (patients that reported adverse symptomatology after 3 weeks on a gluten-containing diet, n = 8). One-way ANOVA was used to evaluate between-group differences as to age and body-mass index; Fisher’s exact test was used to compare categorical variables (i.e., gender distribution and presence of gastrointestinal symptoms) between the groups. The within-group differences of SF-36 and VAS scores before (immediately after 3-weeks on a GFD) and after gluten exposure (i.e., baseline vs. the time when gastrointestinal symptoms returned after the gluten-containing diets) were assessed using a non-parametric Wilcoxon’s rank sum test. The between-group differences were evaluated after gluten exposure using the non-parametric Kruskal-Wallis test. STATA® ver. 13.1 software (StataCorp, College Station, TX, USA) was used for statistics and statistical significance was set at a 5% α-level.
3. Results
3.1. Patients
All patients included in the study obtained a CDAT score from 7 to 13, thus indicating very good adherence to the GFD. As shown in Table 3, the patients were middle-aged, mainly women, and within the normal weight range. Regarding the clinical symptomatology at baseline, symptoms such as constipation, abdominal pain, and dyspepsia were the most frequently reported by the whole sample (46%, 50%, and 38%, respectively). All three groups were comparable regarding both general characteristics and present gastrointestinal symptoms at baseline (Table 3). In regards to the estimated gluten content in the foods used in the intervention diets, the gluten content was 3.5–4 g/day in the low-gluten diet, 6.7–8 g/day in the mid-gluten diet, and 10–13 g/day in the high-gluten diet.

Table 3.
General characteristics of the group of patients at baseline 1.
3.2. Quality of Life
The resulting SF-36 scores are shown in Table 4. There was a significant decrease in several SF-36 sub-scores (role physical, role emotional, bodily pain, mental health, vitality and social interaction, all p < 0.03) after gluten exposure in the group of patients receiving the low-gluten diet but not in the groups receiving mid- and high-gluten content (Table 4). However, when comparing the change in SF-36 scores after dietary gluten exposure between the three groups, we observed a change only in the role emotional score, which was lower in the low-gluten content group. No post-intervention differences were found regarding the general health score among the three groups (Table 4).

Table 4.
SF-36 subscales and global score for quality of life 1.
3.3. Gastrointestinal Symptoms
The within-group comparisons showed no significant changes in most of the evaluated gastrointestinal symptoms before and after dietary intervention (Table 5). However, a decrease in the general perception of well-being was found in the low-gluten group (but not in the mid- and high-gluten groups) after intervention (p = 0.01). In line, when comparing the three groups after dietary gluten exposure, a further decrease of the general well-being level was found in the low-gluten group compared with the mid- and high-gluten groups (p = 0.050, Table 5).

Table 5.
Visual analogue scale scores for gastrointestinal symptoms 1.
4. Discussion
This study evaluated the effects of a short-term re-introduction of gluten on individuals diagnosed with NCGS. Our results show that a level of tolerance is present in patients without showing any adverse signs or gastrointestinal symptoms when consuming gluten. There was a different response among individuals with NCGS when exposed to different amounts of dietary gluten. A subgroup of patients had an immediate response with some worsening of their quality of life and general well-being at a low-gluten dosage, whereas other patients were able to tolerate medium and high doses of gluten, indicating that these latter groups can be administered some gluten without adverse health effects.
At present, it is well known that a GFD is the treatment of choice for patients suffering from NGCS. Several randomized controlled trials (RCTs) [10] have been carried out to identify gluten as the trigger of symptoms (Table 1). Those reports have shown variable results and are not conclusive regarding the cause-effect relationship of gluten and gastrointestinal symptoms [30,32]. We have previously suggested that gluten can be a major trigger of gastrointestinal symptoms in line with other [5,38,39]. Although the data to date suggest a benefit from a GFD for a selected group of patients, it is possible that the improvement in symptoms might not be due to gluten itself. Other components in wheat may trigger the reported symptoms in these patients, suggesting the clinical feature of non-celiac wheat sensitivity. This last entity has not completely been clarified as it is not clear whether patients are suffering from gluten-related symptoms or another component of wheat (such as fructans) [40]. Regardless of the nomenclature, Carroccio et al. [41] provided a clinically useful approach confirming non-celiac wheat sensitivity as a unique clinical condition. Their results suggest the existence of two different groups of patients with this condition: one with characteristics similar to CD and the other with characteristics resembling food allergy [41]. The current nomenclature of gluten sensitivity [1], NCGS [5] and gluten-related disorders does not resolve this problem and may confuse clinicians as to which component in wheat might be triggering patients’ symptomatology. Expert recommendations have proposed a periodic evaluation with re-introduction of gluten for NCGS patients on consideration of the economic costs and quality of life that a lifelong GFD entails [8].
Regarding the quality-of-life perception, previous data of our group from a cross-over study has shown that patients with NCGS treated with a GFD enjoy an improvement in the majority of the SF-36 scores after 7 days [30]. In our study, we observed that the group who tolerated only a low amount of dietary gluten was the only group showing a decrease in several SF-36 sub-scores. On the other hand, it is important to point out that the groups on a mid- and high-gluten diet did not show any significant change in their quality of life. This finding is intriguing because it would suggest that a greater gluten intake by patients with NCGS would not necessarily further affect their quality of life, thus reinforcing the idea of inter-individual variability against gluten in this group.
After the re-introduction of gluten, the gastrointestinal symptomatology showed no main changes against our dietary intervention. Moreover, no differences were found after gluten exposure among the three groups (i.e., at the end of the intervention period). However, we did find a change in the perception of general well-being, which was significantly affected in the group receiving a low level of gluten; such a result is in line with what we found on the patients’ quality of life. Overall, these findings suggest that with regard to quality of life and general well-being the changes observed in the group on a low-gluten diet would be related to a systemic response to gluten consumption rather than only gastrointestinal symptomatology or, at least, a combination of both. Even if our results are interesting per se they require further confirmation in larger samples and with different populations of patients suffering from NCGS.
This was an exploratory study that worked on a small sample of patients to evaluate the re-introduction of gluten through dietary modifications in a homogeneous group of patients correctly diagnosed with NCGS. As to limitations, we would like to point out that all the patients did not receive all their gluten doses in a balanced cross-over design. This was mainly due to ethical considerations since, at the time the clinical picture began to worsen, the patients stopped the administered diet and returned to their established treatment with GFD.
To summarize, we have shown a dissimilar response after the reintroduction of gluten in patients with NCGS who were on GFD for the last 3-weeks. We have also shown that for a group of them the re-introduction of gluten at low dosage affected their quality of life and general well-being, whereas other patients could tolerate higher doses of dietary gluten. Further studies are needed to establish whether NCGS patients require a dietary regimen free of gluten or just a gluten-restricted diet. Therefore, a controlled re-introduction of gluten potentially helps the improvement of selected patients that are able to tolerate gluten intake by developing a personalized diet containing gluten without the reappearance of symptoms. Further research is needed to assess the long-term clinical response of the increase in the dietary gluten content as concerns symptomatology and quality of life for patients with NCGS.
Author Contributions
Conceptualization, L.R., K.A.B. and L.E.; Methodology, L.R., K.A.B., L.D. and L.E.; Investigation, L.R., V.L., A.S., F.B. and L.E.; Data Curation, L.R. and K.A.B.; Formal analysis, K.A.B.; Writing-Original Draft Preparation, K.A.B., L.R. and L.E.; Writing Review & Editing, L.R., K.A.B., L.D. and M.T.B.; M.V. and L.E.; Funding Acquisition, L.E.
Funding
This research was funded by Fondazione IRCCS Ca’ Granda and through grants from the Italian Ministry of Health and Lombardy’s Regional Government Authority (Ministero della Salute and Regione Lombardia, call No. 2011-02348234), and the APC was funded by Fondazione IRCCS Ca’ Granda Ospedale Maggiore Policlinico and Università degli Studi di Milano, Milan, Italy.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analysis, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- Sapone, A.; Bai, J.C.; Ciacci, C.; Dolinsek, J.; Green, P.H.R.; Hadjivassiliou, M.; Kaukinen, K.; Rostami, K.; Sanders, D.S.; Schumann, M.; et al. Spectrum of gluten-related disorders: Consensus on new nomenclature and classification. BMC Med. 2012, 10, 13. [Google Scholar] [CrossRef] [PubMed]
- Bascuñán, K.A.; Roncoroni, L.; Branchi, F.; Doneda, L.; Scricciolo, A.; Ferretti, F.; Aray, M.; Elli, L. The 5 Ws of a gluten challenge for gluten-related disorders. Nutr. Rev. 2018, 76, 79–87. [Google Scholar] [CrossRef] [PubMed]
- Catassi, C.; Bai, J.C.; Bonaz, B.; Bouma, G.; Calabrò, A.; Carroccio, A.; Castillejo, G.; Ciacci, C.; Cristofori, F.; Dolinsek, J.; et al. Non-celiac gluten sensitivity: The new frontier of gluten related disorders. Nutrients 2013, 5, 3839–3853. [Google Scholar] [CrossRef]
- Catassi, C.; Elli, L.; Bonaz, B.; Bouma, G.; Carroccio, A.; Castillejo, G.; Cellier, C.; Cristofori, F.; de Magistris, L.; Dolinsek, J.; et al. Diagnosis of non-celiac gluten sensitivity (NCGS): The salerno experts’ criteria. Nutrients 2015, 7, 4966–4977. [Google Scholar] [CrossRef]
- Biesiekierski, J.R.; Newnham, E.D.; Irving, P.M.; Barrett, J.S.; Haines, M.; Doecke, J.D.; Shepherd, S.J.; Muir, J.G.; Gibson, P.R. Gluten Causes Gastrointestinal Symptoms in Subjects Without Celiac Disease: A Double-Blind Randomized Placebo-Controlled Trial. Am. J. Gastroenterol. 2011, 106, 508. [Google Scholar] [CrossRef] [PubMed]
- Volta, U.; Bardella, M.T.; Calabrò, A.; Troncone, R.; Corazza, G.R.; Bagnato, C.; Belcari, C.; Bellantoni, A.; Caio, G.; Calella, F.; et al. An Italian prospective multicenter survey on patients suspected of having non-celiac gluten sensitivity. BMC Med. 2014, 12, 85. [Google Scholar] [CrossRef] [PubMed]
- Parrish, C.R. Non-Celiac Gluten Sensitivity; Where are we now in 2015? Pract. Gastroenterol. 2015, 142, 40–48. [Google Scholar]
- Fasano, A.; Sapone, A.; Zevallos, V.; Schuppan, D. Nonceliac gluten sensitivity. Gastroenterology 2015, 148, 1195–1204. [Google Scholar] [CrossRef]
- Lionetti, E.; Pulvirenti, A.; Vallorani, M.; Catassi, G.; Verma, A.K.; Gatti, S.; Catassi, C. Re-challenge Studies in Non-celiac Gluten Sensitivity: A Systematic Review and Meta-Analysis. Front. Physiol. 2017, 8, 621. [Google Scholar] [CrossRef]
- Molina-Infante, J.; Carroccio, A. Suspected Nonceliac Gluten Sensitivity Confirmed in Few Patients After Gluten Challenge in Double-Blind, Placebo-Controlled Trials. Clin. Gastroenterol. Hepatol. 2017, 15, 339–348. [Google Scholar] [CrossRef]
- Rubio-Tapia, A.; Hill, I.D.; Kelly, C.P.; Calderwood, A.H.; Murray, J.A. ACG clinical guidelines: Diagnosis and management of celiac disease. Am. J. Gastroenterol. 2013, 108, 656–676, quiz 677. [Google Scholar] [CrossRef] [PubMed]
- Sverker, A.; Hensing, G.; Hallert, C. “Controlled by food”—Lived experiences of coeliac disease. J. Hum. Nutr. Diet. 2005, 18, 171–180. [Google Scholar] [CrossRef] [PubMed]
- Bascuñán, K.A.; Vespa, M.C.; Araya, M. Celiac disease: Understanding the gluten-free diet. Eur. J. Nutr. 2017, 56, 449–459. [Google Scholar] [CrossRef] [PubMed]
- Hill, I.D.; Fasano, A.; Guandalini, S.; Hoffenberg, E.; Levy, J.; Reilly, N.; Verma, R. NASPGHAN clinical report on the diagnosis and treatment of gluten-related disorders. J. Pediatr. Gastroenterol. Nutr. 2016, 63, 156–165. [Google Scholar] [CrossRef] [PubMed]
- Catassi, C.; Alaedini, A.; Bojarski, C.; Bonaz, B.; Bouma, G.; Carroccio, A.; Castillejo, G.; De Magistris, L.; Dieterich, W.; Di Liberto, D.; et al. The overlapping area of non-celiac gluten sensitivity (NCGS) and wheat-sensitive irritable bowel syndrome (IBS): An update. Nutrients 2017, 9, 1268. [Google Scholar] [CrossRef] [PubMed]
- Uhde, M.; Ajamian, M.; Caio, G.; De Giorgio, R.; Indart, A.; Green, P.H.; Verna, E.C.; Volta, U.; Alaedini, A. Intestinal cell damage and systemic immune activation in individuals reporting sensitivity to wheat in the absence of coeliac disease. Gut 2016, 65, 1930–1937. [Google Scholar] [CrossRef] [PubMed]
- Pinto-Sánchez, M.I.; Verdú, E.F. Non-coeliac gluten sensitivity: Are we closer to separating the wheat from the chaff? Gut 2016. [Google Scholar] [CrossRef]
- Junker, Y.; Zeissig, S.; Kim, S.-J.; Barisani, D.; Wieser, H.; Leffler, D.A.; Zevallos, V.; Libermann, T.A.; Dillon, S.; Freitag, T.L.; et al. Wheat amylase trypsin inhibitors drive intestinal inflammation via activation of toll-like receptor 4. J. Exp. Med. 2012, 209, 2395–2408. [Google Scholar] [CrossRef]
- de Punder, K.; Pruimboom, L. The dietary intake of wheat and other cereal grains and their role in inflammation. Nutrients 2013, 5, 771–787. [Google Scholar] [CrossRef]
- Tatham, A.S.; Shewry, P.R. Allergens to wheat and related cereals. Clin. Exp. Allergy 2008, 38, 1712–1726. [Google Scholar]
- Pellegrina, C.D.; Perbellini, O.; Scupoli, M.T.; Tomelleri, C.; Zanetti, C.; Zoccatelli, G.; Fusi, M.; Peruffo, A.; Rizzi, C.; Chignola, R. Effects of wheat germ agglutinin on human gastrointestinal epithelium: Insights from an experimental model of immune/epithelial cell interaction. Toxicol. Appl. Pharmacol. 2009, 237, 146–153. [Google Scholar] [CrossRef] [PubMed]
- Manning, L.P.; Biesiekierski, J.R. Use of dietary interventions for functional gastrointestinal disorders. Curr. Opin. Pharmacol. 2018, 43, 132–138. [Google Scholar] [CrossRef] [PubMed]
- Roncoroni, L.; Bascuñán, K.A.; Doneda, L.; Scricciolo, A.; Lombardo, V.; Branchi, F.; Ferretti, F.; Dell’osso, B.; Montanari, V.; Bardella, M.T.; et al. A low FODMAP gluten-free diet improves functional gastrointestinal disorders and overall mental health of celiac disease patients: A randomized controlled trial. Nutrients 2018, 10, 8. [Google Scholar] [CrossRef]
- Carroccio, A.; Mansueto, P.; Iacono, G.; Soresi, M.; D’Alcamo, A.; Cavataio, F.; Brusca, I.; Florena, A.M.; Ambrosiano, G.; Seidita, A.; et al. Non-celiac wheat sensitivity diagnosed by double-blind placebo-controlled challenge: Exploring a new clinical entity. Am. J. Gastroenterol. 2012, 107, 1898–1906, quiz 1907. [Google Scholar] [CrossRef] [PubMed]
- Peters, S.L.; Biesiekierski, J.R.; Yelland, G.W.; Muir, J.G.; Gibson, P.R. Randomised clinical trial: Gluten may cause depression in subjects with non-coeliac gluten sensitivity—An exploratory clinical study. Aliment. Pharmacol. Ther. 2014, 39, 1104–1112. [Google Scholar] [CrossRef] [PubMed]
- Zanini, B.; Baschè, R.; Ferraresi, A.; Ricci, C.; Lanzarotto, F.; Marullo, M.; Villanacci, V.; Hidalgo, A.; Lanzini, A. Randomised clinical study: Gluten challenge induces symptom recurrence in only a minority of patients who meet clinical criteria for non-coeliac gluten sensitivity. Aliment. Pharmacol. Ther. 2015, 42, 968–976. [Google Scholar] [CrossRef]
- Capannolo, A.; Viscido, A.; Barkad, M.A.; Valerii, G.; Ciccone, F.; Melideo, D.; Frieri, G.; Latella, G. Non-Celiac Gluten Sensitivity among Patients Perceiving Gluten-Related Symptoms. Digestion 2015, 92, 8–13. [Google Scholar] [CrossRef] [PubMed]
- Rosinach, M.; Fernández-Bañares, F.; Carrasco, A.; Ibarra, M.; Temiño, R.; Salas, A.; Esteve, M. Double-Blind Randomized Clinical Trial: Gluten versus Placebo Rechallenge in Patients with Lymphocytic Enteritis and Suspected Celiac Disease. PLoS ONE 2016, 11, e0157879. [Google Scholar] [CrossRef] [PubMed]
- Picarelli, A.; Borghini, R.; Di Tola, M.; Marino, M.; Urciuoli, C.; Isonne, C.; Puzzono, M.; Porowska, B.; Rumi, G.; Lonardi, S.; et al. Intestinal, systemic, and oral gluten-related alterations in patients with nonceliac gluten sensitivity. J. Clin. Gastroenterol. 2016, 50, 849–858. [Google Scholar] [CrossRef]
- Elli, L.; Tomba, C.; Branchi, F.; Roncoroni, L.; Lombardo, V.; Bardella, M.T.; Ferretti, F.; Conte, D.; Valiante, F.; Fini, L.; et al. Evidence for the presence of non-celiac gluten sensitivity in patients with functional gastrointestinal symptoms: Results from a multicenter randomized double-blind placebo-controlled gluten challenge. Nutrients 2016, 8, 84. [Google Scholar] [CrossRef] [PubMed]
- Skodje, G.I.; Sarna, V.K.; Minelle, I.H.; Rolfsen, K.L.; Muir, J.G.; Gibson, P.R.; Veierød, M.B.; Henriksen, C.; Lundin, K.E.A. Fructan, Rather Than Gluten, Induces Symptoms in Patients With Self-Reported Non-Celiac Gluten Sensitivity. Gastroenterology 2018, 154, 529–539.e2. [Google Scholar] [CrossRef] [PubMed]
- Dale, H.F.; Hatlebakk, J.G.; Hovdenak, N.; Ystad, S.O.; Lied, G.A. The effect of a controlled gluten challenge in a group of patients with suspected non-coeliac gluten sensitivity: A randomized, double-blind placebo-controlled challenge. Neurogastroenterol. Motil. 2018, e13332. [Google Scholar] [CrossRef] [PubMed]
- Volta, U.; Caio, G.; Tovoli, F.; De Giorgio, R. Non-celiac gluten sensitivity: Questions still to be answered despite increasing awareness. Cell. Mol. Immunol. 2013, 10, 383. [Google Scholar] [CrossRef] [PubMed]
- Drossman, D.A.; Dumitrascu, D.L. Rome III: New standard for functional gastrointestinal disorders. J. Gastrointest. Liver Dis. 2006, 15, 237–241. [Google Scholar]
- Leffler, D.A.; Dennis, M.; Edwards George, J.B.; Jamma, S.; Magge, S.; Cook, E.F.; Schuppan, D.; Kelly, C.P. A Simple Validated Gluten-Free Diet Adherence Survey for Adults With Celiac Disease. Clin. Gastroenterol. Hepatol. 2009, 7, 530–536.e2. [Google Scholar] [CrossRef]
- Schalk, K.; Lexhaller, B.; Koehler, P.; Scherf, K.A. Isolation and characterization of gluten protein types from wheat, rye, barley and oats for use as reference materials. PLoS ONE 2017, 12, e0172819. [Google Scholar] [CrossRef]
- Ware, J.E.; Sherbourne, C.D. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med. Care 1992, 473–483. [Google Scholar] [CrossRef]
- Di Sabatino, A.; Volta, U.; Salvatore, C.; Biancheri, P.; Caio, G.; De Giorgio, R.; Di Stefano, M.; Corazza, G.R. Small Amounts of Gluten in Subjects With Suspected Nonceliac Gluten Sensitivity: A Randomized, Double-Blind, Placebo-Controlled, Cross-Over Trial. Clin. Gastroenterol. Hepatol. 2015, 13, 1604–1612.e3. [Google Scholar] [CrossRef]
- Shahbazkhani, B.; Sadeghi, A.; Malekzadeh, R.; Khatavi, F.; Etemadi, M.; Kalantri, E.; Rostami-Nejad, M.; Rostami, K. Non-Celiac Gluten Sensitivity Has Narrowed the Spectrum of Irritable Bowel Syndrome: A Double-Blind Randomized Placebo-Controlled Trial. Nutrients 2015, 7, 4542–4554. [Google Scholar] [CrossRef]
- Sanders, D.S.; Aziz, I. Editorial: Non-celiac wheat sensitivity: Separating the wheat from the chat! Am. J. Gastroenterol. 2012, 107, 1908–1912. [Google Scholar] [CrossRef]
- Carroccio, A.; Rini, G.; Mansueto, P. Non-celiac wheat sensitivity is a more appropriate label than non-celiac gluten sensitivity. Gastroenterology 2014, 146, 320–321. [Google Scholar] [CrossRef] [PubMed]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).