Testing the Predictive Validity of the Healthy Eating Index-2015 in the Multiethnic Cohort: Is the Score Associated with a Reduced Risk of All-Cause and Cause-Specific Mortality?
Abstract
:1. Introduction
2. Methods
2.1. Study Population
2.2. Dietary Assessment and Calculation of the Healthy Eating Index-2015 (HEI-2015)
2.3. Healthy Eating Index-2015 (HEI-2015) Scoring
2.4. Case Ascertainment
2.5. Statistical Analysis
3. Results
3.1. Participant Characteristics
3.2. Mortality Analysis
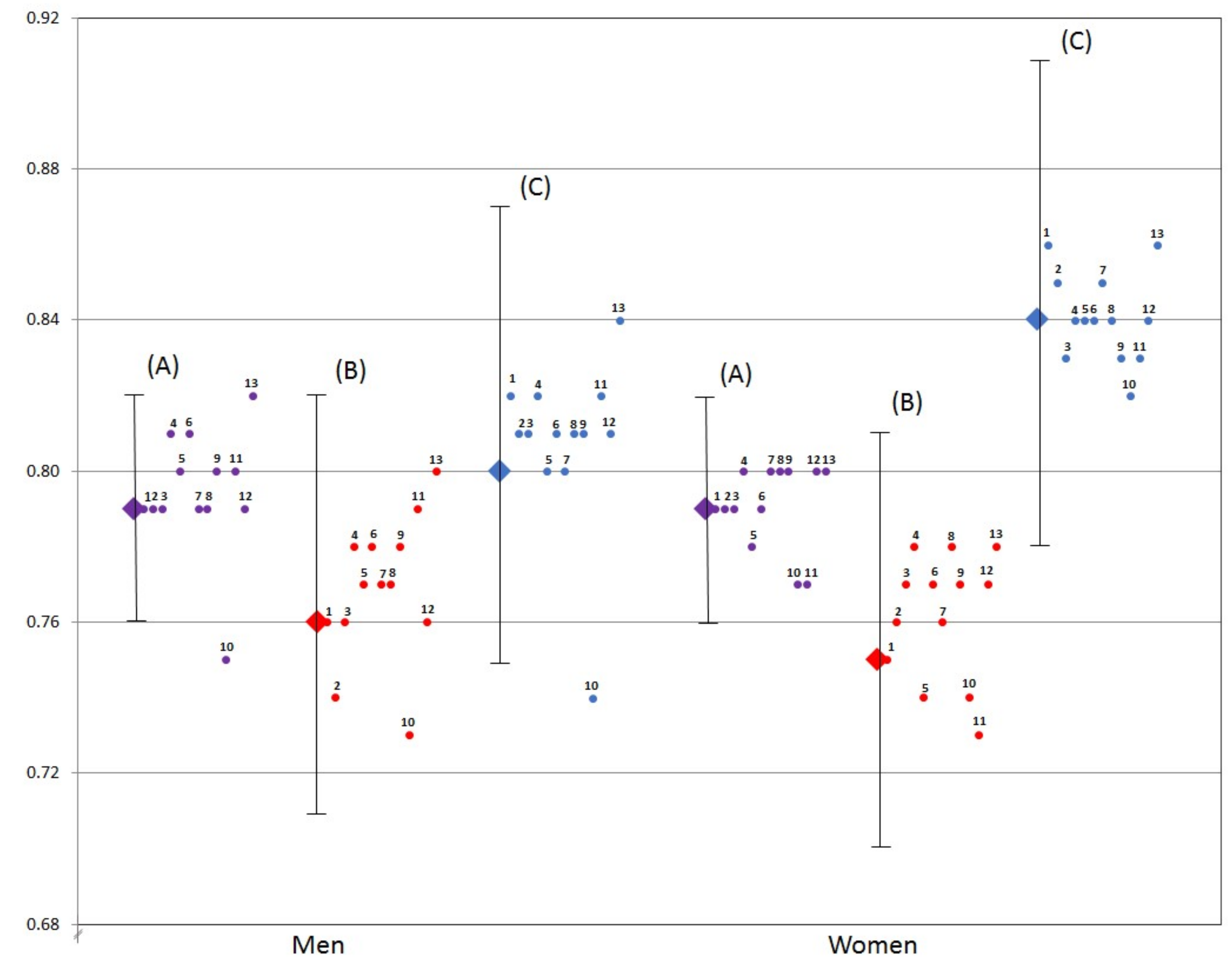
3.3. Radar Graphs
4. Discussion
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
Appendix A
| Ethnic Group | Men | n | Women **** | n |
|---|---|---|---|---|
| White | 67.4 ± 10.4 * | 17,330 | 70.5 ± 10.4 | 20,653 |
| African American | 67.1 ± 10.4 * | 9014 | 70.9 ± 10.3 | 16,072 |
| Native Hawaiian | 63.2 ± 10.6 ** | 4992 | 67.1 ± 10.9 | 6368 |
| Japanese American | 63.7 ± 10.3 *** | 21,239 | 68.3 ± 10.3 | 24,785 |
| Latino | 63.8 ± 9.3 *** | 17,595 | 66.5 ± 9.8 | 18,756 |
| Total group | 65.0 ± 10.3 | 70,170 | 68.8 ± 10.4 | 86,634 |
References
- Schulze, M.B.; Hu, F.B. Dietary patterns and risk of hypertension, type 2 diabetes mellitus, and coronary heart disease. Curr. Atheroscler. Rep. 2002, 4, 462–467. [Google Scholar] [CrossRef] [PubMed]
- Hu, F.B. Dietary pattern analysis: A new direction in nutritional epidemiology. Curr. Opin. Lipidol. 2002, 13, 3–9. [Google Scholar] [CrossRef] [PubMed]
- Guenther, P.M.; Casavale, K.O.; Reedy, J.; Kirkpatrick, S.I.; Hiza, H.A.; Kuczynski, K.J.; Kahle, L.L.; Krebs-Smith, S.M. Update of the Healthy Eating Index: HEI-2010. J. Acad. Nutr. Diet. 2013, 113, 569–580. [Google Scholar] [CrossRef] [PubMed]
- U.S. Department of Health and Human Services; U.S. Department of Agriculture. Dietary Guidelines for Americans 2015–2020, 8th ed.; U.S. Government Printing Office: Washington, DC, USA, 2015. Available online: http://health.gov/dietaryguidelines/2015/guidelines (accessed on 15 April 2017).
- National Research Council (US) Committee on Diet and Health. Diet and Health: Implications for Reducing Chronic Disease Risk; National Academy Press: Washington, DC, USA, 1989.
- Guenther, P.M.; Reedy, J.; Krebs-Smith, S.M. Development of the Healthy Eating Index-2005. J. Am. Diet. Assoc. 2008, 108, 1896–1901. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, E.T.; Ohls, J.; Carlson, S.; Fleming, K. The Healthy Eating Index: Design and applications. J. Am. Diet. Assoc. 1995, 95, 1103–1108. [Google Scholar] [CrossRef]
- Bowman, S.A.; Lino, M.; Gerrior, S.A.; Basiotis, P.P. The Healthy Eating Index: 1994–96; U.S. Department of Agriculture: Washington, DC, USA; Center for Nutrition Policy and Promotion, U.S. Government Printing Office: Washington, DC, USA, 1998.
- Center for Nutrition Policy and Promotion, U.S. Department of Agriculture. The Healthy Eating Index; U.S. Government Printing Office: Washington, DC, USA, 1995.
- U.S. Department of Health and Human Services; U.S. Department of Agriculture. Scientific Report of the 2015 Dietary Guidelines Advisory Committee; U.S. Government Printing Office: Washington, DC, USA, 2015. Available online: https://health.gov/dietaryguidelines/2015-scientific-report/pdfs/scientific-report-of-the-2015-dietary-guidelines-advisory-committee.pdf (accessed on 15 April 2017).
- Basiotis, P.P.; Carlson, A.; Gerrior, S.A.; Juan, W.Y.; Lino, M. The Healthy Eating Index: 1999–2000; U.S. Department of Agriculture: Washington, DC, USA; Center for Nutrition Policy and Promotion, U.S. Government Printing Office: Washington, DC, USA, 2002.
- National Cancer Institute; National Institutes of Health. Overview and Background of the Healthy Eating Index. Available online: https://epi.grants.cancer.gov/hei/ (accessed on 15 April 2017).
- Guenther, P.M.; Reedy, J.; Krebs-Smith, S.M.; Reeve, B.B.; Basiotis, P.P. Development and Evaluation of the Healthy Eating Index-2005: Technical Report; U.S. Department of Agriculture: Washington, DC, USA; Center for Nutrition Policy and Promotion, U.S. Government Printing Office: Washington, DC, USA, 2007.
- National Cancer Institute; National Institutes of Health. Dietary Patterns Methods Project. Available online: https://epi.grants.cancer.gov/dietary-patterns/ (accessed on 15 April 2017).
- Liese, A.D.; Krebs-Smith, S.M.; Subar, A.F.; George, S.M.; Harmon, B.E.; Neuhouser, M.L.; Boushey, C.J.; Schap, T.E.; Reedy, J. The Dietary Patterns Methods Project: Synthesis of findings across cohorts and relevance to dietary guidance. J. Nutr. 2015, 145, 393–402. [Google Scholar] [CrossRef] [PubMed]
- Reedy, J.; Krebs-Smith, S.M.; Miller, P.E.; Liese, A.D.; Kahle, L.L.; Park, Y.; Subar, A.F. Higher diet quality is associated with decreased risk of all-cause, cardiovascular disease, and cancer mortality among older adults. J. Nutr. 2014, 144, 881–889. [Google Scholar] [CrossRef] [PubMed]
- Schatzkin, A.; Subar, A.F.; Thompson, F.E.; Harlan, L.C.; Tangrea, J.; Hollenbeck, A.R.; Hurwitz, P.E.; Coyle, L.; Schussler, N.; Michaud, D.S.; et al. Design and serendipity in establishing a large cohort with wide dietary intake distributions: The National Institutes of Health-American Association of Retired Person Diet and Health Study. Am. J. Epidemiol. 2001, 154, 1119–1125. [Google Scholar] [CrossRef] [PubMed]
- Kolonel, L.N.; Henderson, B.E.; Hankin, J.H.; Nomura, A.M.Y.; Wilkens, L.R.; Pike, M.C.; Stram, D.O.; Monroe, K.R.; Earle, M.E.; Nagamine, F.S. A multiethnic cohort in Hawaii and Los Angeles: Baseline characteristics. Am. J. Epidemiol. 2000, 151, 346–357. [Google Scholar] [CrossRef] [PubMed]
- Harmon, B.E.; Boushey, C.J.; Shvetsov, Y.B.; Etienne, R.; Reedy, J.; Wilkens, L.R.; Le Marchand, L.; Henderson, B.E.; Kolonel, L.N. Associations of key diet-quality indexes with mortality in the Multiethnic Cohort: The Dietary Patterns Methods Project. Am. J. Clin. Nutr. 2015, 101, 587–597. [Google Scholar] [CrossRef] [PubMed]
- Langer, R.D.; White, E.; Lewis, C.E.; Kotchen, J.M.; Hendrix, S.L.; Trevisan, M. The women’s health initiative observational study: Basline characteristics of participants and reliability of baseline measures. Ann. Epidemiol. 2003, 13, S107–S121. [Google Scholar] [CrossRef]
- George, S.M.; Ballard-Barbash, R.; Manson, J.E.; Reedy, J.; Shikany, J.M.; Subar, A.F.; Tinker, L.F.; Vitolins, M.; Neuhouser, M.L. Comparing indices of diet quality with chronic disease mortality risk in postmenopausal women in the Women’s Health Initiative Observational Study: Evidence to inform national dietary guidance. Am. J. Epidemiol. 2014, 180, 616–625. [Google Scholar] [CrossRef] [PubMed]
- Stram, D.O.; Hankin, J.H.; Wilkens, L.R.; Pike, M.C.; Monroe, K.R.; Park, S.; Henderson, B.E.; Nomura, A.M.; Earle, M.E.; Nagamine, F.S.; et al. Calibration of the dietary questionnaire for a multiethnic cohort in Hawaii and Los Angeles. Am. J. Epidemiol. 2000, 151, 358–370. [Google Scholar] [CrossRef] [PubMed]
- Murphy, S.P. Unique nutrition support for research at the Cancer Research Center of Hawaii. Hawaii Med. J. 2002, 61, 15–17. [Google Scholar] [PubMed]
- Bowman, S.A.; Friday, J.E.; Moshfegh, A. MyPyramid Equivalents Database, 2.0 for USDA Survey Foods, 2003–2004; Food Surveys Research Group. Beltsville Human Nutrition Research Center: Beltsville, MD, USA; Agricultural Research Service: Beltsville, MD, USA; U.S. Department of Agriculture: Beltsville, MD, USA, 2008. [Google Scholar]
- Sharma, S.; Murphy, S.P.; Wilkens, L.R.; Au, D.; Shen, L.; Kolonel, L.N. Extending a multiethnic food composition table to include standardized food group servings. J. Food Compost. Anal. 2003, 16, 485–495. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention. International Classification of Diseases, 9th Revision. 2014. Available online: https://www.cdc.gov/nchs/icd/icd9.htm (accessed on 15 April 2017).
- National Center for Health Statistics. International Statistical Classification of Diseases and Related Health Problems, 5th ed.; 10th Revision; World Health Organization: Geneva, Switzerland, 2016; Available online: http://apps.who.int/classifications/icd10/browse/Content/statichtml/ICD10Volume2_en_2016.pdf (accessed on 15 April 2017).
- Grambsch, P.M.; Therneau, T.M. Proportional hazards tests and diagnostics based on weighted residuals. Biometrika 1994, 81, 515–526. [Google Scholar] [CrossRef]
- Krebs-Smith, S.; Wilson, M.; Reedy, J. Visualization of dietary patterns. FASEB J. 2015, 29, 131.2. [Google Scholar]
- Ritenbaugh, C.; Patterson, R.E.; Chlebowski, R.T.; Caan, B.; Fels-Tinker, L.; Howard, B.; Ockene, J. The Women’s Health Initiative Dietary Modification Trial: Overview and baseline characteristics of participants. Ann. Epidemiol. 2003, 13, S87–S97. [Google Scholar] [CrossRef]
- Reedy, J.; Mitrou, P.N.; Krebs-Smith, S.M.; Wirfält, E.; Flood, A.; Kipnis, V.; Leitzmann, M.; Mouw, T.; Hollenbeck, A.; Schatzkin, A.; et al. Index-based dietary patterns and risk of colorectal cancer: The NIH-AARP Diet and Health Study. Am. J. Epidemiol. 2008, 168, 38–48. [Google Scholar] [CrossRef] [PubMed]
- Schwingshackl, L.; Bogensberger, B.; Hoffmann, G. Diet quality as assessed by the Healthy Eating Index, Alternate Healthy Eating Index, Dietary Approaches to Stop Hypertension score, and health outcomes: An updated systematic review and meta-analysis of cohort studies. J. Acad. Nutr. Diet. 2017, in press. [Google Scholar] [CrossRef]
- U.S. Department of Agriculture. Agricultural Research Service Food Surveys Research Group. What We Eat in America, NHANES Dietary Survey. Available online: http://seprl.ars.usda.gov/Services/docs.htm?docid=13793 (accessed on 15 April 2017).
- Sharma, S.; Murphy, S.P.; Wilkens, L.R.; Shen, L.; Hankin, J.H.; Monroe, K.R.; Henderson, B.; Kolonel, L.N. Adherence to the food guide pyramid recommendations among African Americans and Latinos: Results from the Multiethnic Cohort. J. Am. Diet. Assoc. 2004, 104, 1873–1877. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Murphy, S.P.; Wilkens, L.R.; Shen, L.; Hankin, J.H.; Henderson, B.; Kolonel, L.N. Adherence to the food guide pyramid recommendations among Japanese Americans, Native Hawaiians, and whites: Results from the Multiethnic Cohort Study. J. Am. Diet. Assoc. 2003, 103, 1195–1198. [Google Scholar] [CrossRef]
- Willett, W.C.; Stampfer, M.J.; Colditz, G.A.; Rosner, B.A.; Speizer, F.E. Relation of meat, fat, and fiber intake to the risk of colon cancer in a prospective study among women. N. Engl. J. Med. 1990, 323, 1664–1672. [Google Scholar] [CrossRef] [PubMed]
- Manson, J.E.; Colditz, G.A.; Stampfer, M.J.; Willett, W.C.; Rosner, B.; Monson, R.R.; Speizer, F.E.; Hennekens, C.H. A prospective study of obesity and risk of coronary heart disease in women. N. Engl. J. Med. 1990, 322, 882–889. [Google Scholar] [CrossRef] [PubMed]
- Ascherio, A.; Rimm, E.B.; Giovannucci, E.L.; Colditz, G.A.; Rosner, B.; Willett, W.C.; Sacks, F.; Stampfer, M.J. A prospective study of nutritional factors and hypertension among US men. Circulation 1992, 86, 1475–1484. [Google Scholar] [CrossRef] [PubMed]
- Giovannucci, E.; Rimm, E.B.; Stampfer, M.J.; Colditz, G.A.; Ascherio, A.; Willett, W.C. Intake of fat, meat, and fiber in relation to risk of colon cancer in men. Cancer Res. 1994, 54, 2390–2397. [Google Scholar] [PubMed]
- Balfour, P.C.; Ruiz, J.M.; Talavera, G.A.; Allison, M.A.; Rodriguez, C.J. Cardiovascular Disease in Hispanics/Latinos in the United States. J. Lat. Psychol. 2016, 4, 98–113. [Google Scholar] [CrossRef] [PubMed]

| Components | Maximum Scores | Standard for Maximum Scores 3 | Standard for Minimum of Zero |
|---|---|---|---|
| Adequacy | |||
| Total Fruits 4 | 5 | ≥0.8 cup | No Fruits |
| Whole Fruits 5 | 5 | ≥0.4 cup | No Whole Fruits |
| Total Vegetables 6 | 5 | ≥1.1 cup | No Vegetables |
| Greens & Beans 6 | 5 | ≥0.2 cup | No Greens and Beans |
| Whole Grains | 10 | ≥1.5 oz | No Whole Grains |
| Dairy 7 | 10 | ≥1.3 cup | No Dairy |
| Total Protein Foods 6,8 | 5 | ≥2.5 oz | No Protein Foods |
| Seafood & Plant Proteins 6,8 | 5 | ≥0.8 cup | No Seafood & Plant Proteins |
| Fatty Acids 9 | 10 | (PUFAs + MUFAs)/SFAs ≥ 2.5 | (PUFAs + MUFAs)/SFAs ≤ 1.2 |
| Moderation | |||
| Refined Grains | 10 | ≤1.8 oz | ≥4.3 oz |
| Sodium | 10 | ≤1.1 g | ≥2.0 g |
| Added Sugars | 10 | ≤6.5% of energy | ≥26% of energy |
| Saturated Fats | 10 | ≤8% of energy | ≥16% of energy |
| Quintile 1 | Quintile 2 | Quintile 3 | Quintile 4 | Quintile 5 | |
|---|---|---|---|---|---|
| Men (n = 70,170) | |||||
| HEI-2015 scores, range | 17.9–56.1 | 56.2–62.1 | 62.2–67.5 | 67.6–74.0 | 74.1–98.7 |
| Mean HEI-2015 score *** | 50.7 | 59.4 | 64.9 | 70.7 | 79.6 |
| N | 14,034 | 14,034 | 14,035 | 14,033 | 14,034 |
| Mortality, n cases | 5003 | 5177 | 5321 | 5355 | 5520 |
| Cardiovascular disease | 1708 | 1740 | 1864 | 1894 | 1924 |
| Cancer | 1566 | 1598 | 1572 | 1561 | 1515 |
| Age at time of death, years 1 | 74.4 ± 9.6 | 75.9 ± 9.4 | 77.0 ± 9.2 | 77.9 ± 9.1 | 79.3 ± 8.6 |
| Age at time of questionnaire, years 1,*** | 56.8 ± 8.6 | 58.3 ± 8.7 | 59.4 ± 8.7 | 60.0 ± 8.7 | 61.1 ± 8.6 |
| Ethnicity, % of row | |||||
| Japanese American (n = 21,239) | 24.1 | 21.5 | 20.0 | 17.8 | 16.6 |
| Latino (n = 17,595) | 20.7 | 23.1 | 22.8 | 19.8 | 13.7 |
| White (n = 17,330) | 14.9 | 16.3 | 18.0 | 23.0 | 28.0 |
| African American (n = 9014) | 15.1 | 17.0 | 19.2 | 21.7 | 26.9 |
| Native Hawaiian (n = 4992) | 27.0 | 20.9 | 18.7 | 17.2 | 16.3 |
| Body mass index (kg/m2) 1,*** | 26.9 ± 4.5 | 26.9 ± 4.3 | 26.8 ± 4.2 | 26.6 ± 4.1 | 26.1 ± 3.8 |
| Energy intake, kcal 1,*** | 2479 ± 1158 | 2552 ± 1220 | 2524 ± 1181 | 2448 ± 1107 | 2256 ± 978 |
| Physical activity, h/week 1,2,*** | 1.2 ± 1.5 | 1.3 ± 1.6 | 1.4 ± 1.5 | 1.4 ± 1.5 | 1.5 ± 1.5 |
| History of diabetes, % with diabetes *** | 8.4 | 10.0 | 11.4 | 11.7 | 12.5 |
| Smoking, % who never smoked *** | 24.3 | 28.0 | 30.6 | 33.8 | 37.7 |
| Education, % graduated from college *** | 23.9 | 26.2 | 28.6 | 33.3 | 38.5 |
| Marital status, % married | 74.5 | 76.3 | 77.7 | 76.8 | 75.2 |
| Women (n = 86,634) | |||||
| HEI-2015 scores, range | 23.5–59.8 | 59.9–66.3 | 66.4–71.8 | 71.9–78.0 | 78.1–99.8 |
| Mean HEI-2015 score *** | 53.8 | 63.3 | 69.2 | 74.9 | 83.0 |
| N | 17,327 | 17,326 | 17,328 | 17,327 | 17,326 |
| Mortality, n cases | 4603 | 4809 | 5020 | 5119 | 5515 |
| Cardiovascular disease | 1493 | 1637 | 1747 | 1782 | 1873 |
| Cancer | 1398 | 1365 | 1385 | 1353 | 1465 |
| Age at time of death, years | 75.1 ± 9.9 | 77.3 ± 9.6 | 78.3 ± 9.1 | 79.5 ± 9.1 | 80.4 ± 8.6 |
| Age at time of questionnaire, years *** | 56.4 ± 8.6 | 58.2 ± 8.7 | 59.3 ± 8.7 | 60.1 ± 8.8 | 61.5 ± 8.5 |
| Ethnicity, % of row | |||||
| Japanese American (n = 24,785) | 21.1 | 21.5 | 20.1 | 19.0 | 18.3 |
| White (n = 20,653) | 15.8 | 16.6 | 19.7 | 22.9 | 25.0 |
| Latina (n = 18,756) | 25.4 | 24.1 | 21.2 | 17.0 | 12.4 |
| African American (n = 16,072) | 15.0 | 17.0 | 19.3 | 22.3 | 26.5 |
| Native Hawaiian (n = 6368) | 26.1 | 20.8 | 18.8 | 17.8 | 16.5 |
| Body mass index (kg/m2) *** | 27.1 ± 6.2 | 26.7 ± 5.8 | 26.5 ± 5.6 | 26.1 ± 5.4 | 25.5 ± 5.2 |
| Energy intake, kcal *** | 2052 ± 1068 | 2038 ± 1023 | 2003 ± 967 | 1956 ± 915 | 1865 ± 817 |
| Physical activity, h/week 2,*** | 1.0 ± 1.2 | 1.0 ± 1.3 | 1.1 ± 1.3 | 1.2 ± 1.3 | 1.3 ± 1.3 |
| History of diabetes, % with diabetes *** | 8.2 | 9.3 | 9.8 | 9.5 | 10.1 |
| Smoking, % never smoked *** | 50.5 | 55.8 | 56.5 | 57.9 | 58.7 |
| Education, % graduated from college *** | 18.7 | 21.3 | 23.9 | 27.4 | 31.3 |
| Marital status, % married * | 59.1 | 60.9 | 60.5 | 59.4 | 57.6 |
| Hormone replacement therapy, % users *** | 37.4 | 42.7 | 46.1 | 49.2 | 53.1 |
| HEI-2015 Category | n | Any Deaths n | Person-Years of Follow-Up | All-Cause Mortality 1 HR (95% CI) | CVD Deaths n | CVD Mortality 1 HR (95% CI) | Cancer Deaths n | Cancer Mortality 1 HR (95% CI) |
|---|---|---|---|---|---|---|---|---|
| Men 2,3 | ||||||||
| Quintile 1 | 14,034 | 5003 | 252,098 | 1.00 | 1708 | 1.00 | 1566 | 1.00 |
| Quintile 2 | 14,034 | 5177 | 250,512 | 0.93 (0.90, 0.97) | 1740 | 0.90 (0.84, 0.96) | 1598 | 0.97 (0.90, 1.04) |
| Quintile 3 | 14,035 | 5321 | 250,072 | 0.89 (0.85, 0.92) | 1864 | 0.88 (0.82, 0.94) | 1572 | 0.91 (0.85, 0.98) |
| Quintile 4 | 14,033 | 5355 | 250,974 | 0.85 (0.81, 0.88) | 1894 | 0.84 (0.78, 0.90) | 1561 | 0.88 (0.82, 0.95) |
| Quintile 5 | 14,034 | 5520 | 252,489 | 0.79 (0.76, 0.82) | 1924 | 0.76 (0.71, 0.82) | 1515 | 0.80 (0.75, 0.87) |
| Women 3,4 | ||||||||
| Quintile 1 | 17,327 | 4603 | 327,551 | 1.00 | 1493 | 1.00 | 1398 | 1.00 |
| Quintile 2 | 17,326 | 4809 | 327,362 | 0.92 (0.89, 0.96) | 1637 | 0.93 (0.87, 1.00) | 1365 | 0.93 (0.86, 1.00) |
| Quintile 3 | 17,328 | 5020 | 325,938 | 0.87 (0.84, 0.91) | 1747 | 0.88 (0.82, 0.95) | 1385 | 0.90 (0.83, 0.97) |
| Quintile 4 | 17,327 | 5119 | 326,802 | 0.82 (0.79, 0.86) | 1782 | 0.83 (0.77, 0.89) | 1353 | 0.84 (0.78, 0.91) |
| Quintile 5 | 17,326 | 5515 | 325,836 | 0.79 (0.76, 0.82) | 1873 | 0.75 (0.70, 0.81) | 1465 | 0.84 (0.78, 0.91) |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Panizza, C.E.; Shvetsov, Y.B.; Harmon, B.E.; Wilkens, L.R.; Le Marchand, L.; Haiman, C.; Reedy, J.; Boushey, C.J. Testing the Predictive Validity of the Healthy Eating Index-2015 in the Multiethnic Cohort: Is the Score Associated with a Reduced Risk of All-Cause and Cause-Specific Mortality? Nutrients 2018, 10, 452. https://doi.org/10.3390/nu10040452
Panizza CE, Shvetsov YB, Harmon BE, Wilkens LR, Le Marchand L, Haiman C, Reedy J, Boushey CJ. Testing the Predictive Validity of the Healthy Eating Index-2015 in the Multiethnic Cohort: Is the Score Associated with a Reduced Risk of All-Cause and Cause-Specific Mortality? Nutrients. 2018; 10(4):452. https://doi.org/10.3390/nu10040452
Chicago/Turabian StylePanizza, Chloe E., Yurii B. Shvetsov, Brook E. Harmon, Lynne R. Wilkens, Loic Le Marchand, Christopher Haiman, Jill Reedy, and Carol J. Boushey. 2018. "Testing the Predictive Validity of the Healthy Eating Index-2015 in the Multiethnic Cohort: Is the Score Associated with a Reduced Risk of All-Cause and Cause-Specific Mortality?" Nutrients 10, no. 4: 452. https://doi.org/10.3390/nu10040452
APA StylePanizza, C. E., Shvetsov, Y. B., Harmon, B. E., Wilkens, L. R., Le Marchand, L., Haiman, C., Reedy, J., & Boushey, C. J. (2018). Testing the Predictive Validity of the Healthy Eating Index-2015 in the Multiethnic Cohort: Is the Score Associated with a Reduced Risk of All-Cause and Cause-Specific Mortality? Nutrients, 10(4), 452. https://doi.org/10.3390/nu10040452





