Behavioral Intervention in Adolescents Improves Bone Mass, Yet Lactose Maldigestion Is a Barrier
Abstract
1. Introduction
2. Materials and Methods
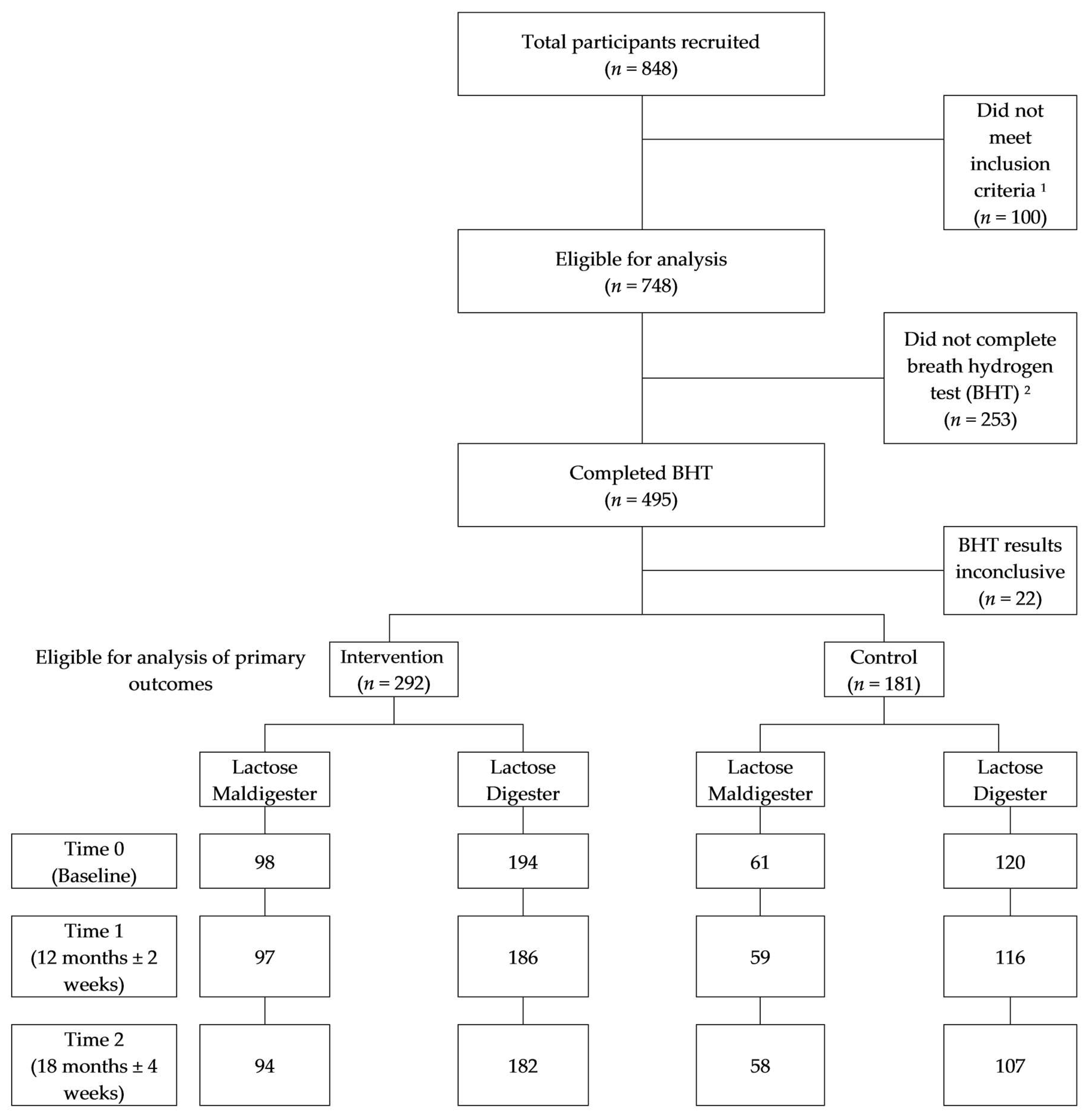
2.1. Study Design and Sample Selection
2.2. Sample Size Estimation
2.3. Intervention Implementation
2.4. Anthropometric Measures and Maturity
2.5. LM Status and PMI Status
2.6. Bone Mineral Assessment
2.7. Dietary Assessment
2.8. Statistical Analyses
3. Results
3.1. Descriptive Characteristics
3.2. Effects of the Intervention on Bone Mass Gains
3.3. Effects of the Intervention on Dietary Calcium Intake
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Appendix A
| Baseline a | 12 Months a | Adjusted Differences between Girls in Maldigester and Digester Groups | 95% Confidence Interval | Group Difference, p | |||
| Lactose Maldigester (n = 55) | Lactose Digester (n = 105) | Lactose Maldigester (n = 53) | Lactose Digester (n = 105) | Δ0–12 Months | |||
| Dietary calcium intake, mg b | |||||||
| Total dietary calcium | 1162 ± 566 | 1102 ± 556 | 958 ± 475 | 1023 ± 518 | 95 | −94 to 285 | 0.321 |
| Total dairy calcium | 956 ± 494 | 942 ± 488 | 788 ± 417 | 870 ± 456 | 80 | −88 to 248 | 0.349 |
| Exclusively dairy calcium | 646 ± 415 | 652 ± 379 | 506 ± 327 | 607 ± 367 | 37 | −102 to 177 | 0.597 |
| Baseline a | 12 Months a | Adjusted Differences between Girls in Maldigester and Digester Groups | 95% Confidence Interval | Group Difference, p | |||
| Maldigester (n = 61) | Digester (n = 120) | Maldigester (n = 59) | Digester (n = 117) | Δ0–12 Months | |||
| Bone mineral content, g c | |||||||
| Total body | 1546 ± 368 | 1619 ± 374 | 1804 ± 416 | 1903 ± 390 | 9.29 | −40.24 to 58.81 | 0.710 |
| Total hip | 23.11 ± 4.77 | 23.72 ± 4.99 | 25.60 ± 4.84 | 26.83 ± 5.02 | −0.41 | −0.96 to 0.13 | 0.136 |
| Spine (L2–L4) | 27.39 ± 7.13 | 27.83 ± 7.12 | 33.06 ± 7.67 | 34.15 ± 7.98 | −0.42 | −1.45 to 0.60 | 0.411 |
| Femoral neck | 3.52 ± 0.66 | 3.62 ± 0.68 | 3.89 ± 0.67 | 4.07 ± 0.74 | −0.08 | −0.17 to 0.01 | 0.09 |
| 12 Months a | 18 Months a | Adjusted Differences between Girls in Maldigester and Digester Groups | 95% Confidence Interval | Group Difference, p | |||
| Lactose Maldisgeter (n = 53) | Lactose Digester (n = 105) | Lactose Maldigester (n = 53) | Lactose Digester (n = 102) | Δ12–18 Months | |||
| Dietary calcium intake, mg b | |||||||
| Total dietary calcium | 958 ± 475 | 1023 ± 518 | 1001± 611 | 976 ± 469 | 107 | −124 to 339 | 0.361 |
| Total dairy calcium | 788 ± 417 | 870 ± 456 | 822 ± 562 | 840 ± 418 | 82 | −125 to 290 | 0.433 |
| Exclusively dairy calcium | 506 ± 327 | 607 ± 367 | 540 ± 430 | 596 ± 368 | 72 | −95 to 238 | 0.395 |
| 12 months a | 18 months a | Adjusted Differences between Girls in Maldigester and Digester Groups | 95% Confidence Interval | Group Difference, p | |||
| Lactose Maldisgeter (n = 59) | Lactose Digester (n = 117) | Lactose Maldisgeter (n = 57) | Lactose Digester (n = 106) | Δ12–18 Months | |||
| Bone mineral content, g c | |||||||
| Total body | 1804 ± 416 | 1903 ± 390 | 1899 ± 388 | 2027 ± 391 | −43.10 | −88.06 to 1.87 | 0.060 |
| Total hip | 25.60 ± 4.84 | 26.83 ± 5.02 | 26.57 ± 4.76 | 28.10 ± 4.99 | −0.04 | −0.44 to 0.36 | 0.837 |
| Spine (L2–L4) | 33.06 ± 7.67 | 34.15 ± 7.98 | 35.59 ± 7.23 | 36.83 ± 8.10 | 0.11 | −0.61 to 0.83 | 0.762 |
| Femoral neck | 3.89 ± 0.67 | 4.07 ± 0.74 | 4.05 ± 0.68 | 4.28 ± 0.75 | −0.03 | −0.10 to 0.04 | 0.354 |
References
- Siris, E.S.; Miller, P.D.; Barrett-Connor, E.; Faulkner, K.G.; Wehren, L.E.; Abbott, T.A.; Berger, M.L.; Santora, A.C.; Sherwood, L.M. Identification and fracture outcomes of undiagnosed low bone mineral density in postmenopausal women: Results from the national osteoporosis risk assessment. JAMA 2001, 286, 2815–2822. [Google Scholar] [CrossRef] [PubMed]
- Heaney, R.P. Nutrition and catch-up bone augmentation in young women. Am. J. Clin. Nutr. 1998, 68, 523–524. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Institute of Medicine. Dietary Reference Intakes for Calcium and Vitamin D; National Academy Press: Washington, DC, USA, 2011. [Google Scholar]
- U.S. Department of Agriculture, Agricultural Research Service. What We Eat in America, 2011–2012: Documentation and Data Files; U.S. Department of Agriculture, Agricultural Research Service: Beltsville, MD, USA, 2014.
- Birge, S.J., Jr.; Keutmann, H.T.; Cuatrecasas, P.; Whedon, G.D. Osteoporosis, intestinal lactase deficiency and low dietary calcium intake. N. Engl. J. Med. 1967, 276, 445–448. [Google Scholar] [CrossRef] [PubMed]
- Horowitz, M.; Wishart, J.; Mundy, L.; Nordin, B.E. Lactose and calcium absorption in postmenopausal osteoporosis. Arch. Intern. Med. 1987, 147, 534–536. [Google Scholar] [CrossRef] [PubMed]
- Obermayer-Pietsch, B.M.; Gugatschka, M.; Reitter, S.; Plank, W.; Strele, A.; Walter, D.; Bonelli, C.; Goessler, W.; Dobnig, H.; Hogenauer, C.; et al. Adult-type hypolactasia and calcium availability: Decreased calcium intake or impaired calcium absorption? Osteoporos. Int. 2007, 18, 445–451. [Google Scholar] [CrossRef] [PubMed]
- Di Stefano, M.; Veneto, G.; Malservisi, S.; Cecchetti, L.; Minguzzi, L.; Strocchi, A.; Corazza, G.R. Lactose malabsorption and intolerance and peak bone mass. Gastroenterology 2002, 122, 1793–1799. [Google Scholar] [CrossRef] [PubMed]
- Corazza, G.R.; Benati, G.; Di Sario, A.; Tarozzi, C.; Strocchi, A.; Passeri, M.; Gasbarrini, G. Lactose intolerance and bone mass in postmenopausal italian women. Br. J. Nutr. 1995, 73, 479–487. [Google Scholar] [CrossRef] [PubMed]
- Matlik, L.; Savaiano, D.; McCabe, G.; VanLoan, M.; Blue, C.L.; Boushey, C.J. Perceived milk intolerance is related to bone mineral content in 10- to 13-year-old female adolescents. Pediatrics 2007, 120, e669–e677. [Google Scholar] [CrossRef] [PubMed]
- Cadogan, J.; Eastell, R.; Jones, N.; Barker, M.E. Milk intake and bone mineral acquisition in adolescent girls: Randomised, controlled intervention trial. BMJ 1997, 315, 1255–1260. [Google Scholar] [CrossRef] [PubMed]
- Cheng, S.; Lyytikainen, A.; Kroger, H.; Lamberg-Allardt, C.; Alen, M.; Koistinen, A.; Wang, Q.J.; Suuriniemi, M.; Suominen, H.; Mahonen, A.; et al. Effects of calcium, dairy product, and vitamin d supplementation on bone mass accrual and body composition in 10–12-y-old girls: A 2-y randomized trial. Am. J. Clin. Nutr. 2005, 82, 1115–1126. [Google Scholar] [CrossRef] [PubMed]
- Bonjour, J.P.; Carrie, A.L.; Ferrari, S.; Clavien, H.; Slosman, D.; Theintz, G.; Rizzoli, R. Calcium-enriched foods and bone mass growth in prepubertal girls: A randomized, double-blind, placebo-controlled trial. J. Clin. Invest. 1997, 99, 1287–1294. [Google Scholar] [CrossRef] [PubMed]
- Chan, G.M.; Hoffman, K.; McMurry, M. Effects of dairy products on bone and body composition in pubertal girls. J. Pediatr. 1995, 126, 551–556. [Google Scholar] [CrossRef]
- Lee, W.T.; Leung, S.S.; Leung, D.M.; Cheng, J.C. A follow-up study on the effects of calcium-supplement withdrawal and puberty on bone acquisition of children. Am. J. Clin. Nutr. 1996, 64, 71–77. [Google Scholar] [CrossRef] [PubMed]
- French, S.A.; Story, M.; Fulkerson, J.A.; Himes, J.H.; Hannan, P.; Neumark-Sztainer, D.; Ensrud, K. Increasing weight-bearing physical activity and calcium-rich foods to promote bone mass gains among 9–11 years old girls: Outcomes of the cal-girls study. Int. J. Behav. Nutr. Phys. Act. 2005, 2, 8. [Google Scholar] [CrossRef] [PubMed]
- Lloyd, T.; Andon, M.B.; Rollings, N.; Martel, J.K.; Landis, J.R.; Demers, L.M.; Eggli, D.F.; Kieselhorst, K.; Kulin, H.E. Calcium supplementation and bone mineral density in adolescent girls. JAMA 1993, 270, 841–844. [Google Scholar] [CrossRef] [PubMed]
- No Bones about It Package. Available online: https://mdc.Itap.Purdue.Edu/mobile/item.Asp?Itemid=18252 (accessed on 9 March 2016).
- Prochaska, J.O.; DiClemente, C.C.; Norcross, J.C. In search of how people change. Applications to addictive behaviors. Am. Psychol. 1992, 47, 1102–1114. [Google Scholar] [CrossRef] [PubMed]
- Schunk, D.H. Learning Theories: An Educational Perspective, 6th ed.; Allyn & Bacon: Boston, MA, USA, 2012. [Google Scholar]
- Lohmann, T.G.; Roche, A.F.; Martorell, R. Anthropometric Standardization Reference Manual; Human Kinetics Books: Champaign, IL, USA, 1988. [Google Scholar]
- Weaver, C.M.; McCabe, L.D.; McCabe, G.P.; Novotny, R.; Van Loan, M.; Going, S.; Matkovic, V.; Boushey, C.; Savaiano, D.A. Bone mineral and predictors of bone mass in white, hispanic, and asian early pubertal girls. Calcif. Tissue Int. 2007, 81, 352–363. [Google Scholar] [CrossRef] [PubMed]
- Tanner, J.M. Growth at Adolescence, 2nd ed.; Blackwell Scientific: Oxford, UK, 1962. [Google Scholar]
- GE Healthcare. Lunar Prodigy enCORE, version 9.1; GE Medical Instruments: Madison, WI, USA, 2000. [Google Scholar]
- Jensen, J.K.; Gustafson, D.; Boushey, C.J.; Auld, G.; Bock, M.A.; Bruhn, C.M.; Gabel, K.; Misner, S.; Novotny, R.; Peck, L.; et al. Development of a food frequency questionnaire to estimate calcium intake of asian, hispanic, and white youth. J. Am. Diet. Assoc. 2004, 104, 762–769. [Google Scholar] [CrossRef] [PubMed]
- Wong, S.S.; Boushey, C.J.; Novotny, R.; Gustafson, D.R. Evaluation of a computerized food frequency questionnaire to estimate calcium intake of asian, hispanic, and non-hispanic white youth. J. Am. Diet. Assoc. 2008, 108, 539–543. [Google Scholar] [CrossRef] [PubMed]
- IBM SPSS Statistics for Windows [Computer Program], version 21.0; IBM Corp: Armonk, NY, USA, 2012.
- Horner, N.K.; Patterson, R.E.; Neuhouser, M.L.; Lampe, J.W.; Beresford, S.A.; Prentice, R.L. Participant characteristics associated with errors in self-reported energy intake from the women’s health initiative food-frequency questionnaire. Am. J. Clin. Nutr. 2002, 76, 766–773. [Google Scholar] [CrossRef] [PubMed]
- DeBar, L.L.; Ritenbaugh, C.; Aickin, M.; Orwoll, E.; Elliot, D.; Dickerson, J.; Vuckovic, N.; Stevens, V.J.; Moe, E.; Irving, L.M. Youth: A health plan-based lifestyle intervention increases bone mineral density in adolescent girls. Arch. Pediatr. Adolesc. Med. 2006, 160, 1269–1276. [Google Scholar] [CrossRef] [PubMed]
- Golden, N.H.; Abrams, S.A. Optimizing bone health in children and adolescents. Pediatrics 2014, 134, e1229–e1243. [Google Scholar] [CrossRef] [PubMed]
- Novotny, R.; Boushey, C.; Bock, M.A.; Peck, L.; Auld, G.; Bruhn, C.M.; Gustafson, D.; Gabel, K.; Jensen, J.K.; Misner, S.; et al. Calcium intake of asian, hispanic and white youth. J. Am. Coll. Nutr. 2003, 22, 64–70. [Google Scholar] [CrossRef] [PubMed]
- Osborne, D.L.; Weaver, C.M.; McCabe, L.D.; McCabe, G.P.; Novotny, R.; Van Loan, M.D.; Going, S.; Matkovic, V.; Boushey, C.J.; Savaiano, D.A. Body size and pubertal development explain ethnic differences in structural geometry at the femur in Asian, Hispanic, and white early adolescent girls living in the U.S. Bone 2012, 51, 888–895. [Google Scholar] [CrossRef]
- Aaron, D.J.; Kriska, A.M.; Dearwater, S.R.; Cauley, J.A.; Metz, K.F.; LaPorte, R.E. Reproducibility and validity of an epidemiologic questionnaire to assess past year physical activity in adolescents. Am. J. Epidemiol. 1995, 142, 191–201. [Google Scholar] [CrossRef] [PubMed]
- Ainsworth, B.E.; Haskell, W.L.; Whitt, M.C.; Irwin, M.S.; Swartz, A.M.; O’Brien, W.L.; Bassett, D.R., Jr.; Schmitz, K.H.; Emplaincourt, P.O.; Jacobs, D.R.; et al. Compendium of physical activities: An update of activity codes and MET intesities. Med. Sci. Sports Exerc. 2000, 32 (Suppl. 9), S498–S504. [Google Scholar] [CrossRef] [PubMed]
| Baseline | 12 Months | 18 Months | ||||
|---|---|---|---|---|---|---|
| Group a | C | I | C | I | C | I |
| Total Participants (n) | 181 | 292 | 176 | 290 | 164 | 274 |
| Race/Ethnic Group (n) | ||||||
| Asian (%) | 45 (25) | 105 (36) | 43 (24) | 104 (36) | 43 (26) | 104 (38) |
| Hispanic (%) | 81 (45) | 99 (34) | 78 (44) | 98 (34) | 68 (41) | 86 (31) |
| Non-Hispanic White (%) | 55 (30) | 88 (30) | 55 (32) | 88 (30) | 53 (33) | 84 (31) |
| Location (n) | ||||||
| Arizona | 51 | 71 | 49 | 70 | 39 | 56 |
| California | 39 | 81 | 38 | 80 | 38 | 78 |
| Hawaii | 30 | 81 | 29 | 81 | 29 | 81 |
| Indiana | 50 | 50 | 49 | 50 | 47 | 50 |
| Nevada | 11 | 9 | 11 | 9 | 11 | 9 |
| Physical Development b | ||||||
| Tanner Score | 2.43 ± 0.97 | 2.41 ± 0.82 | 3.13 ± 0.97 | 3.09 ± 0.97 | 3.32 ± 0.91 | 3.30 ± 0.88 |
| Tanner Upper | 2.25 ± 1.01 | 2.25 ± 0.87 | 2.89 ± 1.07 | 2.88 ± 1.09 | 3.07 ± 1.03 | 3.06 ± 0.98 |
| Tanner Lower | 2.61 ± 1.12 | 2.58 ± 1.01 | 3.37 ± 1.08 | 3.33 ± 1.04 | 3.58 ± 0.94 | 3.55 ± 1.05 |
| Age b (years) | 11.7 ± 0.44 | 11.7 ± 0.46 | 12.8 ± 0.44 | 12.7 ± 0.46 | 13.3 ± 0.45 | 13.2 ± 0.47 |
| BMI b (kg/m2) | 20.6 ± 4.56 | 20.5 ± 4.70 | 21.5 ± 4.79 | 21.4 ± 5.08 | 21.9 ± 4.77 | 21.9 ± 5.18 |
| Complete PMI c questionnaire | 179 | 287 | - | - | - | - |
| Positive for PMI c | 22 | 42 | 21 | 42 | 20 | 39 |
| Completed BHT c | 181 | 292 | - | - | - | - |
| Lactose maldigester d (%) | 61 (34) | 98 (34) | 59 (34) | 98 (34) | 58 (35) | 93 (34) |
| Completed FFQ c | 181 | 290 | 174 | 282 | 163 | 273 |
| FFQ excluding outliers e | 160 | 251 | 157 | 250 | 153 | 253 |
| Control | Intervention | Group Differences b, p | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Baseline | Δ0–12 Months | Δ12–18 Months | Δ0–18 Months | Baseline | Δ0–12 Months | Δ12–18 Months | Δ0–18 Months | Δ0–12 Months | Δ12–18 Months | Δ0–18 Months | |
| Dietary calcium intake c, mg | |||||||||||
| Total dietary calcium | 1123 ± 56 | −92 ± 64 | −40 ± 64 | −76 ± 70 | 1052 ± 566 | −39 ± 51 | −89 ± 54 | −188 ± 54 | 0.42 | 0.47 | 0.17 |
| Total dairy calcium | 947 ± 489 | −85 ± 57 | −32 ± 59 | −72 ± 62 | 895 ± 521 | −39 ± 45 | −75 ± 49 | −176 ± 48 | 0.45 | 0.49 | 0.16 |
| Exclusively dairy calcium | 650 ± 390 | −87 ± 48 | −20 ± 51 | −59 ± 55 | 632 ± 416 | −51 ± 38 | −22 ± 43 | −131 ± 42 | 0.51 | 0.97 | 0.28 |
| Bone mineral content d, g | |||||||||||
| Total body | 1595 ± 372 | 293 ± 19 | 108 ± 14 | 388 ± 21 | 1585 ± 397 | 275 ± 15 | 107 ± 12 | 390 ± 17 | 0.45 | 0.92 | 0.93 |
| Total hip | 24 ± 5 | 2.8 ± 0.2 | 0.9 ± 0.1 | 3.8 ± 0.2 | 23 ± 5 | 3.1 ± 0.2 | 0.9 ± 0.1 | 4.2 ± 0.2 | 0.20 | 0.51 | 0.08 |
| Spine (L2–L4) | 28 ± 7 | 6.4 ± 0.4 | 2.6 ± 0.2 | 9.0 ± 0.4 | 28 ± 8 | 6.4 ± 0.3 | 2.6 ± 0.2 | 9.3 ± 0.3 | 0.92 | 0.95 | 0.58 |
| Femoral neck | 4 ± 0.7 | 0.47 ± 0.03 | 0.20 ± 0.02 | 0.66 ± 0.04 | 4 ± 0.7 | 0.46 ± 0.02 | 0.17 ± 0.02 | 0.66 ± 0.03 | 0.91 | 0.29 | 0.99 |
| Lactose Maldigesters | Lactose Digesters | Group Differences b, p | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Baseline | Δ0–12 Months | Δ12–18 Months | Δ0–18 Months | Baseline | Δ0–12 Months | Δ12–18 Months | Δ0–18 Months | Δ0–12 Months | Δ12–18 Months | Δ0–18 Months | |
| Dietary calcium intake c, mg | |||||||||||
| Total dietary calcium | 1126 ± 580 | −53 ± 74 | −165 ± 77 | −209 ± 77 | 1014 ± 557 | 12 ± 59 | −75 ± 61 | −151 ± 61 | 0.39 | 0.27 | 0.46 |
| Total dairy calcium | 957 ± 530 | −55 ± 66 | −149 ± 71 | −204 ± 68 | 864 ± 516 | −5 ± 52 | −57 ± 56 | −148 ± 54 | 0.46 | 0.29 | 0.42 |
| Exclusively dairy calcium | 653 ± 399 | −59 ± 55 | −84 ± 64 | −156 ± 61 | 622 ± 425 | −41 ± 43 | 12 ± 51 | −110 ± 48 | 0.74 | 0.16 | 0.45 |
| Bone mineral content d, g | |||||||||||
| Total body | 1535 ± 392 | 273 ± 22 | 108 ± 15 | 393 ± 24 | 1610 ± 398 | 279 ± 17 | 115 ± 12 | 404 ± 20 | 0.76 | 0.63 | 0.65 |
| Total hip | 22 ± 5 | 2.9 ± 0.2 | 0.9 ± 0.1 | 4.2 ± 0.2 | 23 ± 5 | 3.3 ± 0.2 | 1.0 ± 0.1 | 4.4 ± 0.2 | 0.09 | 0.51 | 0.33 |
| Spine (L2–L4) | 27 ± 8 | 6.0 ± 0.4 | 2.3 ± 0.2 | 8.7 ± 0.5 | 28 ± 8 | 6.9 ± 0.3 | 3.0 ± 0.2 | 9.9 ± 0.4 | 0.03 * | <0.01 * | <0.01 * |
| Femoral neck | 3 ± 1 | 0.43 ± 0.04 | 0.16 ± 0.02 | 0.64 ± 0.04 | 4 ± 0.7 | 0.49 ± 0.03 | 0.19 ± 0.02 | 0.70 ± 0.03 | 0.09 | 0.16 | 0.14 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, Y.; Savaiano, D.A.; McCabe, G.P.; Pottenger, F.M.; Welshimer, K.; Weaver, C.M.; McCabe, L.D.; Novotny, R.; Read, M.; Going, S.; et al. Behavioral Intervention in Adolescents Improves Bone Mass, Yet Lactose Maldigestion Is a Barrier. Nutrients 2018, 10, 421. https://doi.org/10.3390/nu10040421
Lee Y, Savaiano DA, McCabe GP, Pottenger FM, Welshimer K, Weaver CM, McCabe LD, Novotny R, Read M, Going S, et al. Behavioral Intervention in Adolescents Improves Bone Mass, Yet Lactose Maldigestion Is a Barrier. Nutrients. 2018; 10(4):421. https://doi.org/10.3390/nu10040421
Chicago/Turabian StyleLee, Yujin, Dennis A. Savaiano, George P. McCabe, Francis M. Pottenger, Kathleen Welshimer, Connie M. Weaver, Linda D. McCabe, Rachel Novotny, Marsha Read, Scott Going, and et al. 2018. "Behavioral Intervention in Adolescents Improves Bone Mass, Yet Lactose Maldigestion Is a Barrier" Nutrients 10, no. 4: 421. https://doi.org/10.3390/nu10040421
APA StyleLee, Y., Savaiano, D. A., McCabe, G. P., Pottenger, F. M., Welshimer, K., Weaver, C. M., McCabe, L. D., Novotny, R., Read, M., Going, S., Mason, A., Van Loan, M., & Boushey, C. J. (2018). Behavioral Intervention in Adolescents Improves Bone Mass, Yet Lactose Maldigestion Is a Barrier. Nutrients, 10(4), 421. https://doi.org/10.3390/nu10040421







