Polyphenolic Extract from Tarocco (Citrus sinensis L. Osbeck) Clone “Lempso” Exerts Anti-Inflammatory and Antioxidant Effects via NF-kB and Nrf-2 Activation in Murine Macrophages
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Collection and Characterization
2.2. On-Line HPLC-DPPH Conditions
2.3. Cells Cultures
2.3.1. J774A.1 Macrophage Cell Line
2.3.2. Cell Viability Assay
2.3.3. Nitrite Determination and Cytofluorimetry Evaluation of iNOS, COX-2, HO-1, and NQO1 Expression in J774A.1 Macrophages
2.3.4. TNF-α and IL-6 Determination in Macrophages J774A.1
2.3.5. Immunofluorescence Analysis with Confocal Microscopy in Macrophages J774A.1
2.3.6. Measurement of Intracellular ROS
2.4. Data Analysis
3. Results
3.1. Identification of Main Polyphenols in Lempso Extract and Fast Antioxidant Activity Screening
3.2. Evaluation of the Anti-Inflammatory and Antioxidant Potential of LFE in J774A.1 Murine Macrophages
3.2.1. Cells Viability
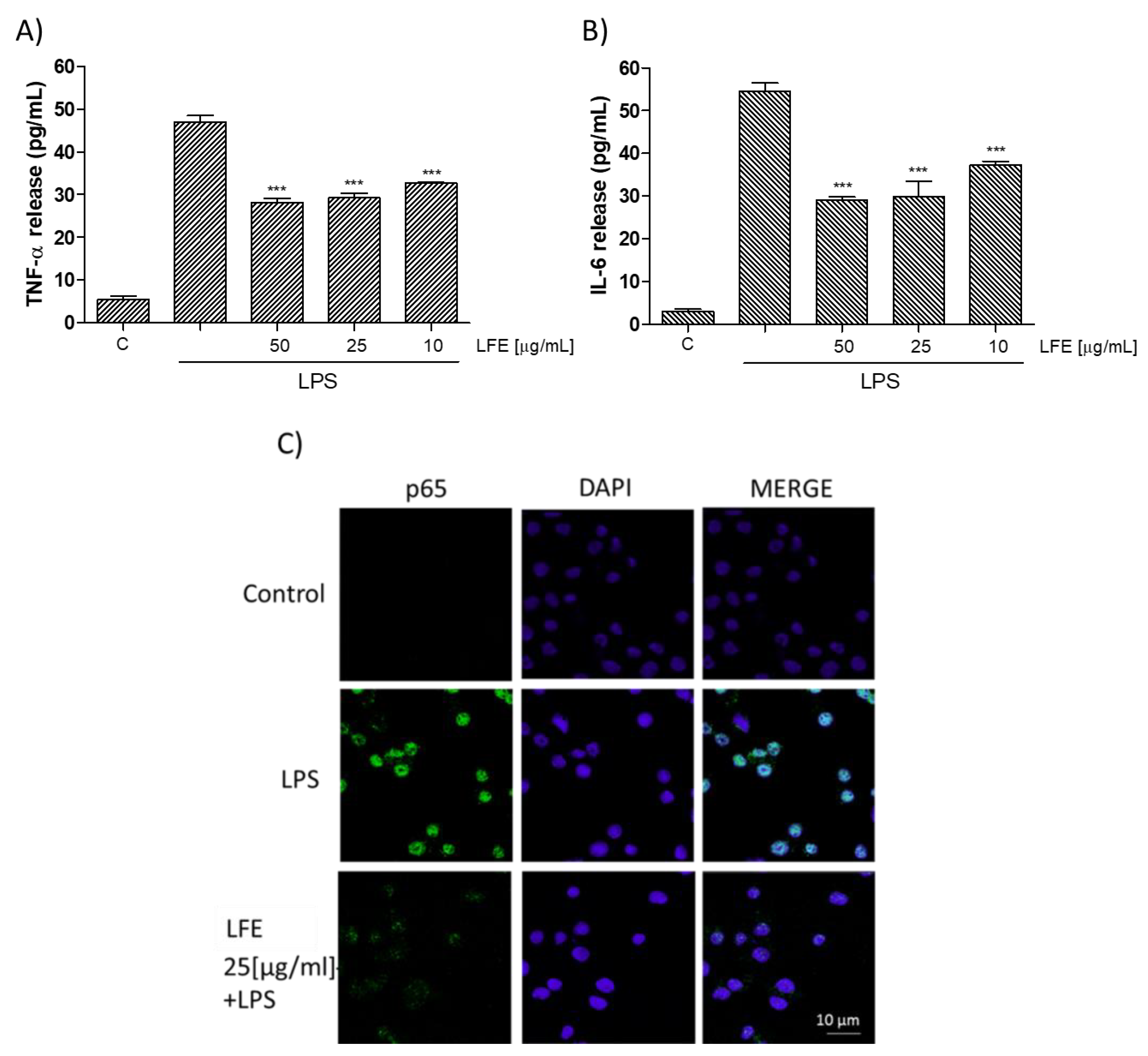
3.2.2. Lempso Extract Inhibited LPS-Induced NO, iNOS, COX-2, TNF-α, and IL-6 Production
3.2.3. Lempso Extract Inhibits p65 NF-kB Nuclear Translocation
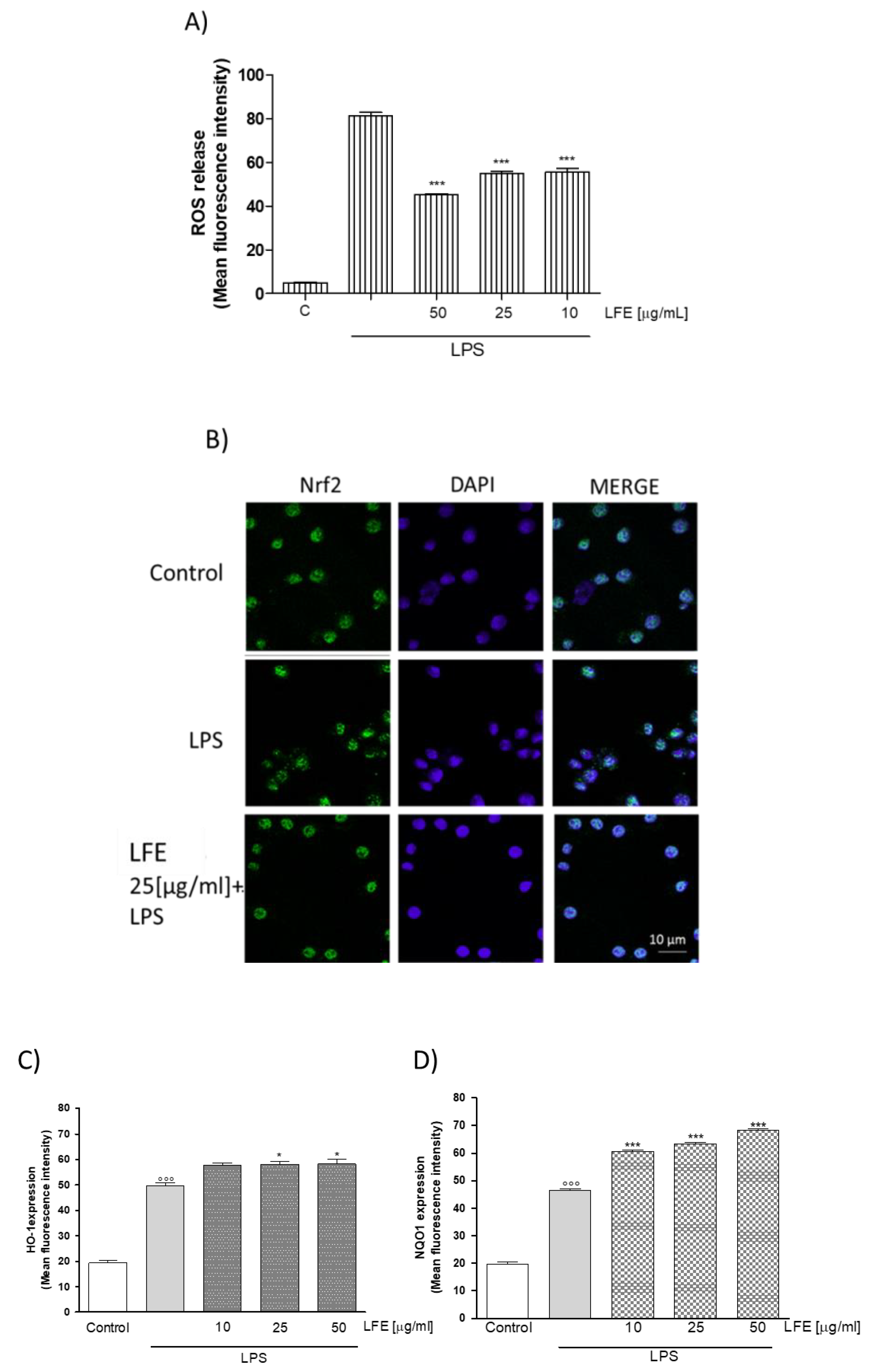
3.2.4. LFE Antioxidant Properties
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Rice Evans, C.A.; Miller, N.J.; Paganga, G. Structure-antioxidant activity relationships of flavonoids and phenolic acids. Free Radic. Biol. Med. 1996, 20, 933–956. [Google Scholar] [CrossRef]
- Favela-Hernández, J.M.; González-Santiago, O.; Ramírez-Cabrera, M.A.; Esquivel-Ferriño, P.C.; Camacho-Corona, M.R. Chemistry and Pharmacology of Citrus sinensis. Molecules 2016, 21, 247. [Google Scholar] [CrossRef] [PubMed]
- Di Donna, L.; Iacopetta, D.; Cappello, A.R.; Galluccia, G.; Martello, E.; Fiorillo, M.; Dolce, V.; Sindona, G. Hypocholesterolaemic activity of 3-hydroxy-3-methyl-glutaryl flavanones enriched fraction from bergamot fruit (Citrus bergamia): ‘‘In vivo’’ studies. J. Funct. Foods 2014, 7. [Google Scholar] [CrossRef]
- Raciti, G.A.; Fiory, F.; Campitelli, M.; Desiderio, A.; Spinelli, R.; Longo, M.; Nigro, C.; Pepe, G.; Sommella, E.; Campiglia, P.; et al. Citrus aurantium L. dry extracts promote C/ebpβ expression and improve adipocyte differentiation in 3T3-L1 cells. PLoS ONE 2018, 13, e0193704. [Google Scholar] [CrossRef] [PubMed]
- Sommella, E.; Pepe, G.; Pagano, F.; Tenore, G.C.; Dugo, P.; Manfra, M.; Campiglia, P. Ultra high performance liquid chromatography with ion-trap TOF-MS for the fast characterization of flavonoids in Citrus bergamia juice. J. Sep. Sci. 2013, 36, 3351–3355. [Google Scholar] [CrossRef]
- Sommella, E.; Pepe, G.; Pagano, F.; Tenore, G.C.; Marzocco, S.; Manfra, M.; Calabrese, G.; Aquino, R.P.; Campiglia, P. UHPLC profiling and effects on LPS-stimulated J774A.1 macrophages of flavonoids from bergamot (Citrus bergamia) juice, an underestimated waste product with high anti-inflammatory potential. J. Funct. Foods 2014, 7, 641–649. [Google Scholar] [CrossRef]
- Pepe, G.; Pagano, F.; Adesso, S.; Sommella, E.; Ostacolo, C.; Manfra, M.; Chieppa, M.; Sala, M.; Russo, M.; Marzocco, S.; et al. Bioavailable Citrus sinensis extract: Polyphenolic composition and biological activity. Molecules 2017, 22, 623. [Google Scholar] [CrossRef] [PubMed]
- Adesso, S.; Pepe, G.; Sommella, E.; Manfra, M.; Scopa, A.; Sofo, A.; Tenore, G.C.; Russo, M.; Di Gaudio, F.; Autore, G.; et al. Anti-inflammatory and antioxidant activity of polyphenolic extracts from Lactuca sativa (var. Maravilla de Verano) under different farming methods. J. Sci. Food Agric. 2016, 96, 4194–4206. [Google Scholar] [CrossRef] [PubMed]
- Sofo, A.; Lundegårdh, B.; Mårtensson, A.; Manfra, M.; Pepe, G.; Sommella, E.; De Nisco, M.; Tenore, G.C.; Campiglia, P.; Scopa, A. Different agronomic and fertilization systems affect polyphenolic profile, antioxidant capacity and mineral composition of lettuce. Sci. Hort. 2016, 204, 106–115. [Google Scholar] [CrossRef]
- Sansone, F.; Mencherini, T.; Picerno, P.; Esposito, T.; Del Gaudio, P.; Russo, P.; Pepe, G.; Lauro, M.R.; Aquino, R.P. Microencapsulation by spray drying of Lannea microcarpa extract: Technological characteristics and antioxidant activity. J. Pharm. Pharmacogn. Res. 2014, 2, 100–109. [Google Scholar]
- Colotta, F.; Allavena, P.; Sica, A.; Garlanda, C.; Mantovani, A. Cancer-related inflammation, the seventh hallmark of cancer: links to genetic instability. Carcinogenesis 2009, 30, 1073–1081. [Google Scholar] [CrossRef] [PubMed]
- Biswas, S.K. Does the Interdependence between Oxidative Stress and Inflammation Explain the Antioxidant Paradox? Oxid. Med. Cell. Longev. 2016, 2016. [Google Scholar] [CrossRef]
- Popolo, A.; Autore, G.; Pinto, A.; Marzocco, S. Oxidative stress in patients with cardiovascular disease and chronic renal failure. Free Radic. Res. 2013, 47, 346–356. [Google Scholar] [CrossRef] [PubMed]
- Sommella, E.; Pagano, F.; Pepe, G.; Ostacolo, C.; Manfra, M.; Chieppa, M.; Di Sanzo, R.; Carabetta, S.; Campiglia, P.; Russo, M. Flavonoid Composition of Tarocco (Citrus sinensis L. Osbeck) Clone “Lempso” and Fast Antioxidant Activity Screening by DPPH-UHPLC-PDA-IT-TOF. Phytochem. Anal. 2017, 28, 521–528. [Google Scholar] [CrossRef] [PubMed]
- Marzocco, S.; Adesso, S.; Alilou, M.; Stuppner, H.; Schwaiger, S. Anti-Inflammatory and anti-oxidant potential of the root extract and constituents of Doronicum austriacum. Molecules 2017, 22. [Google Scholar] [CrossRef]
- Abdallah, M.; Marzocco, S.; Adesso, S.; Zarrouk, M.; Guerfel, M. Olive oil polyphenols extracts inhibit inflammatory markers in J774A.1 murine macrophages and scavenge free radicals. Acta Histochem. 2018, 120, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Adesso, S.; Paterniti, I.; Cuzzocrea, S.; Fujioka, M.; Autore, G.; Magnus, T.; Pinto, A.; Marzocco, S. AST-120 Reduces Neuroinflammation Induced by Indoxyl Sulfate in Glial Cells. J. Clin. Med. 2018, 7, 365. [Google Scholar] [CrossRef]
- Pepe, G.; Sommella, E.; Ventre, G.; Scala, M.C.; Adesso, S.; Ostacolo, C.; Marzocco, S.; Novellino, E.; Campiglia, P. Antioxidant peptides released from gastrointestinal digestion of “Stracchino” soft cheese: Characterization, in vitro intestinal protection and bioavailability. J. Funct. Foods 2016, 26, 494–505. [Google Scholar] [CrossRef]
- Adesso, S.; Magnus, T.; Cuzzocrea, S.; Campolo, M.; Rissiek, B.; Paciello, O.; Autore, G.; Pinto, A.; Marzocco, S. Indoxyl sulfate affects glial function increasing oxidative stress and neuroinflammation in chronic kidney disease: interaction between astrocytes and microglia. Front. Pharmacol. 2017, 8, 370. [Google Scholar] [CrossRef]
- Del Regno, M.; Adesso, S.; Popolo, A.; Quaroni, A.; Autore, G.; Severino, L.; Marzocco, S. Nivalenol induces oxidative stress and increases deoxynivalenol pro-oxidant effect in intestinal epithelial cells. Toxicol. Appl. Pharmacol. 2015, 285, 118–127. [Google Scholar] [CrossRef]
- Adesso, S.; Autore, G.; Quaroni, A.; Popolo, A.; Severino, L.; Marzocco, S. The Food Contaminants Nivalenol and Deoxynivalenol Induce Inflammation in Intestinal Epithelial Cells by Regulating Reactive Oxygen Species Release. Nutrients 2017, 9, 1343. [Google Scholar] [CrossRef] [PubMed]
- Sommella, E.; Ismail, O.H.; Pagano, F.; Pepe, G.; Ostacolo, C.; Mazzoccanti, G.; Russo, M.; Novellino, E.; Gasparrini, F.; Campiglia, P. Development of an improved online comprehensive hydrophilic interaction chromatography × reversed-phase ultra-high-pressure liquid chromatography platform for complex multiclass polyphenolic sample analysis. J. Sep. Sci. 2017, 40, 2188–2197. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.V.; Bell, D.W.; Settleman, J.; Haber, D.A. Epidermal growth factor receptor mutations in lung cancer. Nat. Rev. Cancer 2007, 7, 169–181. [Google Scholar] [CrossRef] [PubMed]
- Sautebin, L.; Ialenti, A.; Ianaro, A.; Di Rosa, M. Modulation by nitric oxide of prostaglandin biosynthesis in the rat. Br. J. Pharmacol. 1995, 114, 323–328. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, N.; Chen, L.C.; Gordon, M.A.; Laskin, J.D.; Laskin, D.L. Regulation of cyclooxygenase-2 by nitric oxide in activated hepatic macrophages during acute endotoxemia. J. Leuk. Biol. 2002, 71, 1005–1011. [Google Scholar]
- Duque, A.G.; Descoteaux, A. Macrophage cytokines: involvement in immunity and infectious diseases. Front. Immunol. 2014, 5, 491. [Google Scholar] [CrossRef]
- Hayden, M.S.; Ghosh, S. Shared principles in NF-κB signaling. Cell 2008, 132, 344–362. [Google Scholar] [CrossRef]
- Tak, P.; Firestein, G.S. NF-kB: A key role in inflammatory diseases. J. Clin. Investig. 2001, 107, 7–11. [Google Scholar] [CrossRef]
- Gattuso, G.; Barreca, D.; Gargiulli, C.; Leuzzi, U.; Caristi, C. Flavonoid composition of Citrus juices. Molecules 2007, 12, 1641. [Google Scholar] [CrossRef]
- Peterson, J.J.; Dwyer, J.T.; Beecher, G.R.; Bhagwat, S.A.; Gebhardt, S.E.; Haytowitz, D.B.; Holden, J.M. Flavanones in oranges, tangerines (mandarins), tangors, and tangelos: A compilation and review of the data from the analytical literature. J. Food Comp. Anal. 2006, 19, S66–S73. [Google Scholar] [CrossRef]
- Barreca, D.; Gattuso, G.; Laganà, G.; Leuzzi, U.; Bellocco, E. C- and O-glycosyl flavonoids in Sanguinello and Tarocco blood orange (Citrus sinensis (L.) Osbeck) juice: Identification and influence on antioxidant properties and acetylcholinesterase activity. Food Chem. 2016, 196, 619–627. [Google Scholar] [CrossRef]
- Parhiz, H.; Roohbakhsh, A.; Soltani, F.; Rezaee, R.; Iranshahi, M. Antioxidant and anti-inflammatory properties of the citrus flavonoids hesperidin and hesperetin: an updated review of their molecular mechanisms and experimental models. Phytother. Res. 2015, 29, 323–331. [Google Scholar] [CrossRef]
- Khan, M.K.; Huma, Z.E.; Dangles, O. A comprehensive review on flavanones, the major citrus polyphenols. J. Food Comp. Anal. 2014, 33, 85–104. [Google Scholar] [CrossRef]
- Ha, S.K.; Park, H.Y.; Eom, H.; Kim, Y.; Choi, I. Narirutin fraction from citrus peels attenuates LPS-stimulated inflammatory response through inhibition of NF-jB and MAPKs activation. Food Chem. Toxicol. 2012, 50, 3498–3504. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.G.; Kim, B.; Yang, Y.; Pham, T.X.; Park, Y.K.; Manatou, J.; Koo, S.I.; Chun, O.K.; Lee, J.Y. Berry anthocyanins suppress the expression and secretion of proinflammatory mediators in macrophages by inhibiting nuclear translocation of NF-κB independent of NRF2-mediated mechanism. J. Nutr. Biochem. 2014, 25, 404–411. [Google Scholar] [CrossRef]
- Wang, Q.; Xia, M.; Liu, C.; Guo, H.; Ye, Q.; Hu, Y.; Zhang, Y.; Hou, M.; Zhu, H.; Ma, J.; et al. Cyanidin-3-O-β-glucoside inhibits iNOS and COX-2 expression by inducing liver X receptor alpha activation in THP-1 macrophages. Life Sci. 2008, 83, 176–184. [Google Scholar] [CrossRef] [PubMed]
- Sommella, E.; Pepe, G.; Pagano, F.; Conte, G.; Carimi, F.; Tenore, G.C.; Novellino, E.; Manfra, M.; Russo, M.T.; Campiglia, P. Rapid Screening of Antioxidant Anthocyanins in Autochthonous Nero d’Avola Grape Clones by Pre-column DPPH Reaction Coupled to UHPLC-UV/Vis-IT-TOF: A Strategy to Combine Chemical data and Genetic Diversity. Food Anal. Methods 2016, 9, 2780–2790. [Google Scholar] [CrossRef]
- Speciale, A.; Anwar, S.; Canali, R.; Chirafisi, J.; Saija, A.; Virgili, F.; Cimino, F. Cyanidin-3-O-glucoside counters the response to TNF-alpha of endothelial cells by activating Nrf2 pathway. Mol. Nutr. Food Res. 2013, 57, 1979–1987. [Google Scholar] [CrossRef]
Sample Availability: Samples of the Lempso extract are available from the authors. |


© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pepe, G.; Sommella, E.; Cianciarulo, D.; Ostacolo, C.; Manfra, M.; Di Sarno, V.; Musella, S.; Russo, M.; Messore, A.; Parrino, B.; et al. Polyphenolic Extract from Tarocco (Citrus sinensis L. Osbeck) Clone “Lempso” Exerts Anti-Inflammatory and Antioxidant Effects via NF-kB and Nrf-2 Activation in Murine Macrophages. Nutrients 2018, 10, 1961. https://doi.org/10.3390/nu10121961
Pepe G, Sommella E, Cianciarulo D, Ostacolo C, Manfra M, Di Sarno V, Musella S, Russo M, Messore A, Parrino B, et al. Polyphenolic Extract from Tarocco (Citrus sinensis L. Osbeck) Clone “Lempso” Exerts Anti-Inflammatory and Antioxidant Effects via NF-kB and Nrf-2 Activation in Murine Macrophages. Nutrients. 2018; 10(12):1961. https://doi.org/10.3390/nu10121961
Chicago/Turabian StylePepe, Giacomo, Eduardo Sommella, Donato Cianciarulo, Carmine Ostacolo, Michele Manfra, Veronica Di Sarno, Simona Musella, Mariateresa Russo, Antonella Messore, Barbara Parrino, and et al. 2018. "Polyphenolic Extract from Tarocco (Citrus sinensis L. Osbeck) Clone “Lempso” Exerts Anti-Inflammatory and Antioxidant Effects via NF-kB and Nrf-2 Activation in Murine Macrophages" Nutrients 10, no. 12: 1961. https://doi.org/10.3390/nu10121961
APA StylePepe, G., Sommella, E., Cianciarulo, D., Ostacolo, C., Manfra, M., Di Sarno, V., Musella, S., Russo, M., Messore, A., Parrino, B., Bertamino, A., Autore, G., Marzocco, S., & Campiglia, P. (2018). Polyphenolic Extract from Tarocco (Citrus sinensis L. Osbeck) Clone “Lempso” Exerts Anti-Inflammatory and Antioxidant Effects via NF-kB and Nrf-2 Activation in Murine Macrophages. Nutrients, 10(12), 1961. https://doi.org/10.3390/nu10121961












