Managing Risk of Non-Communicable Diseases in Women with Bulimia Nervosa or Binge Eating Disorders: A Randomized Trial with 12 Months Follow-Up
Abstract
1. Introduction
2. Materials and Methods
2.1. Subjects
2.2. Treatments
2.3. Outcomes and Procedures
2.3.1. Body Composition
2.3.2. Cardio Respiratory Fitness (CRF)
2.3.3. Maximal Muscle Strength
2.3.4. NCD Risk Profile
2.4. Ethics
2.5. Statistics
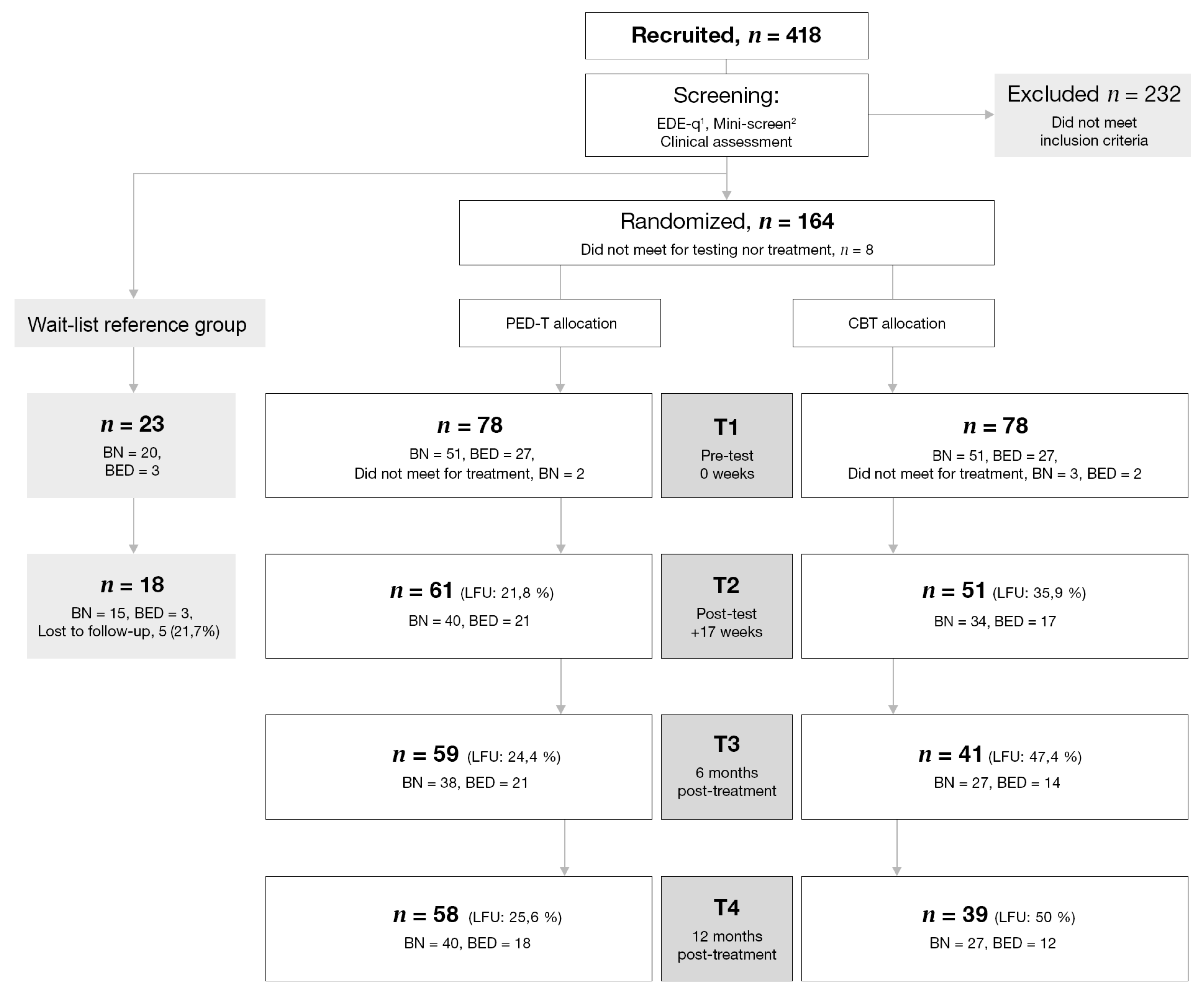
3. Results
3.1. Attrition Analysis
3.2. Demographics
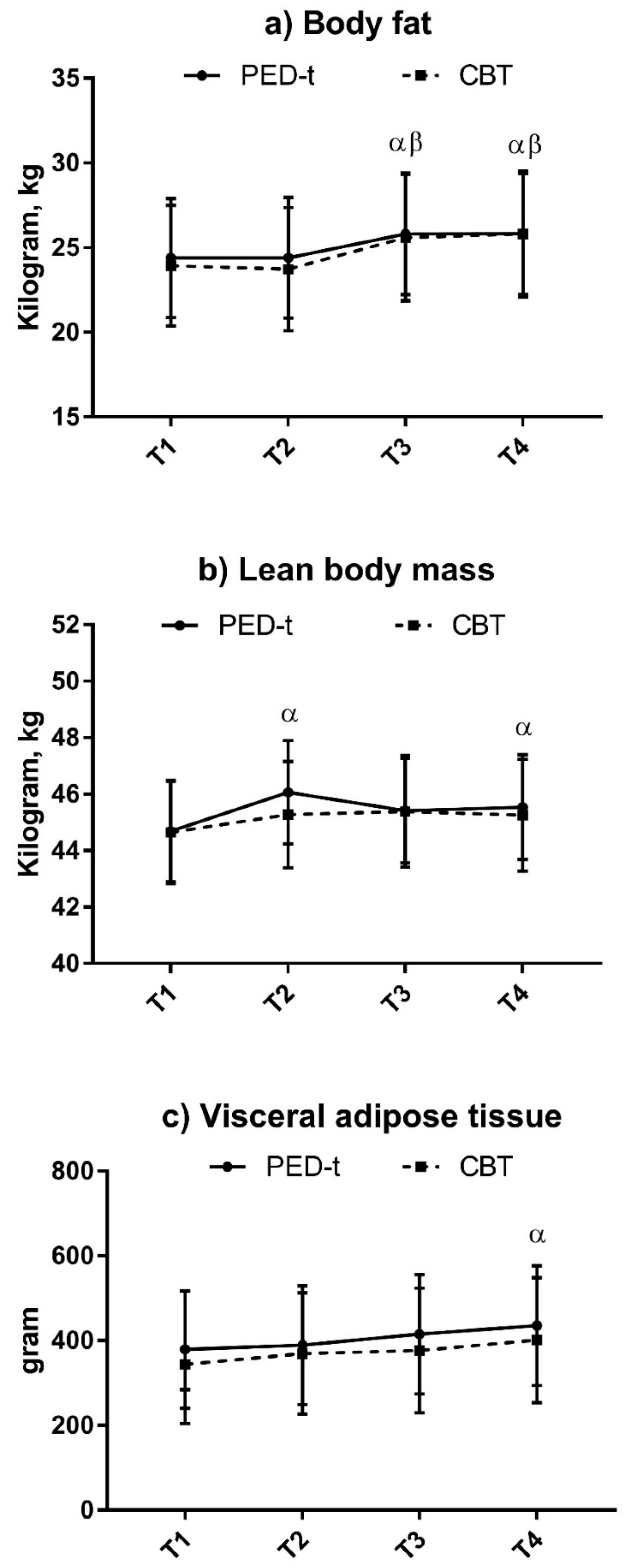
3.3. Body Composition
3.4. Physical Fitness
3.4.1. 1RM
3.4.2. CRF
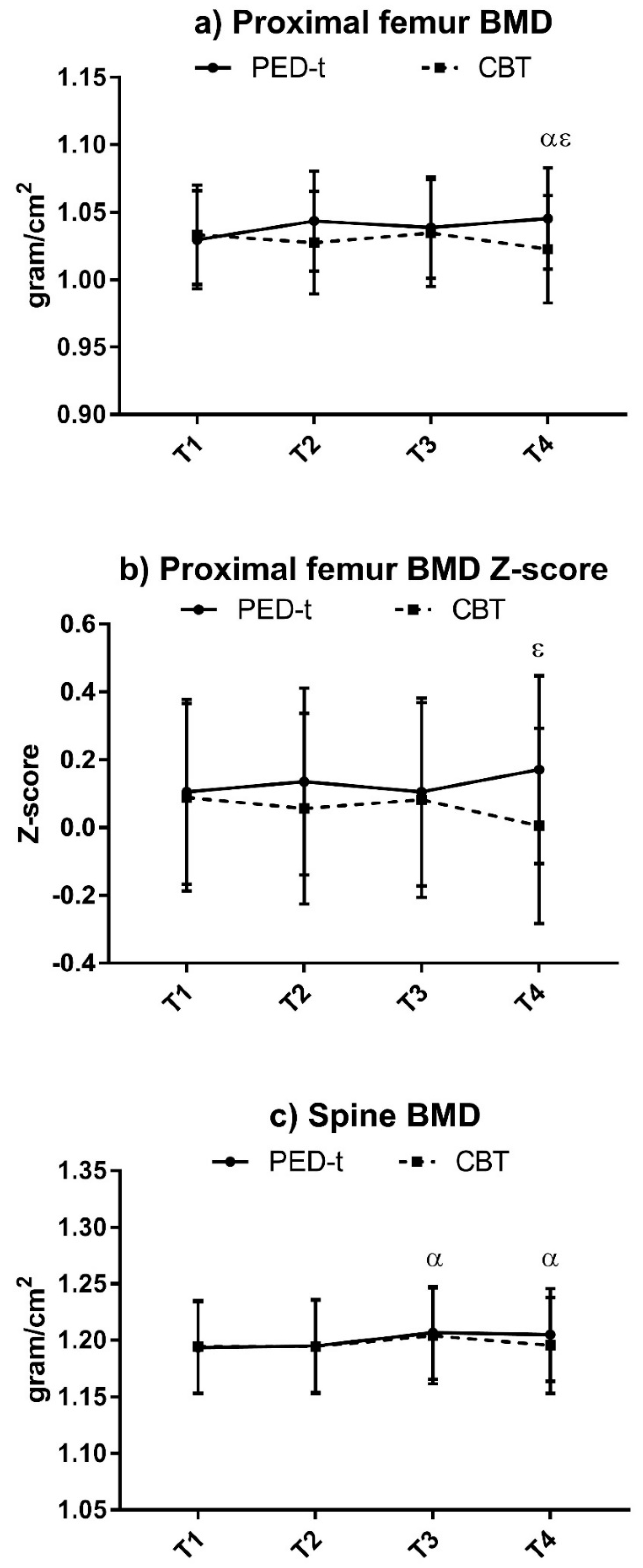
3.5. Risk of NCDs
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kaminsky, L.A.; Arena, R.; Myers, J. Reference Standards for Cardiorespiratory Fitness Measured with Cardiopulmonary Exercise Testing: Data From the Fitness Registry and the Importance of Exercise National Database. Mayo. Clin. Proc. 2015, 90, 1515–1523. [Google Scholar] [CrossRef] [PubMed]
- Mathisen, T.F.; Rosenvinge, J.H.; Friborg, O.; Pettersen, G.; Stensrud, T.; Hansen, B.H.; Underhaug, K.E.; Teinung, E.; Vrabel, K.; Svendsen, M.; et al. Body composition and physical fitness in women with bulimia nervosa or binge-eating disorder. Int. J. Eat. Disord. 2018, 51, 331–342. [Google Scholar] [CrossRef] [PubMed]
- Myers, J.; McAuley, P.; Lavie, C.J.; Despres, J.P.; Arena, R.; Kokkinos, P. Physical activity and cardiorespiratory fitness as major markers of cardiovascular risk: Their independent and interwoven importance to health status. Prog. Cardiovasc. Dis. 2015, 57, 306–314. [Google Scholar] [CrossRef] [PubMed]
- Raevuori, A.; Suokas, J.; Haukka, J.; Gissler, M.; Linna, M.; Grainger, M.; Suvisaari, J. Highly increased risk of type 2 diabetes in patients with binge eating disorder and bulimia nervosa. Int. J. Eat. Disord. 2015, 48, 555–562. [Google Scholar] [CrossRef] [PubMed]
- Hudson, J.I.; Lalonde, J.K.; Coit, C.E.; Tsuang, M.T.; McElroy, S.L.; Crow, S.J. Longitudinal study of the diagnosis of components of the metabolic syndrome in individuals with binge-eating disorder. Am. J. Clin. Nutr. 2010, 91, 1568–1573. [Google Scholar] [CrossRef] [PubMed]
- Nieto-Martinez, R.; González-Rivas, J.P.; Medina-Inojosa, J.R.; Florez, H. Are Eating Disorders Risk Factors for Type 2 Diabetes? A Systematic Review and Meta-analysis. Curr. Diab. Rep. 2017, 17, 138. [Google Scholar] [CrossRef] [PubMed]
- Villarejo, C.; Fernández-Aranda, F.; Jiménez-Murcia, S.; Peñas-Lledó, E.; Granero, R.; Penelo, E. Lifetime obesity in patients with eating disorders: Increasing prevalence, clinical and personality correlates. Eur. Eat. Disord. Rev. 2012, 20, 250–254. [Google Scholar] [CrossRef] [PubMed]
- Nakai, Y.; Noma, S.I.; Fukusima, M.; Taniguchi, A.; Teramukai, S. Serum Lipid Levels in Patients with Eating Disorders. Intern. Med. 2016, 55, 1853–1857. [Google Scholar] [CrossRef]
- Olguin, P.; Fuentes, M.; Gabler, G.; Guerdjikova, A.I.; Keck, P.E.; McElroy, S.L. Medical comorbidity of binge eating disorder. Eat. Weight Disord. Stud. Anorex. Bulim. Obes. 2017, 22, 13–26. [Google Scholar] [CrossRef]
- Pedersen, B.K.; Saltin, B. Exercise as medicine—Evidence for prescribing exercise as therapy in 26 different chronic diseases. Scand. J. Med. Sci. Sports 2015, 25, 1–72. [Google Scholar] [CrossRef]
- da Luz, F.Q.; Hay, P.; Touyz, S.; Sainsbury, A. Obesity with Comorbid Eating Disorders: Associated Health Risks and Treatment Approaches. Nutrients 2018, 10, 829. [Google Scholar] [CrossRef] [PubMed]
- Hilbert, A.; Hoek, H.W.; Schmidt, R. Evidence-based clinical guidelines for eating disorders: International comparison. Curr. Opin. Psychiatry 2017, 30, 423–437. [Google Scholar] [CrossRef] [PubMed]
- Norwegian Directorate of Health. National Guidlines for Early Detection, Progress and Treatment of Eating Disorders; Dahle, K.A., Ed.; Norwegian Directorate of Health: Oslo, Norway, 2016. [Google Scholar]
- Davidson, L.E.; Hunt, S.C.; Adams, T.D. Fitness versus adiposity in cardiovascular disease risk. Eur. J. Clin. Nutr. 2018. [CrossRef]
- Linardon, J.; Fairburn, C.G.; Fitzsimmons-Craft, E.E.; Wilfley, D.E.; Brennan, L. The empirical status of the third-wave behaviour therapies for the treatment of eating disorders: A systematic review. Clin. Psychol. Rev. 2017, 58, 125–140. [Google Scholar] [CrossRef] [PubMed]
- de Jong, M.; Schoorl, M.; Hoek, H.W. Enhanced cognitive behavioural therapy for patients with eating disorders: A systematic review. Curr. Opin. Psychiatry 2018, 31, 436–444. [Google Scholar] [CrossRef] [PubMed]
- Beck, A.T. Cognitive Therapy: Nature and Relation to Behavior Therapy—Republished Article. Behav. Ther. 2016, 47, 776–784. [Google Scholar] [CrossRef] [PubMed]
- Denison, E.; Underland, V.; Mosdøl, A.; Vist, G.E. NIPH Systematic Reviews. In Cognitive Therapies for Increasing Physical Activity; The Norwegian Institute of Public Health (NIPH): Oslo, Norway, 2016. [Google Scholar]
- Bratland-Sanda, S.; Martinsen, E.W.; Sundgot-Borgen, J. Changes in physical fitness, bone mineral density and body composition during inpatient treatment of underweight and normal weight females with longstanding eating disorders. Int. J. Environ. Res. Public Health 2012, 9, 315–330. [Google Scholar] [CrossRef]
- Castelnuovo, G.; Pietrabissa, G.; Manzoni, G.M.; Cattivelli, R.; Rossi, A.; Novelli, M. Cognitive behavioral therapy to aid weight loss in obese patients: Current perspectives. Psychol. Res. Behav. Manag. 2017, 10, 165–173. [Google Scholar] [CrossRef] [PubMed]
- Vancampfort, D.; Vanderlinden, J.; De Hert, M.; Adamkova, M.; Skjaerven, L.H.; Catalán-Matamoros, D. A systematic review on physical therapy interventions for patients with binge eating disorder. Disabil. Rehabil. 2013, 35, 2191–2196. [Google Scholar] [CrossRef] [PubMed]
- Fossati, M.; Amati, F.; Painot, D.; Reiner, M.; Haenni, C.; Golay, A. Cognitive-behavioral therapy with simultaneous nutritional and physical activity education in obese patients with binge eating disorder. Eat. Weight. Disord. 2004, 9, 134–138. [Google Scholar] [CrossRef] [PubMed]
- Levine, M.D.; Marcus, M.D.; Moulton, P. Exercise in the treatment of binge eating disorder. Int. J. Eat. Disord. 1996, 19, 171–177. [Google Scholar] [CrossRef]
- Vancampfort, D.; Vanderlinden, J.; De Hert, M.; Soundy, A.; Adámkova, M.; Skjaerven, L.H. A systematic review of physical therapy interventions for patients with anorexia and bulemia nervosa. Disabil. Rehabil. 2014, 36, 628–634. [Google Scholar] [CrossRef] [PubMed]
- Sundgot-Borgen, J.; Rosenvinge, J.H.; Bahr, R.; Schneider, L.S. The effect of exercise, cognitive therapy, and nutritional counseling in treating bulimia nervosa. Med. Sci. Sports Exerc. 2002, 34, 190–195. [Google Scholar] [CrossRef] [PubMed]
- Hoie, L.H.; Myking, E.; Reine, E.C.; Bruusgaard, D. Diet and exercise in addition to psychotherapy, in the treatment of patients suffering from eating disorders with obesity. Eat. Weight Disord. 1997, 2, 207–210. [Google Scholar] [CrossRef] [PubMed]
- Mathisen, T.F.; Bratland-Sanda, S.; Rosenvinge, J.H.; Friborg, O.; Pettersen, G.; Vrabel, K.A.; Sundgot-Borgen, J. Treatment effects on compulsive exercise and physical activity in eating disorders. J. Eat. Disord. 2018, in press. [Google Scholar]
- Mathisen, T.F. A randomized controlled trial of physical exercise- and dietary therapy versus cognitive behavior therapy: Treatment effects for women with bulimia nervosa or binge eating disorder. Ph.D. Thesis, Norwegian School of Sport Science, Oslo, Norway, September 2018. Available online: https://brage.bibsys.no/xmlui/handle/11250/2562679 (accessed on 28 November 2018).
- Fairburn, C.; Beglin, S. Eating Disorder Examination Questionnaire (EDE-Q 6.0). In Cognitive Behavior Therapy and Eating Disorders; Fairburn, C., Ed.; Guilford Press: New York, NY, USA, 2008. [Google Scholar]
- Sheehan, D.V.; Baker, R.; Dunbar, G.C. The Mini-International Neuropsychiatric Interview (M.I.N.I.): The development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J. Clin. Psychiatry 1998, 59, 34–57. [Google Scholar]
- Mathisen, T.F.; Rosenvinge, J.H.; Pettersen, G.; Friborg, O.; Vrabel, K.; Bratland-Sanda, S. The PED-t trial protocol: The effect of physical exercise –and dietary therapy compared with cognitive behavior therapy in treatment of bulimia nervosa and binge eating disorder. BMC Psychiatry 2017, 17, 180. [Google Scholar] [CrossRef]
- Fairburn, C.G.; Cooper, Z.; Doll, H.A.; O’connor, M.E.; Bohn, K.; Hawker, D.M. Transdiagnostic Cognitive-Behavioral Therapy for Patients with Eating Disorders: A Two-Site Trial with 60-Week Follow-Up. Am. J. Psychiatry 2009, 166, 311–319. [Google Scholar] [CrossRef]
- Ratamess, N.; Alvar, B.; Evetoch, T.; Housh, T.; Kibler, W.; Kraemer, W. Progression Models in Resistance Training for Healthy Adults. Med. Sci. Sports Exerc. 2009, 41, 687–708. [Google Scholar]
- Garber, C.E.; Blissmer, B.; Deschenes, M.R.; Franklin, B.A.; Lamonte, M.J.; Lee, I.M. Quantity and Quality of Exercise for Developing and Maintaining Cardiorespiratory, Musculoskeletal, and Neuromotor Fitness in Apparently Healthy Adults: Guidance for Prescribing Exercise. Med. Sci. Sports Exerc. 2011, 43, 1334–1359. [Google Scholar]
- Nana, A.; Slater, G.J.; Stewart, A.D.; Burke, L.M. Methodology Review: Using Dual-Energy X-Ray Absorptiometry (DXA) for the Assessment of Body Composition in Athletes and Active People. Int. J. Sport Nutr. Exerc. Metab. 2015, 25, 198–215. [Google Scholar] [CrossRef] [PubMed]
- Bosch, T.A.; Steinberger, J.; Sinaiko, A.R.; Moran, A.; Jacobs Jr, D.R.; Kelly, A.S.; Dengel, D.R. Identification of sex-specific thresholds for accumulation of visceral adipose tissue in adults. Obesity 2015, 23, 375–382. [Google Scholar] [CrossRef] [PubMed]
- Imboden, M.T.; Welch, W.A.; Swartz, A.M.; Montoye, A.H.; Finch, H.W.; Harber, M.P.; Kaminsky, L.A. Reference standards for body fat measures using GE dual energy x-ray absorptiometry in Caucasian adults. PLoS ONE 2017, 12, e0175110. [Google Scholar] [CrossRef]
- Edvardsen, E.; Hansen, B.H.; Holme, I.M.; Dyrstad, S.M.; Anderssen, S.A. Reference Values for Cardiorespiratory Response and Fitness on the Treadmill in a 20- to 85-Year-Old Population. Chest 2013, 144, 241–248. [Google Scholar] [CrossRef] [PubMed]
- Edvardsen, E.; Hem, E.; Anderssen, S.A. End Criteria for Reaching Maximal Oxygen Uptake Must Be Strict and Adjusted to Sex and Age: A Cross-Sectional Study. PLoS ONE 2014, 9, e85276. [Google Scholar] [CrossRef]
- Borg, G. Psychophysical bases of perceived exertion. Med. Sci. Sports Exerc. 1982, 14, 377–381. [Google Scholar]
- Haff, G.; Dumke, C. Laboratory Manual for Exercise Physiology, 2nd ed.; Human Kinetics: Champaign, IL, USA, 2012. [Google Scholar]
- Aspenes, S.T.; Nilsen, T.I.; Skaug, E.A.; Bertheussen, G.F.; Ellingsen, Ø.; Vatten, L.; Wisløff, U. Peak Oxygen Uptake and Cardiovascular Risk Factors in 4631 Healthy Women and Men. Med. Sci. Sports Exerc. 2011, 43, 1465–1473. [Google Scholar]
- Twisk, J.; de Boer, M.; de Vente, W.; Heymans, M. Multiple imputation of missing values was not necessary before performing a longitudinal mixed-model analysis. J. Clin. Epidemiol. 2013, 66, 1022–1028. [Google Scholar] [CrossRef]
- Egbewale, B.E.; Lewis, M.; Sim, J. Bias, precision and statistical power of analysis of covariance in the analysis of randomized trials with baseline imbalance: A simulation study. BMC Med. Res. Methodol. 2014, 14, 49. [Google Scholar] [CrossRef]
- Hedges, G.; Olkin, I. Statistical Methods in Meta-Analysis; Academic Press: Cambridge, MA, USA, 1985. [Google Scholar]
- World Health Organization. Physical status: Theuse and interpretation of anthropometry. In Technical Report Series; World Health Organization: Geneva, Switherland, 1995; pp. 427–438. [Google Scholar]
- Barrett, S.; Begg, S.; O’Halloran, P.; Kingsley, M. Integrated motivational interviewing and cognitive behaviour therapy for lifestyle mediators of overweight and obesity in community-dwelling adults: A systematic review and meta-analyses. BMC Public Health 2018, 18, 1160. [Google Scholar] [CrossRef]
- Ryan, R.M.; Deci, E.L. Self-determination theory and the facilitation of intrinsic motivation, social development, and well-being. Am. Psychol. 2000, 55, 68. [Google Scholar] [CrossRef] [PubMed]
- Samdal, G.B.; Eide, G.E.; Barth, T.; Williams, G.; Meland, E. Effective behaviour change techniques for physical activity and healthy eating in overweight and obese adults; systematic review and meta-regression analyses. Int. J. Behav. Nutr. Phys. Act. 2017, 14, 42. [Google Scholar] [CrossRef] [PubMed]
| Supervised Exercise | Unsupervised Exercise | |||
|---|---|---|---|---|
| Week | Micro Cycle | Resistance Exercise | Interval Running | Resistance Exercise |
| 1–3 | 1 | 10RM | Pyramid interval (1–3 min intervals) | 10RM |
| 4–7 | 2 | 8RM | Pyramid interval (1–3 min intervals) | 10RM |
| 8–11 | 3 | 6RM | Pyramid interval (1–3 min intervals) | 10RM |
| 12–14 | 4 | 4RM | Pyramid interval (1–4 min intervals) | 10RM |
| 15–16 | 5 | 2RM | Pyramid interval (1–4 min intervals) | 10RM |
| PED-t | CBT | |
|---|---|---|
| Age, years | 28.3 (6.2) | 27.8 (5.3) |
| BMI, kg × height−1 | 25.3 (5.1) | 25.4 (4.6) |
| EDE-q, total score | 3.7 (0.9) | 3.7 (1.0) |
| Duration of illness, years | 12.9 (7.5) | 11.9 (6.7) |
| Bulimia nervosa, n (%) | 51 (65.4) | 50 (66.7) |
| Binge eating disorder, n (%) | 27 (34.6) | 25 (33.3) |
| Between Effects, p-Value, Effect Size (g) | |||||||
|---|---|---|---|---|---|---|---|
| T1 | T2 | T3 | T4 | T2 | T3 | T4 | |
| VO2peak absolute (L × min−1) | |||||||
| PED-t | 2.67 (2.53, 2.81) | 2.78 * (2.63, 2.93) g = 0.22 | 2.72 (2.57, 2.87) | 2.77 (2.61, 2.92) | |||
| CBT | 2.73 (2.59, 2.88) | 2.77 (2.61, 2.93) | 2.78 (2.59, 2.96) | 2.80 (2.62, 2.98) | n.s. | n.s. | n.s. |
| VO2peak relative (mL × kg−1 × min−1) | |||||||
| PED-t | 38.25 (35.87, 40.63) | 39.47 (36.92, 42.02) | 38.35 (35.70, 41.00) | 39.01 (36.30, 41.72) | |||
| CBT | 39.01 (36.49, 41.54) | 40.35 (37.63, 43.08) | 39.13 (35.95, 42.32) | 39.24 (36.14, 42.33) | n.s. | n.s. | n.s. |
| Squat, 1RM (kg) | |||||||
| PED-t | 63.95 (58.33, 69.56) | 78.60 * (72.81, 84.39) g = −0.83 | 73.89 * (67.94, 79.85) g = −0.56 | 74.98 * (68.96, 81.00) g = −0.71 | |||
| CBT | 64.21 (58.37, 70.06) | 67.15 * (61.06, 73.24) g = −0.15 | 70.07 ** (63.41, 76.72) g = −0.30 | 69.31 * (62.65, 75.97) g = −0.26 | 11.78 (7.42, 16.12) g = 0.65 p < 0.001 | n.s. | 6.10 (0.84, 11.36) g = 0.31 p = 0.003 |
| Bench press, 1RM (kg) | |||||||
| PED-t | 37.02 (34.07, 39.97) | 45.22 * (42.18, 48.26) g = −0.86 | 42.68 * (39.56, 45.79) g = −0.62 | 43.77 * (40.62, 46.93) g = −0.74 | |||
| CBT | 38.32 (35.28, 41.37) | 38.99 (35.80, 42.18) | 39.81 (36.37, 43.26) | 39.35 (35.90, 42.79) | 7.28 (4.92, 9.63) g = 0.63 p < 0.001 | 4.12 (1.41, 6.84) g = 0.30 p < 0.001 | 5.60 (2.85, 8.35) g = 0.43 p < 0.001 |
| Seated row, 1RM (kg) | |||||||
| PED-t | 34.38 (32.41, 36.34) | 38.41 * (36.36, 40.46) g = −0.65 | 37.50 * (35.36, 39.64) g = −0.52 | 37.22 * (35.05, 39.40) g = −0.46 | |||
| CBT | 34.56 (32.54, 36.58) | 35.20 (33.06, 37.34) | 35.03 (32.66, 37.41) | 34.73 (32.31, 37.14) | 3.39 (1.45, 5.34) g = 0.52 p < 0.001 | 2.58 (0.35, 4.81) g = 0.41 p = 0.003 | 2.74 (0.45, 5.02) g = 0.40 p = 0.002 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mathisen, T.F.; Sundgot-Borgen, J.; Rosenvinge, J.H.; Bratland-Sanda, S. Managing Risk of Non-Communicable Diseases in Women with Bulimia Nervosa or Binge Eating Disorders: A Randomized Trial with 12 Months Follow-Up. Nutrients 2018, 10, 1887. https://doi.org/10.3390/nu10121887
Mathisen TF, Sundgot-Borgen J, Rosenvinge JH, Bratland-Sanda S. Managing Risk of Non-Communicable Diseases in Women with Bulimia Nervosa or Binge Eating Disorders: A Randomized Trial with 12 Months Follow-Up. Nutrients. 2018; 10(12):1887. https://doi.org/10.3390/nu10121887
Chicago/Turabian StyleMathisen, Therese Fostervold, Jorunn Sundgot-Borgen, Jan H. Rosenvinge, and Solfrid Bratland-Sanda. 2018. "Managing Risk of Non-Communicable Diseases in Women with Bulimia Nervosa or Binge Eating Disorders: A Randomized Trial with 12 Months Follow-Up" Nutrients 10, no. 12: 1887. https://doi.org/10.3390/nu10121887
APA StyleMathisen, T. F., Sundgot-Borgen, J., Rosenvinge, J. H., & Bratland-Sanda, S. (2018). Managing Risk of Non-Communicable Diseases in Women with Bulimia Nervosa or Binge Eating Disorders: A Randomized Trial with 12 Months Follow-Up. Nutrients, 10(12), 1887. https://doi.org/10.3390/nu10121887





