Peripheral Blood Mononuclear Cell Metabolism Acutely Adapted to Postprandial Transition and Mainly Reflected Metabolic Adipose Tissue Adaptations to a High-Fat Diet in Minipigs
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Experimental Procedure
2.2.1. Postprandial Meal Test
2.2.2. High Fat–High Sucrose Long-Term Trial
2.3. Analytical Procedures
2.3.1. Blood Treatment and PBMC Collection
2.3.2. Plasma Insulin and Metabolite Determination
2.3.3. Measurements of Enzyme Activities
2.3.4. Western Blot Analyses
2.3.5. PCR Analyses
2.4. Statistical Analyses
3. Results
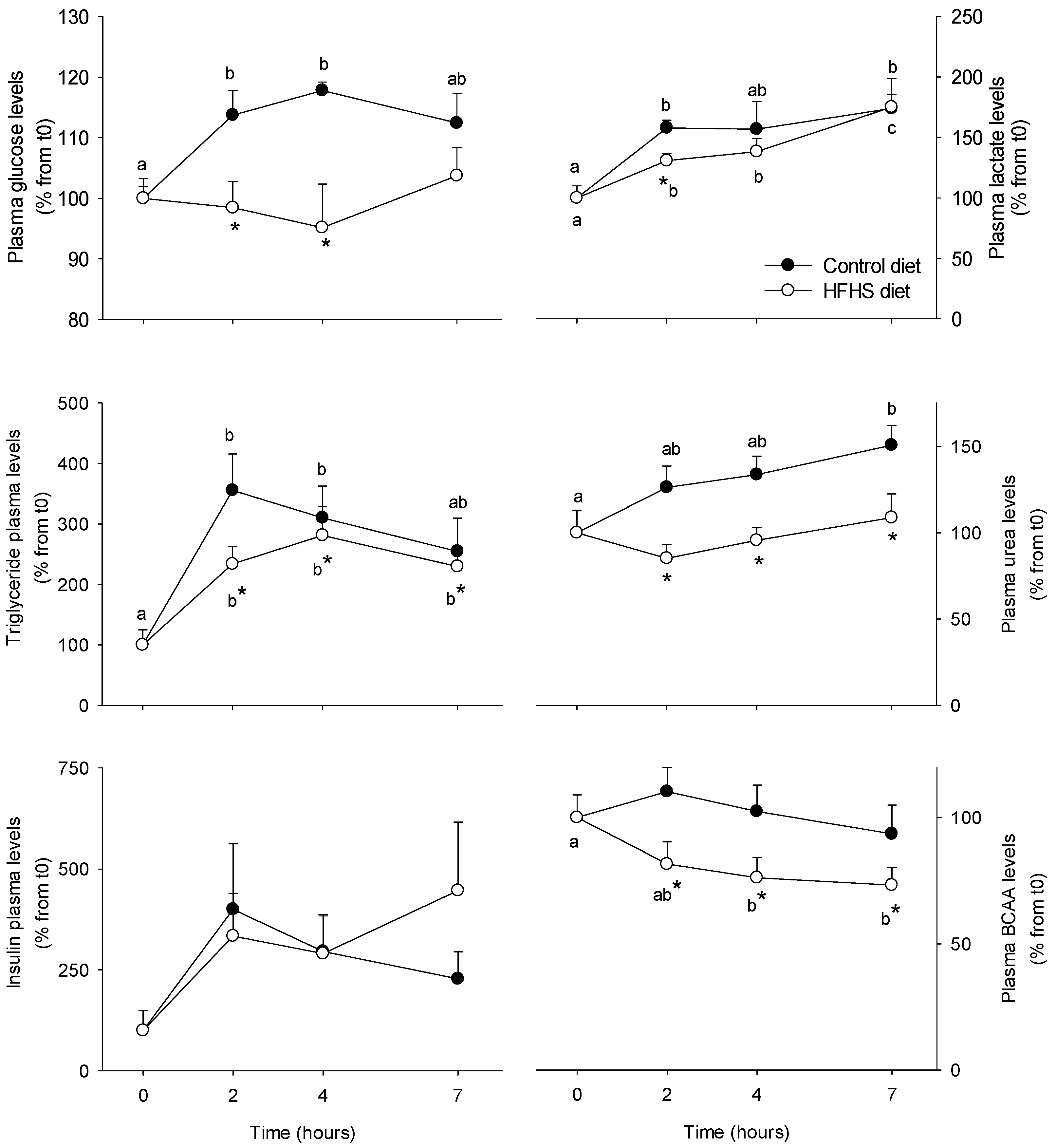
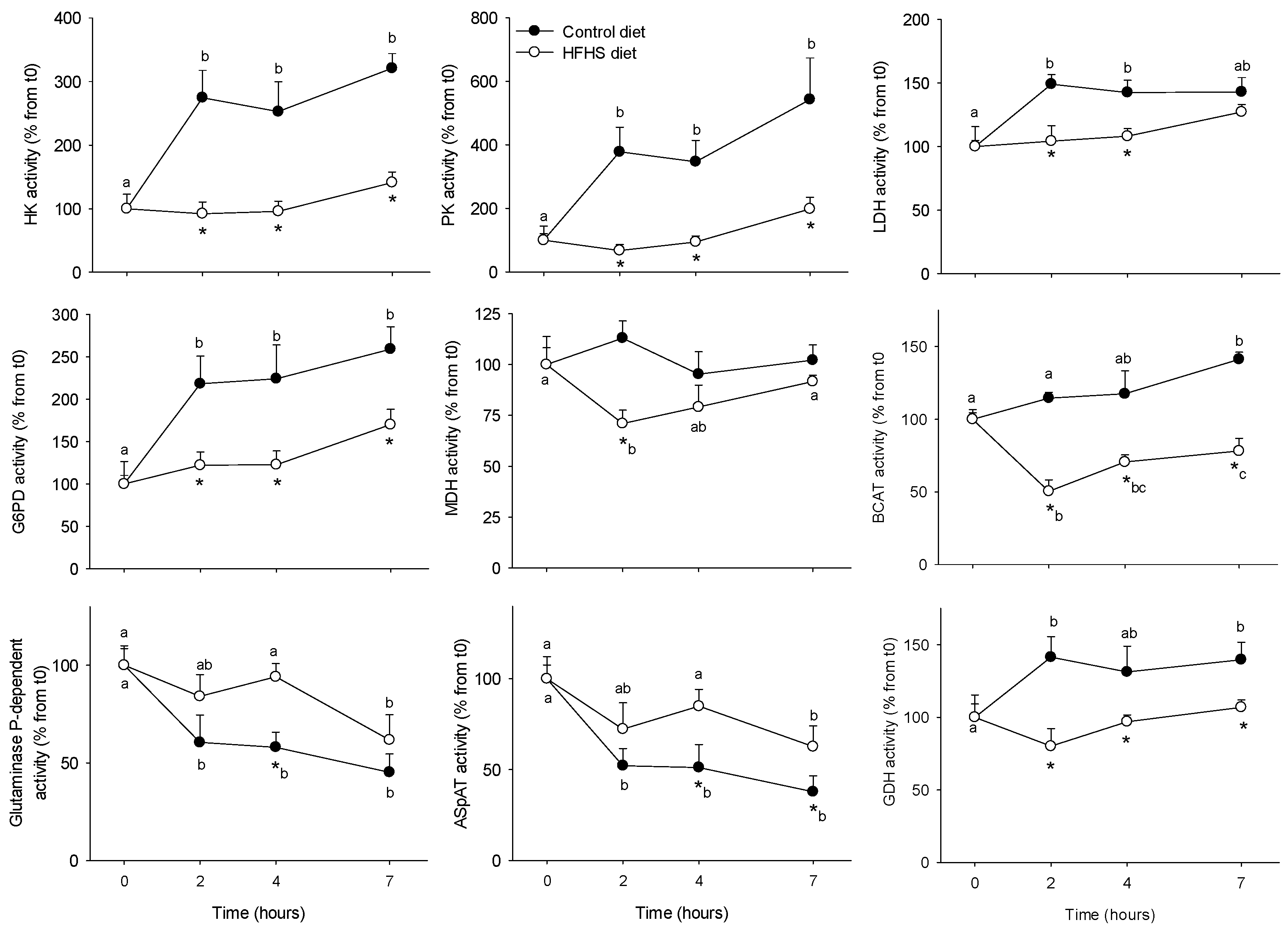
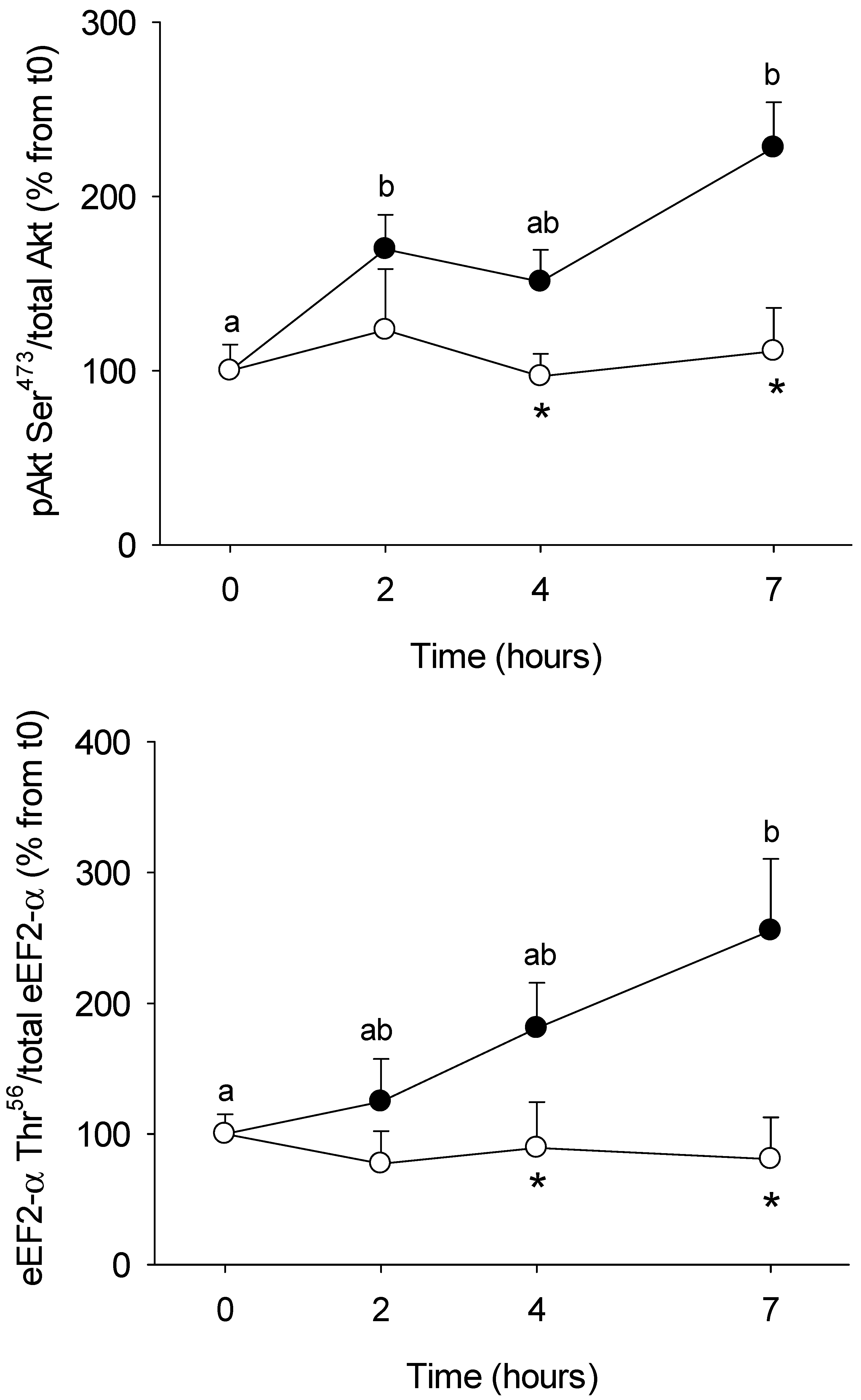
3.1. Short-Term Control vs HFHS Postprandial Trial
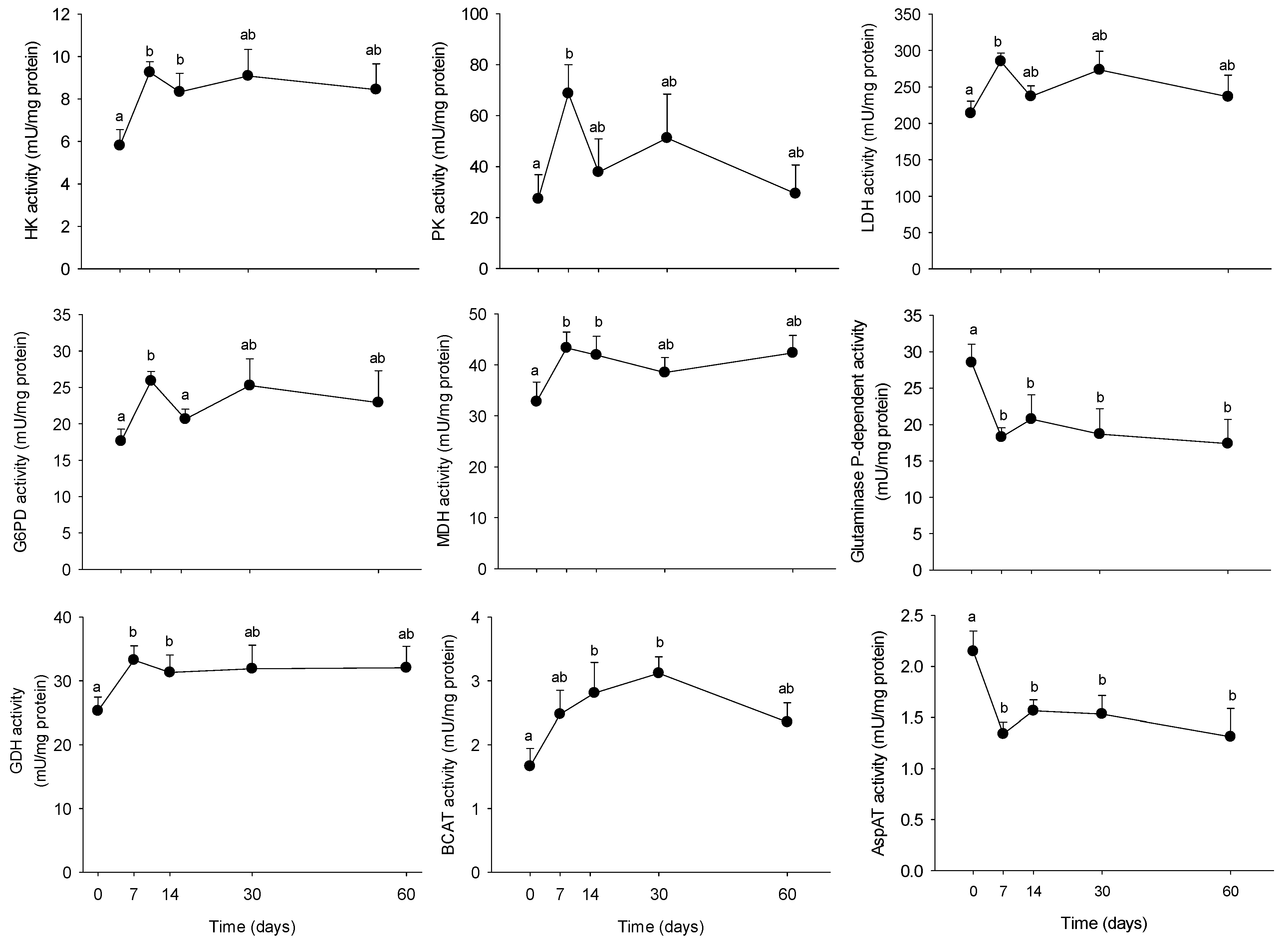
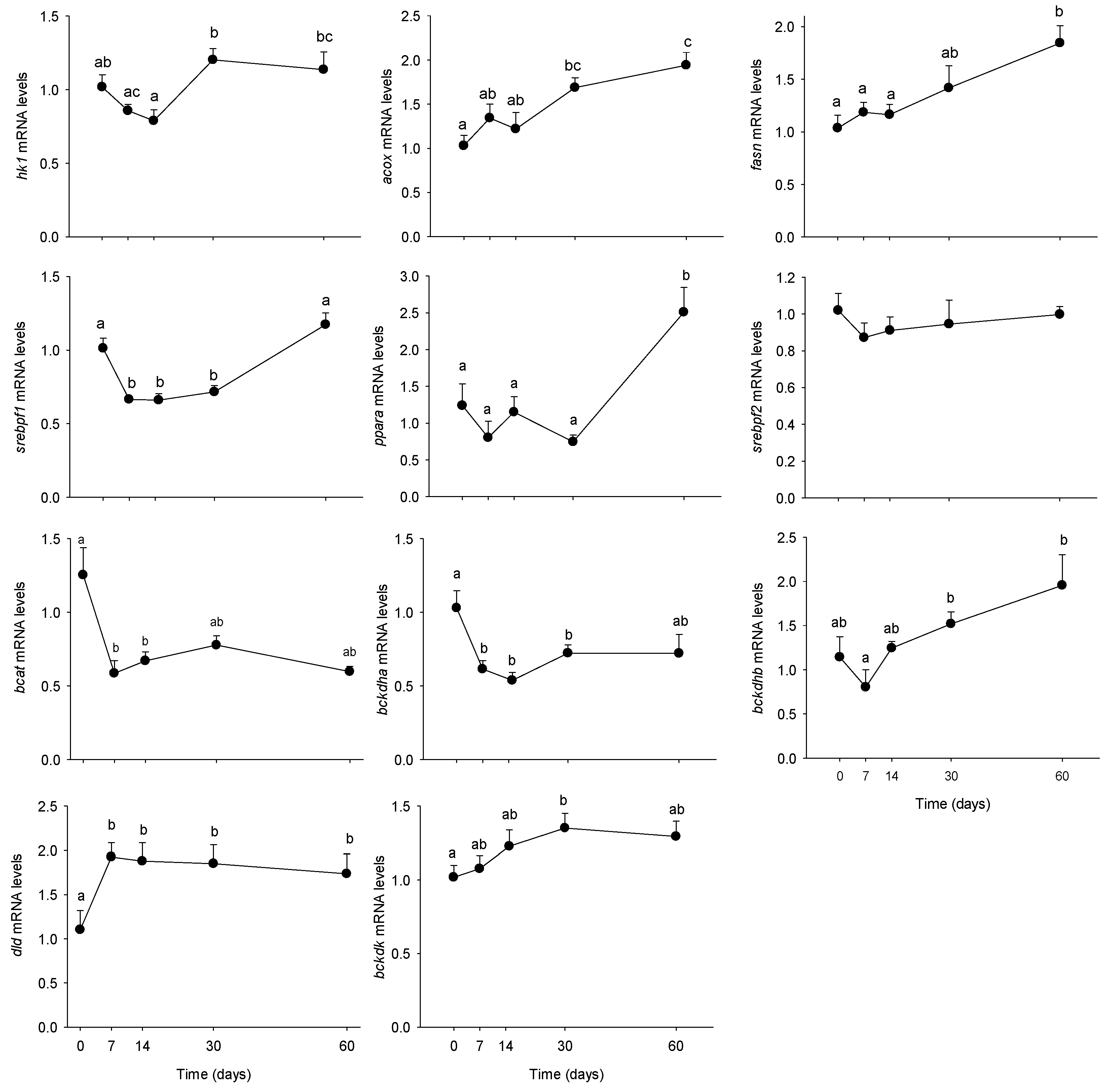
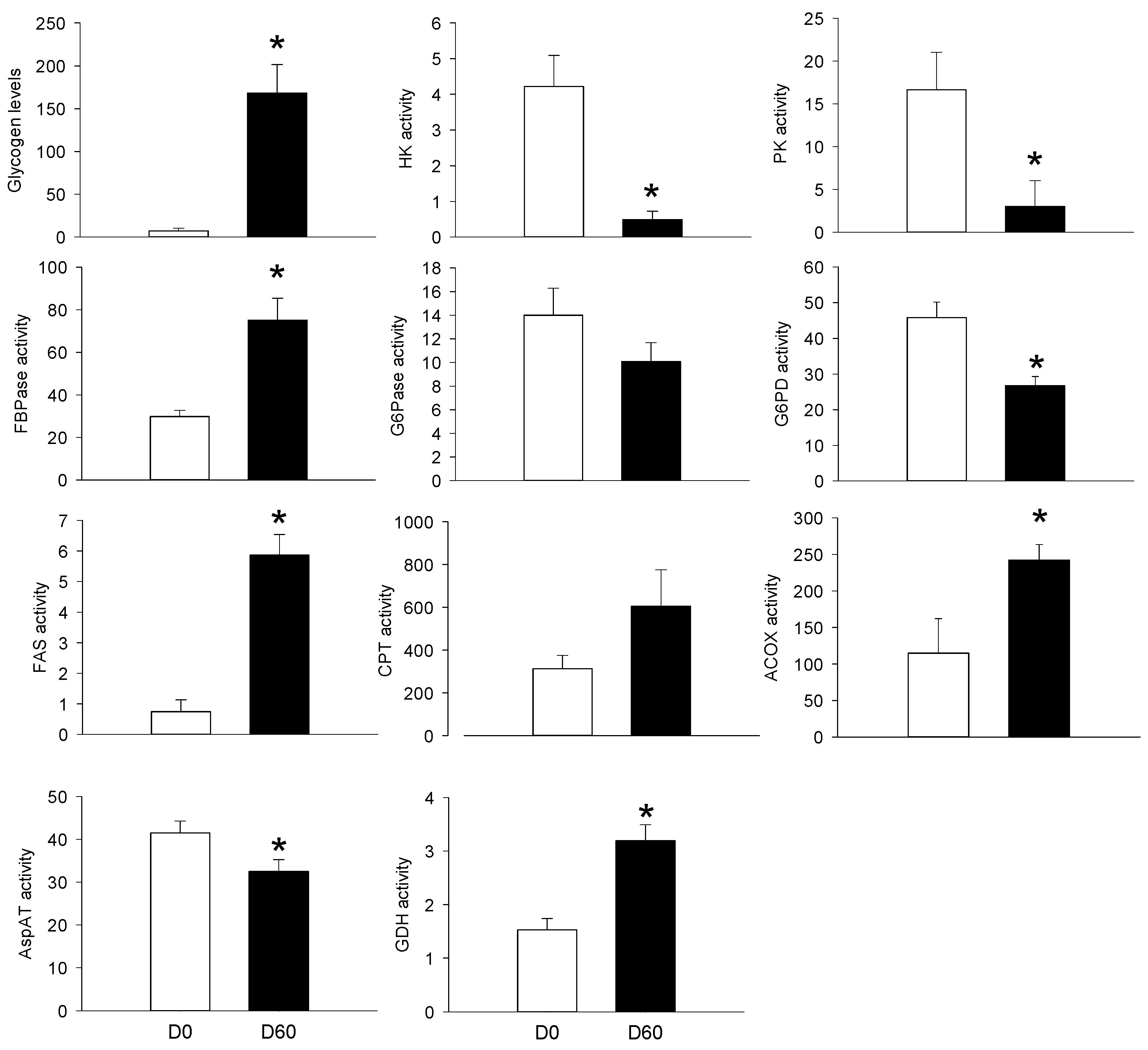
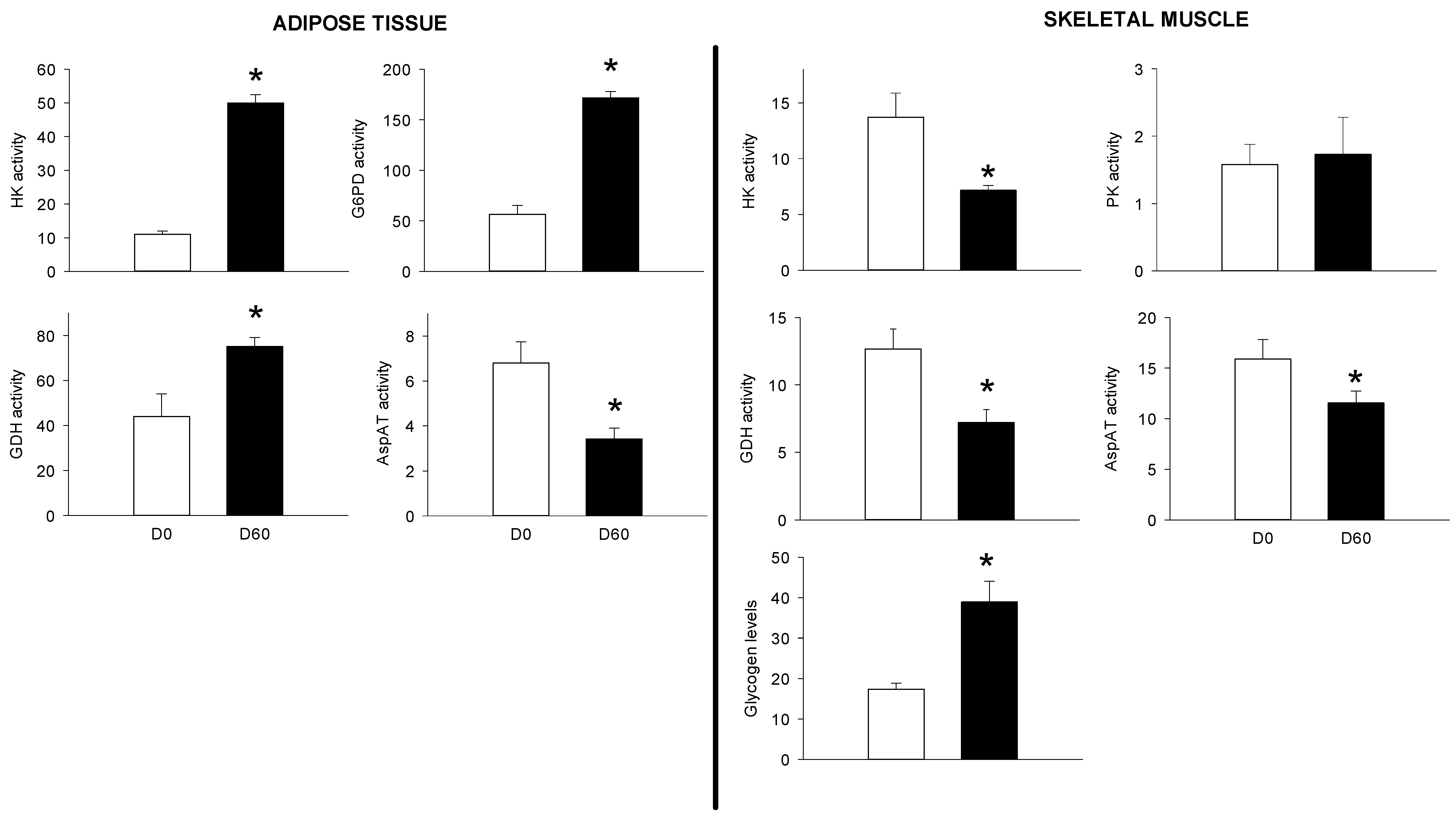
3.2. Long-Term HFHS Trial
4. Discussion
4.1. PBMC Metabolism Is Highly Reactive to the Meal, But Its Response Is Blunted by the Intake of a HFHS Meal
4.2. Time-Course Changes in PBMC Metabolism during Long-Term High Fat Feeding
4.3. The PBMC Metabolism Rather Resembled that of the Adipose Tissue
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Liew, C.C.; Ma, J.; Tang, H.C.; Zheng, R.; Dempsey, A.A. The peripheral blood transcriptome dynamically reflects system wide biology: A potential diagnostic tool. J. Lab. Clin. Med. 2006, 147, 126–132. [Google Scholar] [CrossRef] [PubMed]
- Reynes, B.; Diaz-Rua, R.; Cifre, M.; Oliver, P.; Palou, A. Peripheral blood mononuclear cells as a potential source of biomarkers to test the efficacy of weight-loss strategies. Obesity 2015, 23, 28–31. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S.; Dent, R.; Harper, M.E.; Gorman, S.A.; Stuart, J.S.; McPherson, R. Gene expression profiling in whole blood identifies distinct biological pathways associated with obesity. BMC Med. Genom. 2010, 3, 56. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Kim, M.; Han, J.Y.; Lee, S.H.; Jee, S.H.; Lee, J.H. The metabolites in peripheral blood mononuclear cells showed greater differences between patients with impaired fasting glucose or type 2 diabetes and healthy controls than those in plasma. Diabetes Vasc. Dis. Res. 2017, 14, 130–138. [Google Scholar] [CrossRef] [PubMed]
- Stepien, M.; Nugent, A.P.; Brennan, L. Metabolic profiling of human peripheral blood mononuclear cells: Influence of vitamin D status and gender. Metabolites 2014, 4, 248–259. [Google Scholar] [CrossRef] [PubMed]
- Mohr, S.; Liew, C.C. The peripheral-blood transcriptome: New insights into disease and risk assessment. Trends Mol. Med. 2007, 13, 422–432. [Google Scholar] [CrossRef] [PubMed]
- De Boever, P.; Wens, B.; Forcheh, A.C.; Reynders, H.; Nelen, V.; Kleinjans, J.; Van Larebeke, N.; Verbeke, G.; Valkenborg, D.; Schoeters, G. Characterization of the peripheral blood transcriptome in a repeated measures design using a panel of healthy individuals. Genomics 2014, 103, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Newsholme, E.A.; Crabtree, B.; Ardawi, M.S. Glutamine metabolism in lymphocytes: Its biochemical, physiological and clinical importance. Q. J. Exp. Physiol. 1985, 70, 473–489. [Google Scholar] [CrossRef] [PubMed]
- Washizu, T.; Takahashi, M.; Azakami, D.; Ikeda, M.; Arai, T. Activities of Enzymes in the Malate–Aspartate Shuttle in the Peripheral Leukocytes of Dogs and Cats. Vet. Res. Commun. 2001, 25, 623–629. [Google Scholar] [CrossRef] [PubMed]
- Mori, A.; Kenyon, P.R.; Mori, N.; Yamamoto, I.; Tanaka, Y.; Suzuki, N.; Tazaki, H.; Ozawa, T.; Hayashi, T.; Hickson, R.E.; et al. Changes in metabolite, energy metabolism related enzyme activities and peripheral blood mononuclear cell (PBMC) populations in beef heifers with two differing liveweight change profiles in New Zealand. Vet. Res. Commun. 2008, 32, 159–166. [Google Scholar] [CrossRef] [PubMed]
- Washizu, T.; Tanaka, A.; Sako, T.; Washizu, M.; Arai, T. Comparison of the activities of enzymes related to glycolysis and gluconeogenesis in the liver of dogs and cats. Res. Vet. Sci. 1999, 67, 205–206. [Google Scholar] [CrossRef] [PubMed]
- Wilkinson, J.M.; Dyck, M.K.; Dixon, W.T.; Foxcroft, G.R.; Dhakal, S.; Harding, J.C. Transcriptomic analysis identifies candidate genes and functional networks controlling the response of porcine peripheral blood mononuclear cells to mitogenic stimulation. J. Anim. Sci. 2012, 90, 3337–3352. [Google Scholar] [CrossRef] [PubMed]
- Adler, M.; Murani, E.; Ponsuksili, S.; Wimmers, K. PBMC transcription profiles of pigs with divergent humoral immune responses and lean growth performance. Int. J. Biol. Sci. 2013, 9, 907–916. [Google Scholar] [CrossRef] [PubMed]
- Oster, M.; Murani, E.; Ponsuksili, S.; D’Eath, R.B.; Turner, S.P.; Evans, G.; Tholking, L.; Kurt, E.; Klont, R.; Foury, A.; et al. Transcriptional responses of PBMC in psychosocially stressed animals indicate an alerting of the immune system in female but not in castrated male pigs. BMC Genom. 2014, 15, 967. [Google Scholar] [CrossRef] [PubMed]
- Weber, T.E.; Spurlock, M.E. Leptin alters antibody isotype in the pig in vivo, but does not regulate cytokine expression or stimulate STAT3 signaling in peripheral blood monocytes in vitro. J. Anim. Sci. 2004, 82, 1630–1640. [Google Scholar] [CrossRef] [PubMed]
- Caimari, A.; Oliver, P.; Keijer, J.; Palou, A. Peripheral blood mononuclear cells as a model to study the response of energy homeostasis-related genes to acute changes in feeding conditions. OMICS J. Integr. Biol. 2010, 14, 129–141. [Google Scholar] [CrossRef] [PubMed]
- Manoel-Caetano, F.S.; Xavier, D.J.; Evangelista, A.F.; Takahashi, P.; Collares, C.V.; Puthier, D.; Foss-Freitas, M.C.; Foss, M.C.; Donadi, E.A.; Passos, G.A.; et al. Gene expression profiles displayed by peripheral blood mononuclear cells from patients with type 2 diabetes mellitus focusing on biological processes implicated on the pathogenesis of the disease. Gene 2012, 511, 151–160. [Google Scholar] [CrossRef] [PubMed]
- Tangen, S.E.; Tsinajinnie, D.; Nunez, M.; Shaibi, G.Q.; Mandarino, L.J.; Coletta, D.K. Whole blood gene expression profiles in insulin resistant Latinos with the metabolic syndrome. PLoS ONE 2013, 8, e84002. [Google Scholar] [CrossRef] [PubMed]
- Drew, J.E.; Farquharson, A.J.; Horgan, G.W.; Duthie, S.J.; Duthie, G.G. Postprandial cell defense system responses to meal formulations: Stratification through gene expression profiling. Mol. Nutr. Food Res. 2014, 58, 2066–2079. [Google Scholar] [CrossRef] [PubMed]
- Fazelzadeh, P.; Hangelbroek, R.W.J.; Joris, P.J.; Schalkwijk, C.G.; Esser, D.; Afman, L.; Hankemeier, T.; Jacobs, D.M.; Mihaleva, V.V.; Kersten, S.; et al. Weight loss moderately affects the mixed meal challenge response of the plasma metabolome and transcriptome of peripheral blood mononuclear cells in abdominally obese subjects. Metabolomics 2018, 14, 46. [Google Scholar] [CrossRef] [PubMed]
- Bouwens, M.; Grootte Bromhaar, M.; Jansen, J.; Muller, M.; Afman, L.A. Postprandial dietary lipid-specific effects on human peripheral blood mononuclear cell gene expression profiles. Am. J. Clin. Nutr. 2010, 91, 208–217. [Google Scholar] [CrossRef] [PubMed]
- Bouwens, M.; Afman, L.A.; Muller, M. Fasting induces changes in peripheral blood mononuclear cell gene expression profiles related to increases in fatty acid beta-oxidation: Functional role of peroxisome proliferator activated receptor alpha in human peripheral blood mononuclear cells. Am. J. Clin. Nutr. 2007, 86, 1515–1523. [Google Scholar] [CrossRef] [PubMed]
- Polakof, S.; Remond, D.; Bernalier-Donadille, A.; Rambeau, M.; Pujos-Guillot, E.; Comte, B.; Dardevet, D.; Savary-Auzeloux, I. Metabolic adaptations to HFHS overfeeding: How whole body and tissues postprandial metabolic flexibility adapt in Yucatan mini-pigs. Eur. J. Nutr. 2018, 57, 119–135. [Google Scholar] [CrossRef] [PubMed]
- Polakof, S.; Dardevet, D.; Lyan, B.; Mosoni, L.; Gatineau, E.; Martin, J.F.; Pujos-Guillot, E.; Mazur, A.; Comte, B. Time course of molecular and metabolic events in the development of insulin resistance in fructose-fed rats. J. Proteome Res. 2016, 15, 1862–1874. [Google Scholar] [CrossRef] [PubMed]
- Polakof, S.; Remond, D.; David, J.; Dardevet, D.; Savary-Auzeloux, I. Time-course changes in circulating branched-chain amino acid levels and metabolism in obese Yucatan minipig. Nutrition 2017, 50, 66–73. [Google Scholar] [CrossRef] [PubMed]
- Polakof, S.; Rémond, D.; Rambeau, M.; Pujos-Guillot, E.; Sébédio, J.-L.; Dardevet, D.; Comte, B.; Savary-Auzeloux, I. Postprandial metabolic events in mini-pigs: New insights from a combined approach using plasma metabolomics, tissue gene expression, and enzyme activity. Metabolomics 2015, 11, 964–979. [Google Scholar] [CrossRef]
- Sagaya, F.M.; Hurrell, R.F.; Vergeres, G. Postprandial blood cell transcriptomics in response to the ingestion of dairy products by healthy individuals. J. Nutr. Biochem. 2012, 23, 1701–1715. [Google Scholar] [CrossRef] [PubMed]
- Castro, H.; Pomar, C.A.; Pico, C.; Sanchez, J.; Palou, A. Cafeteria diet overfeeding in young male rats impairs the adaptive response to fed/fasted conditions and increases adiposity independent of body weight. Int. J. Obes. 2014. [Google Scholar] [CrossRef] [PubMed]
- Ardawi, M.S.; Newsholme, E.A. Maximum activities of some enzymes of glycolysis, the tricarboxylic acid cycle and ketone-body and glutamine utilization pathways in lymphocytes of the rat. Biochem. J. 1982, 208, 743–748. [Google Scholar] [CrossRef] [PubMed]
- Coloff, J.L.; Murphy, J.P.; Braun, C.R.; Harris, I.S.; Shelton, L.M.; Kami, K.; Gygi, S.P.; Selfors, L.M.; Brugge, J.S. Differential Glutamate Metabolism in Proliferating and Quiescent Mammary Epithelial Cells. Cell Metab. 2016, 23, 867–880. [Google Scholar] [CrossRef] [PubMed]
- Brand, K.; Fekl, W.; von Hintzenstern, J.; Langer, K.; Luppa, P.; Schoerner, C. Metabolism of glutamine in lymphocytes. Metabolism 1989, 38, 29–33. [Google Scholar] [CrossRef]
- Little, T.J.; Horowitz, M.; Feinle-Bisset, C. Modulation by high-fat diets of gastrointestinal function and hormones associated with the regulation of energy intake: Implications for the pathophysiology of obesity. Am. J. Clin. Nutr. 2007, 86, 531–541. [Google Scholar] [CrossRef] [PubMed]
- Gentilcore, D.; Chaikomin, R.; Jones, K.L.; Russo, A.; Feinle-Bisset, C.; Wishart, J.M.; Rayner, C.K.; Horowitz, M. Effects of Fat on Gastric Emptying of and the Glycemic, Insulin, and Incretin Responses to a Carbohydrate Meal in Type 2 Diabetes. J. Clin. Endocrinol. Metab. 2006, 91, 2062–2067. [Google Scholar] [CrossRef] [PubMed]
- Cunningham, K.M.; Read, N.W. The effect of incorporating fat into different components of a meal on gastric emptying and postprandial blood glucose and insulin responses. Br. J. Nutr. 2007, 61, 285–290. [Google Scholar] [CrossRef]
- Welch, I.M.; Bruce, C.; Hill, S.E.; Read, N.W. Duodenal and ileal lipid suppresses postprandial blood glucose and insulin responses in man: Possible implications for the dietary management of diabetes mellitus. Clin. Sci. 1987, 72, 209–216. [Google Scholar] [CrossRef] [PubMed]
- Eto, K.; Tsubamoto, Y.; Terauchi, Y.; Sugiyama, T.; Kishimoto, T.; Takahashi, N.; Yamauchi, N.; Kubota, N.; Murayama, S.; Aizawa, T.; et al. Role of NADH Shuttle System in Glucose-Induced Activation of Mitochondrial Metabolism and Insulin Secretion. Science 1999, 283, 981–985. [Google Scholar] [CrossRef] [PubMed]
- American Diabetes Association. 2. Classification and diagnosis of diabetes. Diabetes Care 2018, 40, S11–S24. [Google Scholar]
- Oliver, P.; Reynes, B.; Caimari, A.; Palou, A. Peripheral blood mononuclear cells: A potential source of homeostatic imbalance markers associated with obesity development. Pflug. Arch. 2013, 465, 459–468. [Google Scholar] [CrossRef] [PubMed]
- Dufort, F.J.; Gumina, M.R.; Ta, N.L.; Tao, Y.; Heyse, S.A.; Scott, D.A.; Richardson, A.D.; Seyfried, T.N.; Chiles, T.C. Glucose-dependent de novo lipogenesis in B lymphocytes: A requirement for ATP-citrate lyase in lipopolysaccharide-induced differentiation. J. Biol. Chem. 2014, 289, 7011–7024. [Google Scholar] [CrossRef] [PubMed]
- Ferramosca, A.; Conte, A.; Damiano, F.; Siculella, L.; Zara, V. Differential effects of high-carbohydrate and high-fat diets on hepatic lipogenesis in rats. Eur. J. Nutr. 2014, 53, 1103–1114. [Google Scholar] [CrossRef] [PubMed]
- Oosterveer, M.H.; van Dijk, T.H.; Tietge, U.J.F.; Boer, T.; Havinga, R.; Stellaard, F.; Groen, A.K.; Kuipers, F.; Reijngoud, D.-J. High Fat Feeding Induces Hepatic Fatty Acid Elongation in Mice. PLoS ONE 2009, 4, e6066. [Google Scholar] [CrossRef] [PubMed]
- Polakof, S.; Diaz-Rubio, M.E.; Dardevet, D.; Martin, J.F.; Pujos-Guillot, E.; Scalbert, A.; Sebedio, J.L.; Mazur, A.; Comte, B. Resistant starch intake partly restores metabolic and inflammatory alterations in the liver of high-fat-diet-fed rats. J. Nutr. Biochem. 2013, 24, 1920–1930. [Google Scholar] [CrossRef] [PubMed]
- Lynch, C.J.; Adams, S.H. Branched-chain amino acids in metabolic signalling and insulin resistance. Nat. Rev. Endocrinol. 2014, 10, 723–736. [Google Scholar] [CrossRef] [PubMed]
- Caimari, A.; Crescenti, A.; Puiggròs, F.; Boqué, N.; Arola, L.; del Bas, J.M. The intake of a high-fat diet and grape seed procyanidins induces gene expression changes in peripheral blood mononuclear cells of hamsters: Capturing alterations in lipid and cholesterol metabolisms. Genes Nutr. 2014, 10, 438. [Google Scholar] [CrossRef] [PubMed]
- O’Grada, C.M.; Morine, M.J.; Morris, C.; Ryan, M.; Dillon, E.T.; Walsh, M.; Gibney, E.R.; Brennan, L.; Gibney, M.J.; Roche, H.M. PBMCs reflect the immune component of the WAT transcriptome—Implications as biomarkers of metabolic health in the postprandial state. Mol. Nutr. Food Res. 2014, 58, 808–820. [Google Scholar] [CrossRef] [PubMed]
- Konieczna, J.; Sánchez, J.; van Schothorst, E.M.; Torrens, J.M.; Bunschoten, A.; Palou, M.; Picó, C.; Keijer, J.; Palou, A. Identification of early transcriptome-based biomarkers related to lipid metabolism in peripheral blood mononuclear cells of rats nutritionally programmed for improved metabolic health. Genes Nutr. 2013, 9, 366. [Google Scholar] [CrossRef] [PubMed]
| Time (Days) | |||||
|---|---|---|---|---|---|
| 1 | 7 | 14 | 30 | 60 | |
| Glucose (mM) | 3.78 ± 0.28 | 3.54 ± 0.11 | 3.42 ± 0.12 | 3.56 ± 0.15 | 3.54 ± 0.07 |
| Lactate (mM) | 0.53 ± 0.07 | 0.51 ± 0.03 | 0.49 ± 0.03 | 0.48 ± 0.04 | 0.58 ± 0.06 |
| Triglycerides (mM) | 0.19 ± 0.03a | 0.35 ± 0.06b | 0.33 ± 0.07b | 0.21 ± 0.03a | 0.32 ± 0.05ab |
| Insulin (ng/mL) | 0.05 ± 0.03a | 0.17 ± 0.04b | 0.16 ± 0.04b | 0.17 ± 0.05b | 0.14 ± 0.03b |
| Urea (mM) | 5.38 ± 0.43a | 3.41 ± 0.32b | 3.79 ± 0.15b | 3.92 ± 0.31b | 4.06 ± 0.31b |
| Liver | Skeletal Muscle | Adipose Tissue | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Metabolic Pathway | Gene | D0 | D60 | Gene | D0 | D60 | Gene | D0 | D60 |
| Glucose metabolism | gck | 0.63 ± 0.20 | 1.57 ± 0.54 | slc2a4 | 1.03 ± 0.12 | 1.42 ± 0.14 | slc2a4 | 1.03 ± 0.11 | 6.40 ± 1.69 * |
| g6pc | 1.51 ± 0.21 | 0.78 ± 0.15 * | |||||||
| pck1 | 1.09 ± 0.09 | 0.94 ± 0.07 | hk-1 | 0.70 ± 0.08 | 1.50 ± 0.37 | hk-1 | 1.01 ± 0.06 | 1.45 ± 0.16 * | |
| hk1 | 1.23 ± 0.10 | 0.84 ± 0.05 * | |||||||
| Lipid oxidation | cpt1-l | 2.43 ± 0.30 | 0.44 ± 0.04 * | cpt1-l | 1.03 ± 0.11 | 0.50 ± 0.06 * | cpt1-l | 1.27 ± 0.26 | 0.27 ± 0.04 * |
| acox | 1.01 ± 0.06 | 0.86 ± 0.05 | acox | 0.87 ± 0.04 | 0.82 ± 0.09 | acox | 1.03 ± 0.11 | 0.79 ± 0.17 | |
| ppara | 1.25 ± 0.34 | 1.03 ± 0.09 | ppara | 1.02 ± 0.09 | 0.73 ± 0.08 t | ppara | 1.04 ± 0.14 | 1.46 ± 0.18 t | |
| Lipogenesis | acly | 1.01 ± 0.08 | 0.75 ± 0.04 * | - | acly | 1.16 ± 0.34 | 11.88 ± 1.29 * | ||
| fasn | 0.23 ± 0.03 | 5.37 ± 1.19 * | fasn | 1.19 ± 0.36 | 10.34 ± 2.57 * | ||||
| srebf1c | 0.92 ± 0.16 | 1.27 ± 0.21 | srebf1c | 1.29 ± 0.47 | 0.61 ± 0.05 | ||||
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zeng, Y.; David, J.; Rémond, D.; Dardevet, D.; Savary-Auzeloux, I.; Polakof, S. Peripheral Blood Mononuclear Cell Metabolism Acutely Adapted to Postprandial Transition and Mainly Reflected Metabolic Adipose Tissue Adaptations to a High-Fat Diet in Minipigs. Nutrients 2018, 10, 1816. https://doi.org/10.3390/nu10111816
Zeng Y, David J, Rémond D, Dardevet D, Savary-Auzeloux I, Polakof S. Peripheral Blood Mononuclear Cell Metabolism Acutely Adapted to Postprandial Transition and Mainly Reflected Metabolic Adipose Tissue Adaptations to a High-Fat Diet in Minipigs. Nutrients. 2018; 10(11):1816. https://doi.org/10.3390/nu10111816
Chicago/Turabian StyleZeng, Yuchun, Jérémie David, Didier Rémond, Dominique Dardevet, Isabelle Savary-Auzeloux, and Sergio Polakof. 2018. "Peripheral Blood Mononuclear Cell Metabolism Acutely Adapted to Postprandial Transition and Mainly Reflected Metabolic Adipose Tissue Adaptations to a High-Fat Diet in Minipigs" Nutrients 10, no. 11: 1816. https://doi.org/10.3390/nu10111816
APA StyleZeng, Y., David, J., Rémond, D., Dardevet, D., Savary-Auzeloux, I., & Polakof, S. (2018). Peripheral Blood Mononuclear Cell Metabolism Acutely Adapted to Postprandial Transition and Mainly Reflected Metabolic Adipose Tissue Adaptations to a High-Fat Diet in Minipigs. Nutrients, 10(11), 1816. https://doi.org/10.3390/nu10111816






