Stress-Reducing Function of Matcha Green Tea in Animal Experiments and Clinical Trials
Abstract
:1. Introduction
2. Materials and Methods
2.1. Measurement of Tea Components by High-Performance Liquid Chromatography
2.2. Animal Studies
2.2.1. Animals and Stress Experiment
2.2.2. Ingestion of Matcha or Tea Components by Mice
2.3. Human Studies
2.3.1. Participants
2.3.2. Procedure
2.3.3. Measurement of sAA
2.4. Statistical Analyses
3. Results
3.1. Animal Study
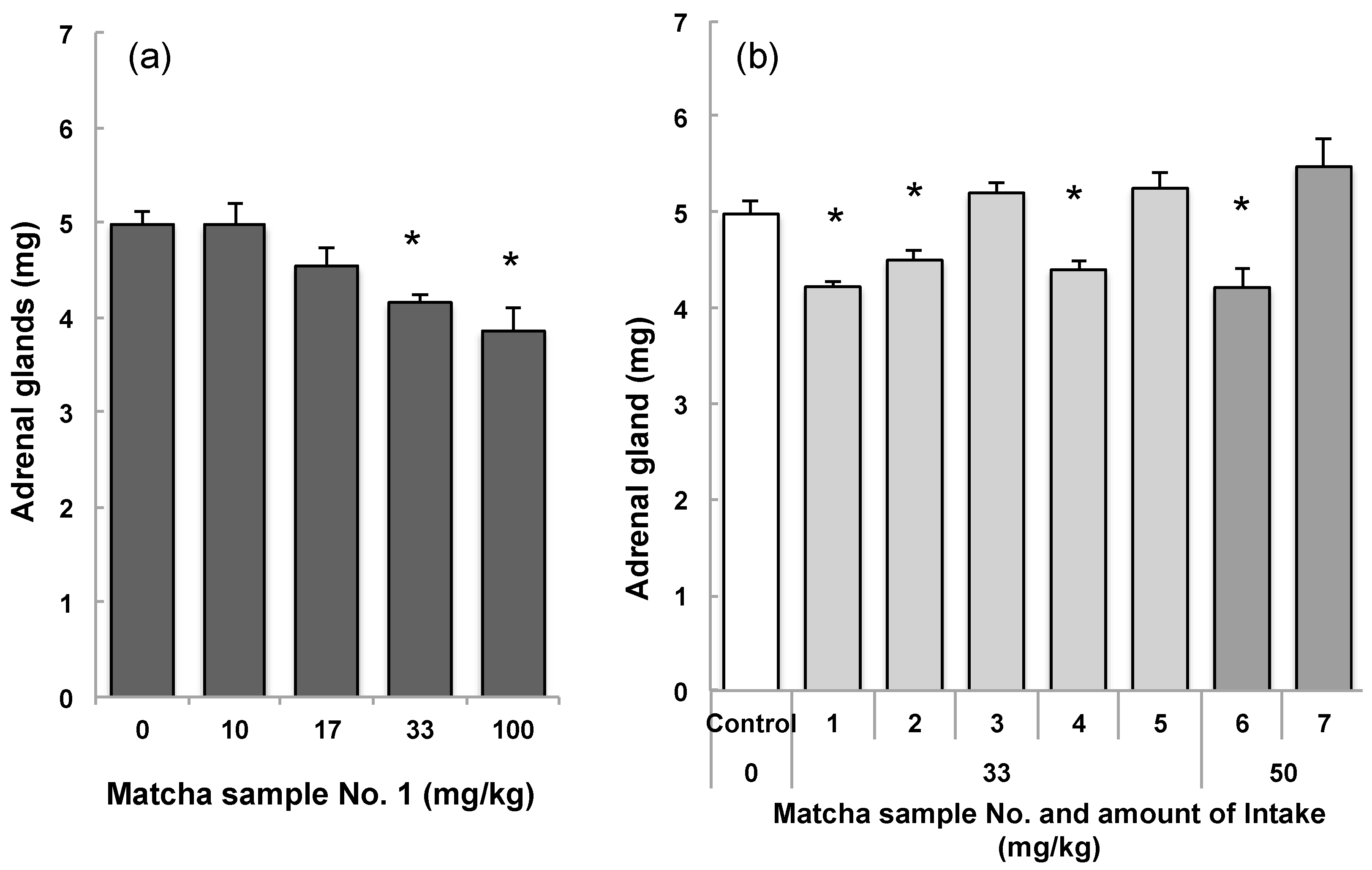
3.1.1. Anti-Stress Effects of Matcha in a Mouse Model of Psychosocial Stress
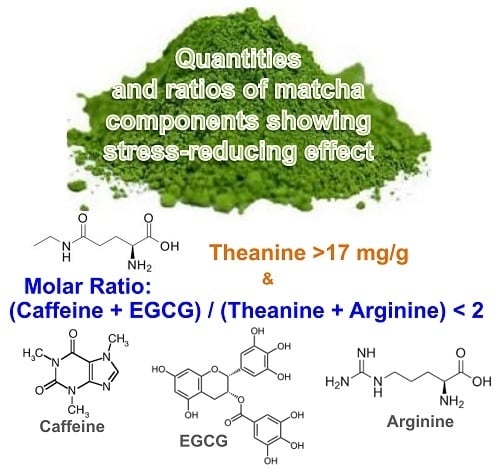
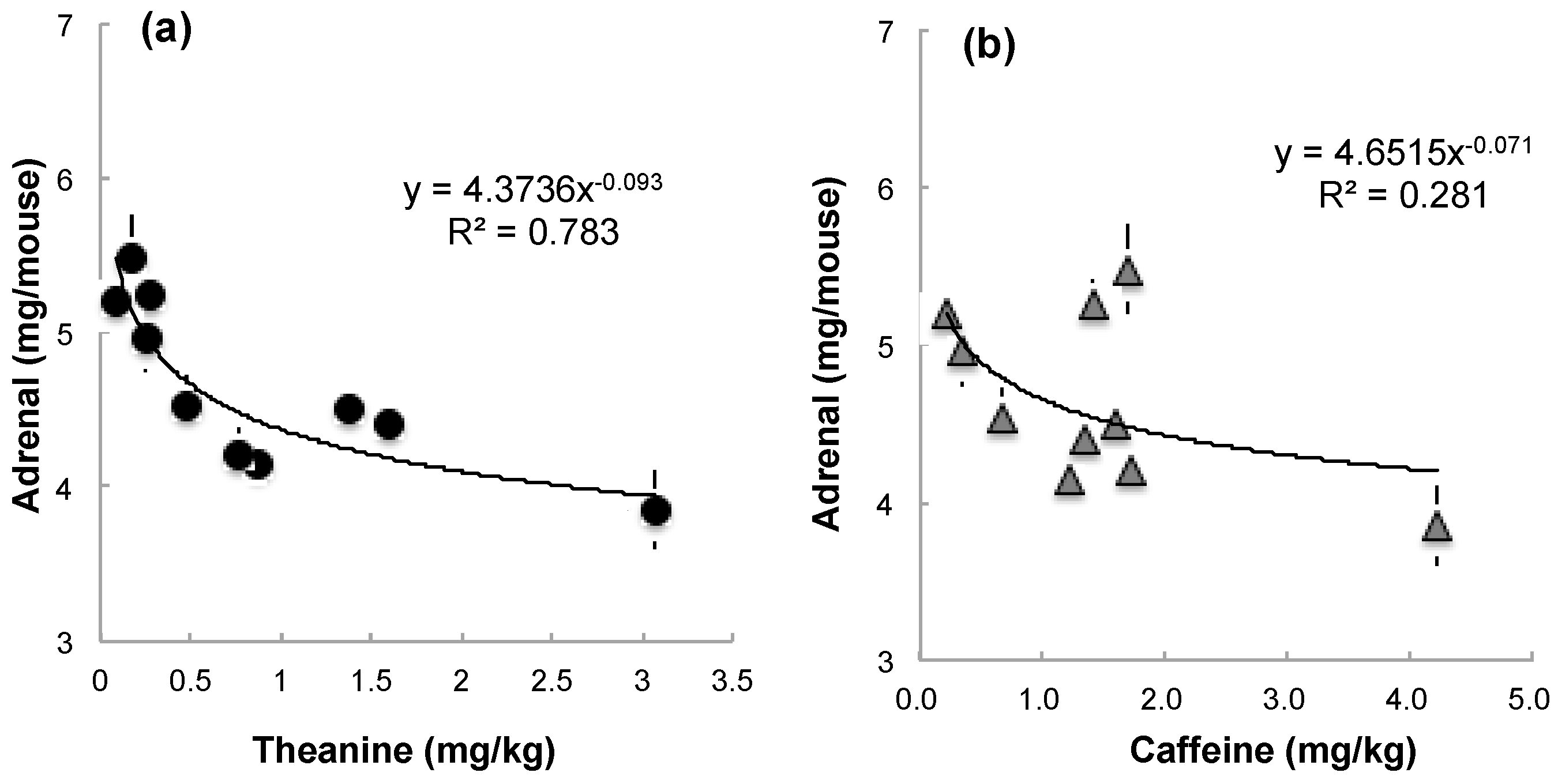
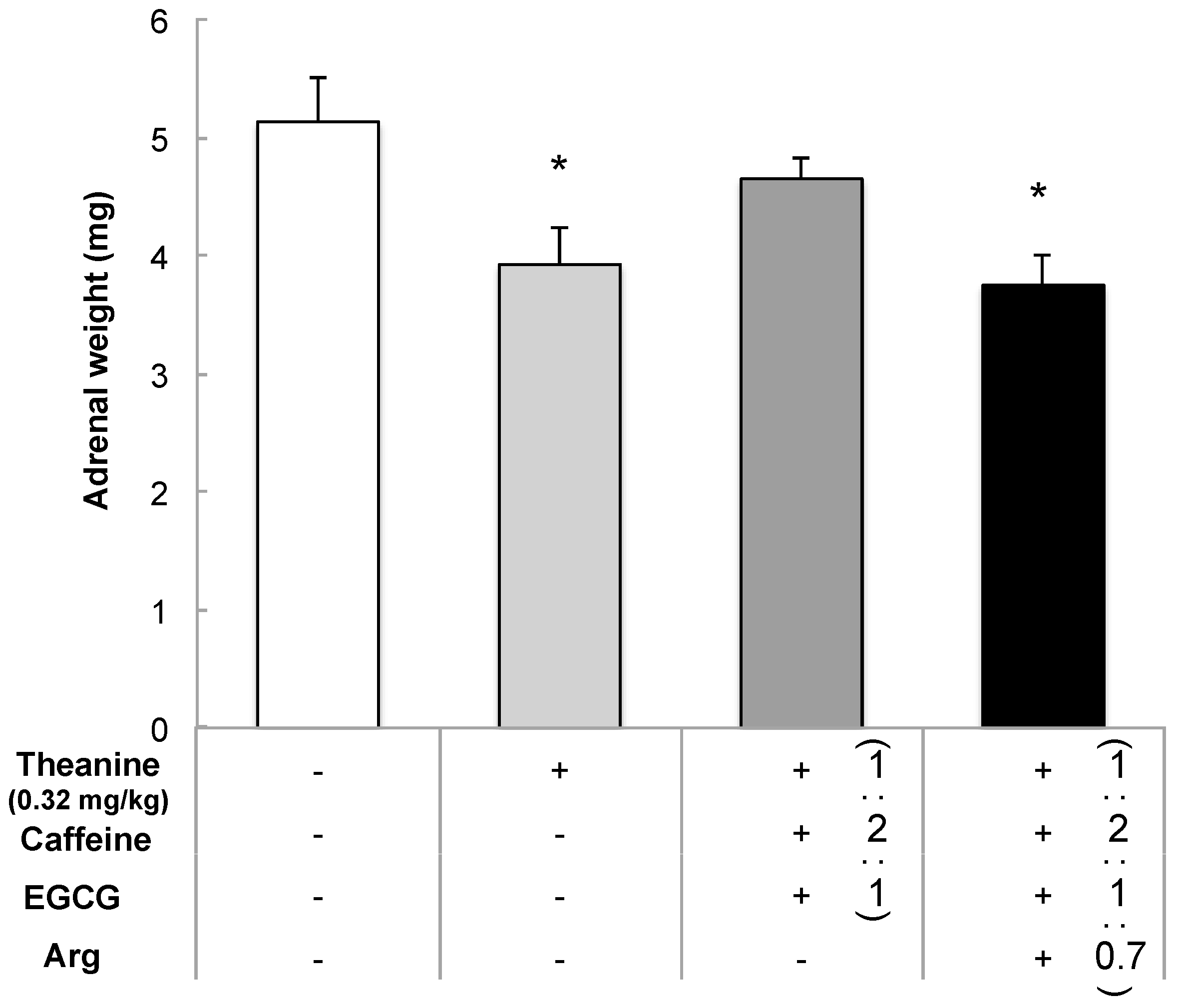
3.1.2. Relationship between Tea Components in Each Matcha Sample and Suppression of Adrenal Hypertrophy
3.2. Human Study
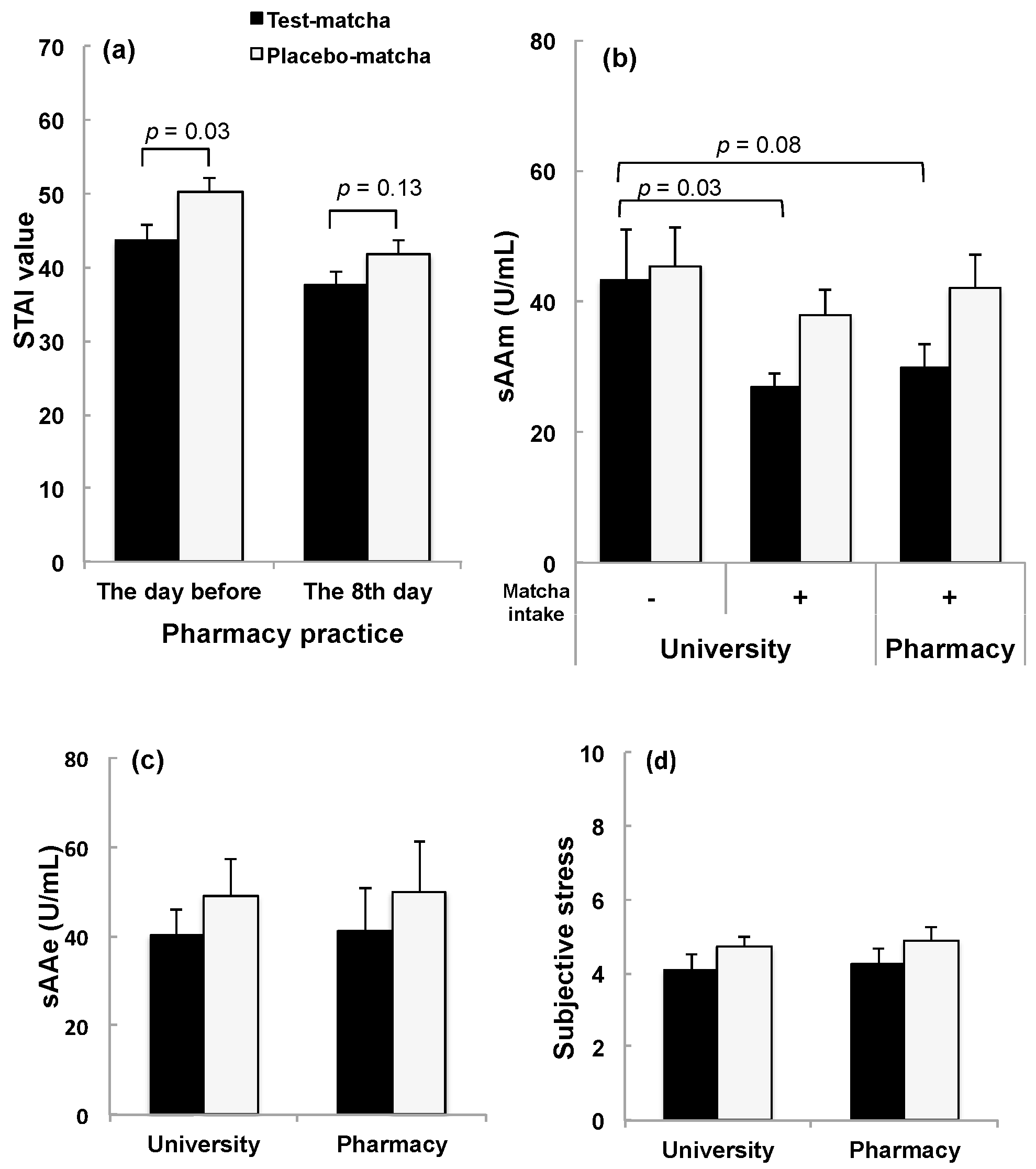
3.2.1. Effect of Matcha Ingestion on Stress in Students Assigned Pharmacy Practice Outside the University
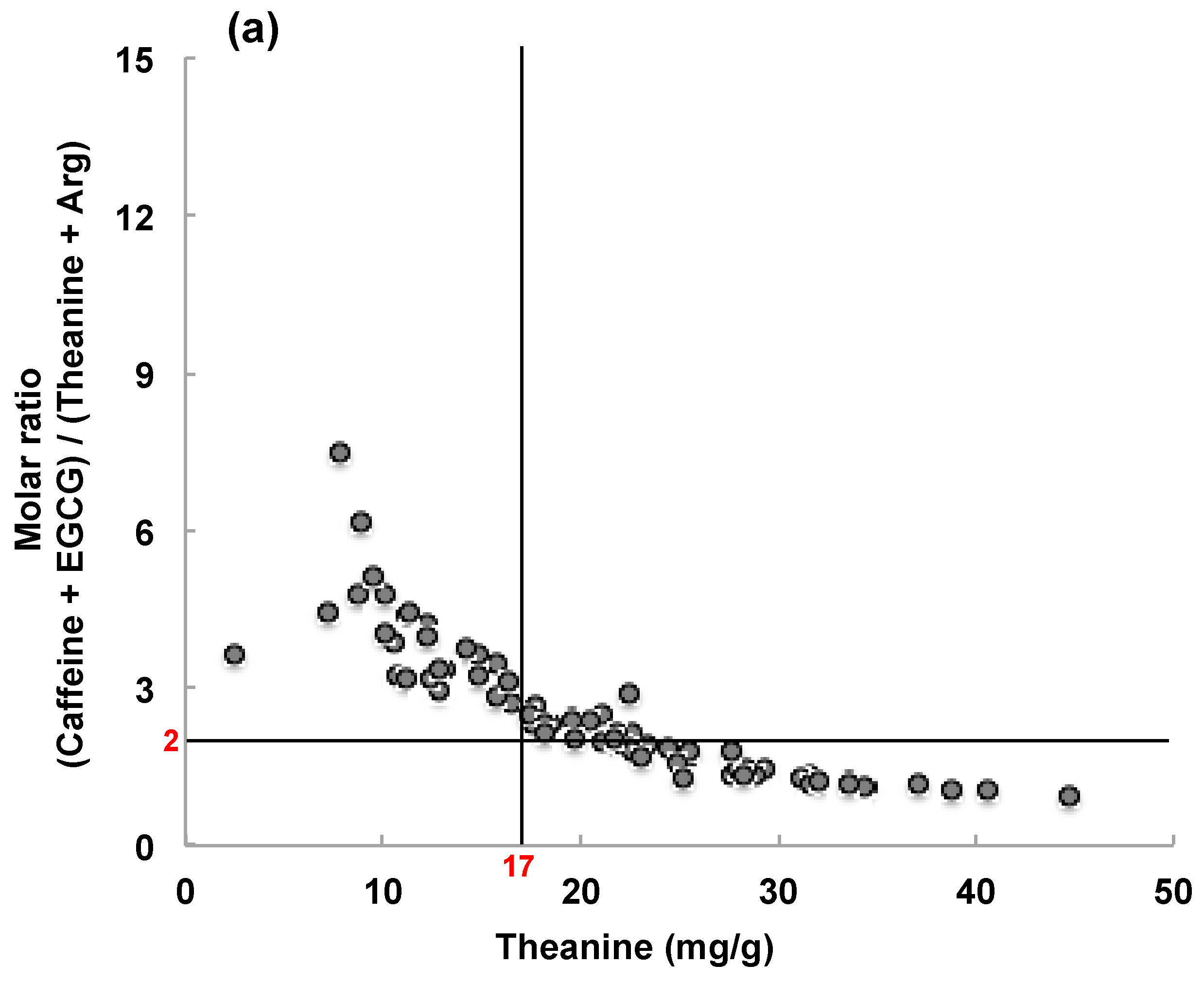
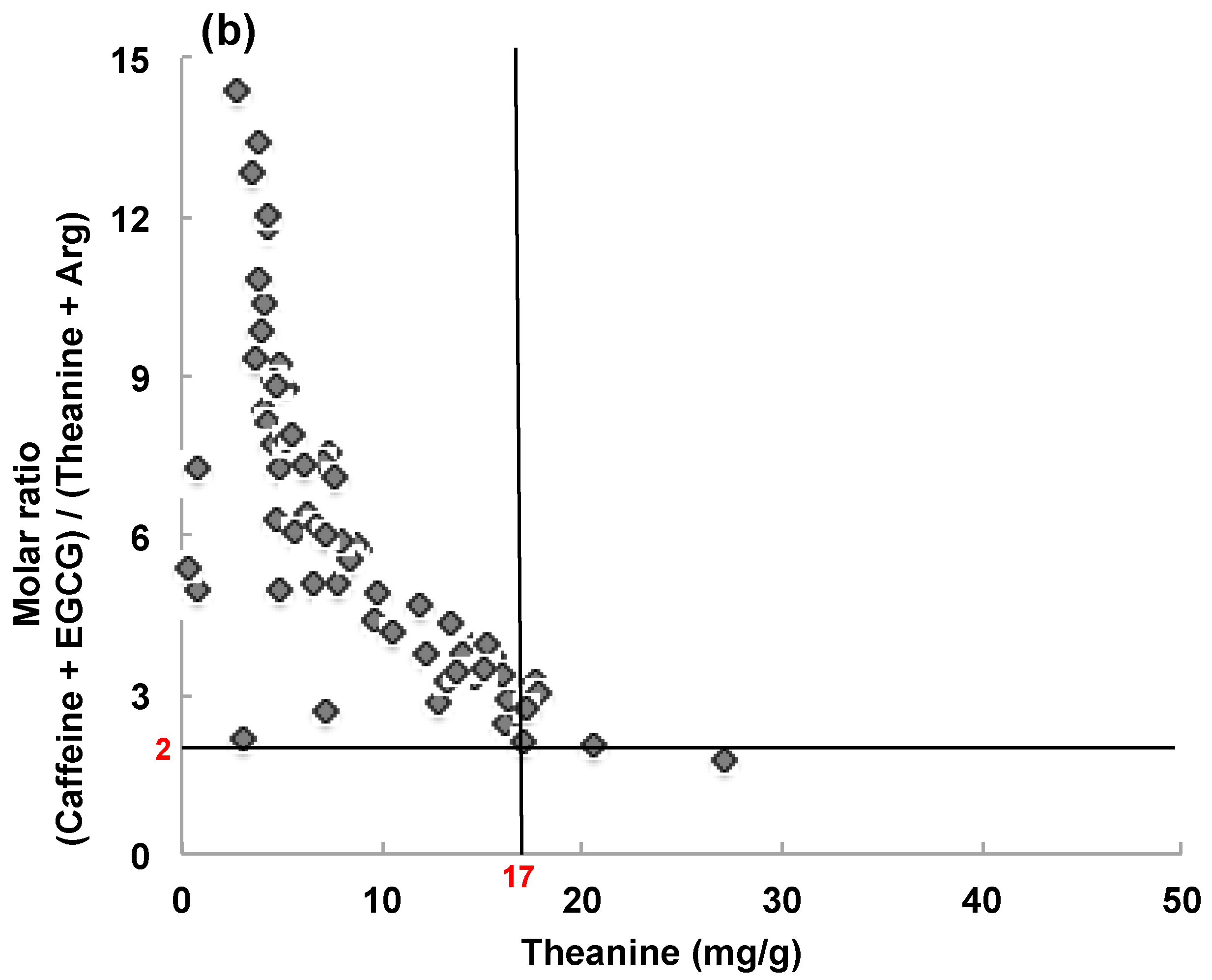
3.2.2. Composition of Matcha Marketed in Japan and Overseas
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Vuong, Q.V.; Bowyer, M.C.; Roach, P.D. L-Theanine: Properties, synthesis and isolation from tea. J. Sci. Food Agric. 2011, 91, 1931–1939. [Google Scholar] [CrossRef] [PubMed]
- Ruan, J.; Haerdter, R.; Gerendás, J. Impact of nitrogen supply on carbon/nitrogen allocation: A case study on amino acids and catechins in green tea [Camellia sinensis (L.) O. Kuntze] plants. Plant Biol. (Stuttg.) 2010, 12, 724–734. [Google Scholar] [CrossRef] [PubMed]
- Ikegaya, K.; Takayanagi, H.; Anan, T. Chemical composition of Matcha. Tea Res. J. (Chagyo Kenkyu Hokoku) 1984, 60, 79–81. [Google Scholar] [CrossRef]
- Ashihara, H. Occurrence, biosynthesis and metabolism of theanine (γ-glutamyl-l-ethylamide) in plants: A comprehensive review. Nat. Prod. Commun. 2015, 10, 803–810. [Google Scholar] [PubMed]
- Horie, H.; Ema, K.; Sumikawa, O. Chemical components of Matcha and powdered green tea. J. Cook. Sci. Jpn. (Nippon Chourikagaku Kaishi) 2017, 50, 182–188. [Google Scholar]
- Goto, T.; Nagashima, H.; Yoshida, Y.; Kiso, M. Contents of individual tea catechins and caffeine in Japanese green tea. Tea Res. J. (Chagyo Kenkyu Hokoku) 1996, 83, 21–28. [Google Scholar] [CrossRef]
- Ashihara, H.; Suzuki, T. Distribution and biosynthesis of caffeine in plants. Front. Biosci. 2004, 9, 1864–1876. [Google Scholar] [CrossRef] [PubMed]
- Munakata, M. Clinical significance of stress-related increase in blood pressure: Current evidence in office and out-of-office settings. Hypertens. Res. 2018. [Google Scholar] [CrossRef] [PubMed]
- Wirtz, P.H.; von Känel, R. Psychological stress, inflammation, and coronary heart disease. Curr. Cardiol. Rep. 2017, 19, 111. [Google Scholar] [CrossRef] [PubMed]
- Piirainen, S.; Youssef, A.; Song, C.; Kalueff, A.V.; Landreth, G.E.; Malm, T.; Tian, L. Psychosocial stress on neuroinflammation and cognitive dysfunctions in Alzheimer’s disease: The emerging role for microglia? Neurosci. Biobehav. Rev. 2017, 77, 148–164. [Google Scholar] [CrossRef] [PubMed]
- Saeed, M.; Naveed, M.; Arif, M.; Kakar, M.U.; Manzoor, R.; Abd El-Hack, M.E.; Alagawany, M.; Tiwari, R.; Khandia, R.; Munjal, A.; et al. Green tea (Camellia sinensis) and L-theanine: Medicinal values and beneficial applications in humans—A comprehensive review. Biomed. Pharmacother. 2017, 95, 1260–1275. [Google Scholar] [CrossRef] [PubMed]
- Kimura, K.; Ozeki, M.; Juneja, L.R.; Ohira, H. L-Theanine reduces psychological and physiological stress responses. Biol. Psychol. 2007, 74, 39–45. [Google Scholar] [CrossRef] [PubMed]
- Unno, K.; Fujitani, K.; Takamori, N.; Takabayashi, F.; Maeda, K.; Miyazaki, H.; Tanida, N.; Iguchi, K.; Shimoi, K.; Hoshino, M. Theanine intake improves the shortened lifespan, cognitive dysfunction and behavioural depression that are induced by chronic psychosocial stress in mice. Free Radic. Res. 2011, 45, 966–974. [Google Scholar] [CrossRef] [PubMed]
- Unno, K.; Iguchi, K.; Tanida, N.; Fujitani, K.; Takamori, N.; Yamamoto, H.; Ishii, N.; Nagano, H.; Nagashima, T.; Hara, A.; et al. Ingestion of theanine, an amino acid in tea, suppresses psychosocial stress in mice. Exp. Physiol. 2013, 98, 290–303. [Google Scholar] [CrossRef] [PubMed]
- Unno, K.; Tanida, H.; Ishii, N.; Yamamoto, H.; Iguchi, K.; Hoshino, M.; Takeda, A.; Ozawa, H.; Ohkubo, T.; Juneja, L.R.; et al. Anti-stress effect of theanine on students during pharmacy practice: Positive correlation among salivary α-amylase activity, trait anxiety and subjective stress. Pharmacol. Biochem. Behav. 2013, 111, 128–135. [Google Scholar] [CrossRef] [PubMed]
- Giles, G.E.; Mahoney, C.R.; Brunyé, T.T.; Taylor, H.A.; Kanarek, R.B. Caffeine and theanine exert opposite effects on attention under emotional arousal. Can. J. Physiol. Pharmacol. 2017, 95, 93–100. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Unno, K.; Hara, A.; Nakagawa, A.; Iguchi, K.; Ohshio, M.; Morita, A.; Nakamura, Y. Anti-stress effects of drinking green tea with lowered caffeine and enriched theanine, epigallocatechin and arginine on psychosocial stress induced adrenal hypertrophy in mice. Phytomedicine 2016, 23, 1365–1374. [Google Scholar] [CrossRef] [PubMed]
- Unno, K.; Yamada, H.; Iguchi, K.; Ishida, H.; Iwao, Y.; Morita, A.; Nakamura, Y. Anti-stress effect of green tea with lowered caffeine on humans: A pilot study. Biol. Pharm. Bull. 2017, 40, 902–909. [Google Scholar] [CrossRef] [PubMed]
- Unno, K.; Noda, S.; Kawasaki, Y.; Yamada, H.; Morita, A.; Iguchi, K.; Nakamura, Y. Ingestion of green tea with lowered caffeine improves sleep quality of the elderly via suppression of stress. J. Clin. Biochem. Nutr. 2017, 61, 210–216. [Google Scholar] [CrossRef] [PubMed]
- Unno, K.; Noda, S.; Kawasaki, Y.; Yamada, H.; Morita, A.; Iguchi, K.; Nakamura, Y. Reduced stress and improved sleep quality caused by green tea are associated with a reduced caffeine content. Nutrients 2017, 9, 777. [Google Scholar] [CrossRef] [PubMed]
- Van Stegeren, A.; Rohleder, N.; Everaerd, W.; Wolf, O.T. Salivary alpha amylase as marker for adrenergic activity during stress: Effect of betablockade. Psychoneuroendocrinology 2006, 31, 137–141. [Google Scholar] [CrossRef] [PubMed]
- Goto, T.; Horie, H.; Mukai, T. Analysis of major amino acids in green tea by high-performance liquid chromatography coupled with OPA precolumn derivatization. Tea Res. J. (Chagyo Kenkyu Hokoku) 1993, 77, 29–33. [Google Scholar]
- Yamaguchi, M.; Kanemori, T.; Kanemaru, M.; Takai, N.; Mizuno, Y.; Yoshida, H. Performance evaluation of salivary amylase activity monitor. Biosens. Bioelectron. 2004, 20, 491–497. [Google Scholar] [CrossRef] [PubMed]
- Niethard, N.; Burgalossi, A.; Born, J. Plasticity during Sleep Is Linked to Specific Regulation of Cortical Circuit Activity. Front. Neural Circuits 2017, 11, 65. [Google Scholar] [CrossRef] [PubMed]
- Pané-Farré, C.A.; Alius, M.G.; Modeß, C.; Methling, K.; Blumenthal, T.; Hamm, A.O. Anxiety sensitivity and expectation of arousal differentially affect the respiratory response to caffeine. Psychopharmacology (Berl) 2015, 232, 1931–1939. [Google Scholar] [CrossRef] [PubMed]
- Vilarim, M.M.; Rocha Araujo, D.M.; Nardi, A.E. Caffeine challenge test and panic disorder: A systematic literature review. Expert Rev. Neurother. 2011, 11, 1185–1195. [Google Scholar] [CrossRef] [PubMed]
- Dias, G.P.; Cavegn, N.; Nix, A.; do Nascimento Bevilaqua, M.C.; Stangl, D.; Zainuddin, M.S.; Nardi, A.E.; Gardino, P.F.; Thuret, S. The role of dietary polyphenols on adult hippocampal neurogenesis: Molecular mechanisms and behavioural effects on depression and anxiety. Oxid. Med. Cell. Longev. 2012, 2012, 541971. [Google Scholar] [CrossRef] [PubMed]
- Catuara-Solarz, S.; Espinosa-Carrasco, J.; Erb, I.; Langohr, K.; Gonzalez, J.R.; Notredame, C.; Dierssen, M. Combined treatment with environmental enrichment and (−)-epigallocatechin-3-gallate ameliorates learning deficits and hippocampal alterations in a mouse model of down syndrome. eNeuro 2016, 3, e0103. [Google Scholar] [CrossRef] [PubMed]
- Chou, C.W.; Huang, W.J.; Tien, L.T.; Wang, S.J. (-)-Epigallocatechin gallate, the most active polyphenolic catechin in green tea, presynaptically facilitates Ca2+-dependent glutamate release via activation of protein kinase C in rat cerebral cortex. Synapse 2007, 61, 889–902. [Google Scholar] [CrossRef] [PubMed]
- Inoue, K.; Miyazaki, Y.; Unno, K.; Min, J.Z.; Todoroki, K.; Toyo’oka, T. Stable isotope dilution HILIC-MS/MS method for accurate quantification of glutamic acid, glutamine, pyroglutamic acid, GABA and theanine in mouse brain tissues. Biomed. Chromatogr. 2016, 30, 55–61. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, S.; Ota, M.; Ogura, J.; Kato, K.; Kunugi, H. Effects of L-theanine on anxiety-like behavior, cerebrospinal fluid amino acid profile, and hippocampal activity in Wistar Kyoto rats. Psychopharmacology (Berl) 2018, 235, 37–45. [Google Scholar] [CrossRef] [PubMed]
- Horie, H.; Ema, K.; Nishikawa, H.; Nakamura, Y. Comparison of the chemical components of powdered green tea sold in the US. JARQ 2018, 52, 143–147. [Google Scholar] [CrossRef]
- Dietz, C.; Dekker, M.; Piqueras-Fiszman, B. An intervention study on the effect of matcha tea, in drink and snack bar formats, on mood and cognitive performance. Food Res. Int. 2017, 99, 72–83. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.S.; Quon, M.J.; Kim, J.A. New insights into the mechanisms of polyphenols beyond antioxidant properties; lessons from the green tea polyphenol, epigallocatechin 3-gallate. Redox Biol. 2014, 2, 187–195. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Practice | University | Pharmacy | ||||
|---|---|---|---|---|---|---|
| Days | 3 | 3–10 | 7 | 8 | ||
| Matcha intake | (−) | (−) | + | + | ||
| sAAm | + | (−) | + | + | ||
| STAI | (−) | (−) | (−) | + | (−) | + |
| Sample | Amino Acids (mg/g) | ||||||||
| No | Theanine | Arg | Glu | Asp | Asn | Ser | Gln | GABA | Total |
| 1 | 28.99 ± 0.10 | 12.50 ± 0.04 | 7.52 ± 0.02 | 6.95 ± 0.02 | 2.90 ± 0.01 | 1.25 ± 0.00 | 1.08 ± 0.01 | 0.25 ± 0.00 | 61.45 ± 0.19 |
| 2 | 38.77 ± 0.16 | 18.19 ± 0.02 | 7.58 ± 0.05 | 8.47 ± 0.05 | 3.36 ± 0.01 | 1.84 ± 0.01 | 1.34 ± 0.01 | 0.22 ± 0.00 | 79.77 ± 0.43 |
| 3 | 2.48 ± 0.03 | 0.36 ± 0.00 | 0.48 ± 0.00 | 0.65 ± 0.01 | 0.09 ± 0.00 | 0.13 ± 0.00 | 0.11 ± 0.00 | 0.05 ± 0.00 | 4.35 ± 0.04 |
| 4 | 44.65 ± 1.73 | 20.39 ± 1.06 | 5.86 ± 0.16 | 7.95 ± 0.19 | 4.01 ± 0.12 | 1.74 ± 0.05 | 3.20 ± 0.11 | 0.23 ± 0.01 | 88.02 ± 3.41 |
| 5 | 7.87 ± 0.17 | 1.41 ± 0.06 | 2.64 ± 0.03 | 2.80 ± 0.02 | 0.59 ± 0.02 | 0.57 ± 0.03 | 0.51 ± 0.01 | 0.14 ± 0.00 | 16.52 ± 0.26 |
| 6 | 17.41 ± 0.18 | 12.33 ± 0.32 | 5.37 ± 0.04 | 7.60 ± 0.14 | 3.18 ± 0.03 | 1.34 ± 0.01 | 0.85 ± 0.01 | 0.13 ± 0.00 | 48.21 ± 0.70 |
| 7 | 3.91 ± 0.09 | 1.43 ± 0.11 | 2.82 ± 0.05 | 2.94 ± 0.06 | 0.82 ± 0.02 | 0.58 ± 0.01 | 0.38 ± 0.01 | 0.26 ± 0.01 | 13.15 ± 0.37 |
| Sample | Caffeine | Catechin (mg/g) | |||||||
| No | (mg/g) | EGCG | ECG | EGC | EC | ||||
| 1 | 39.95 ± 0.19 | 59.34 ± 0.41 | 12.44 ± 0.07 | 14.86 ± 0.06 | 4.26 ± 0.01 | ||||
| 2 | 44.43 ± 0.47 | 57.20 ± 0.84 | 13.81 ± 0.21 | 9.68 ± 0.04 | 3.44 ± 0.02 | ||||
| 3 | 5.95 ± 0.02 | 13.15 ± 0.08 | 2.20 ± 0.01 | 4.09 ± 0.02 | 0.87 ± 0.00 | ||||
| 4 | 37.19 ± 0.36 | 48.44 ± 0.53 | 9.96 ± 0.15 | 8.40 ± 0.06 | 2.50 ± 0.04 | ||||
| 5 | 40.35 ± 0.70 | 86.76 ± 1.47 | 06.09 ± 0.34 | 28.76 ± 0.38 | 6.48 ± 0.13 | ||||
| 6 | 38.95 ± 0.55 | 49.15 ± 0.69 | 25.41 ± 0.69 | 9.91 ± 0.09 | 3.29 ± 0.03 | ||||
| 7 | 37.06 ± 0.23 | 64.61 ± 4.30 | 30.54 ± 1.51 | 31.21 ± 0.40 | 6.75 ± 0.11 | ||||
| Matcha | Concentration | Adrenal | Matcha Intake | Theanine | Arginine | Caffeine | EGCG |
|---|---|---|---|---|---|---|---|
| No. | (mg/kg) | (mg/Mouse) | (mg/kg) | (mg/kg) | (mg/kg) | (mg/kg) | (mg/kg) |
| 1 | 10 | 4.97 ± 0.23 | 8.7 ± 2.2 | 0.25 ± 0.03 | 0.11 ± 0.01 | 0.35 ± 0.04 | 0.51 ± 0.05 |
| 1 | 17 | 4.53 ± 0.19 | 16.7 ± 0.4 | 0.48 ± 0.01 | 0.21 ± 0.01 | 0.67 ± 0.01 | 0.99 ± 0.02 |
| 1 | 33 | 4.15 ± 0.09 | 30.2 ± 1.5 | 0.88 ± 0.04 | 0.38 ± 0.02 | 1.21 ± 0.06 | 1.79 ± 0.09 |
| 1 | 100 | 3.85 ± 0.25 | 105.9 ± 5.0 | 3.07 ± 0.14 | 1.32 ± 0.06 | 4.23 ± 0.20 | 6.28 ± 0.30 |
| 2 | 33 | 4.50 ± 0.09 | 35.5 ± 0.2 | 1.38 ± 0.01 | 0.65 ± 0.00 | 1.58 ± 0.01 | 2.03 ± 0.01 |
| 3 | 33 | 5.20 ± 0.10 | 35.6 ± 1.1 | 0.09 ± 0.00 | 0.01 ± 0.00 | 0.21 ± 0.01 | 0.47 ± 0.01 |
| 4 | 33 | 4.40 ± 0.09 | 35.8 ± 0.3 | 1.60 ± 0.01 | 0.73 ± 0.01 | 1.33 ± 0.01 | 1.73 ± 0.01 |
| 5 | 33 | 5.25 ± 0.17 | 34.8 ± 0.6 | 0.27 ± 0.01 | 0.05 ± 0.00 | 1.40 ± 0.02 | 3.02 ± 0.05 |
| 6 | 50 | 4.21 ± 0.19 | 44.3 ± 1.9 | 0.77 ± 0.03 | 0.55 ± 0.02 | 1.73 ± 0.07 | 1.79 ± 0.09 |
| 7 | 50 | 5.48 ± 0.29 | 45.7 ± 1.7 | 0.18 ± 0.01 | 0.07 ± 0.00 | 1.69 ± 0.06 | 2.95 ± 0.11 |
| Matcha No. | Caffeine/Theanine | EGCG/Theanine | Arg/Theanine | (Caffeine + EGCG)/(Theanine + Arg) |
|---|---|---|---|---|
| 1 | 1.23 | 0.78 | 0.43 | 1.41 |
| 2 | 1.03 | 0.56 | 0.47 | 1.08 |
| 3 | 2.17 | 2.01 | 0.15 | 3.63 |
| 4 | 0.75 | 0.41 | 0.46 | 0.79 |
| 5 | 4.60 | 4.19 | 0.18 | 7.45 |
| 6 | 2.01 | 1.07 | 0.71 | 1.80 |
| 7 | 8.50 | 6.28 | 0.37 | 10.79 |
| Questionnaire Item | University | Pharmacy | ||||
|---|---|---|---|---|---|---|
| Matcha | p | Matcha | p | |||
| Test | Placebo | value | Test | Placebo | value | |
| Physical condition (1−5) | 3.55 ± 0.16 | 3.32 ± 0.13 | 0.30 | 3.44 ± 0.12 | 3.36 ± 0.12 | 0.62 |
| Sleep time (h) | 6.33 ± 0.15 | 6.20 ± 0.18 | 0.71 | 6.15 ± 0.17 | 6.40 ± 0.19 | 0.40 |
| Achievement emotion (1−5) | (−) | (−) | 3.49 ± 0.20 | 3.49 ± 0.16 | 0.82 | |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Unno, K.; Furushima, D.; Hamamoto, S.; Iguchi, K.; Yamada, H.; Morita, A.; Horie, H.; Nakamura, Y. Stress-Reducing Function of Matcha Green Tea in Animal Experiments and Clinical Trials. Nutrients 2018, 10, 1468. https://doi.org/10.3390/nu10101468
Unno K, Furushima D, Hamamoto S, Iguchi K, Yamada H, Morita A, Horie H, Nakamura Y. Stress-Reducing Function of Matcha Green Tea in Animal Experiments and Clinical Trials. Nutrients. 2018; 10(10):1468. https://doi.org/10.3390/nu10101468
Chicago/Turabian StyleUnno, Keiko, Daisuke Furushima, Shingo Hamamoto, Kazuaki Iguchi, Hiroshi Yamada, Akio Morita, Hideki Horie, and Yoriyuki Nakamura. 2018. "Stress-Reducing Function of Matcha Green Tea in Animal Experiments and Clinical Trials" Nutrients 10, no. 10: 1468. https://doi.org/10.3390/nu10101468
APA StyleUnno, K., Furushima, D., Hamamoto, S., Iguchi, K., Yamada, H., Morita, A., Horie, H., & Nakamura, Y. (2018). Stress-Reducing Function of Matcha Green Tea in Animal Experiments and Clinical Trials. Nutrients, 10(10), 1468. https://doi.org/10.3390/nu10101468