Further Evidence on Efficacy of Diet Supplementation with Fatty Acids in Ocular Pathologies: Insights from the EAE Model of Optic Neuritis
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Induction of EAE
2.3. Dietary Supplementation
2.4. Isolation of Total RNA and Proteins
2.5. Quantitative Real Time PCR
2.6. Western Blot
2.7. Electron Microscopy and Quantitative Analysis
2.8. Electroretinography
2.9. Statistical Analysis
3. Results
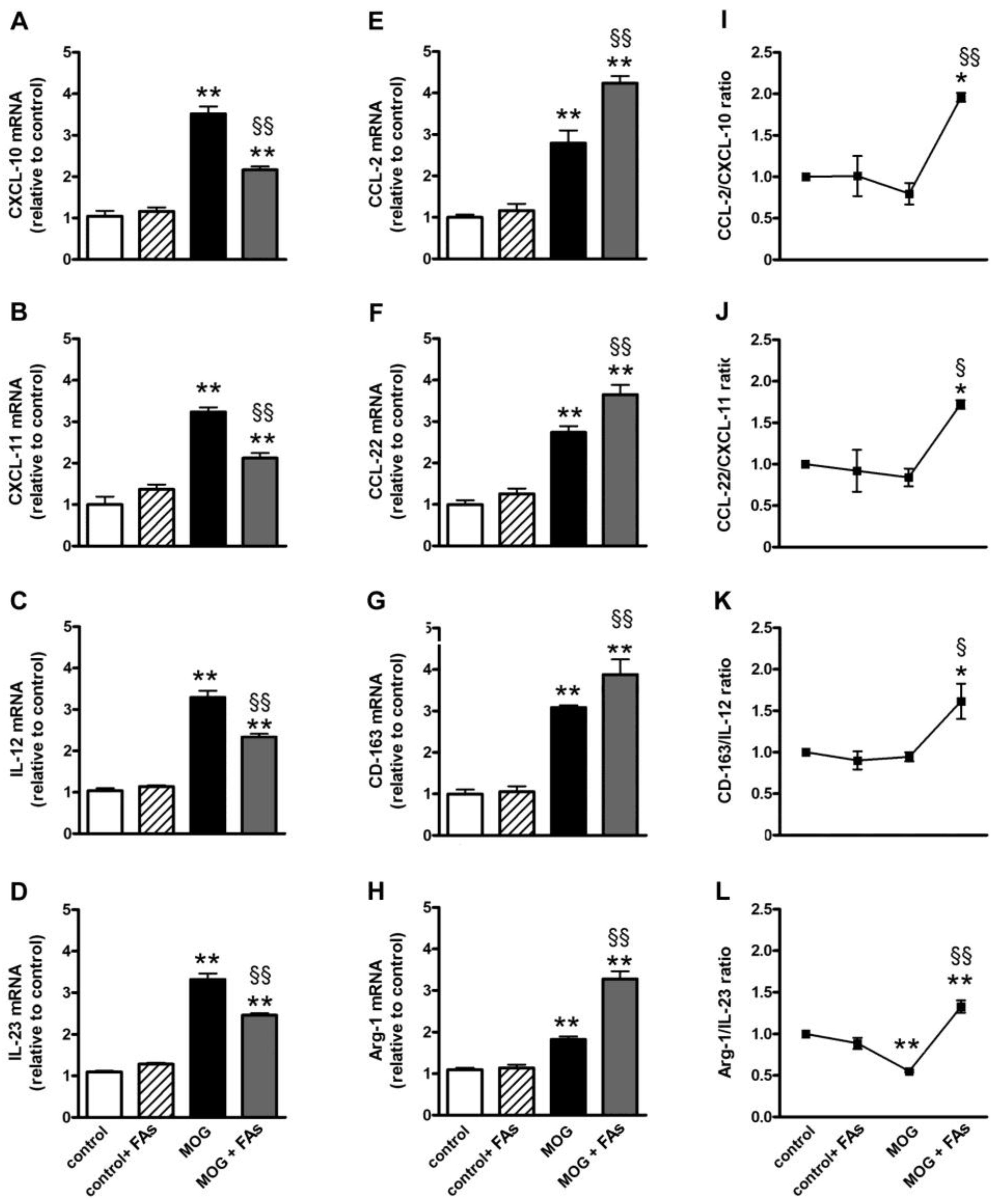
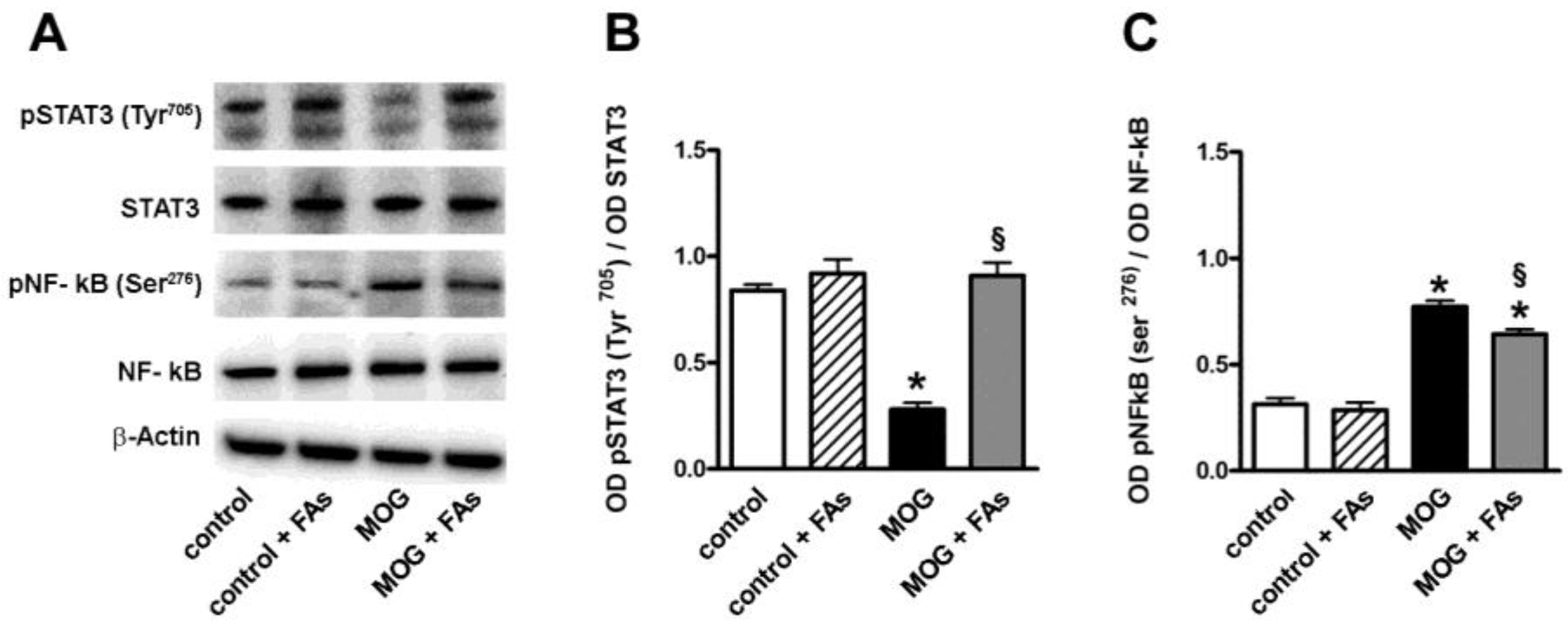
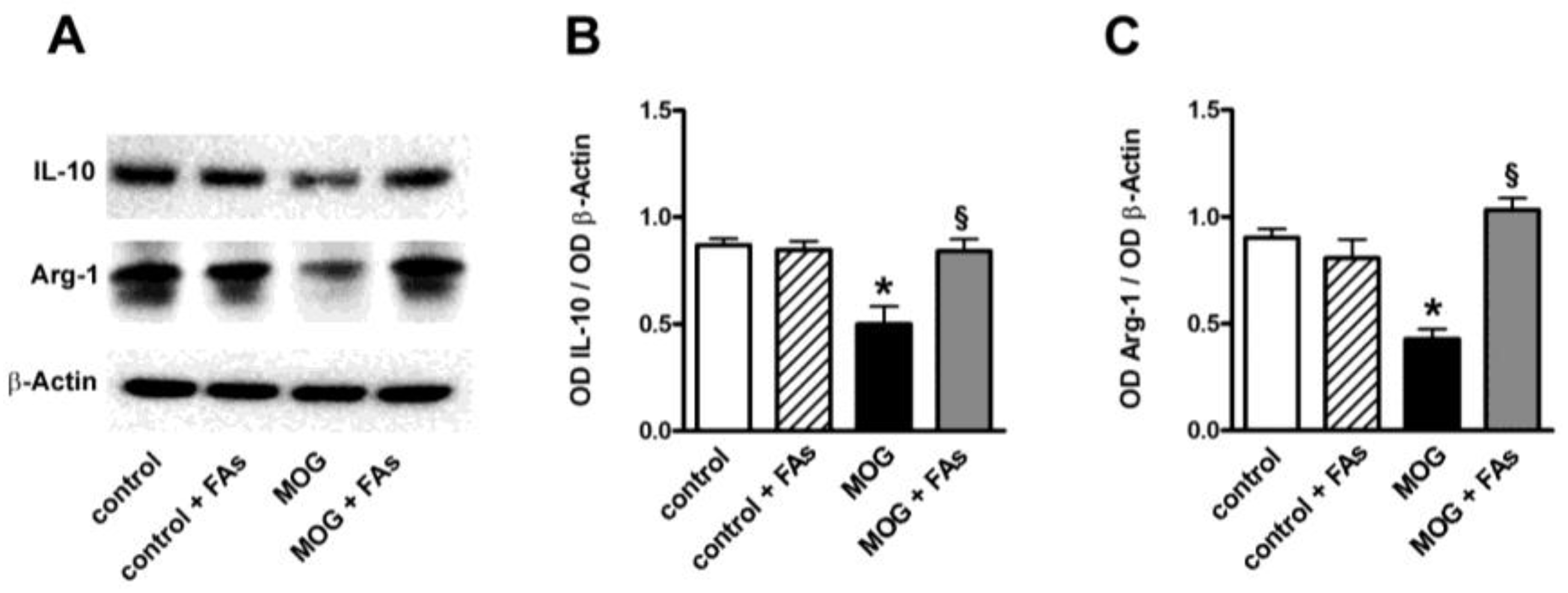
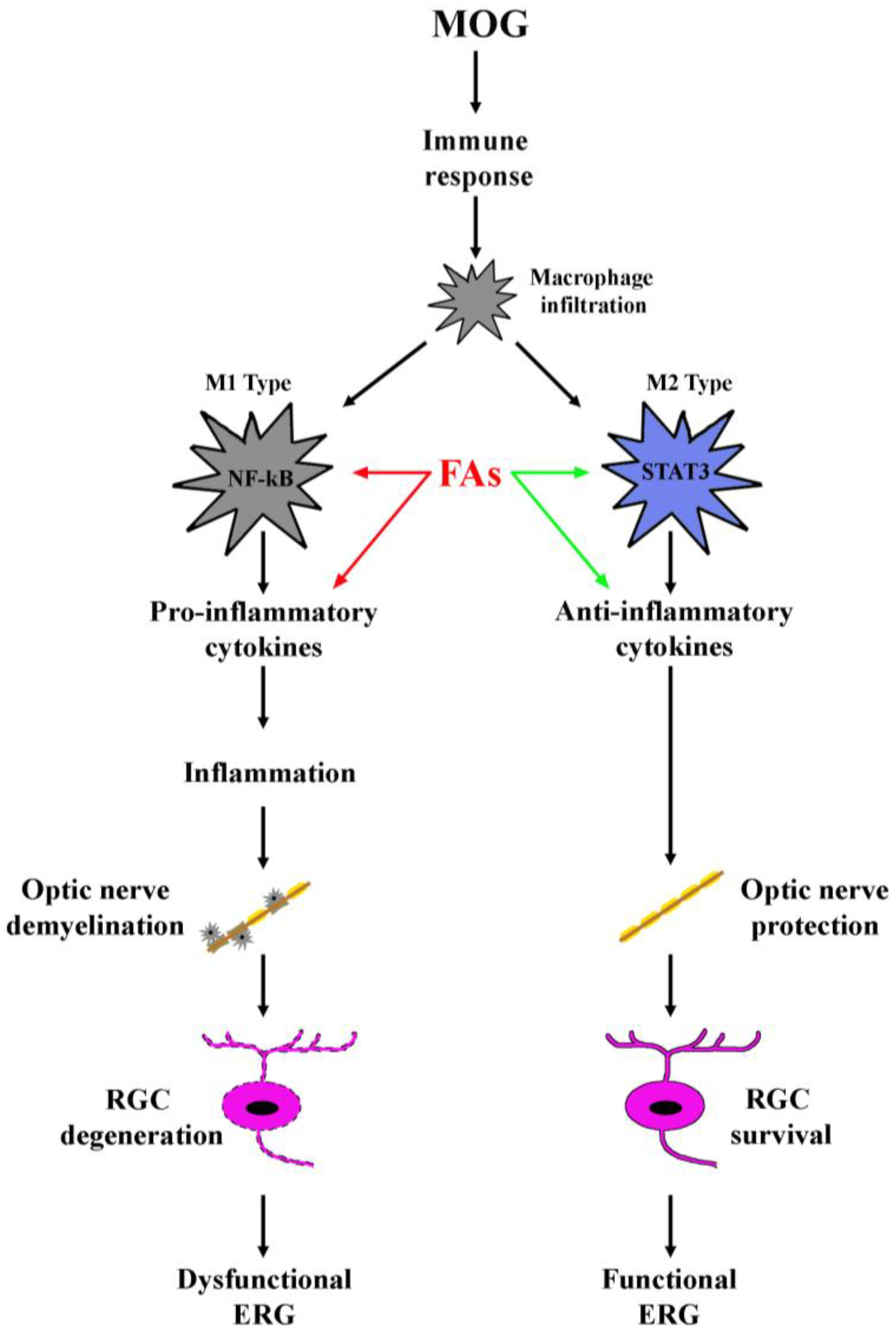
3.1. Supplementation with FAs Shifts M1 Macrophages toward M2 Phenotype
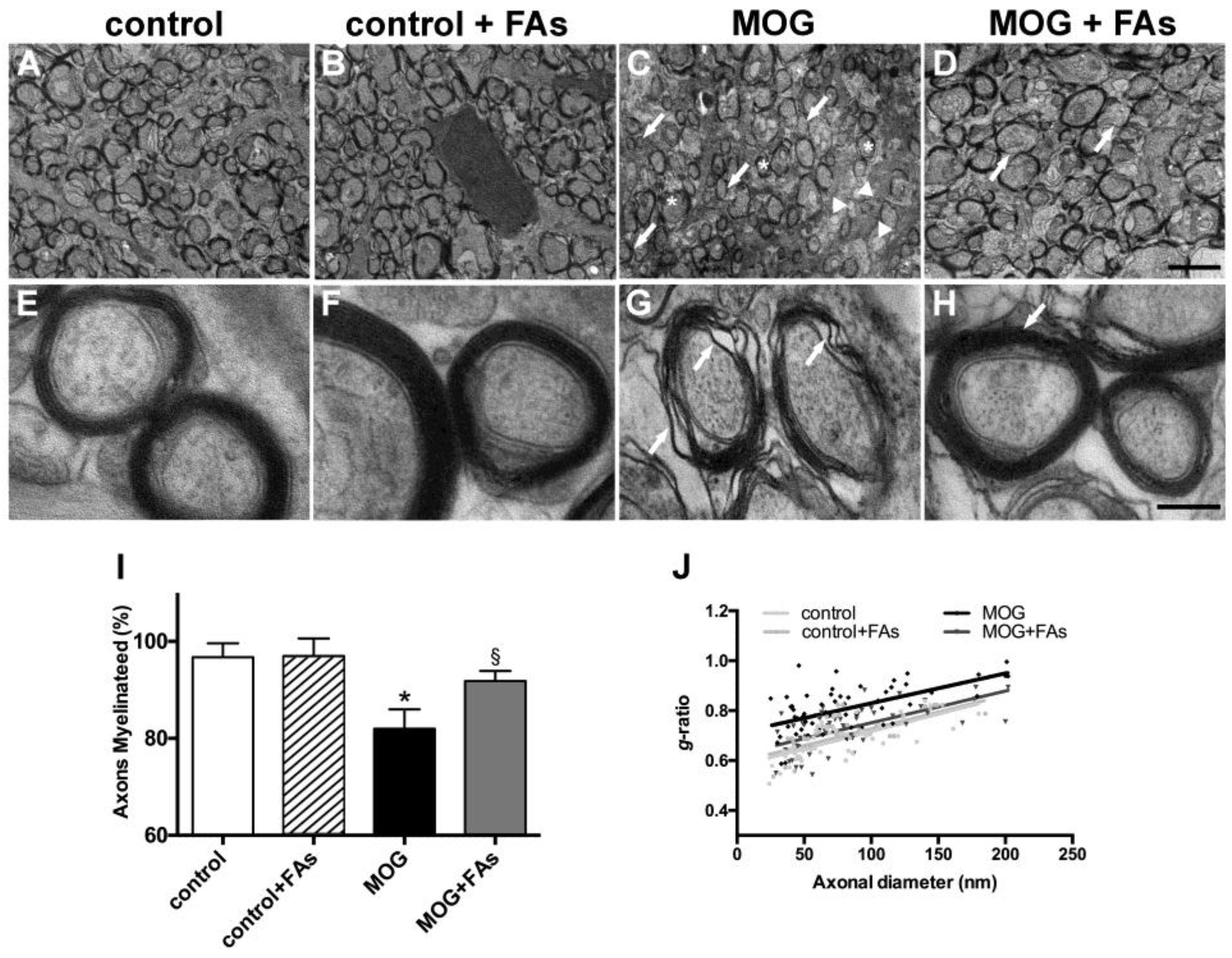
3.2. Supplementation with FAs Counteracts Optic Nerve Damage
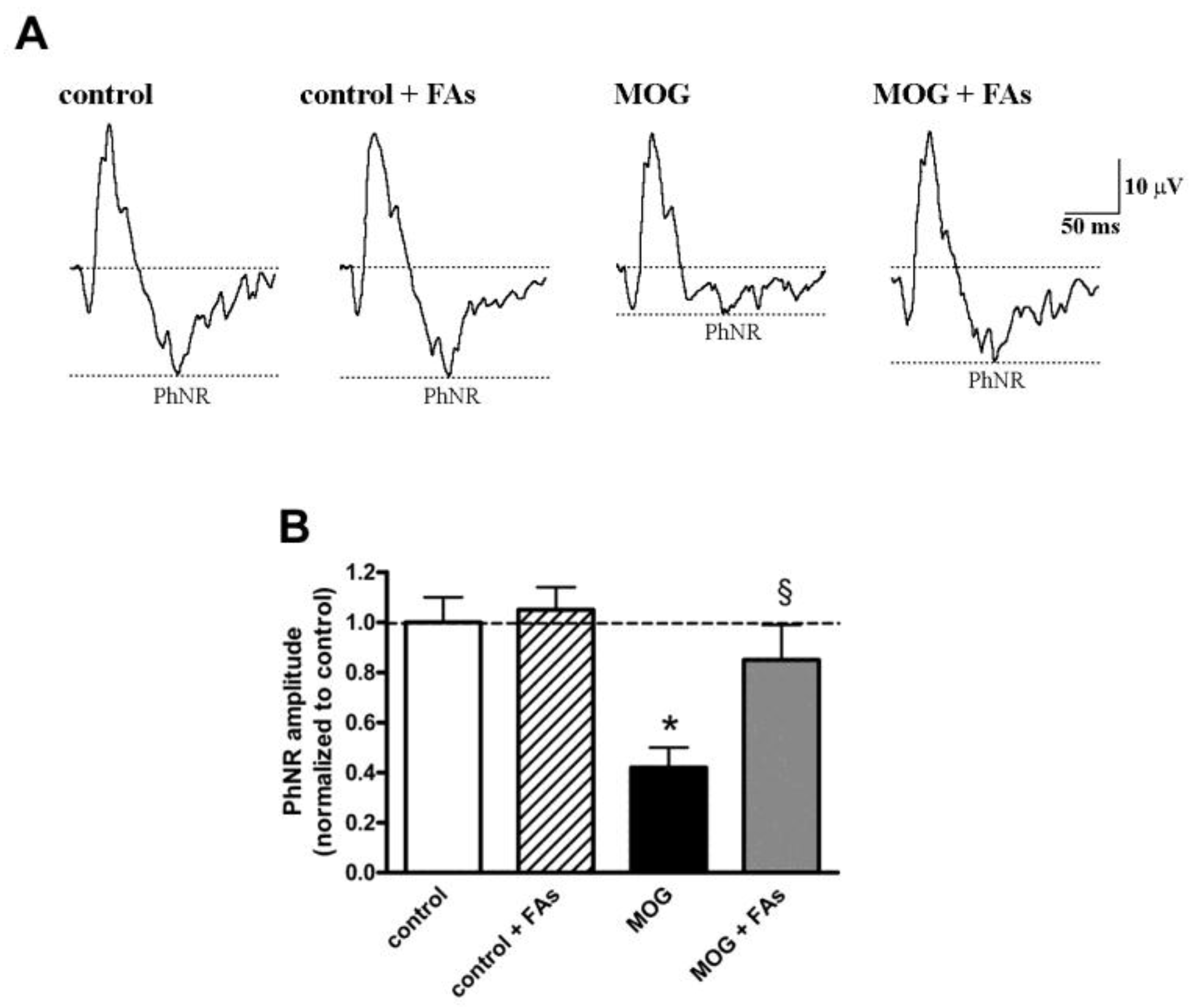
3.3. Supplementation with FAs Prevents the Reduction of PhNR Amplitude
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Abu-Amero, K.K.; Kondkar, A.A.; Chalam, K.V. Resveratrol and ophthalmic diseases. Nutrients 2016, 5, 200. [Google Scholar] [CrossRef] [PubMed]
- Age-Related Eye Disease Study 2 Research Group; Bonds, D.E.; Harringfton, M.; Worral, B.B.; Bertoni, A.G.; Eaton, C.B.; Hsia, J.; Robinson, J.; Clemons, T.E.; Fine, L.J.; et al. Lutein + zeaxanthin and omega-3 fatty acids for age-related macular degeneration: The Age-Related Eye Disease Study 2 (AREDS2) randomized clinical trial. JAMA Intern. Med. 2013, 309, 2005–2015. [Google Scholar] [CrossRef]
- Lyzogubov, V.V.; Bora, N.S.; Tytarenko, R.G.; Bora, P.S. Polyethylene glycol induced mouse model of retinal degeneration. Exp. Eye Res. 2014, 127, 143–152. [Google Scholar] [CrossRef] [PubMed]
- Cammalleri, M.; Dal Monte, M.; Locri, F.; Lardner, E.; Kvanta, A.; Rusciano, D.; André, H.; Bagnoli, P. Efficacy of a fatty acids dietary supplement in a polyethylene glycol-induced mouse model of retinal degeneration. Nutrients 2017, 9, 1079. [Google Scholar] [CrossRef] [PubMed]
- Dal Monte, M.; Cammalleri, M.; Locri, F.; Amato, R.; Marsili, S.; Rusciano, D.; Bagnoli, P. Fatty acids dietary supplements exert anti-inflammatory action and limit ganglion cell degeneration in the retina of the EAE mouse model of multiple sclerosis. Nutrients 2018, 10, 325. [Google Scholar] [CrossRef] [PubMed]
- Benowitz, L.I.; He, Z.; Goldberg, J.L. Reaching the brain: Advances in optic nerve regeneration. Exp. Neurol. 2017, 287, 365–373. [Google Scholar] [CrossRef] [PubMed]
- Mangas, A.; Vecino, E.; David Rodríguez, F.; Geffard, M.; Coveñas, R. GEMSP exerts a myelin-protecting role in the rat optic nerve. Neurol. Res. 2013, 35, 903–911. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Gao, F.; Hu, F.; Huang, J.; Wang, M.; Xu, P.; Zhang, R.; Chen, J.; Sun, X.; Zhang, S.; et al. Asiatic acid prevents retinal ganglion cell apoptosis in a rat model of glaucoma. Front. Neurosci. 2018, 12. [Google Scholar] [CrossRef] [PubMed]
- Huang, R.; Liang, S.; Fang, L.; Wu, M.; Cheng, H.; Mi, X.; Ding, Y. Low-dose minocycline mediated neuroprotection on retinal ischemia-reperfusion injury of mice. Mol. Vis. 2018, 24, 367–378. [Google Scholar] [PubMed]
- Zhou, X.; Cheng, Y.; Zhang, R.; Li, G.; Yang, B.; Zhang, S.; Wu, J. Alpha7 nicotinic acetylcholine receptor agonist promotes retinal ganglion cell function via modulating GABAergic presynaptic activity in a chronic glaucomatous model. Sci. Rep. 2017, 7, 1734. [Google Scholar] [CrossRef] [PubMed]
- Chu, F.; Shi, M.; Zheng, C.; Shen, D.; Zhu, J.; Zheng, X.; Cui, L. The roles of macrophages and microglia in multiple sclerosis and experimental autoimmune encephalomyelitis. J. Neuroimmunol. 2018, 15, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Karanjia, R.; Berezovsky, A.; Sacai, P.Y.; Cavascan, N.N.; Liu, H.Y.; Nazarali, S.; Moraes-Filho, M.N.; Anderson, K.; Tran, J.S.; Watanabe, S.E.; et al. The photopic negative response: An objective measure of retinal ganglion cell function in patients with leber’s hereditary optic neuropathy. Investig. Ophthalmol. Vis. Sci. 2017, 58, BIO300–BIO306. [Google Scholar] [CrossRef] [PubMed]
- Voskuhl, R.R.; Palaszynski, K. Sex hormones in experimental autoimmune encephalomyelitis: Implications for multiple sclerosis. Neuroscientist 2001, 7, 258–270. [Google Scholar] [CrossRef] [PubMed]
- Duquette, P.; Pleines, J.; Girard, M.; Charest, L.; Senecal-Quevillon, M.; Masse, C. The increased susceptibility of women to multiple sclerosis. Can. J. Neurol. Sci. 1992, 19, 466–471. [Google Scholar] [CrossRef] [PubMed]
- Rahn, E.J.; Iannitti, T.; Donahue, R.R.; Taylor, B.K. Sex differences in a mouse model of multiple sclerosis: neuropathic pain behavior in females but not males and protection from neurological deficits during proestrus. Biol. Sex. Differ. 2014, 5, 4. [Google Scholar] [CrossRef] [PubMed]
- Bittner, S.; Afzali, A.M.; Wiendl, H.; Meuth, S.G. Myelin oligodendrocyte glycoprotein (MOG35-55) induced experimental autoimmune encephalomyelitis (EAE) in C57BL/6 mice. J. Vis. Exp. 2014, 86. [Google Scholar] [CrossRef]
- Horstmann, L.; Schmid, H.; Heinen, A.P.; Kurschus, F.C.; Dick, H.B.; Joachim, S.C. Inflammatorydemyelination induces glia alterations and ganglion cell loss in the retina of an experimental autoimmune encephalomyelitis model. J. Neuroinflamm. 2013, 10, 120. [Google Scholar] [CrossRef] [PubMed]
- Quinn, T.A.; Dutt, M.; Shindler, K.S. Optic neuritis and retinal ganglion cell loss in a chronic murine model of multiple sclerosis. Front. Neurol. 2011, 2, 50. [Google Scholar] [CrossRef] [PubMed]
- Koenning, M.; Jackson, S.; Hay, C.M.; Faux, C.; Kilpatrick, T.J.; Willingham, M.; Emery, B. Myelin gene regulatory factor is required for maintenance of myelin and mature oligodendrocyte identity in the adult CNS. J. Neurosci. 2012, 32, 12528–12542. [Google Scholar] [CrossRef] [PubMed]
- Chomiak, T.; Hu, B. What is the optimal value of the g-ratio for myelinated fibers in the rat CNS? A theoretical approach. PLoS ONE 2009, 4, e7754. [Google Scholar] [CrossRef] [PubMed]
- Tran, T.H.; Rastogi, R.; Shelke, J.; Amiji, M.M. Modulation of macrophage functional polarity towards anti-inflammatory phenotype with plasmid DNA delivery in CD44 targeting hyaluronic acid nanoparticles. Sci. Rep. 2015, 5, 16632. [Google Scholar] [CrossRef] [PubMed]
- Cao, X.; Shen, D.; Patel, M.M.; Tuo, J.; Johnson, T.M.; Olsen, T.W.; Chan, C.C. Macrophage polarization in the maculae of age-related macular degeneration: A pilot study. Pathol Int. 2011, 61, 528–535. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.-Y.; Watabe, K. The roles of microglia/macrophages in tumor progression of brain cancer and metastatic disease. Front. Biosci. 2017, 22, 1805–1829. [Google Scholar] [CrossRef]
- Wang, N.; Liang, H.; Zen, K. Molecular mechanisms that influence the macrophage m1-m2 polarization balance. Front. Immunol. 2014, 5, 614. [Google Scholar] [CrossRef] [PubMed]
- Wilsey, L.J; Fortune, B. Electroretinography in glaucoma diagnosis. Curr. Opin. Ophthalmol. 2016, 27, 118–124. [Google Scholar] [CrossRef] [PubMed]
- Saszik, S.M.; Robson, J.G.; Frishman, L.J. The scotopic threshold response of the dark-adapted electroretinogram of the mouse. J. Physiol. 2002, 543, 899–916. [Google Scholar] [CrossRef] [PubMed]
- Frishman, L.J.; Steinberg, R.H. Intraretinal analysis of the threshold dark-adapted ERG of cat retina. J. Neurophysiol. 1989, 61, 1221–1232. [Google Scholar] [CrossRef] [PubMed]
- Preiser, D.; Lagrèze, W.A.; Bach, M.; Poloschek, C.M. Photopic negative response versus pattern electroretinogram in early glaucoma. Investig. Ophthalmol. Vis. Sci. 2013, 54, 1182–1191. [Google Scholar] [CrossRef] [PubMed]
- Niyadurupola, N.; Luu, C.D.; Nguyen, D.Q.; Geddes, K.; Tan, G.X.; Wong, C.C.; Tran, T.; Coote, M.A.; Crowston, J.G. Intraocular pressure lowering is associated with an increase in the photopic negative response (PhNR) amplitude in glaucoma and ocular hypertensive eyes. Investig. Ophthalmol. Vis. Sci. 2013, 54, 1913–1919. [Google Scholar] [CrossRef] [PubMed]
- Yun, H.; Lathrop, K.L.; Yang, E.; Sun, M.; Kagemann, L.; Fu, V.; Stolz, D.B.; Schuman, J.S.; Du, Y. A laser-induced mouse model with long-term intraocular pressure elevation. PLoS ONE 2014, 9, e107446. [Google Scholar] [CrossRef] [PubMed]
- Chrysostomou, V.; Crowston, J.G. The photopic negative response of the mouse electroretinogram: Reduction by acute elevation of intraocular pressure. Investig. Ophthalmol. Vis. Sci. 2013, 54, 4691–4697. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Wang, N.; Peng, X.; Yang, D.; Wang, C.; Zeng, H. Long-term effect of laser-induced ocular hypertension on the cone electroretinogram and central macular thickness in monkeys. Photomed. Laser. Surg. 2014, 32, 371–378. [Google Scholar] [CrossRef] [PubMed]
- Bagur, M.; Murcia, M.A.; Jiménez-Monreal, A.M.; Tur, J.A.; Bibiloni, M.M.; Alonso, G.L.; Martínez-Tomé, M. Influence of diet in multiple sclerosis: A systematic review. Adv. Nutr. 2017, 8, 463–472. [Google Scholar] [CrossRef] [PubMed]
- Peng, S.; Shi, Z.; Su, H.; So, K.F.; Cui, Q. Increased production of omega-3 fatty acids protects retinal ganglion cells after optic nerve injury in mice. Exp. Eye. Res. 2016, 148, 90–96. [Google Scholar] [CrossRef] [PubMed]
- Azuchi, Y.; Kimura, A.; Guo, X.; Akiyama, G.; Noro, T.; Harada, C.; Nishigaki, A.; Namekata, K.; Harada, T. Valproic acid and ASK1 deficiency ameliorate optic neuritis and neurodegeneration in an animal model of multiple sclerosis. Neurosci. Lett. 2017, 639, 82–87. [Google Scholar] [CrossRef] [PubMed]
- Chaudhary, P.; Marracci, G.; Yu, X.; Galipeau, D.; Morris, B.; Bourdette, D. Lipoic acid decreases inflammation and confers neuroprotection in experimental autoimmune optic neuritis. J. Neuroimmunol. 2011, 233, 90–96. [Google Scholar] [CrossRef] [PubMed]
- Antel, J.; Antel, S.; Caramanos, Z.; Arnold, D.L.; Kuhlmann, T. Primary progressive multiple sclerosis: Part of the MS disease spectrum or separate disease entity? Acta Neuropathol. 2012, 123, 627–638. [Google Scholar] [CrossRef] [PubMed]
- Bjørnevik, K.; Chitnis, T.; Asche rio, A.; Munger, K.L. Polyunsaturated fatty acids and the risk of multiple sclerosis. Mult. Scler. 2017, 23, 1830–1838. [Google Scholar] [CrossRef] [PubMed]
- Hoare, S.; Lithander, F.; van der Mei, I.; Ponsonby, A.L.; Lucas, R.; Ausimmune Investigator Group. Higher intake of omega-3 polyunsaturated fatty acids is associated with a decreased risk of a first clinical diagnosis of central nervous system demyelination: Results from the Ausimmune Study. Mult. Scler. 2016, 22, 884–892. [Google Scholar] [CrossRef] [PubMed]
- Bäärnhielm, M.; Olsson, T.; Alfredsson, L. Fatty fish intake is associated with decreased occurrence of multiple sclerosis. Mult. Scler. 2014, 20, 726–732. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.M.; Willett, W.C.; Hernán, M.A.; Olek, M.J.; Ascherio, A. Dietary fat in relation to risk of multiple sclerosis among two large cohorts of women. Am. J. Epidemiol. 2000, 152, 1056–1064. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.L.; Hassan, M.Y.; Zheng, X.Y.; Azimullah, S.; Quezada, H.C.; Amir, N.; Elwasila, M.; Mix, E.; Adem, A.; Zhu, J. Attenuated EAN in TNF-α deficient mice is associated with an altered balance of M1/M2 macrophages. PLoS ONE 2012, 7, e38157. [Google Scholar] [CrossRef] [PubMed]
- Kroenke, M.A.; Carlson, T.J.; Andjelkovic, A.V.; Segal, B.M. IL-12- and IL-23-modulated T cells induce distinct types of EAE based on histology, CNS chemokine profile, and response to cytokine inhibition. J. Exp. Med. 2008, 205, 1535–1541. [Google Scholar] [CrossRef] [PubMed]
- Ha, Y.; Liu, H.; Zhu, S.; Yi, P.; Liu, W.; Nathanson, J.; Kayed, R.; Loucas, B.; Sun, J.; Frishman, L.J.; et al. Critical role of the CXCL10/C-X-C chemokine receptor 3 Axis in promoting leukocyte recruitment and neuronal injury during traumatic optic neuropathy induced by optic nerve crush. Am. J. Pathol. 2017, 187, 352–365. [Google Scholar] [CrossRef] [PubMed]
- Kwon, M.J; Shin, H.Y.; Cui, Y.; Kim, H.; Thi, A.H.; Choi, J.Y.; Kim, E.Y.; Hwang, D.H.; Kim, B.G. CCL2 mediates neuron-macrophage interactions to drive proregenerative macrophage activation following preconditioning injury. J. Neurosci. 2015, 35, 15934–15947. [Google Scholar] [CrossRef] [PubMed]
- Butti, E.; Bergami, A.; Recchia, A.; Brambilla, E.; Del Carro, U.; Amadio, S.; Cattalini, A.; Esposito, M.; Stornaiuolo, A.; Comi, G.; et al. IL4 gene delivery to the CNS recruits regulatory T cells and induces clinical recovery in mouse models of multiple sclerosis. Gene Ther. 2008, 15, 504–515. [Google Scholar] [CrossRef] [PubMed]
- Georgiou, T.; Wen, Y.T.; Chang, C.H.; Kolovos, P.; Kalogerou, M.; Prokopiou, E.; Neokleous, A.; Huang, C.T.; Tsai, R.K. Neuroprotective effects of omega-3 polyunsaturated fatty acids in a rat model of anterior ischemic optic neuropathy. Investig. Ophthalmol. Vis. Sci. 2017, 58, 1603–1611. [Google Scholar] [CrossRef] [PubMed]
- Hossain, D.M.; Panda, A.K.; Manna, A.; Mohanty, S.; Bhattacharjee, P.; Bhattacharyya, S.; Saha, T.; Chakraborty, S.; Kar, R.K.; Das, T.; et al. FoxP3 acts as a cotranscription factor with STAT3 in tumor-induced regulatory T cells. Immunity 2013, 39, 1057–1069. [Google Scholar] [CrossRef] [PubMed]
- Tugal, D.; Liao, X.; Jain, M.K. Transcriptional control of macrophage polarization. Arterioscler. Thromb. Vasc. Biol. 2013, 33, 1135–1144. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Zhang, L.; Joo, D.; Sun, S.C. NF-κB signaling in inflammation. Signal Transduct. Target Ther. 2017, 2. [Google Scholar] [CrossRef] [PubMed]
- Ponath, G.; Park, C.; Pitt, D. The role of astrocytes in multiple sclerosis. Front. Immunol. 2018, 9, 217. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Wang, Z.; Zheng, Z.; Chen, Y.; Khor, S.; Shi, K.; He, Z.; Wang, Q.; Zhao, Y.; Zhang, H.; et al. Neuron and microglia/macrophage-derived FGF10 activate neuronal FGFR2/PI3K/Akt signaling and inhibit microglia/macrophages TLR4/NF-κB-dependent neuroinflammation to improve functional recovery after spinal cord injury. Cell Death Dis. 2017, 8, e3090. [Google Scholar] [CrossRef] [PubMed]
- Iyer, S.S.; Cheng, G. Role of interleukin 10 transcriptional regulation in inflammation and autoimmune disease. Crit. Rev. Immunol. 2012, 32, 23–63. [Google Scholar] [CrossRef] [PubMed]
- Martinez, F.O.; Helming, L.; Gordon, S. Alternative activation of macrophages: an immunologic functional perspective. Annu. Rev. Immunol. 2009, 27, 451–483. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.P.; Chen, Y.G.; Qin, W.D.; Zhang, W.; Wei, S.J.; Wang, J.; Liu, F.Q.; Gong, L.; An, F.S.; Zhang, Y.; et al. Arginase I attenuates inflammatory cytokine secretion induced by lipopolysaccharide in vascular smooth muscle cells. Arterioscler. Thromb. Vasc. Biol. 2011, 31, 1853–1860. [Google Scholar] [CrossRef] [PubMed]
- Talla, V.; Yu, H.; Chou, T.H.; Porciatti, V.; Chiodo, V.; Boye, S.L.; Hauswirth, W.W.; Lewin, A.S.; Guy, J. NADH-dehydrogenase type-2 suppresses irreversible visual loss and neurodegeneration in the EAE animal model of MS. Mol. Ther. 2013, 21, 1876–1888. [Google Scholar] [CrossRef] [PubMed]
- Brambilla, R.; Dvoriantchikova, G.; Barakat, D.; Ivanov, D.; Bethea, J.R.; Shestopalov, V.I. Transgenic inhibition of astroglial NF-κB protects from optic nerve damage and retinal ganglion cell loss in experimental optic neuritis. J. Neuroinflamm. 2012, 9, 213. [Google Scholar] [CrossRef] [PubMed]
- Machida, S. Clinical applications of the photopic negative response to optic nerve and retinal diseases. J. Ophthalmol. 2012, 2012, 397178. [Google Scholar] [CrossRef] [PubMed]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Locri, F.; Cammalleri, M.; Pini, A.; Dal Monte, M.; Rusciano, D.; Bagnoli, P. Further Evidence on Efficacy of Diet Supplementation with Fatty Acids in Ocular Pathologies: Insights from the EAE Model of Optic Neuritis. Nutrients 2018, 10, 1447. https://doi.org/10.3390/nu10101447
Locri F, Cammalleri M, Pini A, Dal Monte M, Rusciano D, Bagnoli P. Further Evidence on Efficacy of Diet Supplementation with Fatty Acids in Ocular Pathologies: Insights from the EAE Model of Optic Neuritis. Nutrients. 2018; 10(10):1447. https://doi.org/10.3390/nu10101447
Chicago/Turabian StyleLocri, Filippo, Maurizio Cammalleri, Alessandro Pini, Massimo Dal Monte, Dario Rusciano, and Paola Bagnoli. 2018. "Further Evidence on Efficacy of Diet Supplementation with Fatty Acids in Ocular Pathologies: Insights from the EAE Model of Optic Neuritis" Nutrients 10, no. 10: 1447. https://doi.org/10.3390/nu10101447
APA StyleLocri, F., Cammalleri, M., Pini, A., Dal Monte, M., Rusciano, D., & Bagnoli, P. (2018). Further Evidence on Efficacy of Diet Supplementation with Fatty Acids in Ocular Pathologies: Insights from the EAE Model of Optic Neuritis. Nutrients, 10(10), 1447. https://doi.org/10.3390/nu10101447







