Annurca Apple Polyphenols Ignite Keratin Production in Hair Follicles by Inhibiting the Pentose Phosphate Pathway and Amino Acid Oxidation
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Nutraceuticals
2.3. Animals
2.4. Histology
2.5. Metabolite and RNA Extraction from Murine Tissues
2.6. Mass Spectrometry-Based Metabolomic, Statistics and Analysis
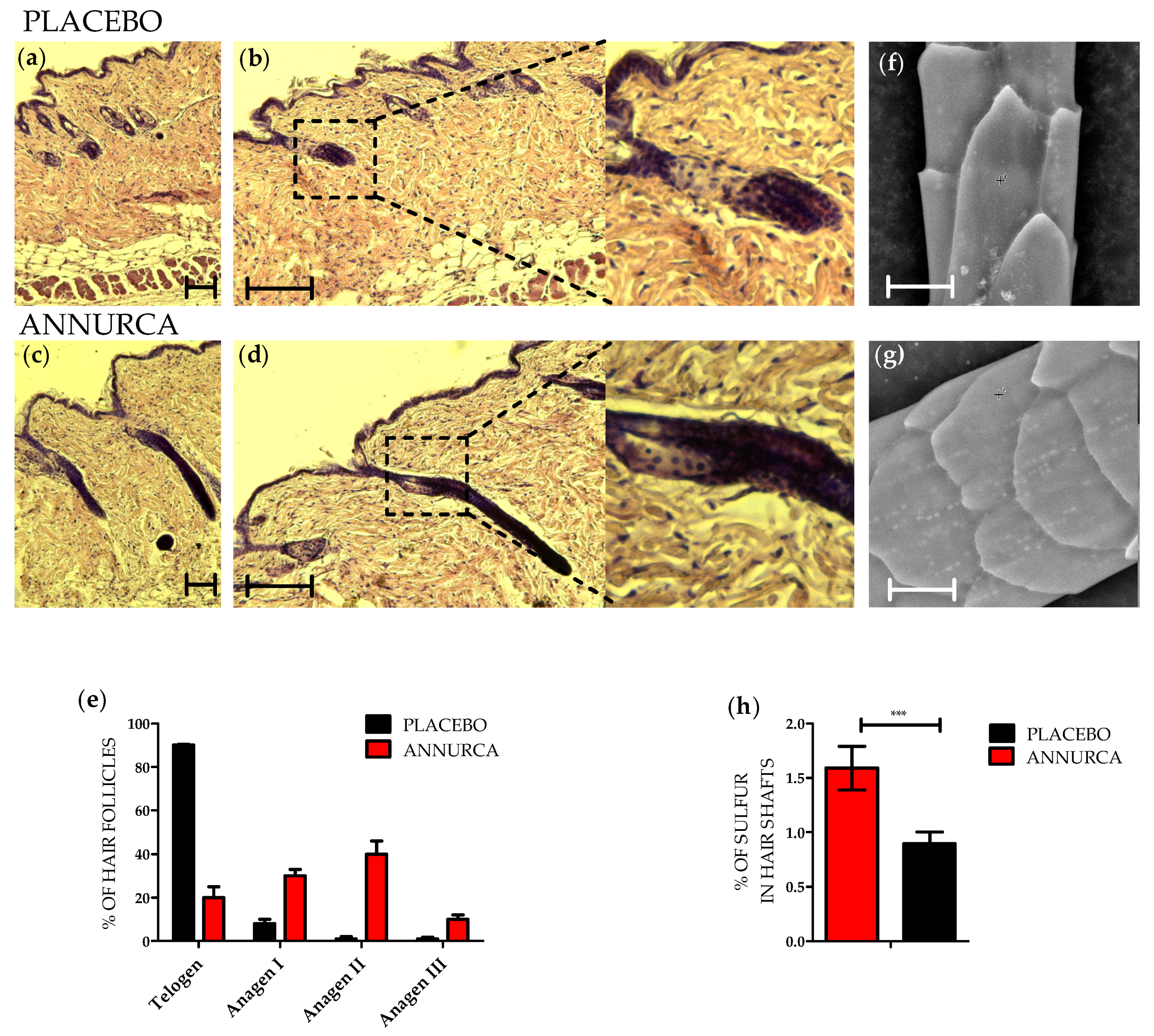
3. Results
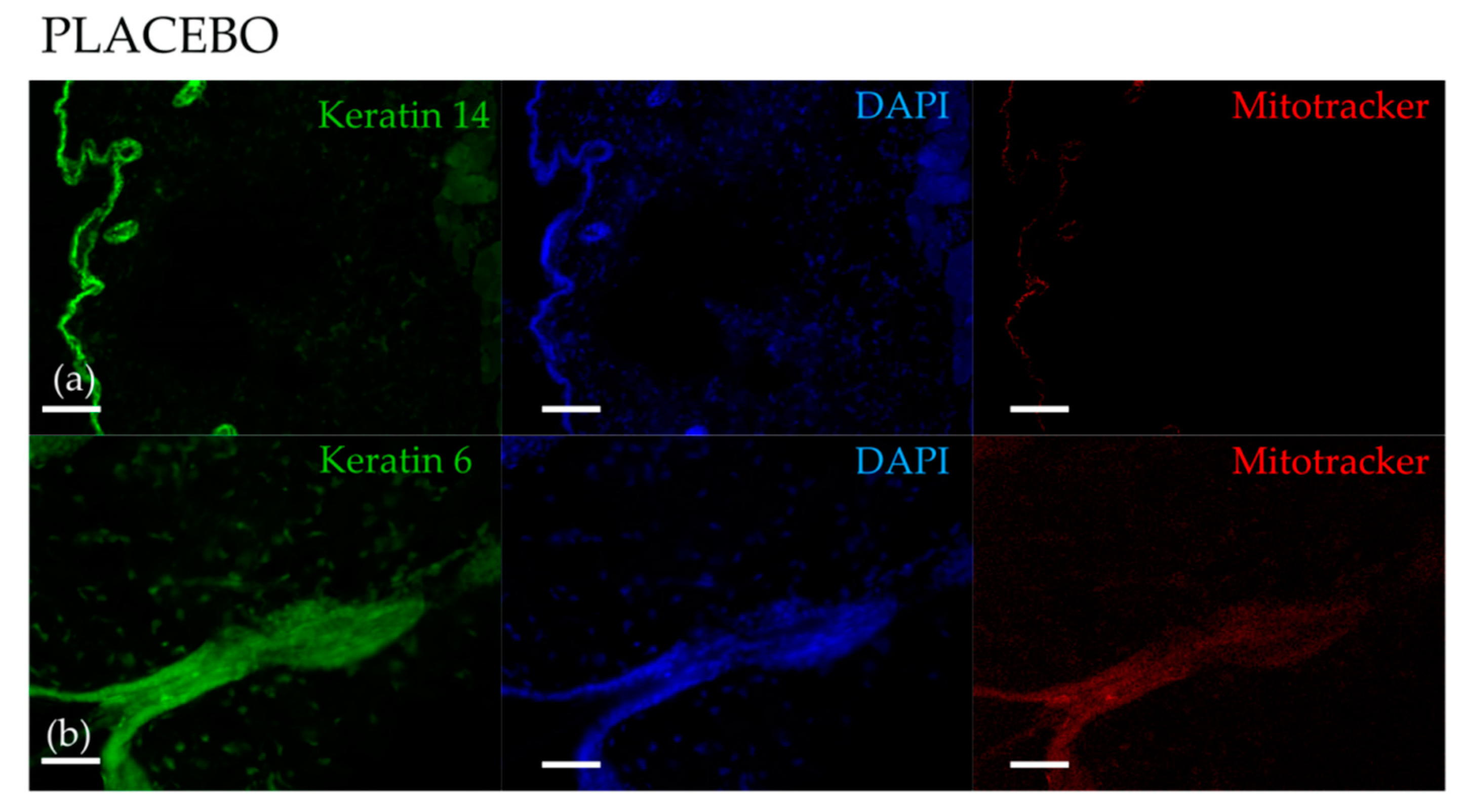
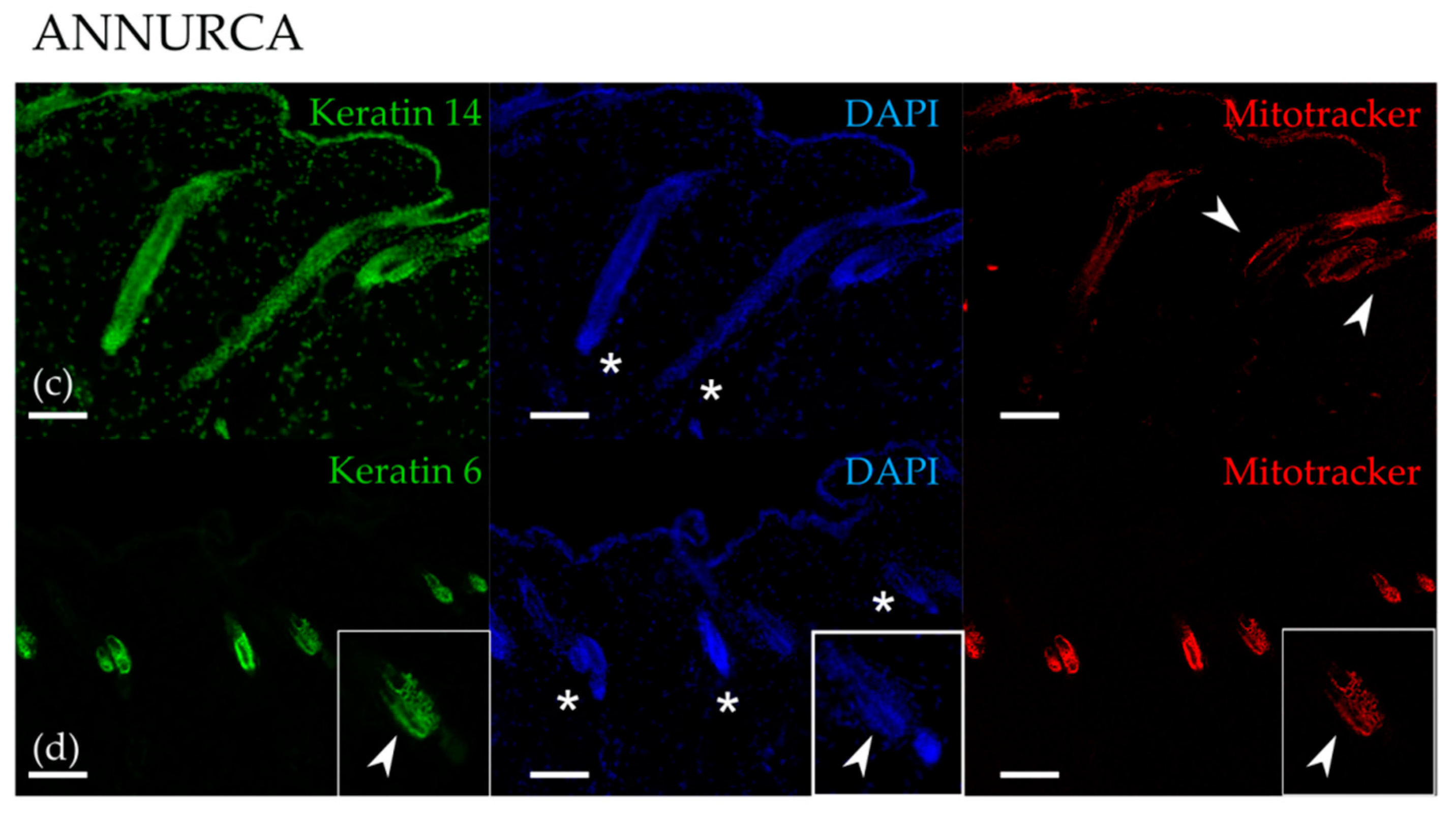
3.1. Topical Treatment with AAE Accellerates Exit of Murine HF from Telogen and Increases Keratin Content in Hair Shafts
3.2. Topical Treatment with AAE Alters the Intracellular Levels of HF Key Metabolites
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Fuchs, E. The Tortoise and the Hair-Slow-Cycling Cells in the Stem Cell Race. Cell 2009, 137, 811–819. [Google Scholar] [CrossRef] [PubMed]
- Fuchs, E.; Merrill, B.J.; Jamora, C.; DasGupta, R. At the roots of a never-ending Cycle. Dev. Cell 2001, 1, 13–25. [Google Scholar] [CrossRef]
- Bernard, B. Advances in Understanding Hair Growth. F1000Research 2016, 5, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Sonthalia, S. Hair Restoration in Androgenetic Alopecia: Looking Beyond Minoxidil, Finasteride and Hair Transplantation. J. Cosmetol. Trichol. 2016, 02, 1–13. [Google Scholar] [CrossRef]
- Cash, T.F. The physiological effects of androgenetic alopecia in man. J. Am. Acad. Dermatol. 1992, 26, 926–931. [Google Scholar] [CrossRef]
- Sinclair, R. Hair shedding in women: How much is too much? Br. J. Dermatol. 2015, 173, 846–848. [Google Scholar] [CrossRef] [PubMed]
- Guo, E.L.; Katta, R. Diet and hair loss: Effects of nutrient deficiency and supplement use. Dermatol. Pract. Concept. 2017, 7, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Su, L.H.; Chen, T.H.H. Association of androgenetic alopecia with metabolic syndrome in men: A community-based survey. Br. J. Dermatol. 2010, 163, 371–377. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, R.; Happle, R. Current understanding of androgenetic alopecia. Part II: Clinical aspects and treatment. Eur. J. Dermatol. 2000, 10, 410–417. [Google Scholar] [PubMed]
- Hoffmann, R. Hormonal interaction and hair growth. Ann. Dermatol. Venereol. 2002, 129, 787–792. [Google Scholar] [PubMed]
- Kelly, Y.; Blanco, A.; Tosti, A. Androgenetic Alopecia: An Update of Treatment Options. Drugs 2016, 76, 1349–1364. [Google Scholar] [CrossRef] [PubMed]
- Valente Duarte de Sousa, I.C.; Tosti, A. New investigational drugs for androgenetic alopecia. Expert Opin. Investig. Drugs 2013, 22, 573–589. [Google Scholar] [CrossRef] [PubMed]
- Sonthalia, S.; Sahaya, K.; Singal, A.; Gupta, K.; Srivastava, A.; Wadhawan, R.; Zartab, H.; Arora, R. Nocebo effect in Dermatology. Indian J. Dermatol. Venereol. Leprol. 2015, 81, 242. [Google Scholar] [CrossRef] [PubMed]
- Le Floc’h, C.; Cheniti, A.; Connétable, S.; Piccardi, N.; Vincenzi, C.; Tosti, A. Effect of a nutritional supplement on hair loss in women. J. Cosmet. Dermatol. 2015, 14, 76–82. [Google Scholar] [CrossRef] [PubMed]
- Lengg, N.; Heidecker, B.; Seifert, B.; Trüeb, R.M. Dietary supplement increases anagen hair rate in women with telogen effluvium: Results of a double-blind, placebo-controlled trial. Therapy 2007, 4, 59–65. [Google Scholar] [CrossRef]
- Yoshizawa, T.; Handa, Y.; Uematsu, Y.; Takeda, S.; Sekine, K.; Yoshihara, Y.; Kawakami, T.; Arioka, K.; Sato, H.; Uchiyama, Y.; et al. Mice lacking the vitamin D receptor exhibit impared bone formation, uterine hyprplasia and growth retardation after weaning. Nat. Genet. 1997, 16, 391–396. [Google Scholar] [CrossRef] [PubMed]
- Hajem, N.; Chapelle, A.; Bignon, J.; Pinault, A.; Liu, J.M.; Salah-Mohellibi, N.; Lati, E.; Wdzieczak-Bakala, J. The regulatory role of the tetrapeptide AcSDKP in skin and hair physiology and the prevention of ageing effects in these tissues—A potential cosmetic role. Int. J. Cosmet. Sci. 2013, 35, 286–298. [Google Scholar] [CrossRef] [PubMed]
- Zempleni, J.; Hassan, Y.I.; Wijeratne, S.S.K. Biotin and biotinidase deficiency. Expert Rev. Endocrinol. Metab. 2008, 3, 715–724. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, T.; Kamimura, A.; Yokoo, Y.; Honda, S.; Watanabe, Y. The first clinical trial of topical application of procyanidin B-2 to investigate its potential as a hair growing agent. Phyther. Res. 2001, 15, 331–336. [Google Scholar] [CrossRef] [PubMed]
- Tenore, G.C.; Campiglia, P.; Ritieni, A.; Novellino, E. In vitro bioaccessibility, bioavailability and plasma protein interaction of polyphenols from Annurca apple (M. pumila Miller cv Annurca). Food Chem. 2013, 141, 3519–3524. [Google Scholar] [CrossRef] [PubMed]
- Tenore, G.C.; Caruso, D.; Buonomo, G.; D’Avino, M.; Santamaria, R.; Irace, C.; Piccolo, M.; Maisto, M.; Novellino, E. Annurca Apple Nutraceutical Formulation Enhances Keratin Expression in a Human Model of Skin and Promotes Hair Growth and Tropism in a Randomized Clinical Trial. J. Med. Food 2018, 21, 90–103. [Google Scholar] [CrossRef] [PubMed]
- Bouayed, J.; Bohn, T. Exogenous antioxidants—Double-edged swords in cellular redox state: Health beneficial effects at physiologic doses versus deleterious effects at high doses. Oxid. Med. Cell. Longev. 2010, 3, 228–237. [Google Scholar] [CrossRef] [PubMed]
- Grosso, G.; Godos, J.; Lamuela-Raventos, R.; Ray, S.; Micek, A.; Pajak, A.; Sciacca, S.; D’Orazio, N.; Del Rio, D.; Galvano, F. A comprehensive meta-analysis on dietary flavonoid and lignan intake and cancer risk: Level of evidence and limitations. Mol. Nutr. Food Res. 2017, 61, 1600930. [Google Scholar] [CrossRef] [PubMed]
- Grosso, G.; Micek, A.; Godos, J.; Pajak, A.; Sciacca, S.; Galvano, F.; Giovannucci, E.L. Dietary Flavonoid and Lignan Intake and Mortality in Prospective Cohort Studies: Systematic Review and Dose-Response Meta-Analysis. Am. J. Epidemiol. 2017, 185, 1304–1316. [Google Scholar] [CrossRef] [PubMed]
- Grosso, G.; Stepaniak, U.; Topor-Madry, R.; Szafraniec, K.; Pajak, A. Estimated dietary intake and major food sources of polyphenols in the Polish arm of the HAPIEE study. Nutrition 2014, 30, 1398–1403. [Google Scholar] [CrossRef] [PubMed]
- Sommella, E.; Ismail, O.H.; Pagano, F.; Pepe, G.; Ostacolo, C.; Mazzoccanti, G.; Russo, M.; Novellino, E.; Gasparrini, F.; Campiglia, P. Development of an improved online comprehensive hydrophilic interaction chromatography × reversed-phase ultra-high-pressure liquid chromatography platform for complex multiclass polyphenolic sample analysis. J. Sep. Sci. 2017, 40, 2188–2197. [Google Scholar] [CrossRef] [PubMed]
- Kamimura, A.; Takahashi, T. Procyanidin B-2, extracted from apples, promotes hair growth: A laboratory study. Br. J. Dermatol. 2002, 146, 41–51. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, T.; Kamiya, T.; Hasegawa, A.; Yokoo, Y. Procyanidin oligomers selectively and intensively promote proliferation of mouse hair epithelial cells in vitro and activate hair follicle growth in vivo. J. Investig. Dermatol. 1999, 112, 310–316. [Google Scholar] [CrossRef] [PubMed]
- Kamimura, A.; Takahashi, T.; Watanabe, Y. Investigation of topical application of procyanidin B-2 from apple to identify its potential use as a hair growing agent. Phytomedicine 2000, 7, 529–536. [Google Scholar] [CrossRef]
- Oshimori, N.; Fuchs, E. The harmonies played by TGF-β in stem cell biology. Cell Stem Cell 2012, 11, 751–764. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.-Y.; Yang, L.-T. Differential response of epithelial stem cell populations in hair follicles to TGF-β signaling. Dev. Biol. 2012, 373, 394–406. [Google Scholar] [CrossRef] [PubMed]
- Sommella, E.; Conte, G.M.; Salviati, E.; Pepe, G.; Bertamino, A.; Ostacolo, C.; Sansone, F.; Prete, F.D.; Aquino, R.P.; Campiglia, P. Fast profiling of natural pigments in different spirulina (arthrospira platensis) dietary supplements by DI-FT-ICR and evaluation of their antioxidant potential by pre-column DPPH-UHPLC assay. Molecules 2018, 23, 1132. [Google Scholar] [CrossRef] [PubMed]
- Want, E.J.; Masson, P.; Michopoulos, F.; Wilson, I.D.; Theodoridis, G.; Plumb, R.S.; Shockcor, J.; Loftus, N.; Holmes, E.; Nicholson, J.K. Global metabolic profiling of animal and human tissues via UPLC MS. Nat. Protoc. 2013, 8, 17–32. [Google Scholar] [CrossRef] [PubMed]
- Johnson, S.; Rabinovitch, P. Ex vivo imaging of excised tissue using vital dyes and confocal microscopy. Curr. Protoc. Cytom. 2012, 61, 9–39. [Google Scholar] [CrossRef]
- Riccio, G.; Bottone, S.; La Regina, G.; Badolati, N.; Passacantilli, S.; Rossi, G.B.; Accardo, A.; Dentice, M.; Silvestri, R.; Novellino, E.; et al. A Negative Allosteric Modulator of WNT Receptor Frizzled 4 Switches into an Allosteric Agonist. Biochemistry 2018, 57, 839–851. [Google Scholar] [CrossRef] [PubMed]
- Forslind, B. Clinical applications of scanning electron microscopy and energy dispersive X-ray analysis in dermatology—An up-date. Scanning Microsc. 1988, 2, 959–976. [Google Scholar] [PubMed]
- Ser, Z.; Liu, X.; Tang, N.N.; Locasale, J.W. Extraction parameters for metabolomics from cell extracts Zheng. Anal. Biochem. 2015, 475, 87–92. [Google Scholar] [CrossRef] [PubMed]
- Carrizzo, A.; Ambrosio, M.; Damato, A.; Madonna, M.; Storto, M.; Capocci, L.; Campiglia, P.; Sommella, E.; Trimarco, V.; Rozza, F.; et al. Morus alba extract modulates blood pressure homeostasis through eNOS signaling. Mol. Nutr. Food Res. 2016, 60, 2304–2311. [Google Scholar] [CrossRef] [PubMed]
- Sundberg, J.P.; Silva, K.A. What color is the skin of a mouse? Vet. Pathol. 2012, 49, 142–145. [Google Scholar] [CrossRef] [PubMed]
- Müller-Röver, S.; Handjiski, B.; Van Der Veen, C.; Eichmüller, S.; Foitzik, K.; McKay, I.A.; Stenn, K.S.; Paus, R. A comprehensive guide for the accurate classification of murine hair follicles in distinct hair cycle stages. J. Investig. Dermatol. 2001, 117, 3–15. [Google Scholar] [CrossRef] [PubMed]
- De Souza, F.H.M.; Fernando, L.; Torres, B. Quantitative evaluation of transverse scalp sections * Avaliação quantitativa em cortes histológicos transversais. An. Bras. Dematol. 2006, 81, 227–232. [Google Scholar] [CrossRef]
- Trempus, C.S.; Morris, R.J.; Ehinger, M.; Elmore, A.; Bortner, C.D.; Ito, M.; Cotsarelis, G.; Nijhof, J.G.W.; Peckham, J.; Flagler, N.; et al. CD34 expression by hair follicle stem cells is required for skin tumor development in mice. Cancer Res. 2007, 67, 4173–4181. [Google Scholar] [CrossRef] [PubMed]
- Kretzschmar, K.; Watt, F.M. Markers of epidermal stem cell subpopulations in adult mammalian skin. Cold Spring Harb. Perspect. Med. 2014, 4. [Google Scholar] [CrossRef] [PubMed]
- Williams, R.; Philpott, M.P.; Kealey, T. Metabolism of freshly isolated human hair follicles capable of hair elongation: A glutaminolytic, aerobic glycolytic tissue. J. Investig. Dermatol. 1993, 100, 834–840. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Wu, Z.; Dai, Z.; Yang, Y.; Wang, J.; Wu, G. Glycine metabolism in animals and humans: Implications for nutrition and health. Amino Acids 2013, 45, 463–477. [Google Scholar] [CrossRef] [PubMed]
- Lemasters, J.J.; Ramshesh, V.K.; Lovelace, G.L.; Lim, J.; Wright, G.D.; Harland, D.; Dawson, T.L. Compartmentation of Mitochondrial and Oxidative Metabolism in Growing Hair Follicles: A Ring of Fire. J. Investig. Dermatol. 2017, 137, 1434–1444. [Google Scholar] [CrossRef] [PubMed]
- Foitzik, K.; Hoting, E.; Heinrich, U.; Tronnier, H.; Paus, R. Indications that topical l-carnitin-l-tartrate promotes human hair growth in vivo. J. Dermatol. Sci. 2007, 48, 141–144. [Google Scholar] [CrossRef] [PubMed]
- Foitzik, K.; Hoting, E.; Förster, T.; Pertile, P.; Paus, R. l-Carnitine-l-tartrate promotes human hair growth in vitro. Exp. Dermatol. 2007, 16, 936–945. [Google Scholar] [CrossRef] [PubMed]
- Wei, P.; Dove, K.K.; Bensard, C.; Schell, J.C.; Rutter, J. The Force Is Strong with This One: Metabolism (Over)powers Stem Cell Fate. Trends Cell Biol. 2018, 28, 551–559. [Google Scholar] [CrossRef] [PubMed]
- Stirpe, M.; Palermo, V.; Bianchi, M.M.; Silvestri, R.; Falcone, C.; Tenore, G.; Novellino, E.; Mazzoni, C. Annurca apple (M. pumila Miller cv Annurca) extracts act against stress and ageing in S. cerevisiae yeast cells. BMC Complement. Altern. Med. 2017, 17, 200. [Google Scholar] [CrossRef] [PubMed]
- Tenore, G.C.; Caruso, D.; Buonomo, G.; D’Urso, E.; D’Avino, M.; Campigli, P.; Marinelli, L.; Novellino, E. Annurca (Malus pumila Miller cv. Annurca) apple as a functional food for the contribution to a healthy balance of plasma cholesterol levels: Results of a randomized clinical trial. J. Sci. Food Agric. 2016, 97, 2107–2115. [Google Scholar] [CrossRef] [PubMed]
- Tenore, G.C.; Calabrese, G.; Stiuso, P.; Ritieni, A.; Giannetti, D.; Novellino, E. Effects of Annurca apple polyphenols on lipid metabolism in HepG2 cell lines: A source of nutraceuticals potentially indicated for the metabolic syndrome. Food Res. Int. 2014, 63, 252–257. [Google Scholar] [CrossRef]
- Sommella, E.; Pepe, G.; Pagano, F.; Ostacolo, C.; Tenore, G.C.; Russo, M.T.; Novellino, E.; Manfra, M.; Campiglia, P. Detailed polyphenolic profiling of Annurca apple (M. pumila Miller cv Annurca) by a combination of RP-UHPLC and HILIC, both hyphenated to IT-TOF mass spectrometry. Food Res. Int. 2015, 76, 466–477. [Google Scholar] [CrossRef] [PubMed]
- Riccio, G.; Maisto, M.; Bottone, S.; Badolati, N.; Rossi, G.B.; Tenore, G.C.; Stornaiuolo, M.; Novellino, E. WNT inhibitory activity of Malus Pumila miller cv annurca and Malus domestica cv limoncella apple extracts on human colon-rectal cells carrying familial adenomatous polyposis mutations. Nutrients 2017, 9, 1262. [Google Scholar] [CrossRef] [PubMed]
- Flores, A.; Schell, J.; Krall, A.S.; Jelinek, D.; Miranda, M.; Grigorian, M.; Braas, D.; White, A.C.; Zhou, J.L.; Graham, N.A.; et al. Lactate dehydrogenase activity drives hair follicle stem cell activation. Nat. Cell Biol. 2017, 19, 1017–1026. [Google Scholar] [CrossRef] [PubMed]
- Kloepper, J.E.; Baris, O.R.; Reuter, K.; Kobayashi, K.; Weiland, D.; Vidali, S.; Tobin, D.J.; Niemann, C.; Wiesner, R.J.; Paus, R. Mitochondrial function in murine skin epithelium is crucial for hair follicle morphogenesis and epithelial-mesenchymal interactions. J. Investig. Dermatol. 2015, 135, 679–689. [Google Scholar] [CrossRef] [PubMed]
- Van Beek, N.; Bodó, E.; Kromminga, A.; Gáspár, E.; Meyer, K.; Zmijewski, M.A.; Slominski, A.; Wenzel, B.E.; Paus, R. Thyroid hormones directly alter human hair follicle functions: Anagen prolongation and stimulation of both hair matrix keratinocyte proliferation and hair pigmentation. J. Clin. Endocrinol. Metab. 2008, 93, 4381–4388. [Google Scholar] [CrossRef] [PubMed]
- Vidali, S.; Knuever, J.; Lerchner, J.; Giesen, M.; Bíró, T.; Klinger, M.; Kofler, B.; Funk, W.; Poeggeler, B.; Paus, R. Hypothalamic-pituitary-thyroid axis hormones stimulate mitochondrial function and biogenesis in human hair follicles. J. Investig. Dermatol. 2014, 134, 33–42. [Google Scholar] [CrossRef] [PubMed]
- Vidali, S.; Chéret, J.; Giesen, M.; Haeger, S.; Alam, M.; Watson, R.E.B.; Langton, A.K.; Klinger, M.; Knuever, J.; Funk, W.; et al. Thyroid Hormones Enhance Mitochondrial Function in Human Epidermis. J. Investig. Dermatol. 2016, 136, 2003–2012. [Google Scholar] [CrossRef] [PubMed]
- Hamanaka, R.B.; Glasauer, A.; Hoover, P.; Yang, S.; Blatt, H.; Mullen, A.R.; Getsios, S.; Gottardi, C.J.; DeBerardinis, R.J.; Lavker, R.M.; et al. Mitochondrial Reactive Oxygen Species Promote Epidermal Differentiation and Hair Follicle Development. Sci. Signal. 2013, 6, ra8. [Google Scholar] [CrossRef] [PubMed]
- Hamanaka, R.B.; Chandel, N.S. Mitochondrial metabolism as a regulator of keratinocyte differentiation. Cell. Logist. 2013, 3, e25456. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Luo, B.; Deng, Z.; Wang, B.; Liu, F.; Li, J.; Shi, W.; Xie, H.; Hu, X.; Li, J. Mitochondrial aerobic respiration is activated during hair follicle stem cell differentiation, and its dysfunction retards hair regeneration. PeerJ 2016, 4, e1821. [Google Scholar] [CrossRef] [PubMed]
- Kopustinskiene, D.M.; Savickas, A.; Vetchý, D.; Masteikova, R.; Kasauskas, A.; Bernatoniene, J. Direct effects of (−)-Epicatechin and procyanidin B2 on the respiration of rat heart mitochondria. BioMed Res. Int. 2015, 2015. [Google Scholar] [CrossRef] [PubMed]
- Kealey, T.; Williams, R.; Philpott, M.P. The human hair follicle engages in glutaminolysis and aerobic glycolysis: Implications for skin, splanchnic and neoplastic metabolism. Skin Pharmacol. Physiol. 1994, 7, 41–46. [Google Scholar] [CrossRef]
- Stenn, K.S.; Paus, R. Controls of Hair Follicle Cycling. Physiol. Rev. 2001, 81, 449–494. [Google Scholar] [CrossRef] [PubMed]
- Leirõs, G.J.; Attorresi, A.I.; Balañá, M.E. Hair follicle stem cell differentiation is inhibited through cross-talk between Wnt/β-catenin and androgen signalling in dermal papilla cells from patients with androgenetic alopecia. Br. J. Dermatol. 2012, 166, 1035–1042. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.-H.; Yoon, J.; Shin, S.H.; Zahoor, M.; Kim, H.J.; Park, P.J.; Park, W.-S.; Min, D.S.; Kim, H.-Y.; Choi, K.-Y. Valproic Acid Induces Hair Regeneration in Murine Model and Activates Alkaline Phosphatase Activity in Human Dermal Papilla Cells. PLoS ONE 2012, 7, e34152. [Google Scholar] [CrossRef] [PubMed]
- Zimber, M.P.; Ziering, C.; Zeigler, F.; Hubka, M.; Mansbridge, J.N.; Baumgartner, M.; Hubka, K.; Kellar, R.; Perez-Meza, D.; Sadick, N.; et al. Hair regrowth following a Wnt- and follistatin containing treatment: Safety and efficacy in a first-in-man phase 1 clinical trial. J. Drugs Dermatol. 2011, 10, 1308–1312. [Google Scholar] [PubMed]
- Kishimoto, J.; Burgeson, R.E.; Morgan, B.A. Wnt signaling maintains the hair-inducing activity of the dermal papilla. Genes Dev. 2000, 14, 1181–1185. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Xing, Y.Z.; Guo, H.; Ma, X.; Li, Y.H. Immunohistochemical study of hair follicle stem cells in regenerated hair follicles induced by Wnt10b. Int. J. Med. Sci. 2016, 13, 765–771. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Liu, S.; Zhang, H.S.; Deng, Z.L.; Zhao, H.S.; Zhao, Q.; Lei, X.H.; Ning, L.N.; Cao, Y.J.; Wang, H.B.; et al. Exogenous R-spondin1 induces precocious telogen-to-anagen transition in mouse hair follicles. Int. J. Mol. Sci. 2016, 17, 582. [Google Scholar] [CrossRef] [PubMed]
- Upton, J.H.; Hannen, R.F.; Bahta, A.W.; Farjo, N.; Farjo, B.; Philpott, M.P. Oxidative stress-associated senescence in dermal papilla cells of men with androgenetic alopecia. J. Investig. Dermatol. 2015, 135, 1244–1252. [Google Scholar] [CrossRef] [PubMed]
- Akar, A.; Arca, E.; Erbil, H.; Akay, C.; Sayal, A.; Gür, A.R. Antioxidant enzymes and lipid peroxidation in the scalp of patients with alopecia areata. J. Dermatol. Sci. 2002, 29, 85–90. [Google Scholar] [CrossRef]
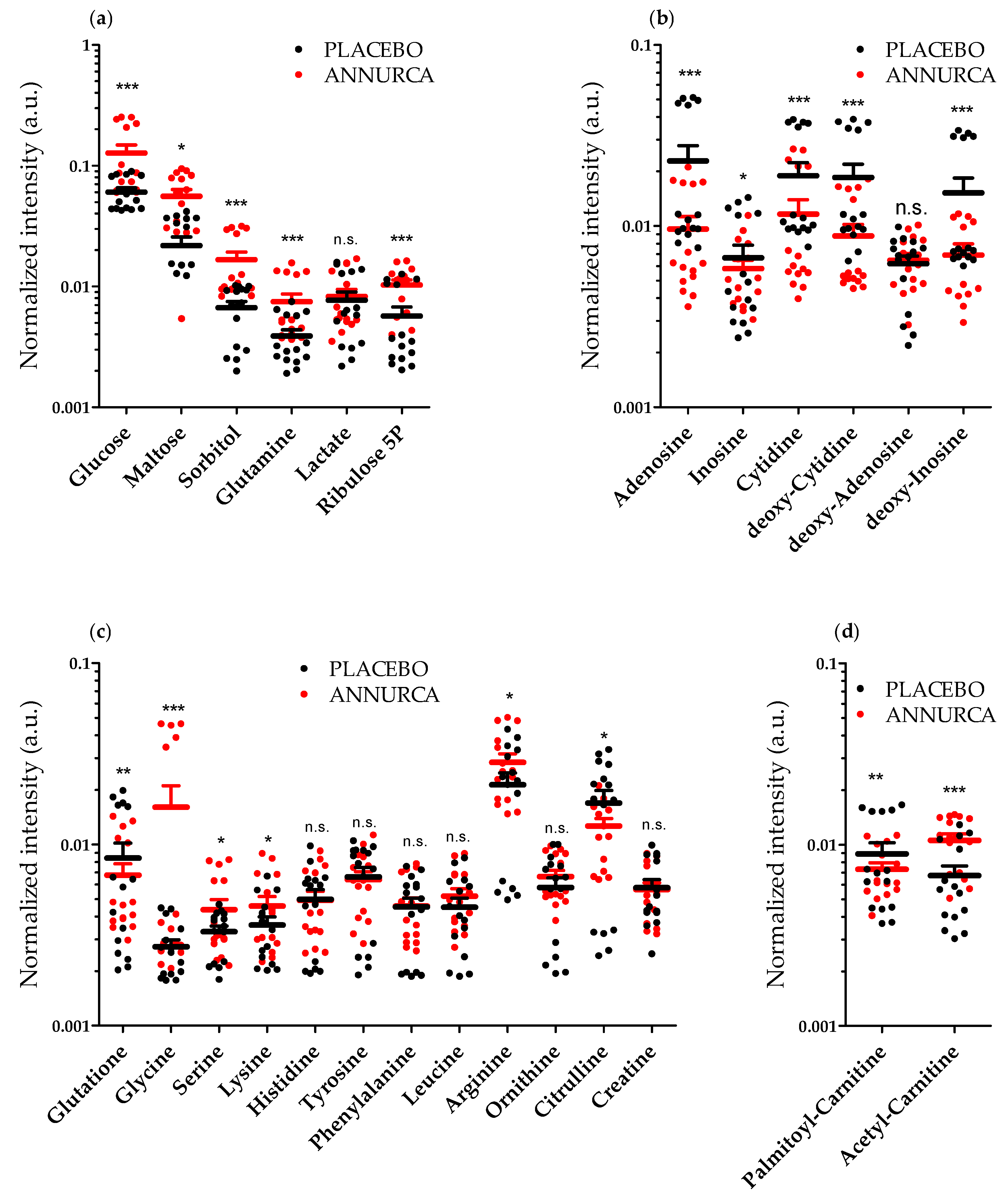
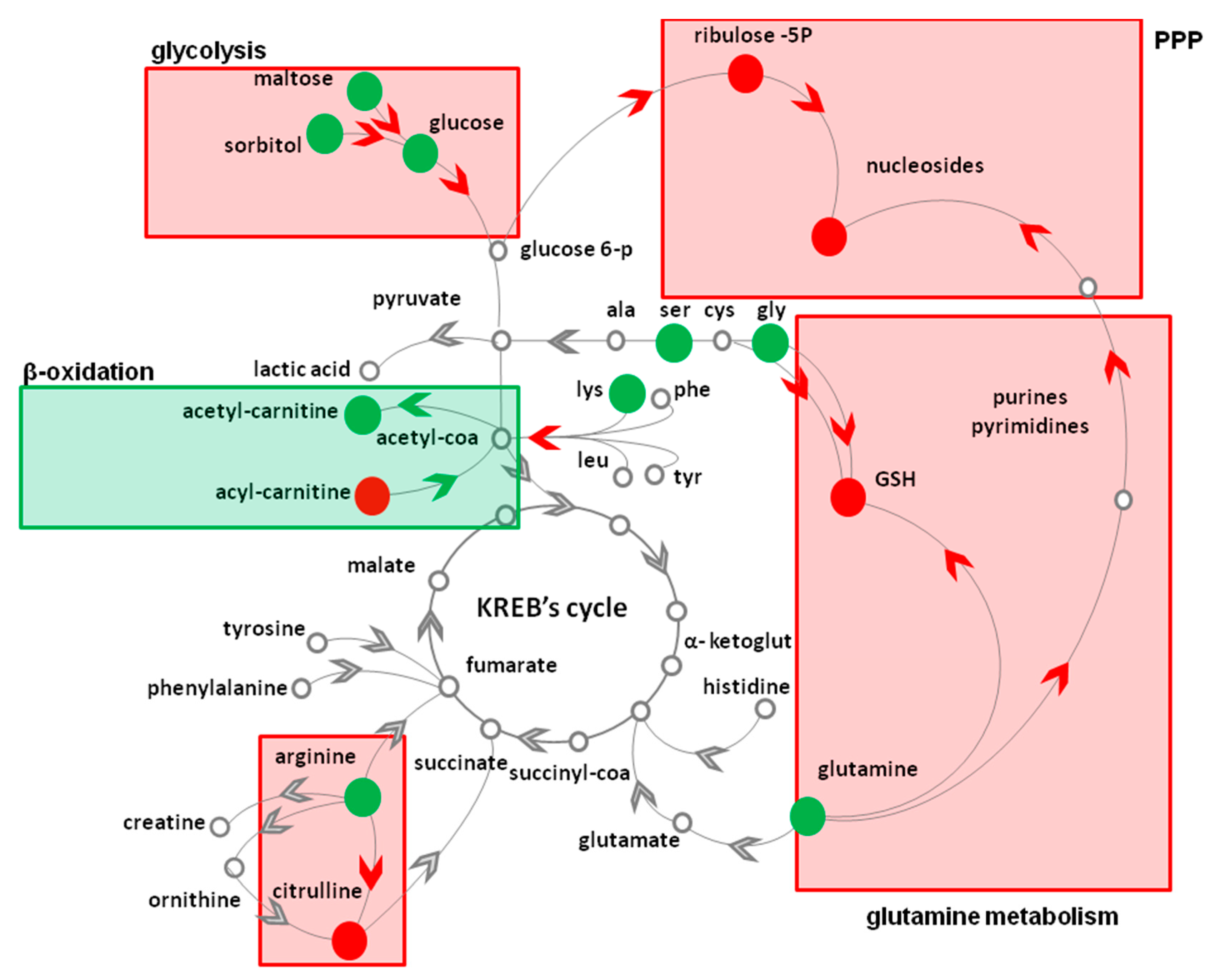
| Metabolic Pathway | Metabolite | Fold Change 1 | Metabolic Pathway | Metabolite | Fold Change 1 |
|---|---|---|---|---|---|
| Glycolysis | PPP | ||||
| Glucose * | 2.4 ± 0.2 | Ribulose 5P * | 3.2 ± 0.1 | ||
| Lactic acid | 1.1 ± 0.2 | ||||
| Glycogenolysis | Nucleotides | Adenosine * | 0.4 ± 0.1 | ||
| Maltose | 2.2 ± 0.1 | Cytidine * | 0.6 ± 0.1 | ||
| Sorbitol | 3.1 ± 0.2 | Deoxy-cytidine * | 0.5 ± 0.1 | ||
| Amino acids | Deoxy-inosine * | 0.5 ± 0.1 | |||
| Glutamine * | 2.4 ± 0.2 | ||||
| Glycine | 6.2 ± 0.2 | β-oxidation | Palmitoyl-carnitine | 0.8 ± 0.1 | |
| Serine | 1.3 ± 0.2 | Acetyl-carnitine | 1.6 ± 0.2 | ||
| Lysine | 1.3 ± 0.2 | ||||
| GSH * | 0.9 ± 0.1 | ||||
| Arginine | 1.3 ± 0.2 | ||||
| Citrulline * | 0.7 ± 0.2 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Badolati, N.; Sommella, E.; Riccio, G.; Salviati, E.; Heintz, D.; Bottone, S.; Di Cicco, E.; Dentice, M.; Tenore, G.; Campiglia, P.; et al. Annurca Apple Polyphenols Ignite Keratin Production in Hair Follicles by Inhibiting the Pentose Phosphate Pathway and Amino Acid Oxidation. Nutrients 2018, 10, 1406. https://doi.org/10.3390/nu10101406
Badolati N, Sommella E, Riccio G, Salviati E, Heintz D, Bottone S, Di Cicco E, Dentice M, Tenore G, Campiglia P, et al. Annurca Apple Polyphenols Ignite Keratin Production in Hair Follicles by Inhibiting the Pentose Phosphate Pathway and Amino Acid Oxidation. Nutrients. 2018; 10(10):1406. https://doi.org/10.3390/nu10101406
Chicago/Turabian StyleBadolati, Nadia, Eduardo Sommella, Gennaro Riccio, Emanuela Salviati, Dimitri Heintz, Sara Bottone, Emery Di Cicco, Monica Dentice, Giancarlo Tenore, Pietro Campiglia, and et al. 2018. "Annurca Apple Polyphenols Ignite Keratin Production in Hair Follicles by Inhibiting the Pentose Phosphate Pathway and Amino Acid Oxidation" Nutrients 10, no. 10: 1406. https://doi.org/10.3390/nu10101406
APA StyleBadolati, N., Sommella, E., Riccio, G., Salviati, E., Heintz, D., Bottone, S., Di Cicco, E., Dentice, M., Tenore, G., Campiglia, P., Stornaiuolo, M., & Novellino, E. (2018). Annurca Apple Polyphenols Ignite Keratin Production in Hair Follicles by Inhibiting the Pentose Phosphate Pathway and Amino Acid Oxidation. Nutrients, 10(10), 1406. https://doi.org/10.3390/nu10101406












