Serum Carotenoids Reveal Poor Fruit and Vegetable Intake among Schoolchildren in Burkina Faso
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Area and Subjects
2.2. Data and Sample Collection
2.3. Serum Retinol and Carotenoid Extraction and Analys
2.4. Statistical Methods
3. Results
3.1. Subject Characteristics
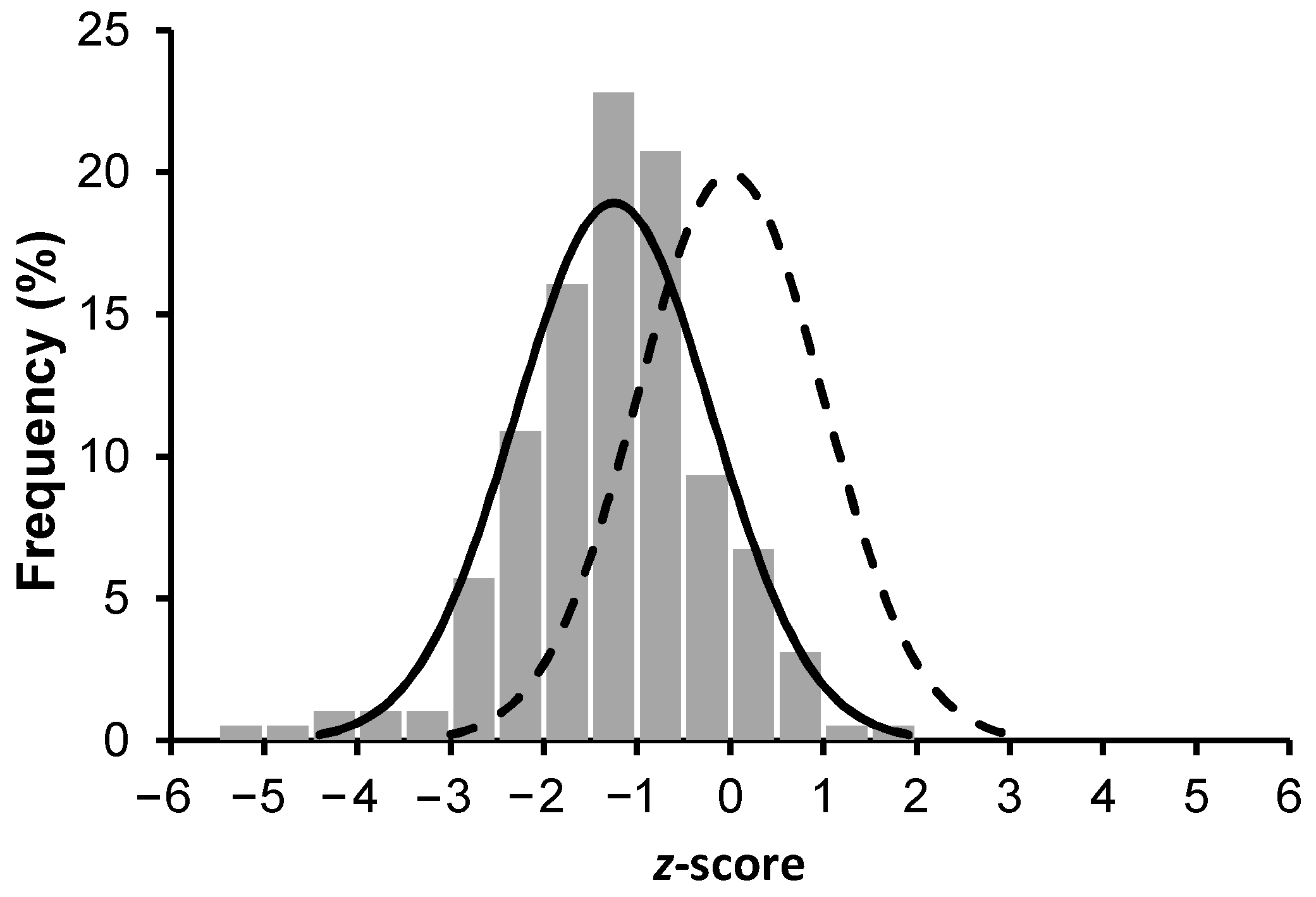
3.2. Serum Retinol and Carotenoid Concentrations
4. Discussion
Author Contributions
Funding
Conflicts of Interest
References
- Essential Nutrition Actions: Improving Maternal, Newborn, Infant and Young Child Health and Nutrition; World Health Organization: Geneva, Switzerland, 2013.
- Zeba, A.N.; Sorgho, H.; Rouamba, N.; Zongo, I.; Rouamba, J.; Guiguemdë, R.T.; Hamer, D.H.; Mokhtar, N.; Ouedraogo, J.B. Major reduction of malaria morbidity with combined vitamin A and zinc supplementation in young children in Burkina Faso: A randomized double blind trial. Nutr. J. 2008, 7, 7. [Google Scholar] [CrossRef] [PubMed]
- Shankar, A.H.; Genton, B.; Semba, R.D.; Baisor, M.; Paino, J.; Tamja, S.; Adiguma, T.; Wu, L.; Rare, L.; Tielsch, J.M.; et al. Effect of vitamin A supplementation on morbidity due to Plasmodium falciparum in young children in Papua New Guinea: A randomised trial. Lancet 1999, 354, 203–209. [Google Scholar] [CrossRef]
- Imdad, A.; Mayo-Wilson, E.; Herzer, K.; Bhutta, Z.A. Vitamin A supplementation for preventing morbidity and mortality in children from six months to five years of age. Cochrane Database Syst. Rev. 2017, 3, CD008524. [Google Scholar] [CrossRef] [PubMed]
- Krause, V.M.; Delisle, H.; Solomons, N.W. Fortified foods contribute one half of recommended vitamin A intake in poor urban Guatemalan toddlers. J. Nutr. 1998, 128, 860–864. [Google Scholar] [CrossRef] [PubMed]
- Engle-Stone, R.; Nankap, M.; Ndjebayi, A.O.; Brown, K.H. Simulations based on representative 24-h recall data predict region-specific differences in adequacy of vitamin A intake among Cameroonian women and young children following large-scale fortification of vegetable oil and other potential food vehicles. J. Nutr. 2014, 144, 1826–1834. [Google Scholar] [CrossRef] [PubMed]
- Nana, C.P.; Brouwer, I.D.; Zagré, N.M.; Kok, F.J.; Traoré, A.S. Impact of promotion of mango and liver as sources of vitamin A for young children: A pilot study in Burkina Faso. Public Health Nutr. 2016, 9, 808–813. [Google Scholar] [CrossRef]
- Plan National d’Action Pour La Nutrition, Version Révisée; Ministère de la Santé: Ouagadougou, Burkina Faso, 2001.
- Daboné, C.; Delisle, H.F.; Receveur, O. Poor nutritional status of schoolchildren in urban and peri-urban areas of Ouagadougou (Burkina Faso). Nutr. J. 2011, 10, 34. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zeba, A.N.; Prével, Y.M.; Somé, I.T.; Delisle, H.F. The positive impact of red palm oil in school meals on vitamin A status: Study in Burkina Faso. Nutr. J. 2006, 5, 17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tanumihardjo, S.A.; Russell, R.M.; Stephensen, C.B.; Gannon, B.M.; Craft, N.E.; Haskell, M.J.; Lietz, G.; Schulze, K.; Raiten, D.J. Biomarkers of nutrition for development (BOND)-Vitamin A review. J. Nutr. 2016, 146, 1816S–1848S. [Google Scholar] [CrossRef] [PubMed]
- Schoenthaler, S.J.; Bier, I.D.; Young, K.; Nichols, D.; Jansenns, S. The effect of vitamin-mineral supplementation on the intelligence of American schoolchildren: A randomized, double-blind placebo-controlled trial. J. Altern. Complement. Med. 2000, 6, 19–29. [Google Scholar] [CrossRef] [PubMed]
- Tanumhardjo, S.A.; Palacios, N.; Pixley, K.V. Provitamin A carotenoid bioavailability: What really matters? Int. J. Vitam. Nutr. Res. 2010, 80, 336–350. [Google Scholar] [CrossRef] [PubMed]
- Blanchette, L.; Brugg, J. Determinants of fruit and vegetable consumption among 6-12-year-old children and effective interventions to increase consumption. J. Hum. Nutr. Diet. 2005, 18, 431–443. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Zhang, Z.; Penniston, K.L.; Binkley, N.; Tanumihardjo, S.A. Serum carotenoid concentrations in postmenopausal women from the United States with and without osteoporosis. Int. J. Vit. Nutr. Res. 2008, 78, 105–111. [Google Scholar] [CrossRef] [PubMed]
- Mondloch, S.; Gannon, B.M.; Davis, C.R.; Chileshe, J.; Kaliwile, C.; Masi, C.; Rios-Avila, L.; Gregory, J.F., 3rd; Tanumihardjo, S.A. High provitamin A carotenoid serum concentrations, elevated retinyl esters, and saturated retinol-binding protein in Zambian preschool children are consistent with the presence of high liver vitamin A stores. Am. J. Clin. Nutr. 2015, 102, 497–504. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vitamin and Mineral Nutrition Information System—Haemoglobin Concentrations for the Diagnosis of Anaemia and Assessment of Severity; World Health Organization: Geneva, Switzerland, 2011.
- Comparison of the World Health Organization (WHO) Child Growth Standards and the National Center for Health Statistics/WHO International Growth Reference: Implications for Child Health Programmes; World Health Organization: Geneva, Switzerland, 2011.
- Rankins, J.; Green, N.R.; Tremper, W.; Stacewitcz-Sapuntzakis, M.; Bowen, P.; Ndiaye, M. Undernutrition and vitamin A deficiency in the Department of Linguère, Louga Region of Sénégal. Am. J. Clin. Nutr. 1993, 58, 91–97. [Google Scholar] [CrossRef] [PubMed]
- Das, B.S.; Thurnham, D.I.; Das, D.B. Plasma α-tocopherol, retinol and carotenoids in children with falciparum malaria. Am. J. Clin. Nutr. 1996, 64, 94–100. [Google Scholar] [CrossRef] [PubMed]
- Ribaya-Mercado, J.D.; Maramag, C.C.; Tengco, L.W.; Blumberg, J.B.; Solon, F.S. Relationships of body mass index with serum carotenoids, tocopherols and retinol at steady-state and in response to a carotenoid rich vegetable diet intervention in Filipino schoolchildren. Biosci. Rep. 2008, 28, 97–106. [Google Scholar] [CrossRef] [PubMed]
- Ford, E.S.; Gillespie, C.; Ballew, C.; Sowell, A.; Mannino, D.M. Serum carotenoid concentrations in US children and adolescents. Am. J. Clin. Nutr. 2002, 76, 818–827. [Google Scholar] [CrossRef] [PubMed]
- Melgar-Quinonez, H.R.; Zubieta, A.C.; MkNelly, B.; Nteziyaremye, A.; Gerardo, M.F.; Dunford, C. Household food insecurity and food expenditure in Bolivia, Burkina Faso and the Philippines. J. Nutr. 2006, 136, 1431S–1437S. [Google Scholar] [CrossRef] [PubMed]
- Belesova, K.; Gasparrini, A.; Sié, A.; Sauerborn, R.; Wilkinson, P. Household cereal crop harvest and children’s nutritional status in rural Burkina Faso. Environ. Health 2017, 16, 65. [Google Scholar] [CrossRef] [PubMed]
- Institute of Medicine, Food and Nutrition Board. Dietary Reference Intakes for Vitamin A, Vitamin K, Arsenic, Boron, Chromium, Copper, Iodine, Iron, Manganese, Molybdenum, Nickel, Silicon, Vanadium, and Zinc; National Academy Press: Washington, DC, USA, 2001; pp. 65–126. [Google Scholar]
- Nana, C.P.; Brouwer, I.D.; Zagré, N.M.; Kok, F.J.; Traoré, A.S. Community assessment of availability, consumption, and cultural acceptability of food sources of (pro)vitamin A: Toward the development of a dietary intervention among preschool children in rural Burkina Faso. Food Nutr. Bull. 2005, 26, 356–365. [Google Scholar] [CrossRef] [PubMed]
- Ayassou, K.; Ouedraogo, M.; Mathieu-Daudé, C.; Alain, B.; Chevalier, P. Amélioration de L’alimentation Burkinabè Avec Des Aliments Riches en Caroténoïdes. Available online: http://horizon.documentation.ird.fr/exl-doc/pleins_textes/divers11-08/010036323.pdf (accessed on 13 July 2018).
- Weber, D.; Grune, T. The contribution of β-carotene to vitamin A supply of humans. Mol. Nutr. Food Res. 2012, 56, 251–258. [Google Scholar] [CrossRef] [PubMed]
- Stuetz, W.; McGready, R.; Cho, T.; Prapamontol, T.; Biesalski, H.K.; Stepniewska, K.; Nosten, F. Relation of DDT residues to plasma retinol, alpha-tocopherol, and beta-carotene during pregnancy and malaria infection: A case-control study in Karen women in northern Thailand. Sci. Total Environ. 2006, 363, 78–86. [Google Scholar] [CrossRef] [PubMed]
| Parameter | Value (mean ± SD) |
|---|---|
| Age, year | 9.3 ± 1.48 |
| Height, cm | 129.1 ± 12.4 |
| Weight, kg | 24.8 ± 5.37 |
| Height-for-age z-score | −0.73 ± 1.25 |
| Weight-for-age z-score | −1.16 ± 1.01 |
| BMI-for-age z-score | −1.24 ± 1.05 |
| Hemoglobin, g/L | 122.4 ± 10.9 |
| Positive malaria blood smear, % (average parasitemia) | 39.5 (1655 parasites) |
| Country | Age Years | n | Retinol µmol/L | β-Carotene µmol/L | α-Carotene µmol/L | β-Cryptoxanthin µmol/L | Lutein µmol/L | Reference |
|---|---|---|---|---|---|---|---|---|
| Burkina Faso | 7–12 | 193 | 0.80 ± 0.35 | 0.12 ± 0.21 | 0.01 ± 0.04 | 0.05 ± 0.06 | 0.05 ± 0.06 | Present study |
| (range) | (0.17–1.92) | (0.004–2.15) | (0–0.4) | (0–0.3) | (0–0.2) | |||
| Zambia | 5–7 | 123 | 0.98 | 0.76 | 0.62 | 0.10 | 0.86 | Mondloch et al. [16] |
| Senegal | 2–4 | 281 | - | 0.16 | 0.030 | 0.020 | 0.46 | Rankins et al. [19] |
| India | 2–11 | 50 | 1.10 | 0.31 | 0.035 | 0.12 | 0.42 | Das et al. [20] |
| Philippines | 9–12 | 27 | 0.87 | 0.23 | 0.03 | 0.07 | 0.23 | Ribaya-Mercado et al. [21] |
| USA | 6–7 | 839 | - | 0.34 | 0.075 | 0.21 | 0.34 a | Ford et al. [22] |
| Characteristic | Normal Diet a | Added Green Leafy Vegetables b | ||
|---|---|---|---|---|
| Age group | 6–9 years | ≥10 years | 6–9 years | ≥10 years |
| Number of meals/day | 2 | 2 | 2 | 2 |
| Amount of sauce ingested/day (g) | 184 | 254 | 184 | 254 |
| Mean retinol activity equivalents (μg RAE/100 g) | 36 | 36 | 55 | 55 |
| Mean intakes of retinol activity (μg RAE/day) | 66 | 91 | 101 | 140 |
| Estimated average requirements [25] (μg RAE/day) | ||||
| Boys (6–13 years) | 275–445 | 445 | 275–445 | 445 |
| Girls (6–13 years) | 275–420 | 420 | 275–420 | 420 |
| Recommended Daily Allowance [25] (g RAE/day) | 400–600 | 600 | 400–600 | 600 |
| % of estimated average requirements met/day for boys and girls aged 6–13 years | 15–24 | 20–22 | 23–37 | 31–33 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bationo, J.F.; Zeba, A.N.; Abbeddou, S.; Coulibaly, N.D.; Sombier, O.O.; Sheftel, J.; Bassole, I.H.N.; Barro, N.; Ouedraogo, J.B.; Tanumihardjo, S.A. Serum Carotenoids Reveal Poor Fruit and Vegetable Intake among Schoolchildren in Burkina Faso. Nutrients 2018, 10, 1422. https://doi.org/10.3390/nu10101422
Bationo JF, Zeba AN, Abbeddou S, Coulibaly ND, Sombier OO, Sheftel J, Bassole IHN, Barro N, Ouedraogo JB, Tanumihardjo SA. Serum Carotenoids Reveal Poor Fruit and Vegetable Intake among Schoolchildren in Burkina Faso. Nutrients. 2018; 10(10):1422. https://doi.org/10.3390/nu10101422
Chicago/Turabian StyleBationo, Jean Fidèle, Augustin N. Zeba, Souheila Abbeddou, Nadine D. Coulibaly, Olivier O. Sombier, Jesse Sheftel, Imael Henri Nestor Bassole, Nicolas Barro, Jean Bosco Ouedraogo, and Sherry A. Tanumihardjo. 2018. "Serum Carotenoids Reveal Poor Fruit and Vegetable Intake among Schoolchildren in Burkina Faso" Nutrients 10, no. 10: 1422. https://doi.org/10.3390/nu10101422
APA StyleBationo, J. F., Zeba, A. N., Abbeddou, S., Coulibaly, N. D., Sombier, O. O., Sheftel, J., Bassole, I. H. N., Barro, N., Ouedraogo, J. B., & Tanumihardjo, S. A. (2018). Serum Carotenoids Reveal Poor Fruit and Vegetable Intake among Schoolchildren in Burkina Faso. Nutrients, 10(10), 1422. https://doi.org/10.3390/nu10101422





