Preparation of a Defined Gluten Hydrolysate for Diagnosis and Clinical Investigations of Wheat Hypersensitivities
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents and Materials
2.2. Standard Analyses
2.3. Contents of Albumins/Globulins, Gliadins and Glutenins in Gluten
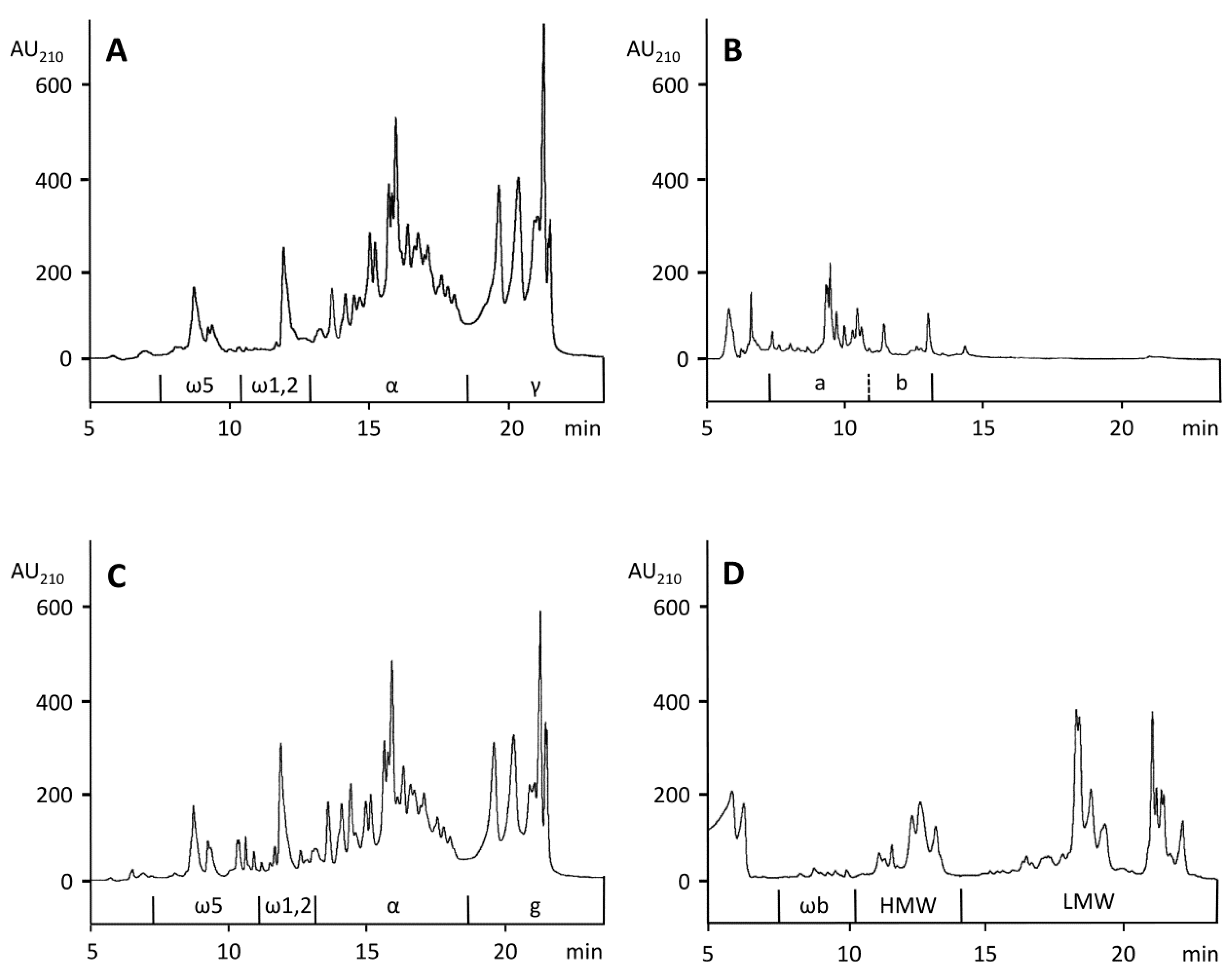
2.4. RP-HPLC for Gluten Proteins
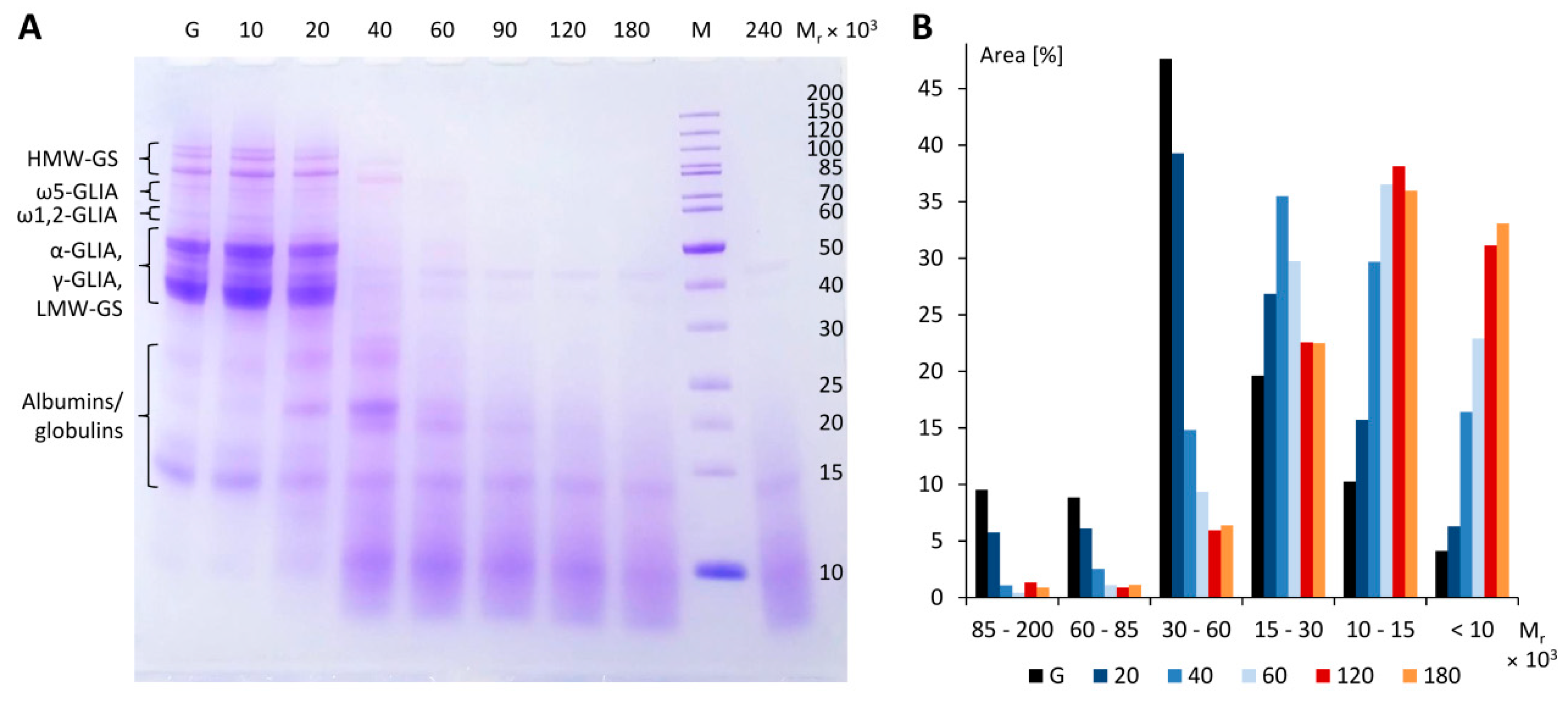
2.5. Optimization of Gluten Hydrolysis
2.6. Preparation of Pepgluten
2.7. RP-HPLC for the Gluten Hydrolysates
2.8. Sodium Dodecyl Sulfate-Polyacrylamide Gel Electrophoresis (SDS-PAGE)
2.9. Enzyme-Linked Immunosorbent Assay (ELISA)
2.10. Quantitation of ATIs
2.11. Descriptive Sensory Analysis
2.12. Statistical Analysis
3. Results
3.1. Composition of Gluten
3.2. Composition of the Protein Fraction in Gluten
3.3. Optimization of Gluten Hydrolysis
3.4. Preparation and Characterization of Pepgluten
3.5. Descriptive Sensory Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sapone, A.; Bai, J.C.; Ciacci, C.; Dolinsek, J.; Green, P.H.; Hadjivassiliou, M.; Kaukinen, K.; Rostami, K.; Sanders, D.S.; Schumann, M.; et al. Spectrum of gluten-related disorders: Consensus on new nomenclature and classification. BMC Med. 2012, 10, 13. [Google Scholar] [CrossRef] [PubMed]
- Catassi, C.; Elli, L.; Bonaz, B.; Bouma, G.; Carroccio, A.; Castillejo, G.; Cellier, C.; Cristofori, F.; De Magistris, L.; Dolinsek, J.; et al. Diagnosis of non-celiac gluten sensitivity (NCGS): The Salerno experts’ criteria. Nutrients 2015, 7, 4966–4977. [Google Scholar] [CrossRef] [PubMed]
- Catassi, C.; Alaedini, A.; Bojarski, C.; Bonaz, B.; Bouma, G.; Carroccio, A.; Castillejo, G.; De Magistris, L.; Dieterich, W.; Di Liberto, D.; et al. The overlapping area of non-celiac gluten sensitivity (NCGS) and wheat-sensitive irritable bowel syndrome (IBS): An update. Nutrients 2017, 9, 1268. [Google Scholar] [CrossRef] [PubMed]
- Vazquez-Roque, M.I.; Camilleri, M.; Smyrk, T.; Murray, J.A.; Marietta, E.; O’Neill, J.; Carlson, P.; Lamsam, J.; Janzow, D.; Eckert, D.; et al. A controlled trial of gluten-free diet in patients with irritable bowel syndrome-diarrhea: Effects on bowel frequency and intestinal function. Gastroenterology 2013, 144, 903.e3–911.e3. [Google Scholar] [CrossRef] [PubMed]
- Schalk, K.; Lexhaller, B.; Koehler, P.; Scherf, K.A. Isolation and characterization of gluten protein types from wheat, rye, barley and oats for use as reference materials. PLoS ONE 2017, 12, e0172819. [Google Scholar] [CrossRef] [PubMed]
- Scherf, K.A.; Koehler, P.; Wieser, H. Gluten and wheat sensitivities—An overview. J. Cereal Sci. 2016, 67, 2–11. [Google Scholar] [CrossRef]
- Wieser, H. Chemistry of gluten proteins. Food Microbiol. 2007, 24, 115–119. [Google Scholar] [CrossRef] [PubMed]
- Junker, Y.; Zeissig, S.; Kim, S.J.; Barisani, D.; Wieser, H.; Leffler, D.A.; Zevallos, V.; Libermann, T.A.; Dillon, S.; Freitag, T.L.; et al. Wheat amylase trypsin inhibitors drive intestinal inflammation via activation of toll-like receptor 4. J. Exp. Med. 2012, 209, 2395–2408. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zevallos, V.; Raker, V.; Tenzer, S.; Jimenez-Calvente, C.; Ashfaq-Khan, M.; Rüssel, N.; Pickert, G.; Schild, H.; Steinbrink, K.; Schuppan, D. Nutritional wheat amylase-trypsin inhibitors promote intestinal inflammation via activation of myeloid cells. Gastroenterology 2017, 152, 1100–1113. [Google Scholar] [CrossRef] [PubMed]
- Leffler, D.A.; Schuppan, D.; Pallav, K.; Najarian, R.; Goldsmith, J.D.; Hansen, J.; Kabbani, T.; Dennis, M.; Kelly, C.P. Kinetics of the histologic, serologic, and symptomatic responses to gluten challenge in adults with coeliac disease. Gut 2013, 62, 996–1004. [Google Scholar] [CrossRef] [PubMed]
- Muraro, A.; Werfel, T.; Hoffmann-Sommergruber, K.; Roberts, G.; Beyer, K.; Bindslev-Jensen, C.; Cardona, V.; Dubois, A.; duToit, G.; Eigenmann, P.; et al. EAACI food allergy and anaphylaxis guidelines: Diagnosis and management of food allergy. Allergy 2014, 69, 1008–1025. [Google Scholar] [CrossRef] [PubMed]
- Sampson, H.A.; Gerth van Wijk, R.; Bindslev-Jensen, C.; Sicherer, S.; Teuber, S.S.; Burks, A.W.; Dubois, A.E.; Beyer, K.; Eigenmann, P.A.; Spergel, J.M.; et al. Standardizing double-blind, placebo-controlled oral food challenges: American Academy of Allergy, Asthma & Immunology-European Academy of Allergy and Clinical Immunology PRACTALL consensus report. J. Allergy Clin. Immunol. 2012, 130, 1260–1274. [Google Scholar] [CrossRef] [PubMed]
- Brockow, K.; Kneissl, D.; Valentini, L.; Zelger, O.; Grosber, M.; Kugler, C.; Werich, M.; Darsow, U.; Matsuo, H.; Morita, E.; et al. Using a gluten oral food challenge protocol to improve diagnosis of wheat-dependent exercise-induced anaphylaxis. J. Allergy Clin. Immunol. 2015, 135, 977–984. [Google Scholar] [CrossRef] [PubMed]
- Makharia, A.; Catassi, C.; Makharia, G.K. The overlap between irritable bowel syndrome and non-celiac gluten sensitivity: A clinical dilemma. Nutrients 2015, 7, 10417–10426. [Google Scholar] [CrossRef] [PubMed]
- Volta, U.; Pinto-Sanchez, M.I.; Boschetti, E.; Caio, G.; De Giorgio, R.; Verdu, E.F. Dietary triggers in irritable bowel syndrome: Is there a role for gluten? J. Neurogastroenterol. Motil. 2016, 22, 547–557. [Google Scholar] [CrossRef] [PubMed]
- Di Sabatino, A.; Volta, U.; Salvatore, C.; Biancheri, P.; Caio, G.; De Giorgio, R.; Di Stefano, M.; Corazza, G.R. Small amounts of gluten in subjects with suspected nonceliac gluten sensitivity: A randomized, double-blind, placebo-controlled, cross-over trial. Clin. Gastroenterol. Hepatol. 2015, 13, 1604.e3–1612.e3. [Google Scholar] [CrossRef] [PubMed]
- Nilsson, N.; Sjolander, S.; Baar, A.; Berthold, M.; Pahr, S.; Vrtala, S.; Valenta, R.; Morita, E.; Hedlin, G.; Borres, M.P.; et al. Wheat allergy in children evaluated with challenge and IgE antibodies to wheat components. Pediatr. Allergy Immunol. 2015, 26, 119–125. [Google Scholar] [CrossRef] [PubMed]
- Camarca, A.; Radano, G.; Di Mase, R.; Terrone, G.; Maurano, F.; Auricchio, S.; Troncone, R.; Greco, L.; Gianfrani, C. Short wheat challenge is a reproducible in-vivo assay to detect immune response to gluten. Clin. Exp. Immunol. 2012, 169, 129–136. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ito, K.; Futamura, M.; Borres, M.P.; Takaoka, Y.; Dahlstrom, J.; Sakamoto, T.; Tanaka, A.; Kohno, K.; Matsuo, H.; Morita, E. IgE antibodies to omega-5 gliadin associate with immediate symptoms on oral wheat challenge in Japanese children. Allergy 2008, 63, 1536–1542. [Google Scholar] [CrossRef] [PubMed]
- Catassi, C.; Fabiani, E.; Iacono, G.; D’Agate, C.; Francavilla, R.; Biagi, F.; Volta, U.; Accomando, S.; Picarelli, A.; De Vitis, I.; et al. A prospective, double-blind, placebo-controlled trial to establish a safe gluten threshold for patients with celiac disease. Am. J. Clin. Nutr. 2007, 85, 160–166. [Google Scholar] [CrossRef] [PubMed]
- Dale, H.F.; Hatlebakk, J.G.; Hovdenak, N.; Ystad, S.O.; Lied, G.A. The effect of a controlled gluten challenge in a group of patients with suspected non-coeliac gluten sensitivity: A randomized, double-blind placebo-controlled challenge. Neurogastroenterol. Motil. 2018, 30, e13332. [Google Scholar] [CrossRef] [PubMed]
- Bindslev-Jensen, C.; Ballmer-Weber, B.K.; Bengtsson, U.; Blanco, C.; Ebner, C.; Hourihane, J.; Knulst, A.C.; Moneret-Vautrin, D.A.; Nekam, K.; Niggemann, B.; et al. Standardization of food challenges in patients with immediate reactions to foods—Position paper from the European Academy of Allergology and Clinical Immunology. Allergy 2004, 59, 690–697. [Google Scholar] [CrossRef] [PubMed]
- Van Eckert, R.; Berghofer, E.; Ciclitira, P.J.; Chirdo, F.; Denery-Papini, S.; Ellis, H.J.; Ferranti, P.; Goodwin, P.; Immer, U.; Mamone, G.; et al. Towards a new gliadin reference material—Isolation and characterisation. J. Cereal Sci. 2006, 43, 331–341. [Google Scholar] [CrossRef]
- McCleary, B.V.; Solah, V.; Gibson, T.S. Quantitative measurement of total starch in cereal flours and products. J. Cereal Sci. 1994, 20, 51–58. [Google Scholar] [CrossRef]
- Schulte, E. Simplified micro method for the gravimetric determination of fat in foods after acid treatment. Deutsch. Lebensm. Rundsch. 2004, 100, 188–189. [Google Scholar]
- Wieser, H.; Antes, S.; Seilmeier, W. Quantitative determination of gluten protein types in wheat flour by high-performance liquid chromatography. Cereal Chem. 1998, 75, 644–650. [Google Scholar] [CrossRef]
- Hajas, L.; Scherf, K.A.; Török, K.; Bugyi, Z.; Schall, E.; Poms, R.E.; Koehler, P.; Tömösközi, S. Variation in protein composition among wheat (Triticum aestivum L.) cultivars to identify cultivars suitable as reference material for wheat gluten analysis. Food Chem. 2018, 267, 387–394. [Google Scholar] [CrossRef] [PubMed]
- Dorum, S.; Arntzen, M.O.; Qiao, S.-W.; Holm, A.; Koehler, C.J.; Thiede, B.; Sollid, L.M.; Fleckenstein, B. The preferred substrates for transglutaminase 2 in a complex wheat gluten digest are peptide fragments harboring celiac disease T-cell epitopes. PLoS ONE 2010, 5, e14056. [Google Scholar] [CrossRef] [PubMed]
- Laemmli, U.K. Cleavage of structural proteins during assembly of head of bacteriophage T4. Nature 1970, 227, 680–685. [Google Scholar] [CrossRef] [PubMed]
- Lagrain, B.; Rombouts, I.; Wieser, H.; Delcour, J.A.; Koehler, P. A reassessment of the electrophoretic mobility of high molecular weight subunits of wheat. J. Cereal Sci. 2012, 56, 726–732. [Google Scholar] [CrossRef]
- Garcia, E.; Llorente, M.; Hernando, A.; Kieffer, R.; Wieser, H.; Mendez, E. Development of a general procedure for complete extraction of gliadins for heat processed and unheated foods. Eur. J. Gastroenterol. Hepatol. 2005, 17, 529–539. [Google Scholar] [PubMed]
- Geisslitz, S.; Ludwig, C.; Scherf, K.A.; Koehler, P. Targeted LC-MS/MS reveals similar contents of α-amylase/trypsin-inhibitors as cofactors of non-celiac gluten sensitivity in all wheat species except einkorn. J. Agric. Food Chem. 2018. revised. [Google Scholar]
- Scherf, K.A.; Pflaum, T.; Koehler, P.; Hofmann, T. Salt taste perception in hydrocolloid systems is affected by sodium ion release and mechanosensory-gustatory cross-modal interactions. Food Hydrocoll. 2015, 51, 486–494. [Google Scholar] [CrossRef]
- Frazer, A.C.; Fletcher, R.F.; Ross, C.A.; Shaw, B.; Sammons, H.G.; Schneider, R. Gluten-induced enteropathy: The effect of partially digested gluten. Lancet 1959, 2, 252–255. [Google Scholar] [CrossRef]
- Pahlavan, A.; Sharma, G.; Pereira, M.; Williams, K.M. Effects of grain species and cultivar, thermal processing, and enzymatic hydrolysis on gluten quantitation. Food Chem. 2016, 208, 264–271. [Google Scholar] [CrossRef] [PubMed]
- Rallabhandi, P.; Sharma, G.M.; Pereira, M.; Williams, K.M. Immunological characterization of the gluten fractions and their hydrolysates from wheat, rye and barley. J. Agric. Food Chem. 2015, 63, 1825–1832. [Google Scholar] [CrossRef] [PubMed]
- Gessendorfer, B.; Koehler, P.; Wieser, H. Preparation and characterization of enzymatically hydrolyzed prolamins from wheat, rye, and barley as references for the immunochemical quantitation of partially hydrolyzed gluten. Anal. Bioanal. Chem. 2009, 395, 1721–1728. [Google Scholar] [CrossRef] [PubMed]
- Scherf, K.A.; Wieser, H.; Koehler, P. Novel approaches for enzymatic gluten degradation to create high-quality gluten-free products. Food Res. Int. 2018, 110, 62–72. [Google Scholar] [CrossRef] [PubMed]
- Lexhaller, B.; Tompos, C.; Scherf, K.A. Comparative analysis of prolamin and glutelin fractions from wheat, rye, and barley with five sandwich ELISA test kits. Anal. Bioanal. Chem. 2016, 408, 6093–6104. [Google Scholar] [CrossRef] [PubMed]
- Matsuo, H.; Morimoto, K.; Akaki, T.; Kaneko, S.; Kusatake, K.; Kuroda, T.; Niihara, H.; Hide, M.; Morita, E. Exercise and aspirin increase levels of circulating gliadin peptides in patients with wheat-dependent exercise-induced anaphylaxis. Clin. Exp. Allergy 2005, 35, 461–466. [Google Scholar] [CrossRef] [PubMed]
- Moreno, M.L.; Cebolla, A.; Munoz-Suano, A.; Carrillo-Carrion, C.; Comino, I.; Pizarro, A.; Leon, F.; Rodriguez-Herrera, A.; Sousa, C. Detection of immunogenic peptides in the urine of patients with coeliac disease reveals transgressions in the gluten-free diet and incomplete mucosal healing. Gut 2017, 66, 250–257. [Google Scholar] [CrossRef] [PubMed]
- Andre, C.; Andre, F.; Colin, L.; Cavagna, S. Measurement of intestinal permeability to mannitol and lactulose as a means of diagnosing food allergy and evaluating therapeutic effectiveness of disodium cromoglycate. Ann. Allergy 1987, 59, 127–130. [Google Scholar] [PubMed]

| Constituent | Gluten | Pepgluten |
|---|---|---|
| (mg/g) 1 | (mg/g) 1 | |
| Crude protein (nitrogen × 5.7) | 718.8 ± 1.8 A | 707.1 ± 2.5 A |
| Starch | 107.7 ± 3.5 A | 104.2 ± 1.0 A |
| Water | 62.3 ± 0.3 A | 59.2 ± 1.9 B |
| Fat | 41.5 ± 2.7 A | 46.6 ± 0.7 A |
| Dietary fiber | 49.6 ± 4.7 A | 40.5 ± 4.3 A |
| Ash | 7.9 ± 0.1 A | 10.9 ± 0.1 B |
| Residue | 12.2 | 31.5 |
| Gluten 2 | 646.6 ± 34.8 A | 372.0 ± 36.3 B |
| ATIs (sum) | 32.6 A | 22.4 B |
| ATI 0.19 + 0.53 | 10.5 ± 1.6 A | 10.6 ± 1.4 A |
| ATI 0.28 | 2.0 ± 0.2 A | 1.9 ± 0.1 A |
| ATI CM2 | 1.7 ± 0.3 A | 1.6 ± 0.1 A |
| ATI CM3 | 11.9 ± 0.4 A | 2.7 ± 0.1 B |
| ATI CM16 | 6.5 ± 1.1 A | 5.6 ± 0.9 A |
| Protein | Gluten | Gluten Digestion Assay | Pepgluten | |
|---|---|---|---|---|
| (mg/g) 1 | (mg/g) 2 | (%) | (mg/g) | |
| ALGL | 27.7 ± 0.8 | 26.3 | 3.6 | 25.4 |
| GLIA | 402.1 ± 5.4 | 382.0 | 52.1 | 368.6 |
| ω5 | 16.3 ± 1.1 | 15.5 | 2.1 | 15.0 |
| ω1,2 | 27.8 ± 0.6 | 26.4 | 3.6 | 25.5 |
| α | 208.8 ± 2.9 | 198.4 | 27.1 | 191.4 |
| γ | 149.2 ± 1.2 | 141.7 | 19.3 | 136.7 |
| GLUT | 213.8 ± 6.1 | 203.1 | 27.7 | 196.0 |
| ωb | 9.7 ± 0.5 | 9.2 | 1.3 | 8.9 |
| HMW-GS | 55.0 ± 1.5 | 52.3 | 7.1 | 50.5 |
| LMW-GS | 149.1 ± 3.9 | 141.6 | 19.3 | 136.6 |
| Gluten 3 | 615.9 ± 2.9 | 585.1 | 79.8 | 564.6 |
| Residue | 75.2 | 71.4 | 9.8 | 68.9 |
| Capsule 4 | - | 50.0 | 6.8 | 48.2 |
| Sum | 718.8 ± 1.8 | 732.8 | 100 | 707.1 ± 2.5 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wieser, H.; Scherf, K.A. Preparation of a Defined Gluten Hydrolysate for Diagnosis and Clinical Investigations of Wheat Hypersensitivities. Nutrients 2018, 10, 1411. https://doi.org/10.3390/nu10101411
Wieser H, Scherf KA. Preparation of a Defined Gluten Hydrolysate for Diagnosis and Clinical Investigations of Wheat Hypersensitivities. Nutrients. 2018; 10(10):1411. https://doi.org/10.3390/nu10101411
Chicago/Turabian StyleWieser, Herbert, and Katharina A. Scherf. 2018. "Preparation of a Defined Gluten Hydrolysate for Diagnosis and Clinical Investigations of Wheat Hypersensitivities" Nutrients 10, no. 10: 1411. https://doi.org/10.3390/nu10101411
APA StyleWieser, H., & Scherf, K. A. (2018). Preparation of a Defined Gluten Hydrolysate for Diagnosis and Clinical Investigations of Wheat Hypersensitivities. Nutrients, 10(10), 1411. https://doi.org/10.3390/nu10101411





