A Review on the Gluten-Free Diet: Technological and Nutritional Challenges
Abstract
:1. Introduction
2. Gluten-Free Products
2.1. Gluten Functionality
2.2. Gluten Replacement Strategies
2.2.1. Ingredients
2.2.2. Processing
- -
- Corn flour has been milled in various instruments. Different corn varieties were selected to explore the varietal effect and the flour’s physical properties’ impact on its potential to produce high quality gluten-free products [83].
- -
- Germination of brown rice was studied as a pre-treatment to alter the functionality of brown rice flour in gluten-free bread baking applications [84]. Rice germination did indeed alter the hydration and pasting properties of the flour. This resulted in increased crumb softness. However, the germination process had to be closely monitored to control the activity of α-amylase.
- -
- Similar to wheat flour-based systems, sourdough fermentation of teff flour products has also been explored [68,85]. The fermentation was shown to have a major impact on the physicochemical properties of teff starch and a more limited effect on the protein fraction. Bread loaves made with this fermented teff flour yielded better gluten-free breads than those produced with unfermented teff flour [85].
- -
- Phosphorylation of rice flour is another strategy that was studied. The resulting gluten-free breads had a lower hardness and an improved bread volume, crumb appearance, and color [86].
- -
- Pre-gelatinization of the starch used as a base ingredient has also been attempted and led to a decreased dough elasticity, but a higher resistance to deformation, assuring a better retention of gas in the dough structure. As such, hardness of the product was decreased [57].
- -
- -
- Extrusion of rice flour increased the dough consistency and hydration of rice flour gluten-free bread, while increasing the crumb hardness and lowering the specific volume. However, these bread quality effects can be counteracted by working with flours with coarser particle sizes [88].
- -
- Particles of whey protein were shown to display elastic and strain hardening characteristics when mixed with starch. Whey protein has been converted to whey protein particles using a cold gelation method prior to being used to produce gluten-free bread [89]. Van Riemsdijk and colleagues [90] found that the effect of whey protein particles on bread quality was heavily governed by the amount of disulfide bonds present in the dough (and the particles).
3. Gluten-Free Diet
3.1. Consumers’ Motivations, Knowledge and Attitudes
3.1.1. Consumers’ Motivations
3.1.2. Consumers’ Knowledge
3.1.3. Consumers’ Attitudes
3.2. Nutritional Implications
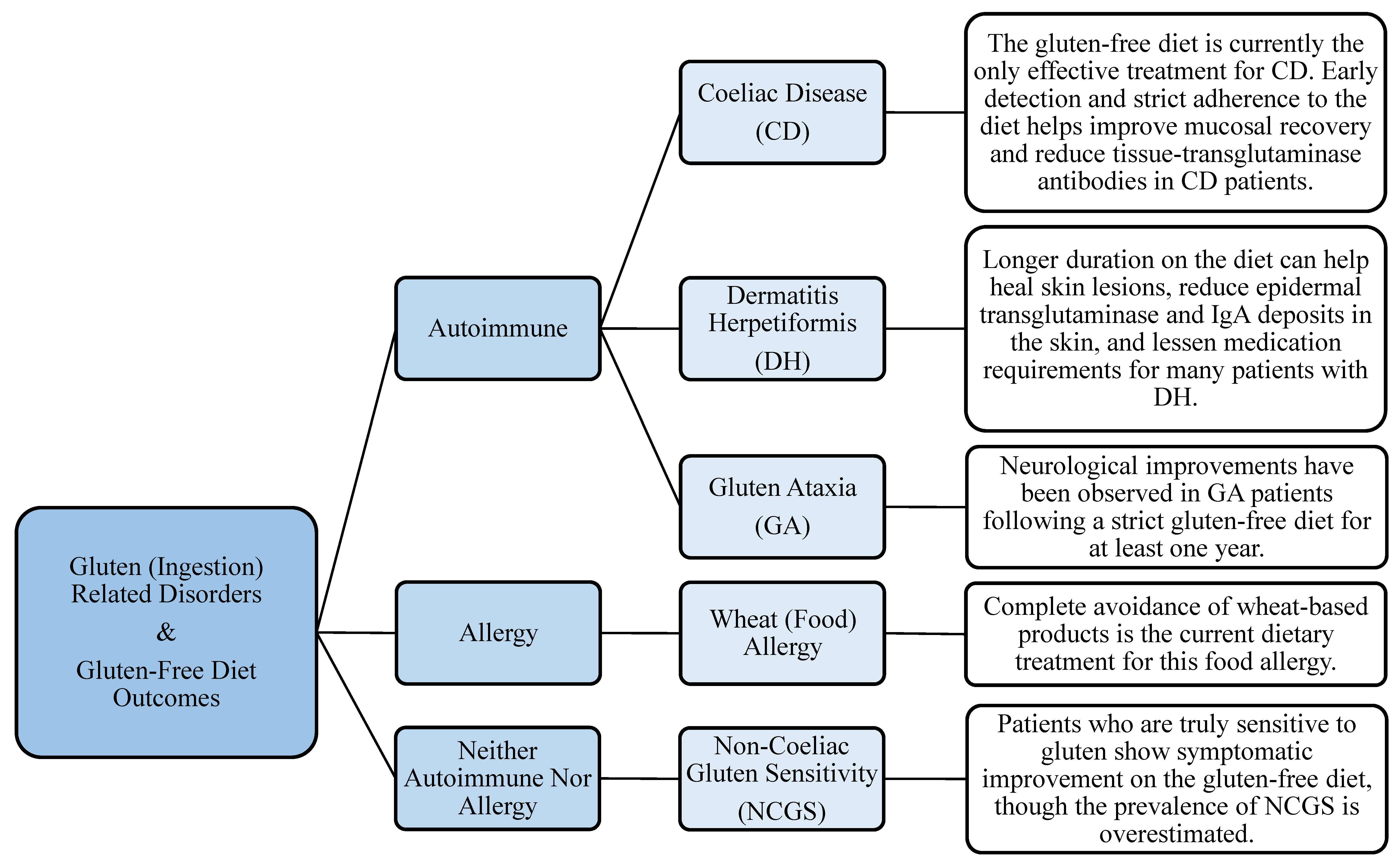
4. Gluten-Related Disorders
4.1. CD, NCGS, GA, and DH
4.1.1. Management of Symptoms
4.1.2. Management of Gut Microflora
4.2. Other Disorders Closely Linked to CD
4.2.1. T1D
4.2.2. Other Autoimmune Diseases
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Wrigley, C.; Bekes, F.; Bushuk, W. Gliadin and Glutenin: The Unique Balance of Wheat Quality; AACC International, Inc.: St. Paul, MN, USA, 2006. [Google Scholar]
- El-Chammas, K.; Danner, E. Gluten-free diet in nonceliac disease. Nutr. Clin. Pract. 2011, 26, 294–299. [Google Scholar] [CrossRef] [PubMed]
- Wieser, H. Chemistry of gluten proteins. Food Microbiol. 2007, 24, 115–119. [Google Scholar] [CrossRef] [PubMed]
- Capriles, V.D.; Arêas, J.A.G. Novel approaches in gluten-free breadmaking: Interface between food science, nutrition, and health. Compr. Rev. Food Sci. Food Saf. 2014, 13, 871–890. [Google Scholar] [CrossRef]
- Market and Markets Gluten-Free Products Market by Type (Bakery Products, Pizzas & Pastas, Cereals & Snacks, Savories, and Others), Source (Oilseeds & Pulses, Rice & Corn, Dairy & Meat Products, and Other Crops), & by Region—Global Trends & Forecast to 2020. Available online: https://www.marketsandmarkets.com/Market-Reports/gluten-free-products-market-738.html (accessed on 31 July 2018).
- European Parliament Regulation (EU) No 609/2013 of the European Parliament and of the Council on food intended for infants and young children, food for special medical purposes, and total diet replacement for weight control. Off. J. Eur. Union 2013, 2012, 35–56.
- U.S Food and Drug Administration Guidance for Industry: Gluten-Free Labeling of Foods; Small Entity Compliance Guide. Available online: https://www.fda.gov/Food/GuidanceRegulation/GuidanceDocumentsRegulatoryInformation/ucm402549.htm (accessed on 31 July 2018).
- Theethira, T.G.; Dennis, M. Celiac disease and the gluten-free diet: Consequences and recommendations for improvement. Dig. Dis. 2015, 33, 175–182. [Google Scholar] [CrossRef] [PubMed]
- Bascuñán, K.A.; Vespa, M.C.; Araya, M. Celiac disease: Understanding the gluten-free diet. Eur. J. Nutr. 2017, 56, 449–459. [Google Scholar] [CrossRef] [PubMed]
- Delcour, J.A.; Joye, I.J.; Pareyt, B.; Wilderjans, E.; Brijs, K.; Lagrain, B. Wheat gluten functionality as quality determinant in cereal-based food products. Annu. Rev. Food Sci. Technol. 2012, 3. [Google Scholar] [CrossRef] [PubMed]
- Wilderjans, E.; Pareyt, B.; Goesaert, H.; Brijs, K.; Delcour, J.A. the role of gluten in a pound cake system: A model approach based on gluten-starch blends. Food Chem. 2008, 110, 909–915. [Google Scholar] [CrossRef] [PubMed]
- Pareyt, B.; Wilderjans, E.; Goesaert, H.; Brijs, K.; Delcour, J.A. The role of gluten in a sugar-snap cookie system: A model approach based on gluten-starch blends. J. Cereal Sci. 2008, 48, 8630869. [Google Scholar] [CrossRef]
- Toufeili, I.; Shawky, D.; Sossy, S.; Abir, N.; Sarakbi, M.; Farran, M.T. Formulation of Gluten-Free Pocket-Type Flat Breads: Optimization of Methylcellulose, Gum Arabic, and Egg Albumen Levels by Response Surface Methodology. Am. Assoc. Cereal Chem. 1994, 71, 594–601. [Google Scholar]
- Pacyński, M.; Wojtasiak, R.Z.; Mildner-Szkudlarz, S. Improving the aroma of gluten-free bread. LWT Food Sci. Technol. 2015. [Google Scholar] [CrossRef]
- Aguilar, N.; Albanell, E.; Miñarro, B.; Capellas, M. Chickpea and tiger nut flours as alternatives to emulsifier and shortening in gluten-free bread. LWT Food Sci. Technol. 2015. [Google Scholar] [CrossRef]
- Kim, M.; Yun, Y.; Jeong, Y. Effects of corn, potato, and tapioca starches on the quality of gluten-free rice bread. Food Sci. Biotechnol. 2015. [Google Scholar] [CrossRef]
- Krupa, U.; Rosell, C.M.; Sadowska, J.; Soral-ŚMietana, M. Bean starch as ingredient for gluten-free bread. J. Food Process. Preserv. 2010. [Google Scholar] [CrossRef] [Green Version]
- Milde, L.B.; Ramallo, L.A.; Puppo, M.C. Gluten-free Bread Based on Tapioca Starch: Texture and Sensory Studies. Food Bioprocess Technol. 2012. [Google Scholar] [CrossRef]
- Onyango, C.; Mutungi, C.; Unbehend, G.; Lindhauer, M.G. Modification of gluten-free sorghum batter and bread using maize, potato, cassava or rice starch. LWT—Food Sci. Technol. 2011. [Google Scholar] [CrossRef]
- Mancebo, C.M.; Merino, C.; Martínez, M.M.; Gómez, M. Mixture design of rice flour, maize starch and wheat starch for optimization of gluten free bread quality. J. Food Sci. Technol. 2015. [Google Scholar] [CrossRef] [PubMed]
- Sarawong, C.; Gutiérrez, Z.R.; Berghofer, E.; Schoenlechner, R. Effect of green plantain flour addition to gluten-free bread on functional bread properties and resistant starch content. Int. J. Food Sci. Technol. 2014. [Google Scholar] [CrossRef]
- Kang, T.Y.; Sohn, K.H.; Yoon, M.R.; Lee, J.S.; Ko, S. Effect of the shape of rice starch granules on flour characteristics and gluten-free bread quality. Int. J. Food Sci. Technol. 2015. [Google Scholar] [CrossRef]
- Ziobro, R.; Korus, J.; Witczak, M.; Juszczak, L. Influence of modified starches on properties of gluten-free dough and bread. Part II: Quality and staling of gluten-free bread. Food Hydrocoll. 2012. [Google Scholar] [CrossRef]
- Hager, A.S.; Wolter, A.; Czerny, M.; Bez, J.; Zannini, E.; Arendt, E.K.; Czerny, M. Investigation of product quality, sensory profile and ultrastructure of breads made from a range of commercial gluten-free flours compared to their wheat counterparts. Eur. Food Res. Technol. 2012. [Google Scholar] [CrossRef]
- Miñarro, B.; Albanell, E.; Aguilar, N.; Guamis, B.; Capellas, M. Effect of legume flours on baking characteristics of gluten-free bread. J. Cereal Sci. 2012. [Google Scholar] [CrossRef]
- Moreira, R.; Chenlo, F.; Torres, M.D. Effect of chia (Sativa hispanica L.) and hydrocolloids on the rheology of gluten-free doughs based on chestnut flour. LWT—Food Sci. Technol. 2013. [Google Scholar] [CrossRef]
- Nyembwe, P.M.; de Kock, H.L.; Taylor, J.R.N. Potential of defatted marama flour-cassava starch composites to produce functional gluten-free bread-type dough. LWT—Food Sci. Technol. 2018. [Google Scholar] [CrossRef]
- Paciulli, M.; Rinaldi, M.; Cirlini, M.; Scazzina, F.; Chiavaro, E. Chestnut flour addition in commercial gluten-free bread: A shelf-life study. LWT—Food Sci. Technol. 2016. [Google Scholar] [CrossRef]
- Rostamian, M.; Milani, J.M.; Maleki, G. Physical properties of gluten-free bread made of corn and chickpea flour. Int. J. Food Eng. 2014. [Google Scholar] [CrossRef]
- Tsatsaragkou, K.; Gounaropoulos, G.; Mandala, I. Development of gluten free bread containing carob flour and resistant starch. LWT—Food Sci. Technol. 2014. [Google Scholar] [CrossRef]
- Trappey, E.F.; Khouryieh, H.; Aramouni, F.; Herald, T. Effect of sorghum flour composition and particle size on quality properties of gluten-free bread. Food Sci. Technol. Int. 2015. [Google Scholar] [CrossRef] [PubMed]
- Korus, J.; Witczak, T.; Ziobro, R.; Juszczak, L. Linseed (Linum usitatissimum L.) mucilage as a novel structure forming agent in gluten-free bread. LWT—Food Sci. Technol. 2015. [Google Scholar] [CrossRef]
- Martínez, M.M.; Díaz, Á.; Gómez, M. Effect of different microstructural features of soluble and insoluble fibres on gluten-free dough rheology and bread-making. J. Food Eng. 2014. [Google Scholar] [CrossRef]
- Pastuszka, D.; Gambuś, H.; Ziobro, R.; Buksa, K.; Sabat, R.; Augustyn, G. Impact of oats β-glucans on properties of gluten-free bread. J. Microbiol. Biotechnol. 2012, 1, 972–979. [Google Scholar]
- Rocha Parra, A.F.; Ribotta, P.D.; Ferrero, C. Apple pomace in gluten-free formulations: Effect on rheology and product quality. Int. J. Food Sci. Technol. 2015. [Google Scholar] [CrossRef]
- Rózyło, R.; Dziki, D.; Gawlik-Dziki, U.; Biernacka, B.; Wójcik, M.; Ziemichód, A. Physical and antioxidant properties of gluten-free bread enriched with carob fibre. Int. Agrophys. 2017. [Google Scholar] [CrossRef]
- Sciarini, L.S.; Bustos, M.C.; Vignola, M.B.; Paesani, C.; Salinas, C.N.; Pérez, G.T. A study on fibre addition to gluten free bread: Its effects on bread quality and in vitro digestibility. J. Food Sci. Technol. 2017. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Quirce, S.; Lazaridou, A.; Biliaderis, C.G.; Ronda, F. Effect of β-glucan molecular weight on rice flour dough rheology, quality parameters of breads and in vitro starch digestibility. LWT Food Sci. Technol. 2017. [Google Scholar] [CrossRef]
- Wolter, A.; Hager, A.S.; Zannini, E.; Czerny, M.; Arendt, E.K. Influence of dextran-producing Weissella cibaria on baking properties and sensory profile of gluten-free and wheat breads. Int. J. Food Microbiol. 2014. [Google Scholar] [CrossRef] [PubMed]
- Demirkesen, I.; Mert, B.; Sumnu, G.; Sahin, S. Utilization of chestnut flour in gluten-free bread formulations. J. Food Eng. 2010. [Google Scholar] [CrossRef]
- Dizlek, H.; Ozer, M.S. The Impacts of Various Ratios of Different Hydrocolloids and Surfactants on Quality Characteristics of Corn Starch Based Gluten-free Bread. Cereal Res. Commun. 2016. [Google Scholar] [CrossRef] [Green Version]
- Hager, A.S.; Arendt, E.K. Influence of hydroxypropylmethylcellulose (HPMC), xanthan gum and their combination on loaf specific volume, crumb hardness and crumb grain characteristics of gluten-free breads based on rice, maize, teff and buckwheat. Food Hydrocoll. 2013. [Google Scholar] [CrossRef]
- Mezaize, S.; Chevallier, S.; Le Bail, A.; De Lamballerie, M. Optimization of gluten-free formulations for French-style breads. J. Food Sci. 2009. [Google Scholar] [CrossRef] [PubMed]
- Morreale, F.; Garzón, R.; Rosell, C.M. Understanding the role of hydrocolloids viscosity and hydration in developing gluten-free bread. A study with hydroxypropylmethylcellulose. Food Hydrocoll. 2018. [Google Scholar] [CrossRef]
- Cappa, C.; Lucisano, M.; Mariotti, M. Influence of Psyllium, sugar beet fibre and water on gluten-free dough properties and bread quality. Carbohydr. Polym. 2013. [Google Scholar] [CrossRef] [PubMed]
- Lazaridou, A.; Duta, D.; Papageorgiou, M.; Belc, N.; Biliaderis, C.G. Effects of hydrocolloids on dough rheology and bread quality parameters in gluten-free formulations. J. Food Eng. 2007. [Google Scholar] [CrossRef]
- Liu, X.; Mu, T.; Sun, H.; Zhang, M.; Chen, J.; Fauconnier, M.L. Influence of different hydrocolloids on dough thermo-mechanical properties and in vitro starch digestibility of gluten-free steamed bread based on potato flour. Food Chem. 2018. [Google Scholar] [CrossRef] [PubMed]
- Nicolae, A.; Radu, G.L.; Belc, N. Effect of sodium carboxymethyl cellulose on gluten-free dough rheology. J. Food Eng. 2016. [Google Scholar] [CrossRef]
- Naji-Tabasi, S.; Mohebbi, M. Evaluation of cress seed gum and xanthan gum effect on macrostructure properties of gluten-free bread by image processing. J. Food Meas. Charact. 2014. [Google Scholar] [CrossRef]
- Ziobro, R.; Juszczak, L.; Witczak, M.; Korus, J. Non-gluten proteins as structure forming agents in gluten free bread. J. Food Sci. Technol. 2016. [Google Scholar] [CrossRef] [PubMed]
- Crockett, R.; Ie, P.; Vodovotz, Y. Effects of soy protein isolate and egg white solids on the physicochemical properties of gluten-free bread. Food Chem. 2011. [Google Scholar] [CrossRef]
- Espinosa-Ramírez, J.; Garzon, R.; Serna-Saldivar, S.O.; Rosell, C.M. Mimicking gluten functionality with β-conglycinin concentrate: Evaluation in gluten free yeast-leavened breads. Food Res. Int. 2018. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez Furlán, L.T.; Pérez Padilla, A.; Campderrós, M.E. Improvement of gluten-free bread properties by the incorporation of bovine plasma proteins and different saccharides into the matrix. Food Chem. 2015. [Google Scholar] [CrossRef] [PubMed]
- Phongthai, S.; D’Amico, S.; Schoenlechner, R.; Rawdkuen, S. Comparative study of rice bran protein concentrate and egg albumin on gluten-free bread properties. J. Cereal Sci. 2016. [Google Scholar] [CrossRef]
- Marco, C.; Rosell, C.M. Breadmaking performance of protein enriched, gluten-free breads. Eur. Food Res. Technol. 2008. [Google Scholar] [CrossRef] [Green Version]
- Mohammadi, M.; Azizi, M.H.; Neyestani, T.R.; Hosseini, H.; Mortazavian, A.M. Development of gluten-free bread using guar gum and transglutaminase. J. Ind. Eng. Chem. 2015. [Google Scholar] [CrossRef]
- Pongjaruvat, W.; Methacanon, P.; Seetapan, N.; Fuongfuchat, A.; Gamonpilas, C. Influence of pregelatinised tapioca starch and transglutaminase on dough rheology and quality of gluten-free jasmine rice breads. Food Hydrocoll. 2014. [Google Scholar] [CrossRef]
- Yano, H. Improvements in the bread-making quality of gluten-free rice batter by glutathione. J. Agric. Food Chem. 2010. [Google Scholar] [CrossRef] [PubMed]
- Hamada, S.; Suzuki, K.; Aoki, N.; Suzuki, Y. Improvements in the qualities of gluten-free bread after using a protease obtained from Aspergillus oryzae. J. Cereal Sci. 2013. [Google Scholar] [CrossRef]
- Hatta, E.; Matsumoto, K.; Honda, Y. Bacillolysin, papain, and subtilisin improve the quality of gluten-free rice bread. J. Cereal Sci. 2015. [Google Scholar] [CrossRef]
- Elgeti, D.; Nordlohne, S.D.; Föste, M.; Besl, M.; Linden, M.H.; Heinz, V.; Jekle, M.; Becker, T. Volume and texture improvement of gluten-free bread using quinoa white flour. J. Cereal Sci. 2014. [Google Scholar] [CrossRef]
- Nunes, M.H.B.; Moore, M.M.; Ryan, L.A.M.; Arendt, E.K. Impact of emulsifiers on the quality and rheological properties of gluten-free breads and batters. Eur. Food Res. Technol. 2009. [Google Scholar] [CrossRef]
- López-Tenorio, J.A.; Rodríguez-Sandoval, E.; Sepúlveda-Valencia, J.U. The influence of different emulsifiers on the physical and textural characteristics of gluten-free cheese bread. J. Texture Stud. 2015. [Google Scholar] [CrossRef]
- Alencar, N.M.M.; de Morais, E.C.; Steel, C.J.; Bolini, H.M.A. Sensory characterisation of gluten-free bread with addition of quinoa, amaranth flour and sweeteners as an alternative for coeliac patients. Int. J. Food Sci. Technol. 2017, 52, 872–879. [Google Scholar] [CrossRef]
- Machado Alencar, N.M.; Steel, C.J.; Alvim, I.D.; de Morais, E.C.; Andre Bolini, H.M. Addition of quinoa and amaranth flour in gluten-free breads: Temporal profile and instrumental analysis. LWT Food Sci. Technol. 2015. [Google Scholar] [CrossRef]
- Alvarez-Jubete, L.; Auty, M.; Arendt, E.K.; Gallagher, E. Baking properties and microstructure of pseudocereal flours in gluten-free bread formulations. Eur. Food Res. Technol. 2009. [Google Scholar] [CrossRef]
- Bernardi, C.; Sánchez, H.; Freyre, M.; Osella, C. Gluten-free bread formulated with Prosopis ruscifolia (vinal) seed and corn flours. Int. J. Food Sci. Nutr. 2010. [Google Scholar] [CrossRef] [PubMed]
- Campo, E.; del Arco, L.; Urtasun, L.; Oria, R.; Ferrer-Mairal, A. Impact of sourdough on sensory properties and consumers’ preference of gluten-free breads enriched with teff flour. J. Cereal Sci. 2016. [Google Scholar] [CrossRef]
- Chakraborty, S.K.; Gupta, S.; Kotwaliwale, N. Quality characteristics of gluten free bread from barnyard millet–soy flour blends. J. Food Sci. Technol. 2016. [Google Scholar] [CrossRef] [PubMed]
- Mariotti, M.; Pagani, M.A.; Lucisano, M. The role of buckwheat and HPMC on the breadmaking properties of some commercial gluten-free bread mixtures. Food Hydrocoll. 2013. [Google Scholar] [CrossRef]
- Sandri, L.T.B.; Santos, F.G.; Fratelli, C.; Capriles, V.D. Development of gluten-free bread formulations containing whole chia flour with acceptable sensory properties. Food Sci. Nutr. 2017. [Google Scholar] [CrossRef] [PubMed]
- Skendi, A.; Mouselemidou, P.; Papageorgiou, M.; Papastergiadis, E. Effect of acorn meal-water combinations on technological properties and fine structure of gluten-free bread. Food Chem. 2018. [Google Scholar] [CrossRef] [PubMed]
- Smith, B.M.; Bean, S.R.; Herald, T.J.; Aramouni, F.M. Effect of HPMC on the Quality of Wheat-Free Bread Made from Carob Germ Flour-Starch Mixtures. J. Food Sci. 2012. [Google Scholar] [CrossRef] [PubMed]
- Andersson, H.; Öhgren, C.; Johansson, D.; Kniola, M.; Stading, M. Extensional flow, viscoelasticity and baking performance of gluten-free zein-starch doughs supplemented with hydrocolloids. Food Hydrocoll. 2011. [Google Scholar] [CrossRef]
- Numfon, R. Effect of different hydrocolloids on properties of gluten-free bread based on small broken broken rice berry flour. Food Sci. Technol. Int. 2017, 23, 310–317. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Sandoval, E.; Cortes-Rodriguez, M.; Manjarres-Pinzon, K. Effect of hydrocolloids on the pasting profiles of tapioca starch mixtures and the baking properties of gluten-free cheese bread. J. Food Process. Preserv. 2015. [Google Scholar] [CrossRef]
- Aprodu, I.; Banu, I. Influence of dietary fiber, water, and glucose oxidase on rheological and baking properties of maize based gluten-free bread. Food Sci. Biotechnol. 2015. [Google Scholar] [CrossRef]
- Föste, M.; Nordlohne, S.D.; Elgeti, D.; Linden, M.H.; Heinz, V.; Jekle, M.; Becker, T. Impact of quinoa bran on gluten-free dough and bread characteristics. Eur. Food Res. Technol. 2014. [Google Scholar] [CrossRef]
- Ronda, F.; Perez-Quirce, S.; Lazaridou, A.; Biliaderis, C.G. Effect of barley and oat β-glucan concentrates on gluten-free rice-based doughs and bread characteristics. Food Hydrocoll. 2015. [Google Scholar] [CrossRef]
- Ziobro, R.; Korus, J.; Juszczak, L.; Witczak, T. Influence of inulin on physical characteristics and staling rate of gluten-free bread. J. Food Eng. 2013. [Google Scholar] [CrossRef]
- Caputo, I.; Lepretti, M.; Martucciello, S.; Esposito, C. Enzymatic strategies to detoxify gluten: Implications for celiac disease. Enzyme Res. 2010. [Google Scholar] [CrossRef] [PubMed]
- Diowksz, A.; Leszczyńska, J. Hypoallergenic wheat bread: Response to an emerging issue. Food Agric. Immunol. 2014. [Google Scholar] [CrossRef]
- Brites, C.; Trigo, M.J.; Santos, C.; Collar, C.; Rosell, C.M. Maize-based gluten-free bread: Influence of processing parameters on sensory and instrumental quality. Food Bioprocess Technol. 2010. [Google Scholar] [CrossRef] [Green Version]
- Cornejo, F.; Rosell, C.M. Influence of germination time of brown rice in relation to flour and gluten free bread quality. J. Food Sci. Technol. 2015. [Google Scholar] [CrossRef] [PubMed]
- Marti, A.; Marengo, M.; Bonomi, F.; Casiraghi, M.C.; Franzetti, L.; Pagani, M.A.; Iametti, S. Molecular features of fermented teff flour relate to its suitability for the production of enriched gluten-free bread. LWT Food Sci. Technol. 2017. [Google Scholar] [CrossRef]
- Kringel, D.H.; da Silva Filipini, G.; de Las Mercedes Salas-Mellado, M. Influence of phosphorylated rice flour on the quality of gluten-free bread. Int. J. Food Sci. Technol. 2017, 52, 1291–1298. [Google Scholar] [CrossRef]
- Shin, D.J.; Kim, W.; Kim, Y. Physicochemical and sensory properties of soy bread made with germinated, steamed, and roasted soy flour. Food Chem. 2013. [Google Scholar] [CrossRef] [PubMed]
- Martínez, M.M.; Oliete, B.; Román, L.; Gómez, M. Influence of the addition of extruded flours on rice bread quality. J. Food Qual. 2014. [Google Scholar] [CrossRef]
- Van Riemsdijk, L.E.; van der Goot, A.J.; Hamer, R.J. The use of whey protein particles in gluten-free bread production, the effect of particle stability. Food Hydrocoll. 2011. [Google Scholar] [CrossRef]
- Van Berge-Henegouwen, G.P.; Mulder, C.J.J. Pioneer in the Gluten Free Diet: Willem-Karel Dicke 1905–1962, over 50 Years of Gluten Free Diet. Gut 1993, 34, 1473–1475. [Google Scholar] [CrossRef] [PubMed]
- Choung, R.S.; Unalp-Arida, A.; Ruhl, C.E.; Brantner, T.L.; Everhart, J.E.; Murray, J.A. Less hidden celiac disease but increased gluten avoidance without a diagnosis in the United States. Mayo Clin. Proc. 2017, 92, 30–38. [Google Scholar] [CrossRef] [PubMed]
- Golley, S.; Corsini, N.; Topping, D.; Morell, M.; Mohr, P. Motivations for avoiding wheat consumption in Australia: Results from a population survey. Public Health Nutr. 2015, 18, 490–499. [Google Scholar] [CrossRef] [PubMed]
- Stevenson, J.C.; Rankin-Sunter, K.; Maki, C.; Bruna, S.; Mosher, M.J.; Barrett, C.E. Community based pilot study of diagnostic paths to the gluten free diet. Int. J. Celiac Dis. 2015, 3, 14–24. [Google Scholar] [CrossRef]
- NSF International NSF International Survey Finds U.S. Consumers Struggle to Define and Identify Gluten. Available online: http://www.nsf.org/newsroom/nsf-survey-finds-us-consumers-struggle-to-define-identify-gluten (accessed on 1 August 2018).
- Singh, P.; Arora, A.; Strand, T.A.; Leffler, D.A.; Catassi, C.; Green, P.H.; Kelly, C.P.; Ahuja, V.; Makharia, G.K. Global Prevalence of Celiac Disease: Systematic Review and Meta-analysis. Clin. Gastroenterol. Hepatol. 2018, 16, 823–836. [Google Scholar] [CrossRef] [PubMed]
- Copelton, D.A.; Valle, G. “You don’t need a prescription to go gluten-free”: The scientific self-diagnosis of celiac disease. Soc. Sci. Med. 2009, 69, 623–631. [Google Scholar] [CrossRef] [PubMed]
- Gaesser, G.A.; Angadi, S.S. Gluten-free diet: Imprudent dietary advice for the general population? J. Acad. Nutr. Diet. 2012, 112, 1330–1333. [Google Scholar] [CrossRef] [PubMed]
- Lebwohl, B.; Cao, Y.; Zong, G.; Hu, F.B.; Green, P.H.R.; Neugut, A.I.; Rimm, E.B.; Sampson, L.; Dougherty, L.W.; Giovannucci, E.; Willett, W.C.; Sun, Q.; Chan, A.T. Long term gluten consumption in adults without celiac disease and risk of coronary heart disease: Prospective cohort study. BMJ 2017, 357, j1892. [Google Scholar] [CrossRef] [PubMed]
- Hartman Group Gluten Free Trend. Available online: https://www.hartman-group.com/hartbeat-acumen/120/gluten-free-trend (accessed on 2 August 2018).
- Gluten-Free Foods in Canada; Packaged Facts: Rockville, MD, USA, 2013.
- Dunn, C.; House, L.; Shelnutt, K.P. Consumer Perceptions of Gluten-Free Products and the Healthfulness of Gluten-Free Diets. J. Nutr. Educ. Behav. 2014, 46, S184–S185. [Google Scholar] [CrossRef]
- Haroldson, A.; Yen, C.-L. (Alan) Consumer Understanding of Nutrition Marketing Terms: A Pilot Study. J. Fam. Consum. Sci. 2016, 108, 24–31. [Google Scholar] [CrossRef]
- Silvester, J.A.; Weiten, D.; Graff, L.A.; Walker, J.R.; Duerksen, D.R. Is it gluten-free? Relationship between self-reported gluten-free diet adherence and knowledge of gluten content of foods. Nutrition 2016, 32, 777–783. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- O’Donnell, B.; Edelstein, S. Dietitians’ perceptions of adherence to a gluten-free diet among low-income individuals with celiac disease. Top. Clin. Nutr. 2009, 24, 82–89. [Google Scholar] [CrossRef]
- Halmos, E.P.; Deng, M.; Knowles, S.R.; Sainsbury, K.; Mullan, B.; Tye-Din, J.A. Food knowledge and psychological state predict adherence to a gluten-free diet in a survey of 5310 Australians and New Zealanders with coeliac disease. Aliment. Pharmacol. Ther. 2018, 48, 78–86. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zarkadas, M.; Cranney, A.; Case, S.; Molloy, M.; Switzer, C.; Graham, I.D.; Butzner, J.D.; Rashid, M.; Warren, R.E.; Burrows, V. The impact of a gluten-free diet on adults with coeliac disease: Results of a national survey. J. Hum. Nutr. Diet. 2006, 19, 41–49. [Google Scholar] [CrossRef] [PubMed]
- Leffler, D.A.; Edwards-George, J.; Dennis, M.; Schuppan, D.; Cook, F.; Franko, D.L.; Blom-Hoffman, J.; Kelly, C.P. Factors that influence adherence to a gluten-free diet in adults with celiac disease. Dig. Dis. Sci. 2008, 53, 1573–1581. [Google Scholar] [CrossRef] [PubMed]
- Villafuerte-Galvez, J.; Vanga, R.R.; Dennis, M.; Hansen, J.; Leffler, D.A.; Kelly, C.P.; Mukherjee, R. Factors governing long-term adherence to a gluten-free diet in adult patients with coeliac disease. Aliment. Pharmacol. Ther. 2015, 42, 753–760. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zarkadas, M.; Dubois, S.; Macisaac, K.; Cantin, I.; Rashid, M.; Roberts, K.C.; La Vieille, S.; Godefroy, S.; Pulido, O.M. Living with coeliac disease and a gluten-free diet: A Canadian perspective. J. Hum. Nutr. Diet. 2013, 26, 10–23. [Google Scholar] [CrossRef] [PubMed]
- Silvester, J.A.; Weiten, D.; Graff, L.A.; Walker, J.R.; Duerksen, D.R. Living gluten-free: Adherence, knowledge, lifestyle adaptations and feelings towards a gluten-free diet. J. Hum. Nutr. Diet. 2016, 29, 374–382. [Google Scholar] [CrossRef] [PubMed]
- Do Nascimento, A.B.; Fiates, G.M.R.; Dos Anjos, A.; Teixeira, E. Gluten-free is not enough-perception and suggestions of celiac consumers. Int. J. Food Sci. Nutr. 2014, 65, 394–398. [Google Scholar] [CrossRef] [PubMed]
- Stevens, L.; Rashid, M. Gluten-free and regular foods: A cost comparison. Can. J. Diet. Pract. Res. 2008, 69, 147–150. [Google Scholar] [CrossRef] [PubMed]
- Singh, J.; Whelan, K. Limited availability and higher cost of gluten-free foods. J. Hum. Nutr. Diet. 2011, 24, 479–486. [Google Scholar] [CrossRef] [PubMed]
- Lambert, K.; Ficken, C. Cost and affordability of a nutritionally balanced gluten-free diet: Is following a gluten-free diet affordable? Nutr. Diet 2016, 73, 36–42. [Google Scholar] [CrossRef]
- Lee, A.R.; Ng, D.L.; Zivin, J.; Green, P.H.R. Economic burden of a gluten-free diet. J. Hum. Nutr. Diet. 2007, 423–430. [Google Scholar] [CrossRef] [PubMed]
- Lee, A.; Newman, J.M. Celiac diet: Its impact on quality of life. J. Am. Diet. Assoc. 2003, 103, 1533–1535. [Google Scholar] [CrossRef] [PubMed]
- Do Nascimento, A.B.; Fiates, G.M.R.; Dos Anjos, A.; Teixeira, E. Analysis of ingredient lists of commercially available gluten-free and gluten-containing food products using the text mining technique. Int. J. Food Sci. Nutr. 2013, 64, 217–222. [Google Scholar] [CrossRef] [PubMed]
- Fry, L.; Madden, A.M.; Fallaize, R. An investigation into the nutritional composition and cost of gluten-free versus regular food products in the UK. J. Hum. Nutr. Diet. 2018, 31, 108–120. [Google Scholar] [CrossRef] [PubMed]
- Miranda, J.; Lasa, A.; Bustamante, M.A.; Churruca, I.; Simon, E. Nutritional Differences Between a Gluten-free Diet and a Diet Containing Equivalent Products with Gluten. Plant Foods Hum. Nutr. 2014, 69, 182–187. [Google Scholar] [CrossRef] [PubMed]
- Kulai, T.; Rashid, M. Assessment of nutritional adequacy of packaged gluten-free food products. Can. J. Diet. Pract. Res. 2014, 75, 186–190. [Google Scholar] [CrossRef] [PubMed]
- Berti, C.; Riso, P.; Monti, L.D.; Porrini, M. In vitro starch digestibility and in vivo glucose response of gluten-free foods and their gluten counterparts. Eur. J. Nutr. 2004, 43, 198–204. [Google Scholar] [CrossRef] [PubMed]
- Thompson, T. Thiamin, riboflavin, and niacin contents of the gluten-free diet: Is there cause for concern? J. Am. Diet. Assoc. 1999, 99, 858–862. [Google Scholar] [CrossRef]
- Thompson, T. Folate, iron, and dietary fiber contents of the gluten-free diet. J. Am. Diet. Assoc. 2000, 100, 1389–1396. [Google Scholar] [CrossRef]
- Barone, M.; Della Valle, N.; Rosania, R.; Facciorusso, A.; Trotta, A.; Cantatore, F.P.; Falco, S.; Pignatiello, S.; Viggiani, M.T.; Amoruso, A.; et al. A comparison of the nutritional status between adult celiac patients on a long-term, strictly gluten-free diet and healthy subjects. Eur. J. Clin. Nutr. 2016, 70, 23–27. [Google Scholar] [CrossRef] [PubMed]
- Wild, D.; Robins, G.G.; Burley, V.J.; Howdle, P.D. Evidence of high sugar intake, and low fibre and mineral intake, in the gluten-free diet. Aliment. Pharmacol. Ther. 2010, 32, 573–581. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Babio, N.; Alcázar, M.; Castillejo, G.; Recasens, M.; Martínez-Cerezo, F.; Gutiérrez-Pensado, V.; Masip, G.; Vaqué, C.; Vila-Martí, A.; Torres-Moreno, M.; et al. Patients with celiac disease reported higher consumption of added sugar and total fat than healthy individuals. J. Pediatr. Gastroenterol. Nutr. 2017, 64, 63–69. [Google Scholar] [CrossRef] [PubMed]
- Öhlund, K.; Olsson, C.; Hernell, O.; Öhlund, I. Dietary shortcomings in children on a gluten-free diet. J. Hum. Nutr. Diet 2010, 23, 294–300. [Google Scholar] [CrossRef] [PubMed]
- Ferrara, P.; Cicala, M.; Tiberi, E.; Spadaccio, C.; Marcella, L.; Gatto, A.; Calzolari, P.; Castellucci, G. High fat consumption in children with celiac disease. Acta Gastroenterol. Belg. 2009, 72, 296–300. [Google Scholar] [PubMed]
- Valitutti, F.; Iorfida, D.; Anania, C.; Trovato, C.M.; Montuori, M.; Cucchiara, S.; Catassi, C. Cereal Consumption among Subjects with Celiac Disease: A Snapshot for Nutritional Considerations. Nutrients 2017, 9. [Google Scholar] [CrossRef] [PubMed]
- Shepherd, S.J.; Gibson, P.R. Nutritional inadequacies of the gluten-free diet in both recently-diagnosed and long-term patients with coeliac disease. J. Hum. Nutr. Diet. 2013, 26, 349–358. [Google Scholar] [CrossRef] [PubMed]
- Hallert, C.; Grant, C.; Grehn, S.; Grännö, C.; Hultén, S.; Midhagen, G.; Ström, M.; Svensson, H.; Valdimarsson, T. Evidence of poor vitamin status in coeliac patients on a gluten-free diet for 10 years. Aliment. Pharmacol. Ther. 2002, 16, 1333–1339. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thompson, T.; Dennis, M.; Higgins, L.A.; Lee, A.R.; Sharrett, M.K. Gluten-free diet survey: Are Americans with coeliac disease consuming recommended amounts of fibre, iron, calcium and grain foods? J. Hum. Nutr. Diet. 2005, 18, 163–169. [Google Scholar] [CrossRef] [PubMed]
- Marcason, W. Is there evidence to support the claim that a gluten-free diet should be used for weight loss? J. Am. Diet Assoc. 2011, 111, 1786. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Demyen, M.F.; Mathew, J.; Kothari, N.; Feurdean, M.; Ahlawat, S.K. Obesity, Metabolic Syndrome, and Cardiovascular Risk in Gluten-Free Followers Without Celiac Disease in the United States: Results from the National Health and Nutrition Examination Survey 2009–2014. Dig. Dis. Sci. 2017, 62, 2440–2448. [Google Scholar] [CrossRef] [PubMed]
- Dickey, W.; Kearney, N. Overweight in celiac disease: Prevalence, clinical characteristics, and effect of a gluten-free diet. Am. J. Gastroenterol. 2006, 101, 2356–2359. [Google Scholar] [CrossRef] [PubMed]
- Kabbani, T.A.; Goldberg, A.; Kelly, C.P.; Pallav, K.; Tariq, S.; Peer, A.; Hansen, J.; Dennis, M.; Leffler, D.A. Body mass index and the risk of obesity in coeliac disease treated with the gluten-free diet. Aliment. Pharmacol. Ther. 2012, 35, 723–729. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Siddh, L.; Sengar, G.; Nagraj, N.; Shyam, R.; Garg, P. Body mass index in celiac disease and effect of a gluten-free diet on body mass index. Int. J. Adv. Med. 2016, 3, 813–815. [Google Scholar] [CrossRef]
- Cheng, J.; Brar, P.S.; Lee, A.R.; Green, P.H.R. Body mass index in celiac disease: Beneficial effect of a gluten-free diet. J. Clin. Gastroenterol. 2010, 44, 267–271. [Google Scholar] [CrossRef] [PubMed]
- Ukkola, A.; Mäki, M.; Kurppa, K.; Collin, P.; Huhtala, H.; Kekkonen, L.; Kaukinen, K. Changes in body mass index on a gluten-free diet in coeliac disease: A nationwide study. Eur. J. Intern. Med. 2012, 23, 384–388. [Google Scholar] [CrossRef] [PubMed]
- Tortora, R.; Capone, P.; De Stefano, G.; Imperatore, N.; Gerbino, N.; Donetto, S.; Monaco, V.; Caporaso, N.; Rispo, A. Metabolic syndrome in patients with coeliac disease on a gluten-free diet. Aliment. Pharmacol. Ther. 2015, 41, 352–359. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sapone, A.; Bai, J.C.; Ciacci, C.; Dolinsek, J.; Green, P.H.R.; Hadjivassiliou, M.; Kaukinen, K.; Rostami, K.; Sanders, D.S.; Schumann, M.; et al. Spectrum of gluten-related disorders: Consensus on new nomenclature and classification. BMC Med. 2012, 10. [Google Scholar] [CrossRef] [PubMed]
- Elli, L.; Villalta, D.; Roncoroni, L.; Barisani, D.; Ferrero, S.; Pellegrini, N.; Bardella, M.T.; Valiante, F.; Tomba, C.; Carroccio, A.; et al. Nomenclature and diagnosis of gluten-related disorders: A position statement by the Italian Association of Hospital Gastroenterologists and Endoscopists (AIGO). Dig. Liver Dis. 2017, 49, 138–146. [Google Scholar] [CrossRef] [PubMed]
- Cianferoni, A. Wheat allergy: Diagnosis and management. J. Asthma Allergy 2016, 9, 13–25. [Google Scholar] [CrossRef] [PubMed]
- Catassi, C.; Bai, J.C.; Bonaz, B.; Bouma, G.; Calabrò, A.; Carroccio, A.; Castillejo, G.; Ciacci, C.; Cristofori, F.; Dolinsek, J.; et al. Non-celiac gluten sensitivity: The new frontier of gluten related disorders. Nutrients 2013, 5, 3839–3853. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Monsuur, A.J.; Wijmenga, C. Understanding the molecular basis of celiac disease: What genetic studies reveal. Ann. Med. 2006, 38, 578–591. [Google Scholar] [CrossRef] [PubMed]
- Fasano, A.; Catassi, C. Celiac Disease. N. Engl. J. Med. 2012, 25367, 2419–2426. [Google Scholar] [CrossRef] [PubMed]
- Reunala, T.; Salmi, T.T.; Hervonen, K. Dermatitis herpetiformis: Pathognomonic transglutaminase IgA deposits in the skin and excellent prognosis on a gluten-free diet. Acta. Derm. Venereol. 2015, 95, 917–922. [Google Scholar] [CrossRef] [PubMed]
- Shahbazkhani, B.; Sadeghi, A.; Malekzadeh, R.; Khatavi, F.; Etemadi, M.; Kalantri, E.; Rostami-Nejad, M.; Rostami, K. Non-celiac gluten sensitivity has narrowed the spectrum of irritable bowel syndrome: A double-blind randomized placebo-controlled trial. Nutrients 2015, 7, 4542–4554. [Google Scholar] [CrossRef] [PubMed]
- Makharia, A.; Catassi, C.; Makharia, G.K. The overlap between irritable bowel syndrome and non-celiac gluten sensitivity: A clinical dilemma. Nutrients 2015, 7, 10417–10426. [Google Scholar] [CrossRef] [PubMed]
- Niland, B.; Cash, B.D. Health benefits and adverse effects of a gluten-free diet in non-celiac disease patients. Gastroenterol. Hepatol. 2018, 14, 82–91. [Google Scholar]
- Sansotta, N.; Amirikian, K.; Guandalini, S.; Jericho, H. Celiac disease symptom resolution: Effectiveness of the gluten-free diet. J. Pediatr. Gastroenterol. Nutr. 2018, 66, 48–52. [Google Scholar] [CrossRef] [PubMed]
- Jericho, H.; Sansotta, N.; Guandalini, S. Extraintestinal manifestations of celiac disease: Effectiveness of the gluten-free diet. J. Pediatr. Gastroenterol. Nutr. 2017, 65, 75–79. [Google Scholar] [CrossRef] [PubMed]
- Pulido, O.; Zarkadas, M.; Dubois, S.; Macisaac, K.; Cantin, I.; La Vieille, S.; Godefroy, S.; Rashid, M. Clinical features and symptom recovery on a gluten-free diet in Canadian adults with celiac disease. Can. J. Gastroenterol. 2012, 27, 449–453. [Google Scholar] [CrossRef]
- Casella, S.; Zanini, B.; Lanzarotto, F.; Villanacci, V.; Ricci, C.; Lanzini, A. Celiac disease in elderly adults: Clinical, serological, and histological characteristics and the effect of a gluten-free diet. J. Am. Geriatr. Soc. 2012, 60, 1064–1069. [Google Scholar] [CrossRef] [PubMed]
- Murray, J.A.; Watson, T.; Clearman, B.; Mitros, F. Effect of a gluten-free diet on gastrointestinal symptoms in celiac disease. Am. J. Clin. Nutr. 2004, 79, 669–673. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bazzigaluppi, E.; Roggero, P.; Parma, B.; Brambillasca, M.F.; Meroni, F.; Mora, S.; Bosi, E.; Barera, G. Antibodies to recombinant human tissue-transglutaminase in coeliac disease: Diagnostic effectiveness and decline pattern after gluten-free diet. Dig. Liver Dis. 2006, 38, 98–102. [Google Scholar] [CrossRef] [PubMed]
- Sugai, E.; Nachman, F.; Váquez, H.; González, A.; Andrenacci, P.; Czech, A.; Niveloni, S.; Mazure, R.; Smecuol, E.; Cabanne, A.; et al. Dynamics of celiac disease-specific serology after initiation of a gluten-free diet and use in the assessment of compliance with treatment. Dig. Liver Dis. 2010, 42, 352–358. [Google Scholar] [CrossRef] [PubMed]
- Galli, G.; Esposito, G.; Lahner, E.; Pilozzi, E.; Corleto, V.D.; Di Giulio, E.; Aloe Spiriti, M.A.; Annibale, B. Histological recovery and gluten-free diet adherence: A prospective 1-year follow-up study of adult patients with coeliac disease. Aliment. Pharmacol. Ther. 2014, 40, 639–647. [Google Scholar] [CrossRef] [PubMed]
- Lanzini, A.; Lanzarotto, F.; Villanacci, V.; Mora, A.; Bertolazzi, S.; Turini, D.; Carella, G.; Malagoli, A.; Ferrante, G.; Cesana, B.M.; Ricci, C. Complete recovery of intestinal mucosa occurs very rarely in adult coeliac patients despite adherence to gluten-free diet. Aliment. Pharmacol. Ther. 2009, 29, 1299–1308. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rubio-Tapia, A.; Rahim, M.W.; See, J.A.; Lahr, B.D.; Wu, T.T.; Murray, J.A. Mucosal recovery and mortality in adults with celiac disease after treatment with a gluten-free diet. Am. J. Gastroenterol. 2010, 105, 1412–1420. [Google Scholar] [CrossRef] [PubMed]
- Bardella, M.T.; Velio, P.; Cesana, B.M.; Prampolini, L.; Casella, G.; Di Bella, C.; Lanzini, A.; Gambarotti, M.; Bassotti, G.; Villanacci, V. Coeliac disease: A histological follow-up study. Histopathology 2007, 50, 465–471. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Lo, W.; Memeo, L.; Rotterdam, H.; Green, P. Duodenal histology in patients with celiac disease after treatment with a gluten-free diet. Gastrointest. Endosc. 2003, 57, 187–191. [Google Scholar] [CrossRef] [PubMed]
- Lebwohl, B.; Murray, J.A.; Rubio-Tapia, A.; Green, P.H.R.; Ludvigsson, J.F. Predictors of persistent villous atrophy in coeliac disease: A population-based study. Aliment. Pharmacol. Ther. 2014, 39, 488–495. [Google Scholar] [CrossRef] [PubMed]
- Lewis, H.M.; Renaula, T.L.; Garioch, J.J.; Leonard, J.N.; Fry, J.S.; Collin, P.; Evans, D.; Fry, L. Protective effect of gluten-free diet against development of lymphoma in dermatitis herpetiformis. Br. J. Dermatol. 1996, 135, 363–367. [Google Scholar] [CrossRef] [PubMed]
- Hietikko, M.; Hervonen, K.; Salmi, T.; Ilus, T.; Zone, J.J.; Kaukinen, K.; Reunala, T.; Lindfors, K. Disappearance of epidermal transglutaminase and IgA deposits from the papillary dermis of patients with dermatitis herpetiformis after a long-term gluten-free diet. Br. J. Dermatol. 2018, 178, e198–e201. [Google Scholar] [CrossRef] [PubMed]
- Reunala, T.; Blomqvist, K.; Tarpila, S.; Halme, H.; Kangas, K. Gluten-free diet in dermatitis herpetiformis. Br. J. Dermatol. 1977, 97, 473–480. [Google Scholar] [CrossRef] [PubMed]
- Garioch, J.J.; Lewis, H.M.; Sargent, S.A.; Leonard, J.N.; Fry, L. 25 Years’ Experience of a Gluten-Free Diet in the Treatment of Dermatitis Herpetiformis. Br. J. Dermatol. 1994, 131, 541–545. [Google Scholar] [CrossRef] [PubMed]
- Fry, L.; Riches, D.J.; Seah, P.P.; Hoffbrand, A.V. Clearance of skin lesions in dermatitis herpetiformis after gluten withdrawal. Lancet 1973, 301, 288–291. [Google Scholar] [CrossRef]
- Pellecchia, M.T.; Scala, R.; Perretti, A.; De Michele, G.; Santoro, L.; Filla, A.; Ciacci, C.; Barone, P. Cerebellar ataxia associated with subclinical celiac disease responding to gluten-free diet. Neurology 1999, 53, 1606. [Google Scholar] [CrossRef] [PubMed]
- Helsing, P.; Frøen, H. Dermatitis herpetiformis presenting as ataxia in a child. Acta Derm. Venereol. 2007, 87, 163–165. [Google Scholar] [CrossRef] [PubMed]
- Hadjivassiliou, M.; Davies-Jones, G.A.B.; Sanders, D.S.; Grünewald, R.A. Dietary treatment of gluten ataxia. J. Neurol. Neurosurg. Psychiatry 2003, 74, 1221–1224. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hadjivassiliou, M.; Grünewald, R.A.; Sanders, D.S.; Shanmugarajah, P.; Hoggard, N. Effect of gluten-free diet on cerebellar MR spectroscopy in gluten ataxia. Neurology 2017, 89, 705–709. [Google Scholar] [CrossRef] [PubMed]
- Tovoli, F.; Masi, C.; Guidetti, E.; Negrini, G.; Paterini, P.; Bolondi, L. Clinical and diagnostic aspects of gluten related disorders. World J. Clin. Cases 2015, 3, 275–284. [Google Scholar] [CrossRef] [PubMed]
- Elli, L.; Tomba, C.; Branchi, F.; Roncoroni, L.; Lombardo, V.; Bardella, M.T.; Ferretti, F.; Conte, D.; Valiante, F.; Fini, L.; et al. Evidence for the presence of non-celiac gluten sensitivity in patients with functional gastrointestinal symptoms: Results from a multicenter randomized double-blind placebo-controlled gluten challenge. Nutrients 2016, 8. [Google Scholar] [CrossRef] [PubMed]
- Di Sabatino, A.; Volta, U.; Salvatore, C.; Biancheri, P.; Caio, G.; De Giorgio, R.; Di Stefano, M.; Corazza, G.R. Small amounts of gluten in subjects with suspected nonceliac gluten sensitivity: a randomized, double-blind, placebo-controlled, cross-over trial. Clin. Gastroenterol. Hepatol. 2015, 13, 1604–1612. [Google Scholar] [CrossRef] [PubMed]
- Dale, H.F.; Hatlebakk, J.G.; Hovdenak, N.; Ystad, S.O.; Lied, G.A. The effect of a controlled gluten challenge in a group of patients with suspected non-coeliac gluten sensitivity: A randomized, double-blind placebo-controlled challenge. Neurogastroenterol. Motil. 2018, e13332. [Google Scholar] [CrossRef] [PubMed]
- Molina-Infante, J.; Carroccio, A. Suspected Nonceliac Gluten Sensitivity Confirmed in Few Patients After Gluten Challenge in Double-Blind, Placebo-Controlled Trials. Clin. Gastroenterol. Hepatol. 2017, 15, 339–348. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aziz, I.; Trott, N.; Briggs, R.; North, J.R.; Hadjivassiliou, M.; Sanders, D.S. Efficacy of a gluten-free diet in subjects with irritable bowel syndrome-diarrhea unaware of their HLA-DQ2/8 genotype. Clin. Gastroenterol. Hepatol. 2016, 14, 696–703.e1. [Google Scholar] [CrossRef] [PubMed]
- Vazquez-Roque, M.I.; Camilleri, M.; Smyrk, T.; Murray, J.A.; Marietta, E.; O’Neill, J.; Carlson, P.; Lamsam, J.; Janzow, D.; Eckert, D.; et al. A controlled trial of gluten-free diet in patients with irritable bowel syndrome-diarrhea: Effects on bowel frequency and intestinal function. Gastroenterology 2013, 144, 903–911. [Google Scholar] [CrossRef] [PubMed]
- Junker, Y.; Zeissig, S.; Kim, S.-J.; Barisani, D.; Wieser, H.; Leffler, D.A.; Zevallos, V.; Libermann, T.A.; Dillon, S.; Freitag, T.L.; et al. Wheat amylase trypsin inhibitors drive intestinal inflammation via activation of toll-like receptor 4. J. Exp. Med. 2012, 209, 2395–2408. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McIntosh, K.; Reed, D.E.; Schneider, T.; Dang, F.; Keshteli, A.H.; De Palma, G.; Madsen, K.; Bercik, P.; Vanner, S. FODMAPs alter symptoms and the metabolome of patients with IBS: A randomised controlled trial. Gut 2017, 66, 1241–1251. [Google Scholar] [CrossRef] [PubMed]
- Gibson, P.R.; Muir, J.G. Not all effects of a gluten-free diet are due to removal of gluten. Gastroenterology 2013, 145, 693. [Google Scholar] [CrossRef] [PubMed]
- Nistal, E.; Caminero, A.; Herrán, A.R.; Arias, L.; Vivas, S.; Ruiz De Morales, J.M.; Calleja, S.; Sáenz De Miera, L.E.; Arroyo, P.; Casqueiro, J. Differences of small intestinal bacteria populations in adults and children with/without celiac disease: Effect of age, gluten diet, and disease. Inflamm. Bowel Dis. 2012, 18, 649–656. [Google Scholar] [CrossRef] [PubMed]
- Tjellström, B.; Högberg, L.; Stenhammar, L.; Fälth-Magnusson, K.; Magnusson, K.-E.; Norin, E.; Sundqvist, T.; Midtvedt, T. Faecal short-chain fatty acid pattern in childhood coeliac disease is normalised after more than one year’s gluten-free diet. Microb. Ecol. Heal. Dis. 2013, 24. [Google Scholar] [CrossRef] [PubMed]
- De Palma, G.; Nadal, I.; Collado, M.C.; Sanz, Y. Effects of a gluten-free diet on gut microbiota and immune function in healthy adult human subjects. Br. J. Nutr. 2009, 102, 1154–1160. [Google Scholar] [CrossRef] [PubMed]
- Wacklin, P.; Laurikka, P.; Lindfors, K.; Collin, P.; Salmi, T.; Lähdeaho, M.L.; Saavalainen, P.; Mäki, M.; Mättö, J.; Kurppa, K.; et al. Altered Duodenal Microbiota Composition in Celiac Disease Patients Suffering From Persistent Symptoms on a Long-Term Gluten-Free Diet. Am. J. Gastroenterol. 2014, 109, 1933–1941. [Google Scholar] [CrossRef] [PubMed]
- Rewers, M.; Liu, E.; Simmons, J.; Redondo, M.J.; Hoffenberg, E.J. Celiac disease associated with type 1 diabetes mellitus. Endocrinol. Metab. Clin. North Am. 2004, 33, 197–214. [Google Scholar] [CrossRef] [PubMed]
- Viljamaa, M.; Kaukinen, K.; Huhtala, H.; Kyrönpalo, S.; Rasmussen, M.; Collin, P. Coeliac disease, autoimmune diseases and gluten exposure. Scand. J. Gastroenterol. 2005, 40, 437–443. [Google Scholar] [CrossRef] [PubMed]
- Ventura, A.; Magazzù, G.; Greco, L. Duration of exposure to gluten and risk for autoimmune disorders in patients with celiac disease. Gastroenterology 1999, 117, 297–303. [Google Scholar] [CrossRef] [PubMed]
- Antvorskov, J.C.; Josefsen, K.; Engkilde, K.; Funda, D.P.; Buschard, K. Dietary gluten and the development of type 1 diabetes. Diabetologia 2014, 57, 1770–1780. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abid, N.; McGlone, O.; Cardwell, C.; McCallion, W.; Carson, D. Clinical and metabolic effects of gluten free diet in children with type 1 diabetes and coeliac disease. Pediatr. Diabetes 2011, 12, 322–325. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Albisua, I.; Wolf, J.; Neu, A.; Geiger, H.; Wäscher, I.; Stern, M. Coeliac disease in children with Type 1 diabetes mellitus: The effect of the gluten-free diet. Diabet. Med. 2005, 22, 1079–1082. [Google Scholar] [CrossRef] [PubMed]
- Amin, R.; Murphy, N.; Edge, J.; Ahmed, M.L.; Acerini, C.L.; Dunger, D.B. A longitudinal study of the effects of a gluten-free diet on glycemic control and weight gain in subjects with type 1 diabetes and celiac disease. Diabetes Care 2002, 25, 1117–1122. [Google Scholar] [CrossRef] [PubMed]
- Kaukinen, K.; Salmi, J.; Lahtela, J.; Siljamaki-Ojansuu, U.; Koivisto, A.-M.; Oksa, H.; Collin, P. No effect of gluten-free diet on the metabolic control of type 1 diabetes in patients with diabetes and celiac disease. Diabetes Care 1999, 22, 1747–1748. [Google Scholar] [CrossRef] [PubMed]
- Taler, I.; Phillip, M.; Lebenthal, Y.; de Vries, L.; Shamir, R.; Shalitin, S. Growth and metabolic control in patients with type 1 diabetes and celiac disease: A longitudinal observational case-control study. Pediatr. Diabetes 2012, 13, 597–606. [Google Scholar] [CrossRef] [PubMed]
- Acerini, C.L.; Ahmed, M.L.; Ross, K.M.; Sullivan, P.B.; Bird, G.; Dunger, D.B. Coeliac disease in children and adolescents with IDDM: Clinical characteristics and response to gluten-free diet. Diabet. Med. 1998, 15, 38–44. [Google Scholar] [CrossRef]
- Scaramuzza, A.E.; Mantegazza, C.; Bosetti, A.; Zuccotti, G.V. Type 1 diabetes and celiac disease: The effects of gluten free diet on metabolic control. World J. Diabetes 2013, 4, 130–134. [Google Scholar] [CrossRef] [PubMed]
- Sategna Guidetti, C.; Solerio, E.; Scaglione, N.; Aimo, G.; Mengozzi, G. Duration of gluten exposure in adult coeliac disease does not correlate with the risk for autoimmune disorders. Gut 2001, 49, 502–505. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ouaka-Kchaou, A.; Ennaifer, R.; Elloumi, H.; Gargouri, D.; Hefaiedh, R.; Kochlef, A.; Romani, M.; Kilani, A.; Kharrat, J.; Ghorbel, A. Autoimmune diseases in coeliac disease: Effect of gluten exposure. Therap. Adv. Gastroenterol. 2008, 1, 169–172. [Google Scholar] [CrossRef] [PubMed]
- Cosnes, J.; Cellier, C.; Viola, S.; Colombel, J.F.; Michaud, L.; Sarles, J.; Hugot, J.P.; Ginies, J.L.; Dabadie, A.; Mouterde, O.; et al. Incidence of Autoimmune diseases in celiac disease: protective effect of the gluten-free diet. Clin. Gastroenterol. Hepatol. 2008, 6, 753–758. [Google Scholar] [CrossRef] [PubMed]
| Formulation | Main Conclusions | References |
|---|---|---|
| Base of corn starch with chickpea and tiger nut flour | Replacement of emulsifier and shortening by the chickpea protein and tiger nut lipids: the combination of both maintains baking characteristics of bread loaves with eliminated shortening and emulsifier. | [15] |
| Base of rice flour, potato, tapioca and cassava starch and xanthan gum with amaranth and quinoa flour | Amaranth and quinoa flour do not affect texture and volume, and final bread loaves are considered ‘moderately acceptable’ in sensory trials. | [64,65] |
| Base of rice flour and xanthan gum with amaranth, quinoa and buckwheat flour | Replacement of potato starch with buckwheat and quinoa flour increases bread volume and softens crumb. Amaranth flour only decreases the crumb firmness. None of the three pseudocereal flours adversely affects the sensory properties. | [66] |
| Base of corn starch with vinal seed and corn flour | Acceptable bread loaves are made with regard to volume and crumb structure. | [67] |
| Base of rice flour, maize starch and HPMC with teff flour and dried rice- and buckwheat based sourdough | Bread aroma is enhanced and visual appearance is good. Buckwheat-based sourdough has a bitter taste. | [68] |
| Soy flour-barnyard millet blends | Soy flour alters the textural properties and color of the bread. | [69] |
| Base of rice flour, shortening, gum blend (xanthan, guar and locust bean gum) and DATEM with partial replacement of the rice flour with chestnut flour | Partial replacement of rice flour with chestnut flour results in lower hardness, increased specific volume, and better color and sensory properties. High chestnut flour recipes had low quality. | [40] |
| Base of rice and corn flour, corn starch, HPMC with gradual replacement of rice/corn flour by quinoa flour | Quinoa flour increases loaf volume and yields a more homogeneous crumb structure, whilst not affecting product taste. | [61] |
| Base of oats, rice, buckwheat, maize, quinoa, sorghum and teff flour | Only oats bread is somewhat comparable to wheat bread. All other loaves are of inferior quality in terms of loaf volume, physical crumb texture, shelf life and aroma profile. | [24] |
| Base of commercial gluten-free mixtures including corn starch, psyllium fiber, guar gum or corn starch, tapioca starch, potato starch and rice flour, HPMC with partial replacement of the flours by buckwheat flour | Dehulled buckwheat flour improved the baking performance of commercial mixtures, whilst puffed buckwheat flour had a clear effect on water availability and the interaction between the matrix biopolymers. | [70] |
| Base of corn starch and xanthan gum with soy and chickpea flour, pea isolate and carob germ flour | Carob germ flour loaves have the lowest volume, whilst chickpea flour yields the highest volume and the softest crumb. | [25] |
| Base of marama bean flour with cassava starch | Marama bean and cassava starch produce strong dough, similar to wheat flour dough that can hold gas in its structure. | [27] |
| Base of potato starch and rice flour with whole chia flour | Chia flour does not adversely affect loaf volume and crumb firmness. | [71] |
| Base of rice flour, gluten-free wheat starch, albumin, HPMC with green plantain flour | Green plantain flour produces good volume bread loaves, and soft crumb firmness breads having a regular porosity. | [21] |
| Base of rice flour and corn starch with acorn meal | Sensory and nutritional properties are improved with acorn supplementation, whilst the specific volume is decreased, and the crumb hardness is increased. | [72] |
| Base of corn starch, HPMC with carob germ flour | Carob germ flour is a good alternative to wheat flour to produce viscoelastic dough and high quality gluten-free bread. | [73] |
| Formulation | Main Conclusions | References |
|---|---|---|
| Zein-starch base with HPMC and high β-glucan oat bran | Hydrocolloid and β-glucan improve bread volume and aid zein to more closely resemble gluten in terms of structural and rheological properties. | [74] |
| Base of soybean flour and corn starch with HPMC, xanthan gum and emulsifiers | HPMC increases volume and softness more than xanthan gum, but xanthan gum gives a better crumb structure. | [41] |
| Base of teff, buckwheat, corn or rice flour with HPMC and xanthan gum (combinations) | Xanthan gum increases the crumb hardness of teff and buckwheat breads, whilst corn breads become softer. HPMC increases loaf volume of teff and corn breads, while xanthan adversely affects the loaf volumes in all different recipes. | [42] |
| Base of rice flour, corn starch and sodium caseinate with pectin, carboxymethyl cellulose, agarose, xanthan gum and oats β-glucan | Except for xanthan, all gums result in a loaf volume increase. | [46] |
| Base of potato flour with HPMC, carboxymethyl cellulose, xanthan gum and apple pectin | Gums yield loaves with higher specific volume and reduced hardness. | [47] |
| Base of rice flour, corn starch, soy flour with guar gum and transglutaminase | Guar gum increases the specific volume and decreases crumb hardness, while transglutaminase increases crumb hardness but yields a good texture. | [56] |
| Base of chestnut and chia flour with guar gum, HPMC and tragacanth gum | All hydrocolloids increase “dough” elasticity. | [26] |
| Base of rice flour, corn starch and sodium caseinate with carboxymethyl cellulose | Carboxymethyl cellulose increases bread volume and sensorial properties. | [48] |
| Base of broken rice berry flour with guar, locust bean or xanthan gum | Hydrocolloids increase loaf volume, texture, microstructure and sensory properties. | [75] |
| Base of tapioca starch, precooked corn flour with guar gum and HPMC | Guar gum and HPMC reduce dough stickiness and soften the crumb. | [76] |
| Base of rice and corn flour and corn starch with cress seed and xanthan gum | Both gums improve crumb color and porosity, cress seed gum triggers the formation of more regular and solid pores. | [51] |
| Formulation | Main Conclusions | References |
|---|---|---|
| Base of corn flour, corn starch, dried eggs and carrageenan with psyllium and pea fiber and oat bran and glucose oxidase | Addition of dietary fiber alters dough cohesion and starch pasting properties. (Glucose oxidase increased the specific loaf volume). | [77] |
| Base of corn starch, rice flour, starch and protein, HPMC, locust bean gum, guar gum and alfa-amylase with psyllium and sugar beet fiber | Both psyllium and sugar beet fiber improve dough workability. Psyllium fiber is superior in its film forming ability and has an antistaling effect due to higher water binding capacity. | [45] |
| Base of rice and corn flour, corn starch, HPMC with quinoa bran or quinoa wholemeal addition | Quinoa bran increases carbon dioxide production, while the gas retention is reduced. Bread volume can be increased without adversely affecting the taste. | [78] |
| Base of corn and potato starch, pectin, guar gum with replacement of pectin and guar gum with linseed mucilage (predominantly arabino-xylan) | Replacement of pectin or guar gum with linseed mucilage improves the sensory acceptance and does not affect texture and bread staling. | [32] |
| Base of rice flour, corn starch and HPMC with insoluble fiber (oat and bamboo, pea and potato fiber) and soluble fiber (barley and polydextrose) | Soluble fiber decreases dough consistency, increases bread volume and decreases crumb hardness. The fine insoluble fibers also increase bread volume and decrease the crumb hardness, the coarse insoluble fibers decrease bread volume and increase hardness. In general, soluble fiber increases the structural stability, while insoluble fiber disrupts the structure. | [33] |
| Base of rice flour, HPMC with β-glucan derived from barley (low molecular weight) and oats (high molecular weight) | Low molecular weight β-glucan develops a gel network structure, whilst high molecular weight β-glucan predominantly increases viscosity. | [79] |
| Base of white rice, corn and buckwheat flour with carob fiber | Carob fiber improves volume, color and crumb texture whilst increasing the antioxidant activity of the breads. | [36] |
| Base of rice flour, cassava starch, full-fat active soy flour with inulin (soluble fiber) and resistant starch and oat bran (insoluble fiber) | Insoluble fiber increases dough firmness and decreases loaf volume, whilst soluble fiber decreases dough firmness. | [37] |
| Base of corn and potato starch, guar gum and pectin with inulin | Inulin addition leads to an increased loaf volume and reduces crumb hardness, whilst the internal structure is more polydisperse. | [80] |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
El Khoury, D.; Balfour-Ducharme, S.; Joye, I.J. A Review on the Gluten-Free Diet: Technological and Nutritional Challenges. Nutrients 2018, 10, 1410. https://doi.org/10.3390/nu10101410
El Khoury D, Balfour-Ducharme S, Joye IJ. A Review on the Gluten-Free Diet: Technological and Nutritional Challenges. Nutrients. 2018; 10(10):1410. https://doi.org/10.3390/nu10101410
Chicago/Turabian StyleEl Khoury, Dalia, Skye Balfour-Ducharme, and Iris J. Joye. 2018. "A Review on the Gluten-Free Diet: Technological and Nutritional Challenges" Nutrients 10, no. 10: 1410. https://doi.org/10.3390/nu10101410
APA StyleEl Khoury, D., Balfour-Ducharme, S., & Joye, I. J. (2018). A Review on the Gluten-Free Diet: Technological and Nutritional Challenges. Nutrients, 10(10), 1410. https://doi.org/10.3390/nu10101410





