Purification and Identification of Angiotensin I-Converting Enzyme Inhibitory Peptides and the Antihypertensive Effect of Chlorella sorokiniana Protein Hydrolysates
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Hot Water Extract and Hydrolysate of C. sorokiniana
2.3. Chemical Analyses
2.4. Measurement of Peptide Content
2.5. In Vitro Assay for ACE-Inhibitory Activity
2.6. In Vitro Gastrointestinal Digestion
2.7. Size Exclusion Chromatography
2.8. Purification of ACE-Inhibitory Peptide
2.9. Sequence Analysis
2.10. Animals and In Vivo Measurement of Blood Pressure
2.11. Statistical Analysis
3. Results and Discussion
3.1. Soluble Protein Content, Peptide Content, Yield, and IC50
3.2. In Vitro Stability of C. sorokiniana–Derived ACE-Inhibitory Peptides
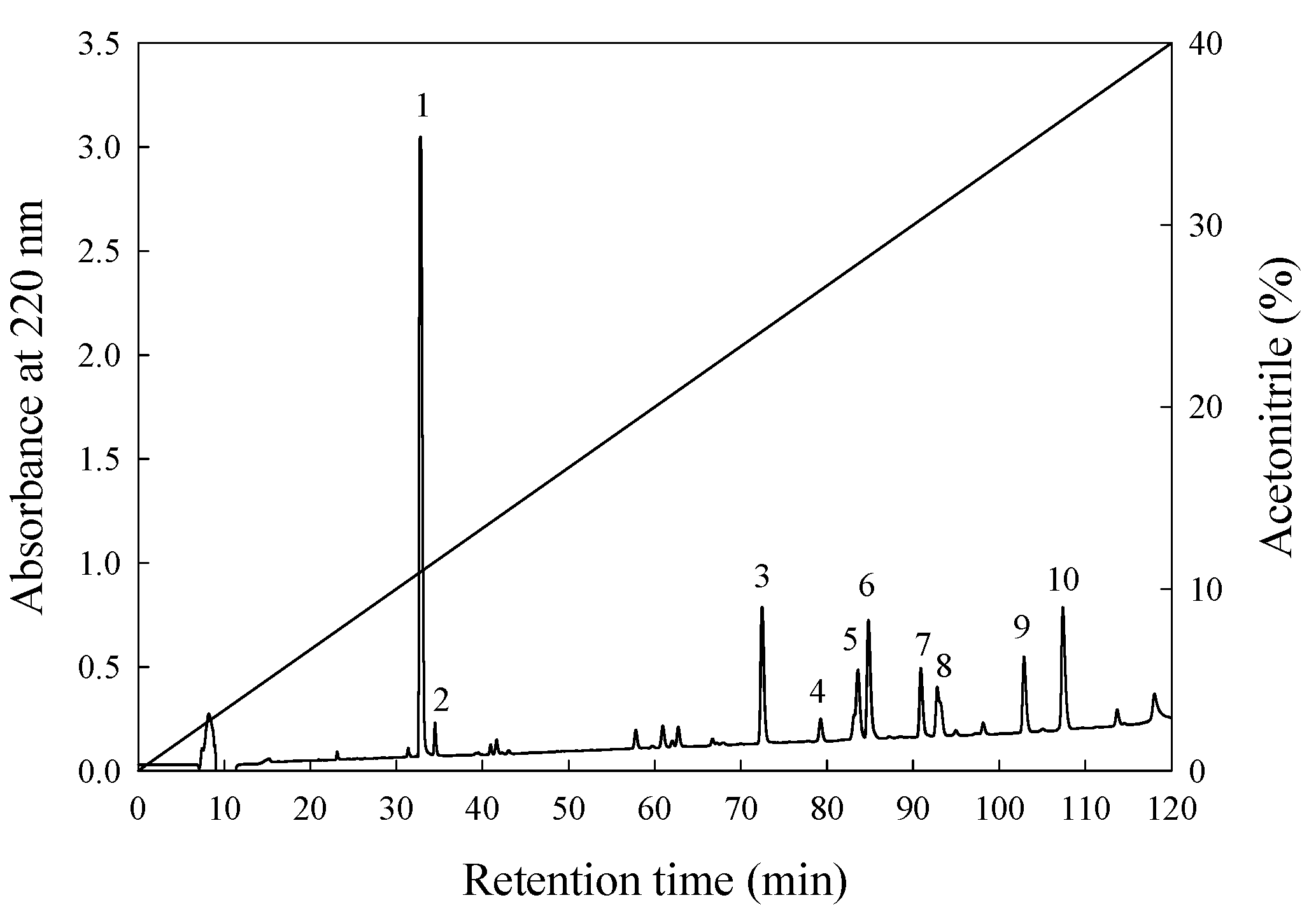
3.3. Isolation and Purification of ACE-Inhibitory Peptide
3.4. Amino Acid Sequences and ACE-Inhibitory Activity
3.5. Antihypertensive Effect of C. sorokiniana Protein Hydrolysate
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sheih, I.C.; Fang, T.J.; Wu, T.K. Isolation and characterisation of a novel angiotensin I-converting enzyme (ACE) inhibitory peptide from the algae protein waste. Food Chem. 2009, 115, 279–284. [Google Scholar] [CrossRef]
- Wang, J.; Hu, J.; Cui, J.; Bai, X.; Du, A.Y.; Miyaguchi, Y.; Lin, B. Purification and identification of a ACE inhibitory peptide from oyster proteins hydrolysate and the antihypertensive effect of hydrolysate in spontaneously hypertensive rats. Food Chem. 2008, 111, 302–308. [Google Scholar] [CrossRef] [PubMed]
- Ondetti, M.A. Design of specific inhibitors of angiotensin-converting enzyme: New class of orally active antihypertensive agents. Science 1977, 196, 441–444. [Google Scholar] [CrossRef] [PubMed]
- Sawayama, T.; Itokawa, A.; Shimada, K.; Doi, Y.; Kimura, Y.; Nishimura, H. Synthesis of 1-[(S)-3-acetylthio-2-methylpropanoyl]-l-phenylalanine (Alacepril) and one of its active metabolites, the desacetyl derivative (DU-1227). Chem. Pharm. Bull. 1990, 38, 1767–1771. [Google Scholar] [CrossRef]
- Julius, S.; Nesbitt, S.D.; Egan, B.M.; Weber, M.A.; Michelson, E.L.; Kaciroti, N.; Black, H.R.; Grimm, R.H.; Messerli, F.H.; Oparil, S.; et al. Feasibility of treating prehypertension with an angiotensin-receptor blocker. N. Engl. J. Med. 2006, 354, 1685–1697. [Google Scholar] [CrossRef] [PubMed]
- Bhuyan, B.J.; Mugesh, G. Synthesis, characterization and antioxidant activity of angiotensin converting enzyme inhibitors. Org. Biomol. Chem. 2011, 9, 1356–1365. [Google Scholar] [CrossRef] [PubMed]
- Al Shohaib, S.; Raweily, E. Acute tubular necrosis due to captopril. Am. J. Nephrol. 2000, 20, 149–152. [Google Scholar] [CrossRef] [PubMed]
- Bhuyan, B.J.; Mugesh, G. Antioxidant activity of peptide-based angiotensin converting enzyme inhibitors. Org. Biomol. Chem. 2012, 10, 2237–2247. [Google Scholar] [CrossRef] [PubMed]
- Samarakoon, K.W.; O-Nam, K.; Ko, J.Y.; Lee, J.H.; Kang, M.C.; Kim, D.; Lee, J.B.; Lee, J.S.; Jeon, Y.J. Purification and identification of novel angiotensin-I converting enzyme (ACE) inhibitory peptides from cultured marine microalgae (Nannochloropsis oculata) protein hydrolysate. J. Appl. Phycol. 2013, 25, 1595–1606. [Google Scholar] [CrossRef]
- Yamada, A.; Sakurai, T.; Ochi, D.; Mitsuyama, E.; Yamauchi, K.; Abe, F. Novel angiotensin I-converting enzyme inhibitory peptide derived from bovine casein. Food Chem. 2013, 141, 3781–3789. [Google Scholar] [CrossRef] [PubMed]
- Pan, S.; Wang, S.; Jing, L.; Yao, D. Purification and characterisation of a novel angiotensin-I converting enzyme (ACE)-inhibitory peptide derived from the enzymatic hydrolysate of Enteromorpha clathrata protein. Food Chem. 2016, 211, 423–430. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.W.; Tsai, J.S.; Sun, P.B. Purification of Angiotensin I-converting enzyme inhibitory peptides and antihypertensive effect of milk produced by protease-facilitated lactic fermentation. Int. Dairy J. 2007, 17, 641–647. [Google Scholar] [CrossRef]
- Chen, G.W.; Tsa, J.S.; Sun, P.B. Cardiovascular effects of whey from prozyme 6-facilitated lactic acid bacteria fermentation of milk. J. Food Biochem. 2007, 31, 639–655. [Google Scholar] [CrossRef]
- Tsai, J.S.; Chen, T.J.; Pan, B.S.; Gong, S.D.; Chung, M.Y. Antihypertensive effect of bioactive peptides produced by protease-facilitated lactic acid fermentation of milk. Food Chem. 2008, 106, 552–558. [Google Scholar] [CrossRef]
- Tsai, J.S.; Lin, T.C.; Chen, J.L.; Pan, B.S. The inhibitory effect of freshwater clam (Corbicula fluminea, Muller) muscle protein hydrolysates on angiotensin I converting enzyme. Process Biochem. 2006, 41, 2276–2281. [Google Scholar] [CrossRef]
- Tsai, J.S.; Chen, J.L.; Pan, B.S. ACE-inhibitory peptides identified from the muscle protein hydrolysate of hard clam (Meretrix lusoria). Process Biochem. 2008, 43, 743–747. [Google Scholar] [CrossRef]
- Terashima, M.; Baba, T.; Ikenmoto, N.; Katayama, M.; Morimoto, T.; Matsumura, S. Novel angiotensin-concerting enzyme (ACE) inhibitory peptides derived from boneless chicken leg meat. J. Agric. Food Chem. 2010, 58, 7432–7436. [Google Scholar] [CrossRef] [PubMed]
- Jang, J.H.; Jeong, S.C.; Kim, J.H.; Lee, Y.H.; Ju, Y.C.; Lee, J.S. Characterisation of a new antihypertensive angiotensin I-converting enzyme inhibitory peptide from Pleurotus cornucopiae. Food Chem. 2011, 127, 412–418. [Google Scholar] [CrossRef] [PubMed]
- Wijesekara, I.; Qian, Z.J.; Ryu, B.; Ngo, D.H.; Kim, S.K. Purification and identification of antihypertensive peptides from seaweed pipefish (Syngnathus schlegeli) muscle protein hydrolysate. Food Res Int. 2011, 44, 703–707. [Google Scholar] [CrossRef]
- Lin, H.C.; Alashi, A.M.; Aluko, R.E.; Sun, P.B.; Chang, Y.W. Antihypertensive properties of tilapia (Oreochromis spp.) frame and skin enzymatic protein hydrolysates. Food Nutr. Res. 2018, 61. Available online: https://www.tandfonline.com/doi/abs/10.1080/16546628.2017.1391666 (accessed on 20 August 2018). [CrossRef] [PubMed]
- Suetsuna, K.; Chen, J.R. Identification of antihypertensive peptides from peptic digest of two microalgae, Chlorella vulgaris and Spirulina platensis. Mar. Biotechnol. 2001, 3, 305–309. [Google Scholar] [CrossRef] [PubMed]
- Olivares-Molina, A.; Fernández, K. Comparison of different extraction techniques for obtaining extracts from brown seaweeds and their potential effects as angiotensin I-converting enzyme (ACE) inhibitors. J. Appl. Phycol. 2016, 28, 1295–1302. [Google Scholar] [CrossRef]
- Xie, J.; Chen, X.; Wu, J.; Zhang, Y.; Zhou, Y.; Zhang, L.; Tang, Y.J.; Wei, D. Antihypertensive effects, molecular docking study, and isothermal titration calorimetry assay of angiotensin I-converting enzyme inhibitory peptides from Chlorella vulgaris. J. Agric. Food Chem. 2018, 66, 1359–1368. [Google Scholar] [CrossRef] [PubMed]
- Okamoto, K.; Suzuki, T.; Ito, H.; Mitachi, Y.; Morita, N. Spontaneous Hypertension: Its Pathogenesis and Complications, 2nd ed.; DHEW Publication: Washington, WA, USA, 1976; pp. 1177–1179. [Google Scholar]
- Murakami, T.; Okamoto, K.; Ogaki, M.; Iizuka, Y. Effect of Chlorella on blood pressure, cerebral stroke lesions, hypertensive vascular change and life-span in spontaneously hypertensive rats. J. Jpn. Soc. Nutr. Food Sci. 1987, 40, 351–359. [Google Scholar] [CrossRef]
- Miyakoshi, M.; Tanaka, M.; Miyazawa, K.; Nara, H.; Takemoto, Y.; Maki, T.; Fukui, S.; Antoku, E.; Shinpo, K.; Shimizu, K. Study of Chlorella producted from the Chikugo area. Clin. Rep. 1980, 14, 3931–3941. [Google Scholar]
- Suetsuna, K. Purification and identification of angiotensin I–converting enzyme inhibitors from the red alga Porphyra yezoensis. J. Mar. Biotechnol. 1998, 6, 163–167. [Google Scholar] [PubMed]
- Suetsuna, K. Separation and identification of angiotensin I–converting enzyme inhibitory peptides from peptic digest of Hizikia fusiformis protein. Nippon Suisan Gakk. 1998, 64, 862–866. [Google Scholar] [CrossRef]
- Suetsuna, K.; Nakano, T. Identification of an antihypertensive peptide from peptic digest of wakame (Undaria pinnatifida). J. Nutr. Biochem. 2000, 11, 450–454. [Google Scholar] [CrossRef]
- Lu, J.; Ren, D.F.; Xue, U.L.; Sawano, Y.; Miyakawa, T.; Tanokura, M. Isolation of an antihypertensive peptide fome alcalase digest of Spirulina platensis. J. Agric. Food Chem. 2010, 58, 7166–7171. [Google Scholar] [CrossRef] [PubMed]
- Spolaore, P.; Joannis-Cassan, C.; Duran, E.; Isambert, A. Commercial applications of microalgae. J. Biosci. Bioeng. 2006, 101, 87–96. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lowry, O.H.; Resebrough, N.J.; Farr, A.L.; Randall, R.J. Protein measurement with the folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [PubMed]
- Cooper, T.G. Spectrophotometry. In The Tools of Biochemistry, 1st ed.; Wiley-Interscience: Hoboken, NJ, USA, 1977; pp. 53–55. ISBN 0471171166. [Google Scholar]
- Church, F.C.; Swaisgood, H.E.; Porter, H.D.; Catignani, G.L. Spectrophotometric assay using o-phthaldialdehyde for determination of proteolysis in milk and isolated milk proteins. J. Dairy Sci. 1983, 66, 1219–1227. [Google Scholar] [CrossRef]
- Cushman, D.W.; Cheung, H.S. Spectrophotometric assay and properties of the angiotensin-converting enzyme of rabbit lung. Biochem. Parmacol. 1971, 20, 1637–1648. [Google Scholar] [CrossRef]
- Wu, J.; Ding, X. Characterization of inhibition and stability of soy-protein-derived angiotensin I-converting enzyme inhibitory peptides. Food Res. Int. 2002, 35, 367–375. [Google Scholar] [CrossRef]
- UniProt Database. Available online: http://www.uniprot.org (accessed on 15 May 2018).
- Pairwise Sequence Alignment Software. Available online: https://www.ebi.ac.uk/Tools/psa/ (accessed on 15 May 2018).
- SAS Institute Inc. SAS/STAT User’s Guide; SAS Institute Press: Cary, NC, USA, 1988; p. 584. [Google Scholar]
- Ruiz, J.Á.G.; Ramos, M.; Recio, J. Angiotensin converting enzyme inhibitory activity of peptides isolated from Manchego cheese. Stability under simulated gastrointestinal digestion. Int. Dairy J. 2004, 14, 1075–1080. [Google Scholar] [CrossRef]
- Sato, M.; Oba, T.; Yamaguchi, T.; Nakano, T.; Kahara, T.; Funayama, K.; Kobayashi, A.; Nakano, T. Antihypertensive effects of hydrolysates of wakame (Undar pinnatifida) and their angiotensin-I-converting enzyme inhibitory activity. Ann. Nutr. MeTab. 2002, 46, 259–267. [Google Scholar] [CrossRef] [PubMed]
- Sekiya, S.; Kobayashi, Y.; Kita, E.; Imamura, Y.; Toyama, S. Antihypertensive effects of tryptic hydrolysate of casein on normotensive and hypertensive volunteers. J. Jpn. Soc. Nutr. Food Sci. 1992, 45, 513–517. [Google Scholar] [CrossRef]
- Ben, H.Y.; Labidi, A.; Arnaudin, I.; Bridiau, N.; Delatouche, R.; Maugard, T.; Piot, J.M.; Sannier, F.; Thiéry, V.; Bordenave-Juchereau, S. Measuring angiotensin-I converting enzyme inhibitory activity by micro plate assays: Comparison using marine cryptides and tentative threshold determinations with captopril and losartan. J. Agric. Food Chem. 2013, 61, 10685–10690. [Google Scholar]
- Okamoto (Kainuma), A.; Matsumoto, E.; Iwashita, A.; Yasuhara, T.; Kawamura, Y.; Koizumi, Y.; Yanagida, F. Angiotensin I-converting enzyme inhibitory action of fish sauce. Food Sci. Technol. Int. 1995, 1, 101–106. [Google Scholar] [CrossRef]
- Saito, S.; Wanezaki (Nakamura), K.; Kawato, A.; Imayasu, S. Structure and activity of angiotensin I converting enzyme inhibitory peptides from sake and sake lees. Biosci. Biotechnol. Biochem. 1994, 58, 1767–1771. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Bao, T.; Han, W.; Zheng, X.; Wang, J. Purification and identification of an angiotensin I-converting enzyme inhibitory peptide from cauliflower byproducts protein hydrolysate. Process Biochem. 2016, 51, 1299–1305. [Google Scholar] [CrossRef]
- Fujita, H.; Yokoyama, K.; Yoshikawa, M. Classification and antihypertensive activity of angiotensin I-converting enzyme inhibitory peptides derived from food proteins. J. Food Sci. 2000, 65, 564–569. [Google Scholar]
- Martin, M.; Wellner, A.; Ossowski, I.; Henle, T. Identification and quantification of inhibitors for angiotensin-converting enzyme in hypoallergenic infant milk formulas. J. Agric. Food Chem. 2008, 56, 6333–6338. [Google Scholar] [CrossRef] [PubMed]
- Ono, S.; Hosokawa, M.; Miyashita, K.; Takahashi, K. Inhibition properties of dipeptides from salmon muscle hydrolysate on angiotensin I-converting enzyme. Int. J. Food Sci. Technol. 2006, 41, 383–386. [Google Scholar] [CrossRef]
- Wu, J.; Aluko, R.E.; Nakai, S. Structural requirements of angiotensin I-converting enzyme inhibitory peptides: Quantitative structure−activity relationship study of di- and tripeptides. J. Agric. Food Chem. 2006, 54, 732–738. [Google Scholar] [CrossRef] [PubMed]
- Sato, M.; Hosokawa, T.; Yamaguchi, T.; Nakano, T.; Muramoto, K.; Kahara, T.; Funayama, K.; Kobayashi, A.; Nakano, T. Angiotensin I-converting enzyme inhibitory peptides derived from wakame (Undaria pinnatifida) and their antihypertensive effect in spontaneously hypertensive rats. J. Agric. Food Chem. 2002, 50, 6245–6252. [Google Scholar] [CrossRef] [PubMed]
- He, R.; Ma, H.; Zhao, W.; Qu, W.; Zhao, J.; Luo, L.; Zhu, W. Modeling the QSAR of ACE-inhibitory peptides with ANN and its applied illustration. Int. J. Pept. 2012. [Google Scholar] [CrossRef] [PubMed]
- Li, G.H.; Le, G.W.; Shi, Y.H.; Shrestha, S. Angiotensin-I-converting enzyme inhibitory peptides derived from food proteins and their physiological and pharmacological effects. Nutr. Res. 2004, 24, 469–486. [Google Scholar] [CrossRef]
- Cheung, H.S.; Wang, F.L.; Ondetti, M.A.; Sabo, E.F.; Cushman, D.W. Binding of peptide substrates and inhibitors of angiotensin-converting enzyme. J. Biol. Chem. 1980, 255, 401–407. [Google Scholar] [PubMed]
- Xiao, G.; Yanhan, H.; Jing, L.; Jing, L.; Zhu, S.; Fei, H. Binding modes between C-domain selective angiotensin-converting enzyme (ACE) inhibitory dipeptides and ACE domains. Food Sci. 2017, 38, 160–166. [Google Scholar] [CrossRef]
- Ono, S.; Hosokawa, M.; Miyashita, K.; Takahashi, K. Isolation of peptides with angiotensin I-converting enzyme inhibitory effect derived from hydrolysate of upstream chum salmon muscle. J. Food Sci. 2003, 68, 1611–1614. [Google Scholar] [CrossRef]


| Sample | Soluble Protein (mg/g) | Peptide Content (mg/g) | Yield 1 | IC50 2 (mg/mL) |
|---|---|---|---|---|
| HWE 3 | 379.9 ± 1.5 | 179.7 ± 2.1 | 4.0 | 1.070 ± 0.020 |
| PX-1 4 | 482.0 ± 2.2 | 260.4 ± 2.0 | 19.1 | 0.043 ± 0.001 |
| PX-2 5 | 566.6 ± 3.5 | 298.6 ± 2.0 | 22.7 | 0.043 ± 0.002 |
| PN-1 6 | 574.8 ± 2.3 | 332.8 ± 3.0 | 28.1 | 0.035 ± 0.002 |
| PN-2 7 | 610.6 ± 3.8 | 341.6 ± 3.1 | 31.2 | 0.042 ± 0.001 |
| Protease | IC50 (mg Peptide/mL) |
|---|---|
| Control | 0.035 ± 0.002 |
| Pepsin 1 | 0.044 ± 0.001 |
| Pepsin + Pancreatin 2 | 0.044 ± 0.001 |
| Fraction | Molecular Weight (Da) | Inhibition (%) | Peptide Content (mg/mL) | IER 1 (%/mg/mL) | IC50 (mg/mL) |
|---|---|---|---|---|---|
| A | 1400–1180 | 74.0 | 0.210 | 350 | — 2 |
| B | 1180–910 | 30.6 | 0.027 | 1130 | 0.0450 |
| C | 910–740 | 58.2 | 0.025 | 2230 | 0.0187 |
| D | 680–590 | 48.0 | 0.064 | 750 | — |
| E | 460–370 | 73.2 | 0.057 | 1280 | 0.0160 |
| F | 340–270 | 68.6 | 0.034 | 2020 | 0.0150 |
| G | 200–250 | 40.0 | 0.053 | 760 | — |
| Peak | ACE Inhibitory (%) | Peptide Content (mg/mL) | IER (%/mg/mL) |
|---|---|---|---|
| F1 | 14.3 | 0.01 | 1430 |
| F2 | 19.1 | 0.01 | 1910 |
| F3 | 41.2 | 0.02 | 2060 |
| F4 | 36.8 | 0.01 | 3680 |
| F5 | 72.4 | 0.02 | 3621 |
| F6 | 75.7 | 0.03 | 2523 |
| F7 | 54.3 | 0.01 | 5425 |
| F8 | 86.1 | 0.01 | 8613 |
| F9 | 95.1 | 0.01 | 9510 |
| F10 | 87.7 | 0.01 | 8770 |
| Peak | Sequence | IC50 (μM) |
|---|---|---|
| F7 | Trp–Val | 307.61 ± 0.01 |
| F8 | Val–Trp | 0.58 ± 0.02 |
| F9 | Ile–Trp | 0.50 ± 0.01 |
| F10 | Leu–Trp | 1.11 ± 0.02 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, Y.-H.; Chen, G.-W.; Yeh, C.H.; Song, H.; Tsai, J.-S. Purification and Identification of Angiotensin I-Converting Enzyme Inhibitory Peptides and the Antihypertensive Effect of Chlorella sorokiniana Protein Hydrolysates. Nutrients 2018, 10, 1397. https://doi.org/10.3390/nu10101397
Lin Y-H, Chen G-W, Yeh CH, Song H, Tsai J-S. Purification and Identification of Angiotensin I-Converting Enzyme Inhibitory Peptides and the Antihypertensive Effect of Chlorella sorokiniana Protein Hydrolysates. Nutrients. 2018; 10(10):1397. https://doi.org/10.3390/nu10101397
Chicago/Turabian StyleLin, Yu-Hsin, Guan-Wen Chen, Chin Hsi Yeh, Helena Song, and Jenn-Shou Tsai. 2018. "Purification and Identification of Angiotensin I-Converting Enzyme Inhibitory Peptides and the Antihypertensive Effect of Chlorella sorokiniana Protein Hydrolysates" Nutrients 10, no. 10: 1397. https://doi.org/10.3390/nu10101397
APA StyleLin, Y.-H., Chen, G.-W., Yeh, C. H., Song, H., & Tsai, J.-S. (2018). Purification and Identification of Angiotensin I-Converting Enzyme Inhibitory Peptides and the Antihypertensive Effect of Chlorella sorokiniana Protein Hydrolysates. Nutrients, 10(10), 1397. https://doi.org/10.3390/nu10101397





