Effect of A Very Low-Calorie Ketogenic Diet on Food and Alcohol Cravings, Physical and Sexual Activity, Sleep Disturbances, and Quality of Life in Obese Patients
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cohort of Patients
2.2. Very-Low-Calorie Ketogenic Diet Protocol
2.3. Anthropometric Assessment
2.4. Total Body Composition
2.5. Determination of Levels of Ketone Bodies and Biochemical Parameters
2.6. Assessment of Food Cravings and Psychological Well-Being by Self-Questionnaires
2.7. Food Craving Questionnaires
2.8. Physical Activity
2.9. Sexual Functionality Questionnaire
2.10. Epworth Daytime Sleepiness Scale (ESS)
2.11. Pittsburgh Sleep Quality Index (PSQI)
2.12. Impact of Weight on Quality of Life-Lite (IWQOL-LITE)
2.13. Statistical Analysis
3. Results
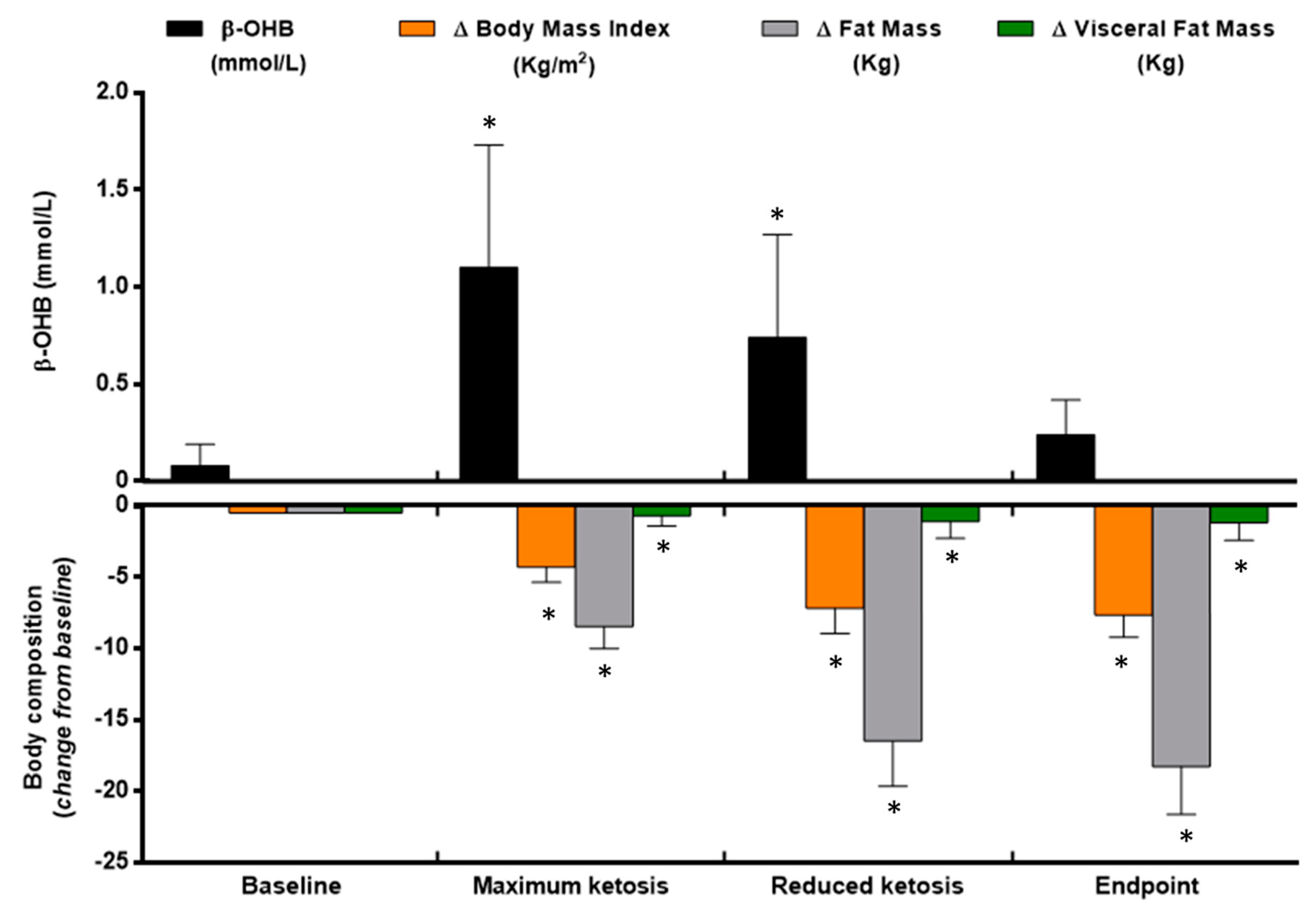
3.1. Changes in Anthropometrical and Body Composition Measurements
3.2. Changes in Craving and Well-Being Self-Reported Test
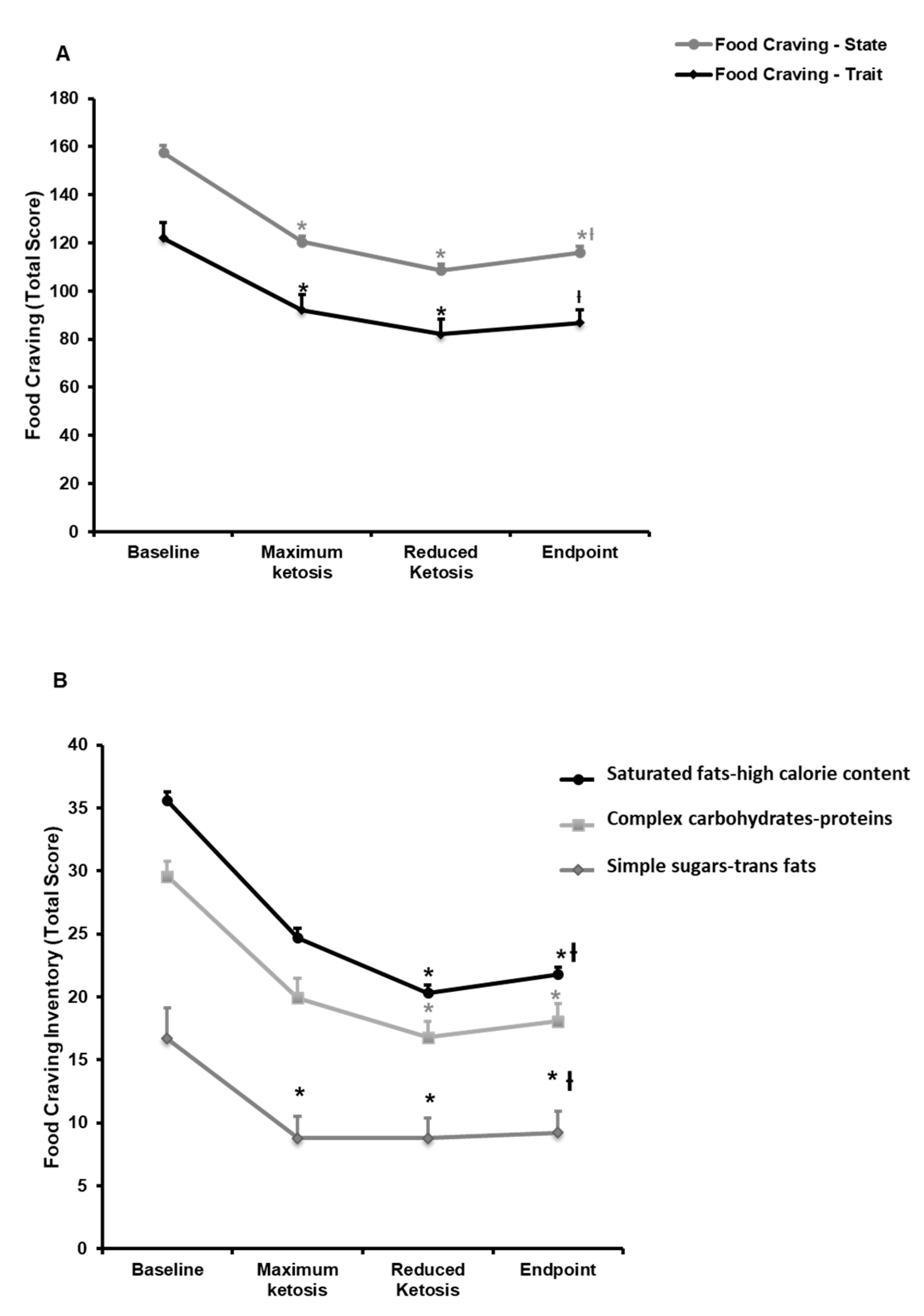
3.2.1. Effect of the Nutritional Intervention on Food and Alcohol Cravings
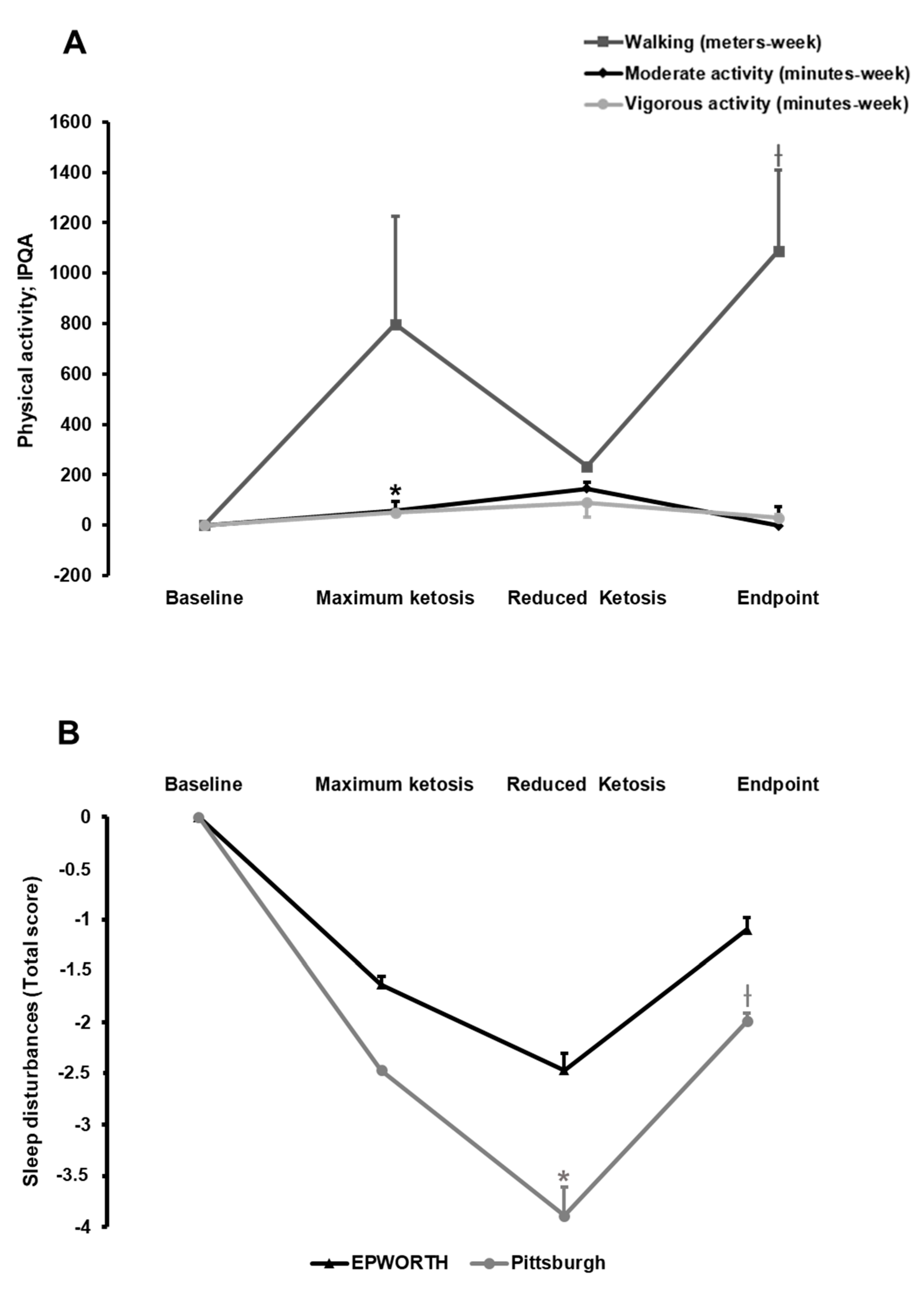
3.2.2. Effect of the Nutritional Intervention on Physical Activity
3.2.3. Effect of the Nutritional Intervention on Sleep Quality
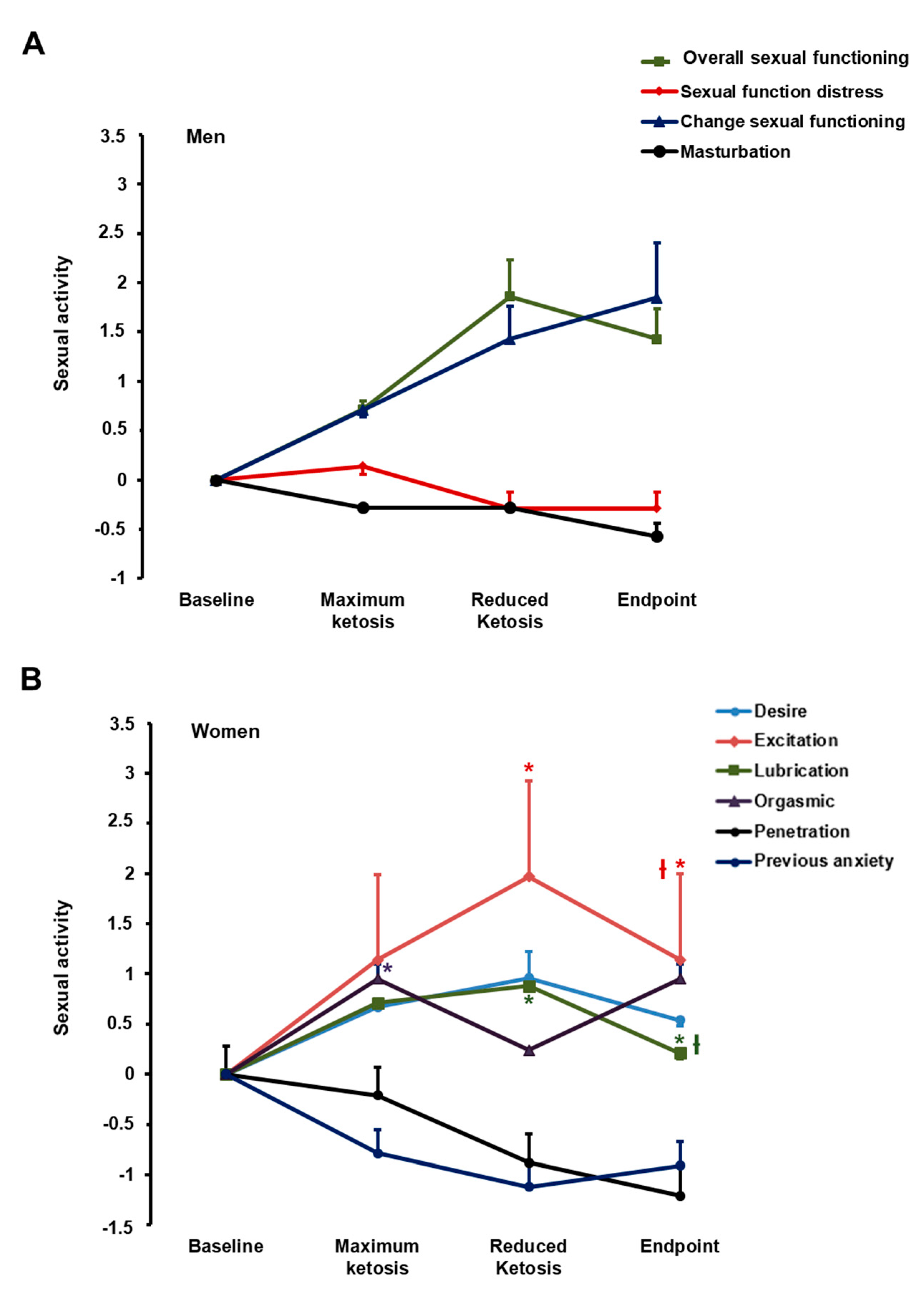
3.2.4. Effect of the Nutritional Intervention on Sexual Function
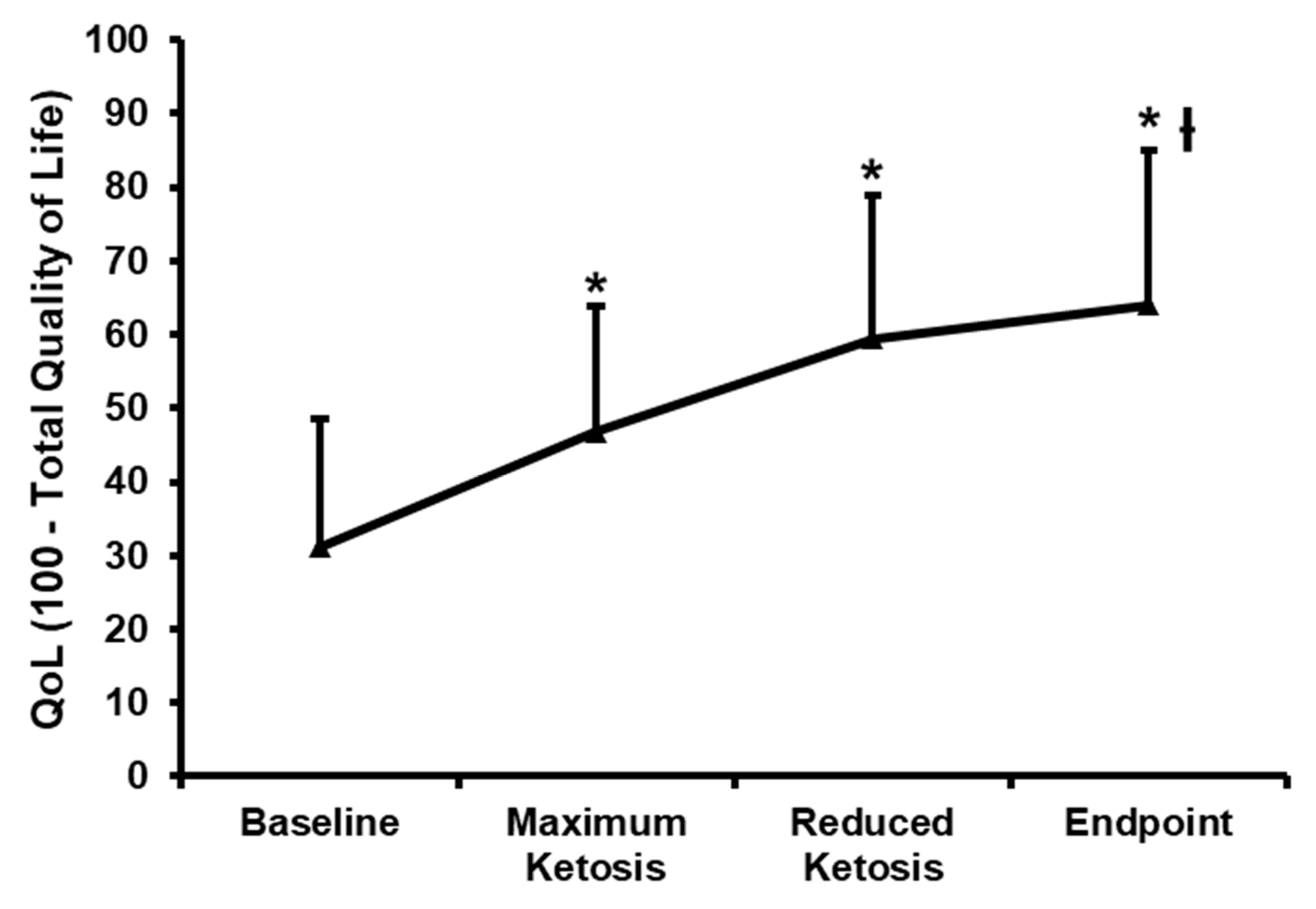
3.2.5. Effect of the Nutritional Intervention on the Impact of Weight on Quality of Life-Lite (IWQOL-Lite)
3.3. Association between the Physical and Psychological Changes during the Follow-Up
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Apovian, C.M.; Aronne, L.J.; Bessesen, D.H.; McDonnell, M.E.; Murad, M.H.; Pagotto, U.; Ryan, D.H.; Still, C.D.; Endocrine, S. Pharmacological management of obesity: An endocrine society clinical practice guideline. J. Clin. Endocrinol. Metab. 2015, 100, 342–362. [Google Scholar] [CrossRef] [PubMed]
- Bray, G.A.; Heisel, W.E.; Afshin, A.; Jensen, M.D.; Dietz, W.H.; Long, M.; Kushner, R.F.; Daniels, S.R.; Wadden, T.A.; Tsai, A.G.; et al. The science of obesity management: An endocrine society scientific statement. Endocr. Rev. 2018, 39, 79–132. [Google Scholar] [CrossRef] [PubMed]
- Gadde, K.M.; Martin, C.K.; Berthoud, H.R.; Heymsfield, S.B. Obesity: Pathophysiology and management. J. Am. Coll. Cardiol. 2018, 71, 69–84. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Arbelaez, D.; Bellido, D.; Castro, A.I.; Ordonez-Mayan, L.; Carreira, J.; Galban, C.; Martinez-Olmos, M.A.; Crujeiras, A.B.; Sajoux, I.; Casanueva, F.F. Body composition changes after very-low-calorie ketogenic diet in obesity evaluated by 3 standardized methods. J. Clin. Endocrinol. Metab. 2017, 102, 488–498. [Google Scholar] [CrossRef] [PubMed]
- Merra, G.; Gratteri, S.; De Lorenzo, A.; Barrucco, S.; Perrone, M.A.; Avolio, E.; Bernardini, S.; Marchetti, M.; Di Renzo, L. Effects of very-low-calorie diet on body composition, metabolic state, and genes expression: A randomized double-blind placebo-controlled trial. Eur. Rev. Med. Pharmacol. Sci. 2017, 21, 329–345. [Google Scholar] [PubMed]
- Moreno, B.; Crujeiras, A.B.; Bellido, D.; Sajoux, I.; Casanueva, F.F. Obesity treatment by very low-calorie-ketogenic diet at two years: Reduction in visceral fat and on the burden of disease. Endocrine 2016, 54, 681–690. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Arbelaez, D.; Crujeiras, A.B.; Castro, A.I.; Martinez-Olmos, M.A.; Canton, A.; Ordonez-Mayan, L.; Sajoux, I.; Galban, C.; Bellido, D.; Casanueva, F.F. Resting metabolic rate of obese patients under very low calorie ketogenic diet. Nutr. Metab. (Lond.) 2018, 15, 18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heymsfield, S.B.; Wadden, T.A. Mechanisms, pathophysiology, and management of obesity. N. Engl. J. Med. 2017, 376, 254–266. [Google Scholar] [CrossRef] [PubMed]
- Crujeiras, A.B.; Diaz-Lagares, A.; Stefansson, O.A.; Macias-Gonzalez, M.; Sandoval, J.; Cueva, J.; Lopez-Lopez, R.; Moran, S.; Jonasson, J.G.; Tryggvadottir, L.; et al. Obesity and menopause modify the epigenomic profile of breast cancer. Endocr. Relat. Cancer 2017, 24, 351–363. [Google Scholar] [CrossRef] [PubMed]
- Crujeiras, A.B.; Morcillo, S.; Diaz-Lagares, A.; Sandoval, J.; Castellano-Castillo, D.; Torres, E.; Hervas, D.; Moran, S.; Esteller, M.; Macias-Gonzalez, M.; et al. Identification of an episignature of human colorectal cancer associated with obesity by genome-wide DNA methylation analysis. Int. J. Obes. (Lond.) 2018. [Google Scholar] [CrossRef] [PubMed]
- Upadhyay, J.; Farr, O.; Perakakis, N.; Ghaly, W.; Mantzoros, C. Obesity as a disease. Med. Clin. N. Am. 2018, 102, 13–33. [Google Scholar] [CrossRef] [PubMed]
- Ferrer-Garcia, M.; Pla-Sanjuanelo, J.; Dakanalis, A.; Vilalta-Abella, F.; Riva, G.; Fernandez-Aranda, F.; Sanchez, I.; Ribas-Sabate, J.; Andreu-Gracia, A.; Escandon-Nagel, N.; et al. Eating behavior style predicts craving and anxiety experienced in food-related virtual environments by patients with eating disorders and healthy controls. Appetite 2017, 117, 284–293. [Google Scholar] [CrossRef] [PubMed]
- Frigolet, M.E.; Ramos Barragan, V.E.; Tamez Gonzalez, M. Low-carbohydrate diets: A matter of love or hate. Ann. Nutr. Metab. 2011, 58, 320–334. [Google Scholar] [CrossRef] [PubMed]
- Paoli, A. Ketogenic diet for obesity: Friend or foe? Int. J. Environ. Res. Public Health 2014, 11, 2092–2107. [Google Scholar] [CrossRef] [PubMed]
- Cahill, G.F., Jr. Fuel metabolism in starvation. Annu. Rev. Nutr. 2006, 26, 1–22. [Google Scholar] [CrossRef] [PubMed]
- McGarry, J.D.; Foster, D.W. Regulation of hepatic fatty acid oxidation and ketone body production. Annu. Rev. Biochem. 1980, 49, 395–420. [Google Scholar] [CrossRef] [PubMed]
- Sumithran, P.; Proietto, J. Ketogenic diets for weight loss: A review of their principles, safety and efficacy. Obes. Res. Clin. Pract. 2008, 2, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Arbelaez, D.; Crujeiras, A.B.; Castro, A.I.; Goday, A.; Mas-Lorenzo, A.; Bellon, A.; Tejera, C.; Bellido, D.; Galban, C.; Sajoux, I.; et al. Acid-base safety during the course of a very low-calorie-ketogenic diet. Endocrine 2017, 58, 81–90. [Google Scholar] [CrossRef] [PubMed]
- Soejima, E.; Ohki, T.; Kurita, Y.; Yuan, X.; Tanaka, K.; Kakino, S.; Hara, K.; Nakayama, H.; Tajiri, Y.; Yamada, K. Protective effect of 3-hydroxybutyrate against endoplasmic reticulum stress-associated vascular endothelial cell damage induced by low glucose exposure. PLoS ONE 2018, 13, e0191147. [Google Scholar] [CrossRef] [PubMed]
- Cavaleri, F.; Bashar, E. Potential synergies of beta-hydroxybutyrate and butyrate on the modulation of metabolism, inflammation, cognition, and general health. J. Nutr. Metab. 2018, 2018. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Miao, Z.; Xu, X. Beta-hydroxybutyrate alleviates depressive behaviors in mice possibly by increasing the histone3-lysine9-beta-hydroxybutyrylation. Biochem. Biophys. Res. Commun. 2017, 490, 117–122. [Google Scholar] [CrossRef] [PubMed]
- EFSA Panel on Dietetic Products; Nutrition and Allergies (NDA). Scientific opinion on the essential composition of total diet replacements for weight control. EFSA J. 2015, 13, 3957. [Google Scholar] [CrossRef] [Green Version]
- SCOOP-VLCKD task 7.3. Reports on Tasks for Scientific Cooperation. Collection of Data on Products Intendend for Use in Verylow-Calorie-Diets; European Comission: Brussels, Belgium, 2002. [Google Scholar]
- Watson, N.A.; Dyer, K.A.; Buckley, J.D.; Brinkworth, G.D.; Coates, A.M.; Parfitt, G.; Howe, P.R.C.; Noakes, M.; Dye, L.; Chadwick, H.; et al. A randomised trial comparing low-fat diets differing in carbohydrate and protein ratio, combined with regular moderate intensity exercise, on glycaemic control, cardiometabolic risk factors, food cravings, cognitive function and psychological wellbeing in adults with type 2 diabetes: Study protocol. Contemp. Clin. Trials 2015, 45, 217–225. [Google Scholar] [PubMed] [Green Version]
- Guardia, J.S.; Segura, L.G.; Gonzalvo, B.C.; Trujols, J.A.; Tejero, A.P.; Suarez, A.G.; Marti, A.G. Validation study of the multidimensional alcohol craving scale (MACS). Med. Clin. (Barc.) 2004, 123, 211–216. [Google Scholar] [CrossRef]
- Craig, C.L.; Marshall, A.L.; Sjostrom, M.; Bauman, A.E.; Booth, M.L.; Ainsworth, B.E.; Pratt, M.; Ekelund, U.; Yngve, A.; Sallis, J.F.; et al. International physical activity questionnaire: 12-country reliability and validity. Med. Sci. Sports Exerc. 2003, 35, 1381–1395. [Google Scholar] [CrossRef] [PubMed]
- O’Connor, D.B.; Corona, G.; Forti, G.; Tajar, A.; Lee, D.M.; Finn, J.D.; Bartfai, G.; Boonen, S.; Casanueva, F.F.; Giwercman, A.; et al. Assessment of sexual health in aging men in Europe: Development and validation of the european male ageing study sexual function questionnaire. J. Sex. Med. 2008, 5, 1374–1385. [Google Scholar] [CrossRef] [PubMed]
- Wiegel, M.; Meston, C.; Rosen, R. The female sexual function index (fsfi): Cross-validation and development of clinical cutoff scores. J. Sex. Marital. Ther. 2005, 31, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Johns, M.W. A new method for measuring daytime sleepiness: The epworth sleepiness scale. Sleep 1991, 14, 540–545. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Kim, J.E.; Armstrong, C.L.; Chen, N.; Campbell, W.W. Higher-protein diets improve indexes of sleep in energy-restricted overweight and obese adults: Results from 2 randomized controlled trials. Am. J. Clin. Nutr. 2016, 103, 766–774. [Google Scholar] [CrossRef] [PubMed]
- Kolotkin, R.L.; Crosby, R.D.; Williams, G.R. Health-related quality of life varies among obese subgroups. Obes. Res. 2002, 10, 748–756. [Google Scholar] [CrossRef] [PubMed]
- Twells, L.K.; Driscoll, S.; Gregory, D.M.; Lester, K.; Fardy, J.M.; Pace, D. Morbidity and health-related quality of life of patients accessing laparoscopic sleeve gastrectomy: A single-centre cross-sectional study in one province of Canada. BMC Obes. 2017, 4, 40. [Google Scholar] [CrossRef] [PubMed]
- Kelley, K.; Preacher, K.J. On effect size. Psychol. Methods 2012, 17, 137–152. [Google Scholar] [CrossRef] [PubMed]
- Rosenthal, R.; Rubin, D.B. R equivalent: A simple effect size indicator. Psychol. Methods 2003, 8, 492–496. [Google Scholar] [CrossRef] [PubMed]
- Crujeiras, A.B.; Gomez-Arbelaez, D.; Zulet, M.A.; Carreira, M.C.; Sajoux, I.; de Luis, D.; Castro, A.I.; Baltar, J.; Baamonde, I.; Sueiro, A.; et al. Plasma FGF21 levels in obese patients undergoing energy-restricted diets or bariatric surgery: A marker of metabolic stress? Int. J. Obes. (Lond.) 2017, 41, 1570–1578. [Google Scholar] [CrossRef] [PubMed]
- NCD Risk Factor Collaboration (NCD-RisC). Worldwide trends in body-mass index, underweight, overweight, and obesity from 1975 to 2016: A pooled analysis of 2416 population-based measurement studies in 128.9 million children, adolescents, and adults. Lancet 2017, 390, 2627–2642. [Google Scholar] [CrossRef]
- Davis, R.A.H.; Plaisance, E.P.; Allison, D.B. Complementary hypotheses on contributors to the obesity epidemic. Obesity 2018, 26, 17–21. [Google Scholar] [CrossRef] [PubMed]
- Schur, E.A.; Cummings, D.E.; Callahan, H.S.; Foster-Schubert, K.E. Association of cognitive restraint with ghrelin, leptin, and insulin levels in subjects who are not weight-reduced. Physiol. Behav. 2008, 93, 706–712. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schwimmer, J.B.; Burwinkle, T.M.; Varni, J.W. Health-related quality of life of severely obese children and adolescents. JAMA 2003, 289, 1813–1819. [Google Scholar] [CrossRef] [PubMed]
- Eisenberg, M.E.; Neumark-Sztainer, D.; Story, M. Associations of weight-based teasing and emotional well-being among adolescents. Arch. Pediatr. Adolesc. Med. 2003, 157, 733–738. [Google Scholar] [CrossRef] [PubMed]
- Lennerz, B.; Lennerz, J.K. Food addiction, high-glycemic-index carbohydrates, and obesity. Clin. Chem. 2018, 64, 64–71. [Google Scholar] [CrossRef] [PubMed]
- Paoli, A.; Bosco, G.; Camporesi, E.M.; Mangar, D. Ketosis, ketogenic diet and food intake control: A complex relationship. Front. Psychol. 2015, 6, 27. [Google Scholar] [CrossRef] [PubMed]
- Kahathuduwa, C.N.; Binks, M.; Martin, C.K.; Dawson, J.A. Extended calorie restriction suppresses overall and specific food cravings: A systematic review and a meta-analysis. Obes. Rev. 2017, 18, 1122–1135. [Google Scholar] [CrossRef] [PubMed]
- Martin, C.K.; O’Neil, P.M.; Pawlow, L. Changes in food cravings during low-calorie and very-low-calorie diets. Obesity 2006, 14, 115–121. [Google Scholar] [CrossRef] [PubMed]
- Kahathuduwa, C.N.; Davis, T.; O’Boyle, M.; Boyd, L.A.; Chin, S.H.; Paniukov, D.; Binks, M. Effects of 3-week total meal replacement vs. typical food-based diet on human brain functional magnetic resonance imaging food-cue reactivity and functional connectivity in people with obesity. Appetite 2018, 120, 431–441. [Google Scholar] [CrossRef] [PubMed]
- Johnstone, A.M.; Horgan, G.W.; Murison, S.D.; Bremner, D.M.; Lobley, G.E. Effects of a high-protein ketogenic diet on hunger, appetite, and weight loss in obese men feeding ad libitum. Am. J. Clin. Nutr. 2008, 87, 44–55. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McClernon, F.J.; Yancy, W.S., Jr.; Eberstein, J.A.; Atkins, R.C.; Westman, E.C. The effects of a low-carbohydrate ketogenic diet and a low-fat diet on mood, hunger, and other self-reported symptoms. Obesity 2007, 15, 182–187. [Google Scholar] [CrossRef] [PubMed]
- Stubbs, B.J.; Cox, P.J.; Evans, R.D.; Cyranka, M.; Clarke, K.; de Wet, H. A ketone ester drink lowers human ghrelin and appetite. Obesity 2018, 26, 269–273. [Google Scholar] [CrossRef] [PubMed]
- Nymo, S.; Coutinho, S.R.; Jorgensen, J.; Rehfeld, J.F.; Truby, H.; Kulseng, B.; Martins, C. Timeline of changes in appetite during weight loss with a ketogenic diet. Int. J. Obes. (Lond.) 2017, 41, 1224–1231. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rowland, D.L.; McNabney, S.M.; Mann, A.R. Sexual function, obesity, and weight loss in men and women. Sex. Med. Rev. 2017, 5, 323–338. [Google Scholar] [CrossRef] [PubMed]
- Sarwer, D.B.; Spitzer, J.C.; Wadden, T.A.; Rosen, R.C.; Mitchell, J.E.; Lancaster, K.; Courcoulas, A.; Gourash, W.; Christian, N.J. Sexual functioning and sex hormones in persons with extreme obesity and seeking surgical and nonsurgical weight loss. Surg. Obes. Relat. Dis. 2013, 9, 997–1007. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Broussard, J.; Brady, M.J. The impact of sleep disturbances on adipocyte function and lipid metabolism. Best Pract. Res. Clin. Endocrinol. Metab. 2010, 24, 763–773. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cappuccio, F.P.; Cooper, D.; D’Elia, L.; Strazzullo, P.; Miller, M.A. Sleep duration predicts cardiovascular outcomes: A systematic review and meta-analysis of prospective studies. Eur. Heart J. 2011, 32, 1484–1492. [Google Scholar] [CrossRef] [PubMed]
- Cappuccio, F.P.; D’Elia, L.; Strazzullo, P.; Miller, M.A. Quantity and quality of sleep and incidence of type 2 diabetes: A systematic review and meta-analysis. Diabetes Care 2010, 33, 414–420. [Google Scholar] [CrossRef] [PubMed]
- Chaput, J.P. Sleep patterns, diet quality and energy balance. Physiol. Behav. 2014, 134, 86–91. [Google Scholar] [CrossRef] [PubMed]
- Dean, D.A.; Wang, R.; Jacobs, D.R.; Duprez, D.; Punjabi, N.M.; Zee, P.C.; Shea, S.; Watson, K.; Redline, S. A systematic assessment of the association of polysomnographic indices with blood pressure: The multi-ethnic study of atherosclerosis (MESA). Sleep 2015, 38, 587–596. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Zhai, L.; Zhang, D. Sleep duration and obesity among adults: A meta-analysis of prospective studies. Sleep Med. 2014, 15, 1456–1462. [Google Scholar] [CrossRef] [PubMed]
- Kolotkin, R.L.; Binks, M.; Crosby, R.D.; Ostbye, T.; Mitchell, J.E.; Hartley, G. Improvements in sexual quality of life after moderate weight loss. Int. J. Impot. Res. 2008, 20, 487–492. [Google Scholar] [CrossRef] [PubMed]
- Lasikiewicz, N.; Myrissa, K.; Hoyland, A.; Lawton, C.L. Psychological benefits of weight loss following behavioural and/or dietary weight loss interventions. A systematic research review. Appetite 2014, 72, 123–137. [Google Scholar] [CrossRef] [PubMed]
- Rothberg, A.E.; McEwen, L.N.; Kraftson, A.T.; Neshewat, G.M.; Fowler, C.E.; Burant, C.F.; Herman, W.H. The impact of weight loss on health-related quality-of-life: Implications for cost-effectiveness analyses. Qual. Life Res. 2014, 23, 1371–1376. [Google Scholar] [CrossRef] [PubMed]
- Thomson, C.A.; Morrow, K.L.; Flatt, S.W.; Wertheim, B.C.; Perfect, M.M.; Ravia, J.J.; Sherwood, N.E.; Karanja, N.; Rock, C.L. Relationship between sleep quality and quantity and weight loss in women participating in a weight-loss intervention trial. Obesity 2012, 20, 1419–1425. [Google Scholar] [CrossRef] [PubMed]
- Verhoef, S.P.; Camps, S.G.; Gonnissen, H.K.; Westerterp, K.R.; Westerterp-Plantenga, M.S. Concomitant changes in sleep duration and body weight and body composition during weight loss and 3-mo weight maintenance. Am. J. Clin. Nutr. 2013, 98, 25–31. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seidelmann, S.B.; Claggett, B.; Cheng, S.; Henglin, M.; Shah, A.; Steffen, L.M.; Folsom, A.R.; Rimm, E.B.; Willett, W.C.; Solomon, S.D. Dietary carbohydrate intake and mortality: A prospective cohort study and meta-analysis. Lancet Public Health 2018, 3, e419–e428. [Google Scholar] [CrossRef]
| Variable | Baseline |
|---|---|
| Age (years) | 47.2 (10.2) |
| Gender (M/F) | 8/12 |
| β-Hydroxy-Butyrate (mmol/L) | 0.08 (0.1) |
| Body Mass Index (kg/m2) | 35.5 (4.4) |
| Waist Circumference (cm) | 109.4 (12.8) |
| Fat Mass (kg) | 42.2 (9.1) |
| Fat Mass Percentage (%) | 45.6 (5.4) |
| Visceral Fat Mass (kg) | 2.18 (1.28) |
| Fat-Free Mass (kg) | 52.8 (10.2) |
| Bone Mineral Content (kg) | 2.59 (0.49) |
| Bone Mineral Density (g/cm2) | 1.24 (0.13) |
| Systolic Blood Pressure (mmHg) | 125.6 (12.5) |
| Diastolic Blood Pressure (mmHg) | 86.1 (7.6) |
| Glucose (mg/dL) | 96.0 (11.7) |
| Insulin (mUI/L) | 20.4 (10.7) |
| HOMA-IR | 5.0 (2.8) |
| HbA1c (%) | 5.7 (0.4) |
| TSH (pg/dL) | 1.9 (1.0) |
| Free T4 (pg/dL) | 1.1 (0.1) |
| Free T3 (ng/dL) | 3.4 (0.3) |
| Change between Assessments 1–2 | Change between Assessments 1–3 | Change between Assessments 1–4 | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| BMI | FM | FFM | Weight | Ketosis | BMI | FM | FFM | Weight | Ketosis | BMI | FM | FFM | Weight | Ketosis | |
| Food Craving Questionnaire (FCQ)-Trait | |||||||||||||||
| Positive Reinforcement | 0.424 † | 0.380 † | 0.102 | 0.392 † | −0.157 | 0.463 † | 0.358 † | 0.391 † | 0.416 † | −0.136 | 0.326 † | 0.269 † | 0.371 † | 0.327 † | −0.122 |
| Negative Reinforcement | 0.312 † | 0.144 | 0.545 † | 0.334 † | −0.178 | 0.459 † | 0.265† | 0.489 † | 0.365 † | −0.134 | 0.408 † | 0.388 † | 0.463 † | 0.456 † | −0.078 |
| Intentions to Eat | 0.177 | 0.025 | 0.465 † | 0.201 | −0.455 † | 0.317 † | 0.324 † | 0.312 † | 0.371 † | −0.293 † | 0.266 † | 0.315 † | 0.278 † | 0.348 † | −0.030 |
| Cue-Dependent Eating | 0.078 | −0.085 | 0.297 † | 0.031 | −0.195 | 0.211 | −0.005 | 0.178 | 0.044 | −0.347 † | 0.329 † | 0.107 | 0.206 | 0.147 | −0.253 † |
| Thoughts-Preoccupations with Food | 0.271 † | 0.108 | 0.234 | 0.186 | −0.056 | 0.337 † | 0.229 | 0.290 † | 0.278 † | −0.052 | 0.331 † | 0.206 | 0.512 † | 0.317 † | −0.027 |
| Feelings of Hunger | 0.212 | 0.110 | 0.353 † | 0.235 | −0.303 † | 0.214 | 0.214 | 0.245 † | 0.258 † | −0.141 | 0.206 | 0.230 | 0.250 † | 0.268 † | −0.007 |
| Lack of Control | 0.294 † | −0.007 | 0.645 † | 0.244 † | −0.149 | 0.485 † | 0.342 † | 0.393 † | 0.410 † | −0.156 | 0.546 † | 0.448 † | 0.518 † | 0.524 † | −0.199 |
| Negative Affect | 0.284 † | −0.016 | 0.483 † | 0.166 | −0.129 | 0.505 † | 0.290 † | 0.473 † | 0.386 † | −0.195 | 0.352 † | 0.207 | 0.281 † | 0.251 † | −0.031 |
| Guilty Feelings | 0.131 | −0.001 | 0.111 | 0.029 | −0.173 | 0.325 † | 0.179 | −0.070 | 0.130 | −0.340 † | 0.393 † | 0.243 † | 0.159 | 0.246 † | −0.256 † |
| Total Trait Score | 0.309 † | 0.093 | 0.443† | 0.252 † | −0.229 | 0.466 † | 0.309 † | 0.376 † | 0.373 † | −0.229 | 0.464 † | 0.353 † | 0.458 † | 0.426 † | −0.148 |
| Food Craving Questionnaire (FCQ)-State | |||||||||||||||
| Intense Desire to Eat | 0.003 | 0.007 | −0.299 † | −0.105 | 0.077 | 0.185 | 0.126 | −0.168 | 0.057 | −0.561 † | 0.236 | 0.152 | 0.288 † | 0.210 | −0.543 † |
| Anticipation Positive Reinforcement | 0.331 † | 0.214 | 0.285 † | 0.303 † | −0.023 | 0.197 | 0.114 | 0.207 | 0.158 | −0.365 † | 0.424 † | 0.380 † | 0.410 † | 0.434 † | −0.467 † |
| Anticipation Relief from Negative States | 0.196 | 0.145 | 0.362 † | 0.266 † | −0.287 † | 0.267 † | 0.234 | 0.212 | 0.262 † | −0.294 † | 0.275 † | 0.285 † | 0.233 | 0.305 † | −0.143 |
| Preoccupation with Food-Lack Control | 0.160 | 0.011 | 0.391 † | 0.154 | −0.415 † | 0.381 † | 0.241 † | 0.408 † | 0.323 † | −0.260 † | 0.387 † | 0.314 † | 0.431 † | 0.388 † | −0.049 |
| Craving as Physiological State | −0.376 † | −0.195 | −0.368 † | −0.325 † | −0.113 | −0.134 | −0.021 | −0.313 † | −0.111 | −0.542 † | −0.055 | −0.031 | −0.009 | −0.030 | −0.083 |
| Total State Score | 0.117 | 0.071 | 0.114 | 0.104 | −0.208 | 0.209 | 0.165 | 0.063 | 0.158 | −0.514 † | 0.349 † | 0.307 † | 0.366 † | 0.361 † | −0.353 † |
| Food Craving Inventory (FCI, SP) | |||||||||||||||
| Simple Sugars/Trans Fats | −0.327 † | −0.487 † | 0.155 | −0.391 † | −0.454 † | −0.377 † | −0.556 † | 0.174 | −0.446 † | −0.227 | −0.171 | −0.371 † | 0.136 | −0.278 † | −0.121 |
| Complex Carbohydrates/Proteins | −0.079 | −0.203 | 0.092 | −0.160 | −0.420 † | −0.228 | −0.346 † | 0.364 † | −0.207 | −0.075 | −0.178 | −0.306 † | 0.317 † | −0.175 | −0.084 |
| Saturated Fat/High Calorie Content | 0.418 † | 0.198 | 0.310 † | 0.281 † | −0.239 | 0.190 | −0.024 | 0.520 † | 0.121 | −0.063 | 0.062 | −0.084 | 0.326 † | 0.017 | 0.230 |
| Multidimentsional Alcohol Craving Scale (MACS) | |||||||||||||||
| Desire | −0.058 | −0.015 | 0.293 † | 0.097 | −0.273 † | 0.135 | 0.111 | 0.284 † | 0.172 | 0.101 | 0.253 † | 0.240 | 0.285 † | 0.280 † | 0.191 |
| Lack of Inhibition | 0.138 | 0.271 † | −0.104 | 0.210 | 0.338 † | 0.111 | 0.188 | 0.041 | 0.178 | 0.029 | 0.154 | 0.145 | 0.158 | 0.163 | 0.124 |
| Total Score | 0.039 | 0.166 | 0.192 | 0.225 | −0.019 | 0.204 | 0.231 | 0.305 † | 0.284 † | 0.118 | 0.302 † | 0.285 † | 0.332 † | 0.330 † | 0.232 |
| Impact of Weight on Quality of Life-Lite | |||||||||||||||
| Physical | 0.311 † | 0.327 † | 0.229 | 0.384 † | 0.354 † | 0.319 † | 0.426 † | 0.303 † | 0.461 † | 0.422 † | 0.414 † | 0.478 † | 0.360 † | 0.502 † | 0.274 † |
| Sexual Life | −0.072 | −0.177 | 0.211 | −0.078 | −0.133 | 0.159 | −0.061 | 0.580 † | 0.110 | −0.038 | 0.379 † | 0.215 | 0.525 † | 0.325 † | 0.039 |
| Self-Steam | −0.201 | −0.214 | −0.077 | −0.224 | 0.122 | −0.155 | −0.266 † | −0.003 | −0.233 | −0.083 | 0.062 | −0.108 | 0.181 | −0.045 | 0.044 |
| Social Anxiety | 0.215 | 0.086 | 0.235 | 0.168 | 0.137 | 0.603 † | 0.347 † | 0.666 † | 0.488 † | −0.327 † | 0.640 † | 0.405 † | 0.740 † | 0.540 † | 0.209 |
| Work Area | 0.295 † | 0.068 | 0.417 † | 0.205 | 0.447 † | 0.197 | 0.130 | 0.217 | 0.177 | −0.143 | 0.458 † | 0.313 † | 0.449 † | 0.386 † | 0.042 |
| Total Score | 0.147 | 0.086 | 0.231 | 0.165 | 0.313 † | 0.330 † | 0.217 | 0.520 † | 0.338 † | 0.118 | 0.488 † | 0.351 † | 0.556 † | 0.446 † | 0.188 |
| EPWORTH Daily Sleep; Total Score | 0.274 † | 0.350† | 0.235 | 0.401 † | −0.033 | 0.313 † | 0.406 † | 0.123 | 0.392 † | −0.379 † | 0.431 † | 0.595 † | 0.122 | 0.542 † | −0.385 † |
| Pittsburgh Sleep Quality Index; Total Score | 0.362 † | 0.066 | 0.543 † | 0.252 † | 0.037 | 0.489 † | 0.240 | 0.536 † | 0.354 † | −0.049 | 0.456 † | 0.236 | 0.504 † | 0.332 † | 0.434 † |
| Physical Activity; IPQA | 0.076 | 0.102 | 0.002 | 0.095 | 0.493 † | −0.163 | −0.115 | −0.189 | −0.156 | 0.379 † | −0.029 | 0.003 | −0.167 | −0.043 | 0.284 † |
| Walking (meters/week) | 0.118 | 0.072 | 0.115 | 0.111 | 0.132 | −0.380 † | −0.059 | −0.637 † | −0.239 † | 0.096 | 0.273 † | 0.459 † | −0.019 | 0.381 † | −0.062 |
| Moderate Activity (min-week) | −0.224 | −0.093 | −0.328 † | −0.206 | −0.213 | 0.350 † | 0.342 † | −0.059 | 0.295 † | −0.100 | −0.113 | −0.329 † | 0.231 | −0.211 | 0.025 |
| Vigorous Activity (min-week) | |||||||||||||||
| Sexual Activity (women, n = 12) | 0.602 † | 0.644 † | 0.409 † | 0.578 † | 0.256 † | −0.159 | 0.101 | −0.544 † | −0.128 | −0.453 † | 0.808 † | 0.842 † | 0.774 † | 0.869 † | 0.276 † |
| Desire | 0.662 † | 0.752 † | 0.251 † | 0.579 † | 0.790 † | 0.492 † | 0.593 † | 0.001 | 0.452 † | −0.031 | 0.594 † | 0.580 † | 0.728 † | 0.660 † | 0.153 |
| Excitation | 0.140 | 0.284 † | −0.206 | 0.082 | 0.628 † | 0.152 | 0.173 | 0.015 | 0.137 | 0.518 † | −0.246 † | −0.102 | −0.371 † | −0.199 | 0.413 † |
| Lubrication | −0.129 | −0.073 | −0.018 | −0.062 | −0.296 † | 0.640 † | 0.747 † | 0.292 † | 0.684 † | −0.114 | −0.527 † | −0.363 † | −0.721 † | −0.505 † | −0.378 † |
| Orgasmic | 0.767 † | 0.679 † | 0.726 † | 0.747 † | 0.537 † | 0.691 † | 0.466 † | 0.801 † | 0.660 † | 0.448 † | 0.692 † | 0.593 † | 0.664 † | 0.653 † | 0.609 † |
| Penetration | 0.537 † | 0.575 † | 0.193 | 0.446 † | 0.661 † | 0.625 † | 0.477 † | 0.492 † | 0.557 † | 0.093 | 0.662 † | 0.574 † | 0.459 † | 0.570† | 0.320 † |
| Previous Anxiety | |||||||||||||||
| Sexual activity (men, n = 8) | 0.549 † | 0.406 † | 0.071 | 0.512 † | 0.093 | 0.383 † | 0.362 † | −0.734 † | 0.252 † | −0.236 | 0.380 † | 0.368† | 0.088 | 0.351 † | 0.142 |
| Overall Sexual Functioning | −0.129 | −0.299 † | 0.831 † | 0.006 | −0.083 | 0.119 | 0.123 | 0.868 † | 0.255 † | 0.283 † | 0.072 | 0.194 | −0.096 | 0.181 | 0.212 |
| Sexual Function Distress | 0.573 † | 0.593 † | −0.682 † | 0.412 † | 0.263 † | 0.027 | 0.037 | −0.900 † | −0.099 | −0.198 | 0.010 | −0.110 | 0.194 | −0.091 | −0.119 |
| Change Sexual Functioning | −0.054 | −0.176 | 0.815† | 0.136 | −0.389 † | 0.119 | 0.123 | 0.868 † | 0.255 † | 0.283 † | −0.249 † | −0.226 | −0.322 † | −0.237 | 0.656 † |
| Masturbation | 0.076 | 0.102 | 0.002 | 0.095 | 0.493 † | −0.163 | −0.115 | −0.189 | −0.156 | 0.379 † | −0.029 | 0.003 | −0.167 | −0.043 | 0.284 † |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Castro, A.I.; Gomez-Arbelaez, D.; Crujeiras, A.B.; Granero, R.; Aguera, Z.; Jimenez-Murcia, S.; Sajoux, I.; Lopez-Jaramillo, P.; Fernandez-Aranda, F.; Casanueva, F.F. Effect of A Very Low-Calorie Ketogenic Diet on Food and Alcohol Cravings, Physical and Sexual Activity, Sleep Disturbances, and Quality of Life in Obese Patients. Nutrients 2018, 10, 1348. https://doi.org/10.3390/nu10101348
Castro AI, Gomez-Arbelaez D, Crujeiras AB, Granero R, Aguera Z, Jimenez-Murcia S, Sajoux I, Lopez-Jaramillo P, Fernandez-Aranda F, Casanueva FF. Effect of A Very Low-Calorie Ketogenic Diet on Food and Alcohol Cravings, Physical and Sexual Activity, Sleep Disturbances, and Quality of Life in Obese Patients. Nutrients. 2018; 10(10):1348. https://doi.org/10.3390/nu10101348
Chicago/Turabian StyleCastro, Ana I., Diego Gomez-Arbelaez, Ana B. Crujeiras, Roser Granero, Zaida Aguera, Susana Jimenez-Murcia, Ignacio Sajoux, Patricio Lopez-Jaramillo, Fernando Fernandez-Aranda, and Felipe F. Casanueva. 2018. "Effect of A Very Low-Calorie Ketogenic Diet on Food and Alcohol Cravings, Physical and Sexual Activity, Sleep Disturbances, and Quality of Life in Obese Patients" Nutrients 10, no. 10: 1348. https://doi.org/10.3390/nu10101348
APA StyleCastro, A. I., Gomez-Arbelaez, D., Crujeiras, A. B., Granero, R., Aguera, Z., Jimenez-Murcia, S., Sajoux, I., Lopez-Jaramillo, P., Fernandez-Aranda, F., & Casanueva, F. F. (2018). Effect of A Very Low-Calorie Ketogenic Diet on Food and Alcohol Cravings, Physical and Sexual Activity, Sleep Disturbances, and Quality of Life in Obese Patients. Nutrients, 10(10), 1348. https://doi.org/10.3390/nu10101348









