Monitoring Leaf Rust and Yellow Rust in Wheat with 3D LiDAR Sensing
Abstract
1. Introduction
2. Materials and Methods
2.1. Field Experiment Description
2.2. Collection of Crop Parameters Information
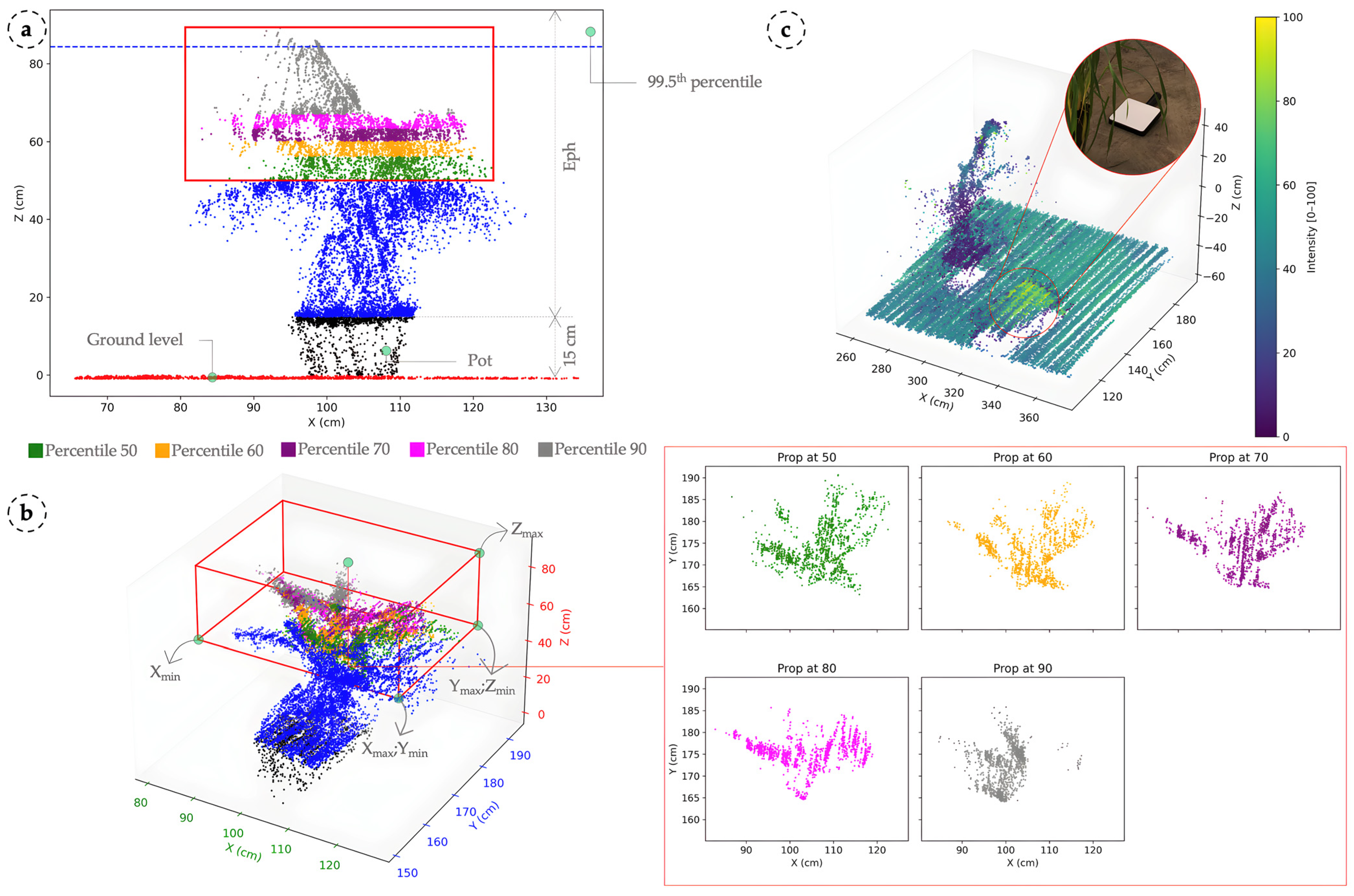
2.3. LiDAR Scanning of Wheat Plants for 3D Point Cloud Generation
2.4. LiDAR-Based Analysis and Crop Parameter Extraction
2.5. Statistical Analysis
3. Results
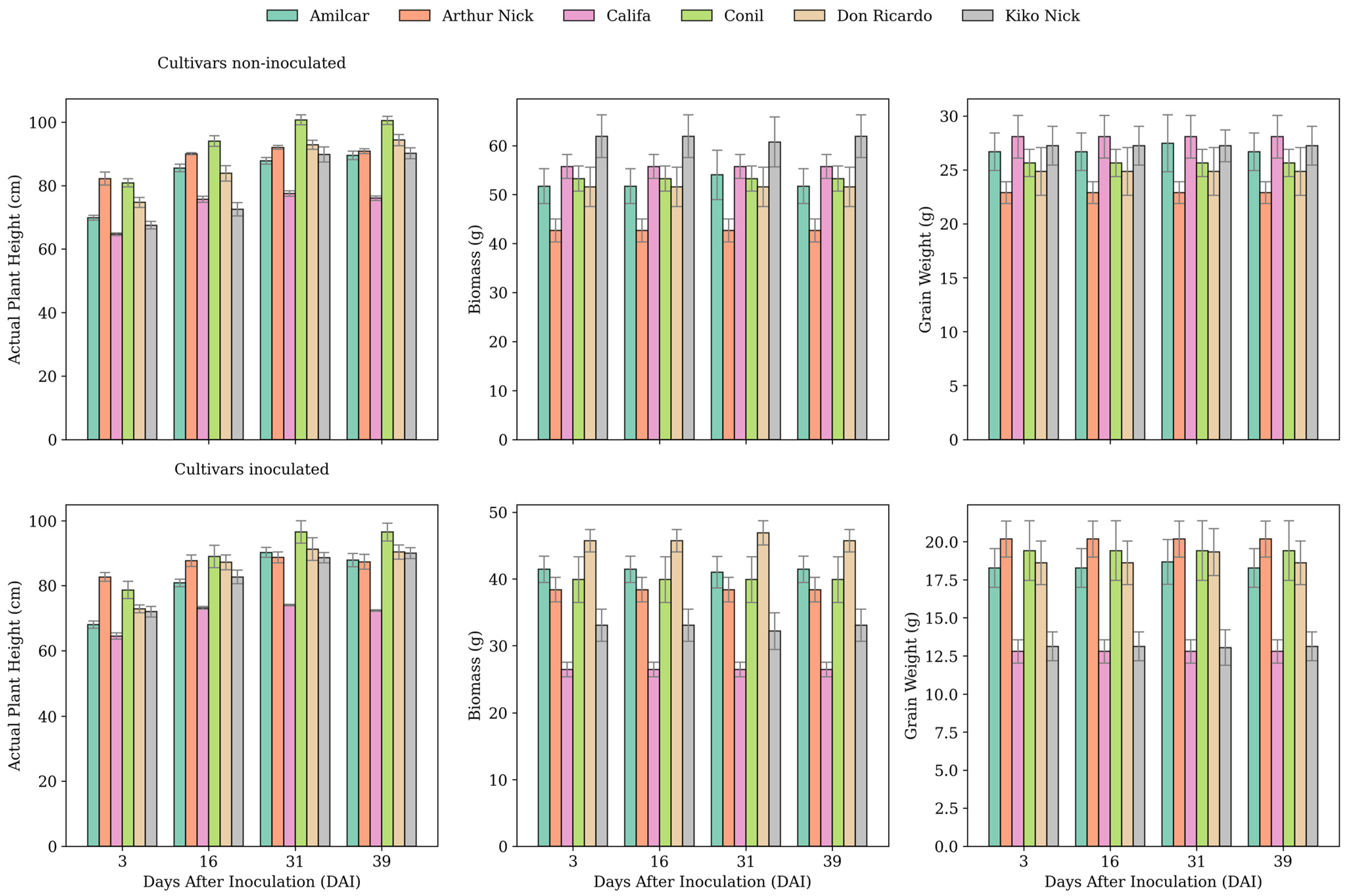
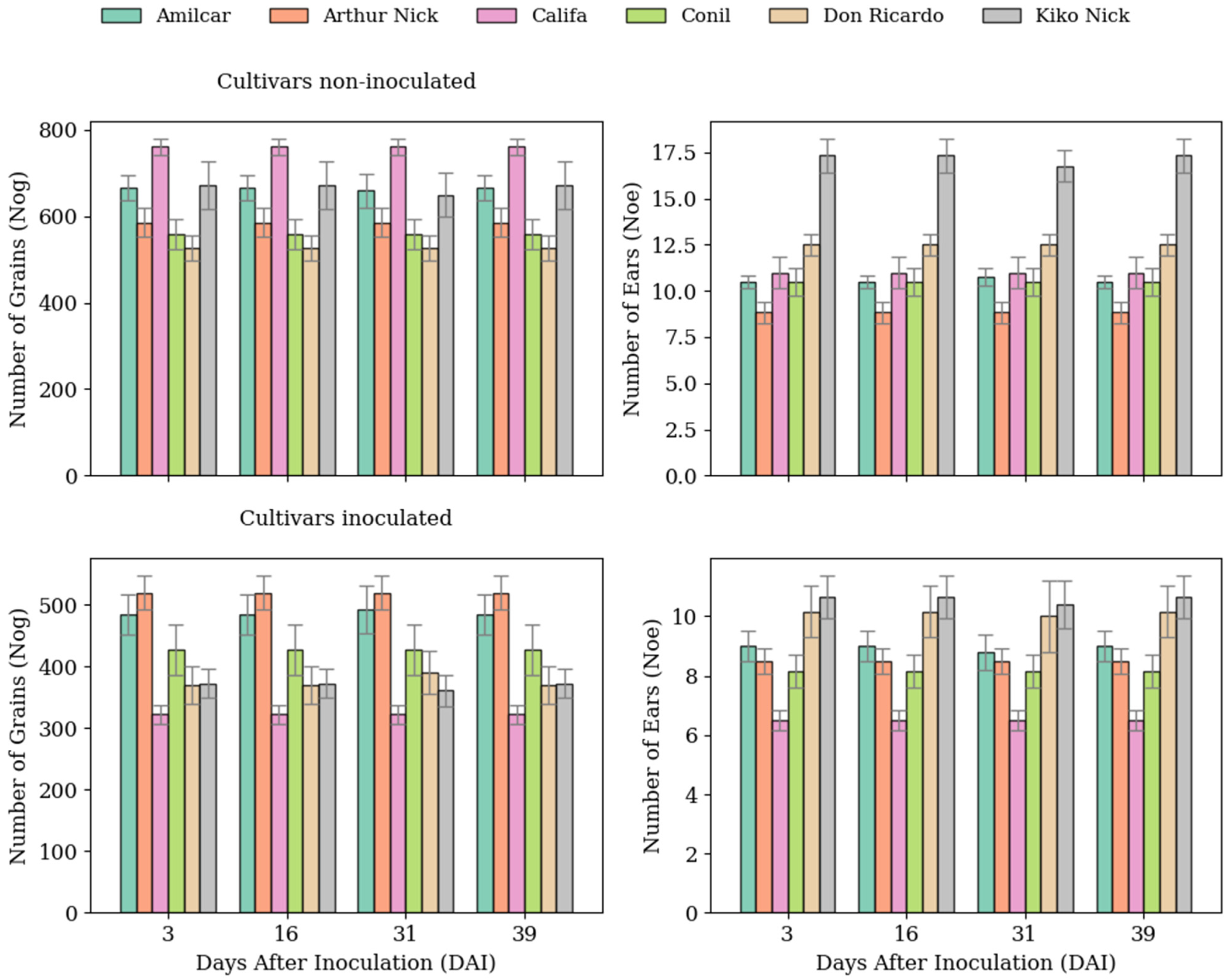
3.1. Ground-Truth Values Obtained Evolution
3.2. Correlations Between All the Crop Parameters Measured Manually and the Severity
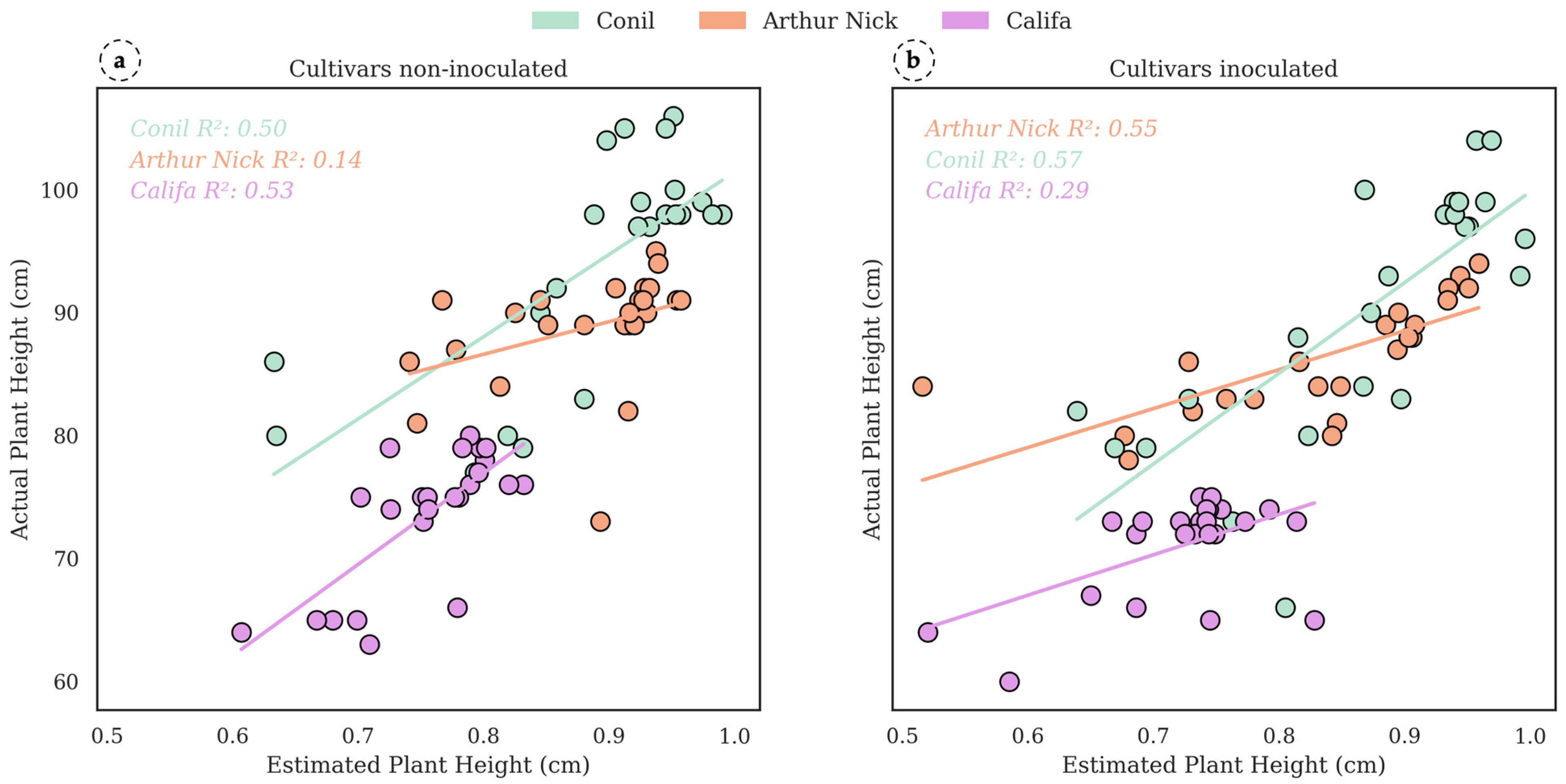
3.3. Estimation of Crop Parameters of Interest in Leaf Rust Detection
3.4. Severity Estimation
4. Discussion
4.1. LiDAR-Based Wheat Rust Severity Estimation Across Cultivars
4.2. Influence of Canopy Structure on LiDAR Reflectance Intensity
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Igrejas, G.; Ikeda, T.M.; Guzmán, C. Wheat Quality for Improving Processing and Human Health; Springer International Publishing: Berlin/Heidelberg, Germany, 2020; ISBN 9783030341633. [Google Scholar]
- Le Gouis, J.; Oury, F.X.; Charmet, G. How Changes in Climate and Agricultural Practices Influenced Wheat Production in Western Europe. J. Cereal Sci. 2020, 93, 102960. [Google Scholar] [CrossRef]
- FAO (Food and Agriculture Organization). Crop Prospects and Food Situation—Triannual Global Report No. 3, November 2023; FAO: Rome, Italy, 2023. [Google Scholar] [CrossRef]
- Choudhary, M.; Yadav, M.; Get, S.; Saran, R. Advanced Screening and Breeding Approaches for Heat Tolerance in Wheat. J. Pharmacogn. Phytochem. 2020, 9, 1047. [Google Scholar]
- Jasrotia, P.; Lal Kashyap, P.; Kumar Bhardwaj, A.; Kumar, S.; Pratap Singh, G.; Kashyap, P.; Bhardwaj, A.; Kumar, S.; Singh, G. Wheat and Barley Research Scope and Applications of Nanotechnology for Wheat Production: A Review of Recent Advances Article History Citation Scope and Applications of Nanotechnology for Wheat Production: A Review of Recent Advances. Wheat Barley Res. 2018, 10, 1–14. [Google Scholar] [CrossRef]
- Bahar, N.H.A.; Lo, M.; Sanjaya, M.; Van Vianen, J.; Alexander, P.; Ickowitz, A.; Sunderland, T. Meeting the Food Security Challenge for Nine Billion People in 2050: What Impact on Forests? In Global Environmental Change; Elsevier Ltd.: Amsterdam, The Netherlands, 2020; Volume 62. [Google Scholar] [CrossRef]
- De La Fuente, G.N.; Frei, U.K.; Lübberstedt, T. Accelerating Plant Breeding. Trends Plant Sci. 2013, 18, 667–672. [Google Scholar] [CrossRef] [PubMed]
- Ray, D.K.; Mueller, N.D.; West, P.C.; Foley, J.A. Yield Trends Are Insufficient to Double Global Crop Production by 2050. PLoS ONE 2013, 8, e66428. [Google Scholar] [CrossRef]
- Vergara-Diaz, O.; Kefauver, S.C.; Elazab, A.; Nieto-Taladriz, M.T.; Araus, J.L. Grain Yield Losses in Yellow-Rusted Durum Wheat Estimated Using Digital and Conventional Parameters under Field Conditions. Crop J. 2015, 3, 200–210. [Google Scholar] [CrossRef]
- Kolmer, J.A.; Hughes, M.E. Physiologic Specialization of Puccinia triticina on Wheat in the United States in 2016. Plant Dis. 2018, 102, 1066–1071. [Google Scholar] [CrossRef]
- Yuan, W.; Li, J.; Bhatta, M.; Shi, Y.; Baenziger, P.S.; Ge, Y. Wheat Height Estimation Using LiDAR in Comparison to Ultrasonic Sensor and UAS. Sensors 2018, 18, 3731. [Google Scholar] [CrossRef]
- Kumar, D.; Kukreja, V. Deep Learning in Wheat Diseases Classification: A Systematic Review. Multimed. Tools Appl. 2022, 81, 10143–10187. [Google Scholar] [CrossRef]
- Nigus, E.A.; Taye, G.B.; Girmaw, D.W.; Salau, A.O. Development of a Model for Detection and Grading of Stem Rust in Wheat Using Deep Learning. Multimed. Tools Appl. 2024, 83, 47649–47676. [Google Scholar] [CrossRef]
- Winterhalter, L.; Mistele, B.; Schmidhalter, U. Evaluation of Active and Passive Sensor Systems in the Field to Phenotype Maize Hybrids with High-Throughput. Field Crops Res. 2013, 154, 236–245. [Google Scholar] [CrossRef]
- Araus, J.L.; Cairns, J.E. Field High-Throughput Phenotyping: The New Crop Breeding Frontier. Trends Plant Sci. 2014, 19, 52–61. [Google Scholar] [CrossRef] [PubMed]
- Yao, Z.F.; He, D.J.; Lei, Y. Thermal Imaging for Early Nondestructive Detection of Wheat Stripe Rust. In Proceedings of the ASABE 2018 Annual International Meeting, Detroit, MI, USA, 29 July–1 August 2018; American Society of Agricultural and Biological Engineers: St. Joseph, MI, USA, 2018. [Google Scholar] [CrossRef]
- Montes, J.M.; Technow, F.; Dhillon, B.S.; Mauch, F.; Melchinger, A.E. High-Throughput Non-Destructive Biomass Determination during Early Plant Development in Maize under Field Conditions. Field Crops Res. 2011, 121, 268–273. [Google Scholar] [CrossRef]
- Jay, S.; Rabatel, G.; Hadoux, X.; Moura, D.; Gorretta, N. In-Field Crop Row Phenotyping from 3D Modeling Performed Using Structure from Motion. Struct. Motion. Comput. Electron. Agric. 2015, 110, 70–77. [Google Scholar] [CrossRef]
- Li, L.; Ma, Z.; Niinemets, Ü.; Guo, D. Three Key Sub-Leaf Modules and the Diversity of Leaf Designs. Front. Plant Sci. 2017, 8, e01542. [Google Scholar] [CrossRef] [PubMed]
- Holman, F.H.; Riche, A.B.; Michalski, A.; Castle, M.; Wooster, M.J.; Hawkesford, M.J. High Throughput Field Phenotyping of Wheat Plant Height and Growth Rate in Field Plot Trials Using UAV Based Remote Sensing. Remote Sens. 2016, 8, 1031. [Google Scholar] [CrossRef]
- Boogaard, F.P.; van Henten, E.J.; Kootstra, G. Boosting Plant-Part Segmentation of Cucumber Plants by Enriching Incomplete 3D Point Clouds with Spectral Data. Biosyst. Eng. 2021, 211, 167–182. [Google Scholar] [CrossRef]
- Rebetzke, G.J.; Jimenez-Berni, J.; Fischer, R.A.; Deery, D.M.; Smith, D.J. Review: High-Throughput Phenotyping to Enhance the Use of Crop Genetic Resources. Plant Sci. 2019, 282, 40–48. [Google Scholar] [CrossRef]
- Luo, S.; Wen, S.; Zhang, L.; Lan, Y.; Chen, X. Extraction of Crop Canopy Features and Decision-Making for Variable Spraying Based on Unmanned Aerial Vehicle LiDAR Data. Comput. Electron. Agric. 2024, 224, 109197. [Google Scholar] [CrossRef]
- Minhui, L.; Shamshiri, R.R.; Schirrmann, M.; Weltzien, C. Impact of Camera Viewing Angle for Estimating Leaf Parameters of Wheat Plants from 3d Point Clouds. Agriculture 2021, 11, 563. [Google Scholar] [CrossRef]
- Whetton, R.L.; Waine, T.W.; Mouazen, A.M. Evaluating Management Zone Maps for Variable Rate Fungicide Application and Selective Harvest. Comput. Electron. Agric. 2018, 153, 202–212. [Google Scholar] [CrossRef]
- Devadas, R.; Lamb, D.W.; Backhouse, D.; Simpfendorfer, S. Sequential Application of Hyperspectral Indices for Delineation of Stripe Rust Infection and Nitrogen Deficiency in Wheat. Precis. Agric. 2015, 16, 477–491. [Google Scholar] [CrossRef]
- Rodríguez-Moreno, V.M.; Jiménez-Lagunes, A.; Estrada-Avalos, J.; Mauricio-Ruvalcaba, J.E.; Padilla-Ramírez, J.S. Weather-Data-Based Model: An Approach for Forecasting Leaf and Stripe Rust on Winter Wheat. Meteorol. Appl. 2020, 27, e1896. [Google Scholar] [CrossRef]
- Gené-Mola, J.; Gregorio, E.; Guevara, J.; Auat, F.; Sanz-Cortiella, R.; Escolà, A.; Llorens, J.; Morros, J.R.; Ruiz-Hidalgo, J.; Vilaplana, V.; et al. Fruit Detection in an Apple Orchard Using a Mobile Terrestrial Laser Scanner. Biosyst. Eng. 2019, 187, 171–184. [Google Scholar] [CrossRef]
- Lu, J.; Wang, H.; Qin, S.; Cao, L.; Pu, R.; Li, G.; Sun, J. Estimation of Aboveground Biomass of Robinia Pseudoacacia Forest in the Yellow River Delta Based on UAV and Backpack LiDAR Point Clouds. Int. J. Appl. Earth Obs. Geoinf. 2020, 86, 102014. [Google Scholar] [CrossRef]
- Peterson, R.F.; Campbell, A.B.; Hannah, A.E. A Diagrammatic Scale for Estimating Rust Intensity on Leaves and Steins of Cereals. Can. J. Res. 1948, 26, 496–500. [Google Scholar] [CrossRef]
- Cassanelli, D.; Cattini, S.; Di Loro, G.; Di Cecilia, L.; Ferrari, L.; Rovati, L. Comparison of VLP-16 and MRS-1000 LiDAR Systems with Absolute Interferometer. In Proceedings of the 2021 IEEE International Workshop on Metrology for Automotive, MetroAutomotive 2021-Proceedings, Catania, Italy, 22–24 June 2021; Institute of Electrical and Electronics Engineers Inc.: Piscataway, NJ, USA, 2021; pp. 54–59. [Google Scholar] [CrossRef]
- Roelfs, A.P.; Singh, R.P.; Saari, E.E. Rust Diseases of Wheat: Concepts and Methods of Disease Management; CIMMYT, Ed.; Springer: Berlin/Heidelberg, Germany, 1992. [Google Scholar]
- Jimenez-Berni, J.A.; Deery, D.M.; Rozas-Larraondo, P.; Condon, A.T.G.; Rebetzke, G.J.; James, R.A.; Bovill, W.D.; Furbank, R.T.; Sirault, X.R.R. High Throughput Determination of Plant Height, Ground Cover, and above-Ground Biomass in Wheat with LiDAR. Front. Plant Sci. 2018, 9, 237. [Google Scholar] [CrossRef]
- Shakoor, N.; Lee, S.; Mockler, T.C. High Throughput Phenotyping to Accelerate Crop Breeding and Monitoring of Diseases in the Field. Curr. Opin. Plant Biol. 2017, 38, 184–192. [Google Scholar] [CrossRef]
- Zhang, J.; Huang, Y.; Pu, R.; Gonzalez-Moreno, P.; Yuan, L.; Wu, K.; Huang, W. Monitoring Plant Diseases and Pests through Remote Sensing Technology: A Review. Comput. Electron. Agric. 2019, 165, 104943. [Google Scholar] [CrossRef]
- Fahey, T.; Pham, H.; Gardi, A.; Sabatini, R.; Stefanelli, D.; Goodwin, I.; Lamb, D.W. Active and Passive Electro-Optical Sensors for Health Assessment in Food Crops. Sensors 2021, 21, 171. [Google Scholar] [CrossRef]
- Khaled, A.Y.; Abd Aziz, S.; Bejo, S.K.; Nawi, N.M.; Seman, I.A.; Onwude, D.I. Early Detection of Diseases in Plant Tissue Using Spectroscopy–Applications and Limitations. Appl. Spectrosc. Rev. 2018, 53, 36–64. [Google Scholar] [CrossRef]
- Azadbakht, M.; Ashourloo, D.; Aghighi, H.; Radiom, S.; Alimohammadi, A. Wheat Leaf Rust Detection at Canopy Scale under Different LAI Levels Using Machine Learning Techniques. Comput. Electron. Agric. 2019, 156, 119–128. [Google Scholar] [CrossRef]
- Oblinger, B.W.; Bright, B.C.; Hanavan, R.P.; Simpson, M.; Hudak, A.T.; Cook, B.D.; Corp, L.A. Identifying Conifer Mortality Induced by Armillaria Root Disease Using Airborne Lidar and Orthoimagery in South Central Oregon. For. Ecol. Manag. 2022, 511, 120126. [Google Scholar] [CrossRef]
- Paulus, S.; Mahlein, A.K. Technical Workflows for Hyperspectral Plant Image Assessment and Processing on the Greenhouse and Laboratory Scale. Gigascience 2020, 9, giaa090. [Google Scholar] [CrossRef]
- Khan, N.; Ray, R.L.; Sargani, G.R.; Ihtisham, M.; Khayyam, M.; Ismail, S. Current Progress and Future Prospects of Agriculture Technology: Gateway to Sustainable Agriculture. Sustainability 2021, 13, 4883. [Google Scholar] [CrossRef]
- Hu, P.; Huang, H.; Chen, Y.; Qi, J.; Li, W.; Jiang, C.; Wu, H.; Tian, W.; Hyyppä, J. Analyzing the Angle Effect of Leaf Reflectance Measured by Indoor Hyperspectral Light Detection and Ranging (LiDAR). Remote Sens. 2020, 12, 919. [Google Scholar] [CrossRef]
- Choudhury, M.R.; Das, S.; Christopher, J.; Apan, A.; Chapman, S.; Menzies, N.W.; Dang, Y.P. Improving Biomass and Grain Yield Prediction of Wheat Genotypes on Sodic Soil Using Integrated High-Resolution Multispectral, Hyperspectral, 3d Point Cloud, and Machine Learning Techniques. Remote Sens. 2021, 13, 3482. [Google Scholar] [CrossRef]
- Ni-Meister, W.; Yang, W.; Kiang, N.Y. A Clumped-Foliage Canopy Radiative Transfer Model for a Global Dynamic Terrestrial Ecosystem Model. I: Theory. Agric. For. Meteorol. 2010, 150, 881–894. [Google Scholar] [CrossRef]
- Husin, N.A.; Khairunniza-Bejo, S.; Abdullah, A.F.; Kassim, M.S.M.; Ahmad, D.; Azmi, A.N.N. Application of Ground-Based LiDAR for Analysing Oil Palm Canopy Properties on the Occurrence of Basal Stem Rot (BSR) Disease. Sci. Rep. 2020, 10, 4883. [Google Scholar] [CrossRef]
- Zhao, X.; Qi, J.; Xu, H.; Yu, Z.; Yuan, L.; Chen, Y.; Huang, H. Evaluating the Potential of Airborne Hyperspectral LiDAR for Assessing Forest Insects and Diseases with 3D Radiative Transfer Modeling. Remote Sens. Environ. 2023, 297, 113759. [Google Scholar] [CrossRef]
- Shafi, U.; Mumtaz, R.; Shafaq, Z.; Zaidi, S.M.H.; Kaifi, M.O.; Mahmood, Z.; Zaidi, S.A.R. Wheat Rust Disease Detection Techniques: A Technical Perspective. J. Plant Dis. Prot. 2022, 129, 489–504. [Google Scholar] [CrossRef]
- Zheng, Q.; Huang, W.; Cui, X.; Dong, Y.; Shi, Y.; Ma, H.; Liu, L. Identification of Wheat Yellow Rust Using Optimal Three-Band Spectral Indices in Different Growth Stages. Sensors 2019, 19, 35. [Google Scholar] [CrossRef] [PubMed]








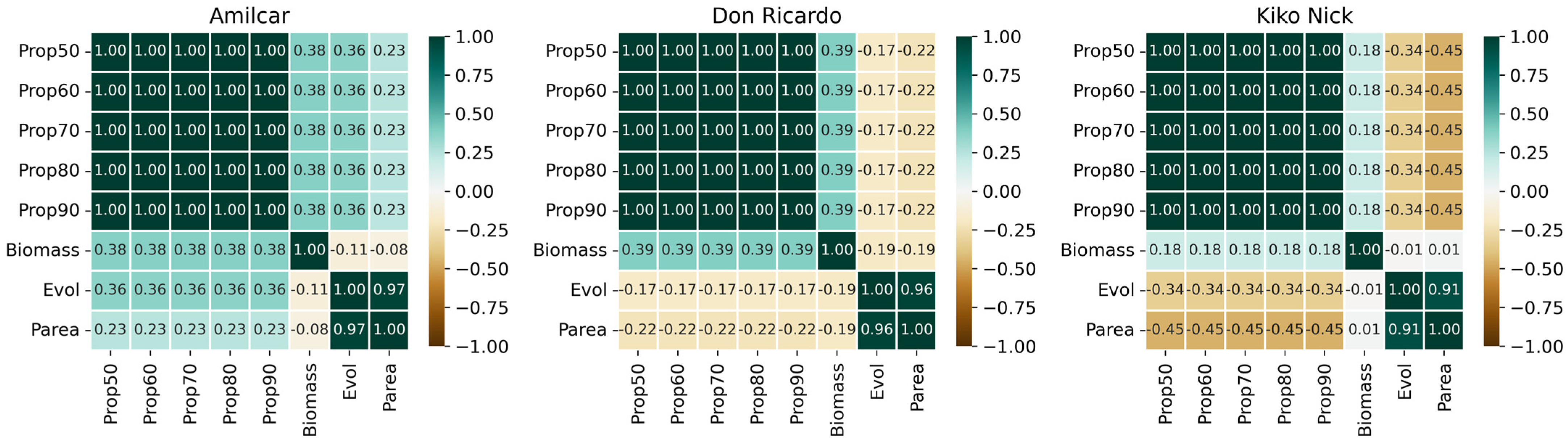
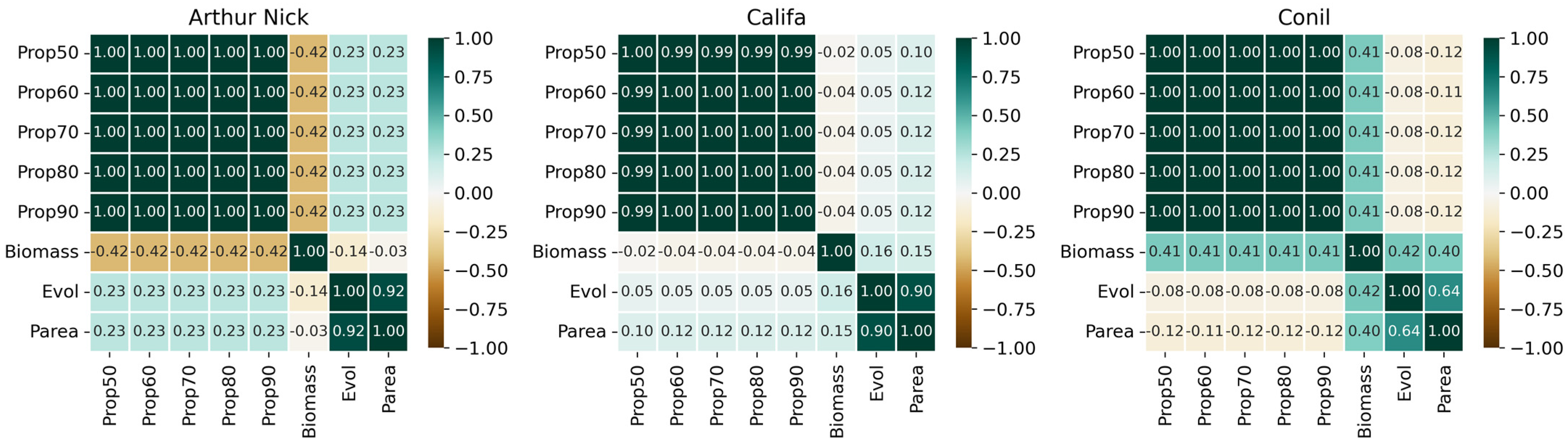
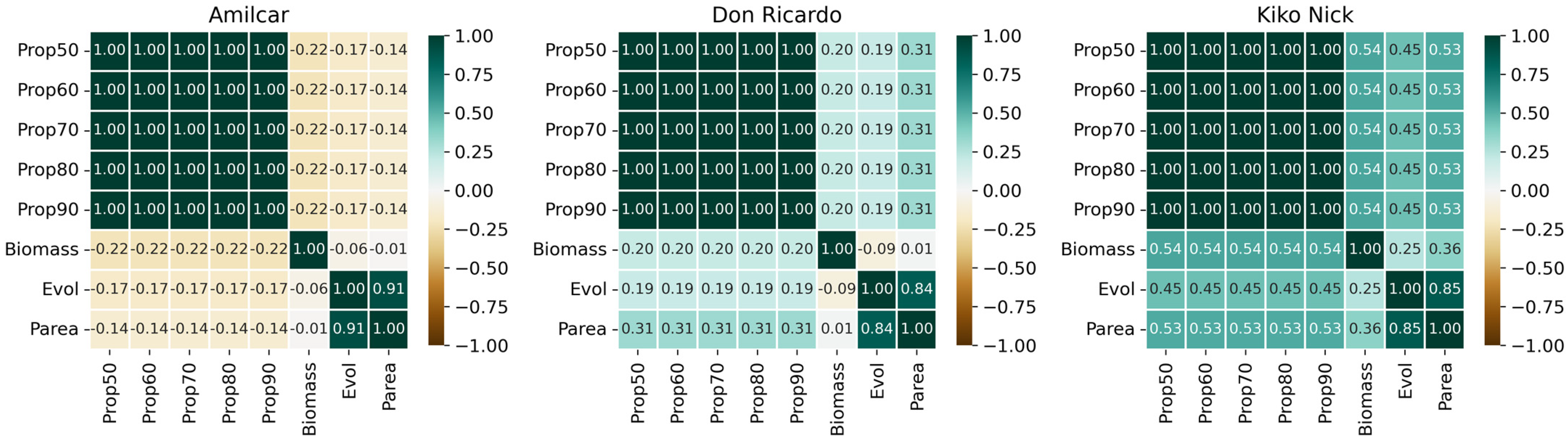

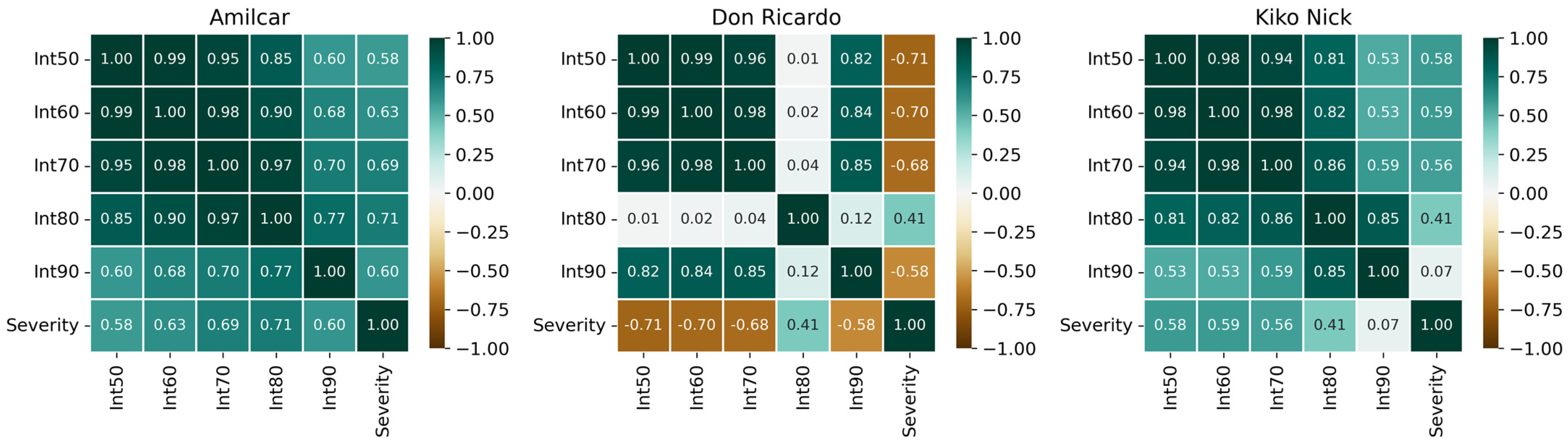


| Wheat Type | Cultivars | Biomass Influence | Aph Influence |
|---|---|---|---|
| Durum | ‘Don Ricardo’ | −0.2556 | 0.0118 |
| ‘Kiko Nick’ | −0.7509 | 2.2690 | |
| ‘Amilcar’ | −1.0070 | 1.1495 | |
| Bread | ‘Conil’ | −0.5753 | 1.0719 |
| ‘Arthur Nick’ | −1.1679 | 0.2611 | |
| ‘Califa’ | −2.5226 | −6.2537 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rodríguez-Vázquez, J.N.; Apolo-Apolo, O.E.; Martínez-Moreno, F.; Sánchez-Fernández, L.; Pérez-Ruiz, M. Monitoring Leaf Rust and Yellow Rust in Wheat with 3D LiDAR Sensing. Remote Sens. 2025, 17, 1005. https://doi.org/10.3390/rs17061005
Rodríguez-Vázquez JN, Apolo-Apolo OE, Martínez-Moreno F, Sánchez-Fernández L, Pérez-Ruiz M. Monitoring Leaf Rust and Yellow Rust in Wheat with 3D LiDAR Sensing. Remote Sensing. 2025; 17(6):1005. https://doi.org/10.3390/rs17061005
Chicago/Turabian StyleRodríguez-Vázquez, Jaime Nolasco, Orly Enrique Apolo-Apolo, Fernando Martínez-Moreno, Luis Sánchez-Fernández, and Manuel Pérez-Ruiz. 2025. "Monitoring Leaf Rust and Yellow Rust in Wheat with 3D LiDAR Sensing" Remote Sensing 17, no. 6: 1005. https://doi.org/10.3390/rs17061005
APA StyleRodríguez-Vázquez, J. N., Apolo-Apolo, O. E., Martínez-Moreno, F., Sánchez-Fernández, L., & Pérez-Ruiz, M. (2025). Monitoring Leaf Rust and Yellow Rust in Wheat with 3D LiDAR Sensing. Remote Sensing, 17(6), 1005. https://doi.org/10.3390/rs17061005









