PhenoCam Guidelines for Phenological Measurement and Analysis in an Agricultural Cropping Environment: A Case Study of Soybean
Abstract
1. Introduction
- Evaluate the variations in existing RGB-based CVIs and other novel combinations of alternative color space-based CVIs across image acquisition times (10:00 –14:00 at 30 min intervals for ≈140 days; 2018 growing season).
- Determine a set of best CVIs with a lower variation than the GCC curve and validate the CVI curve against the visually assessed soybean phenological stages.
- Assess the effect of image acquisition time on the selected CVIs within the PhenoCam’s field of view.
- Assess the effect of object position on the selected CVIs within the PhenoCam’s field of view.
2. Materials and Methods
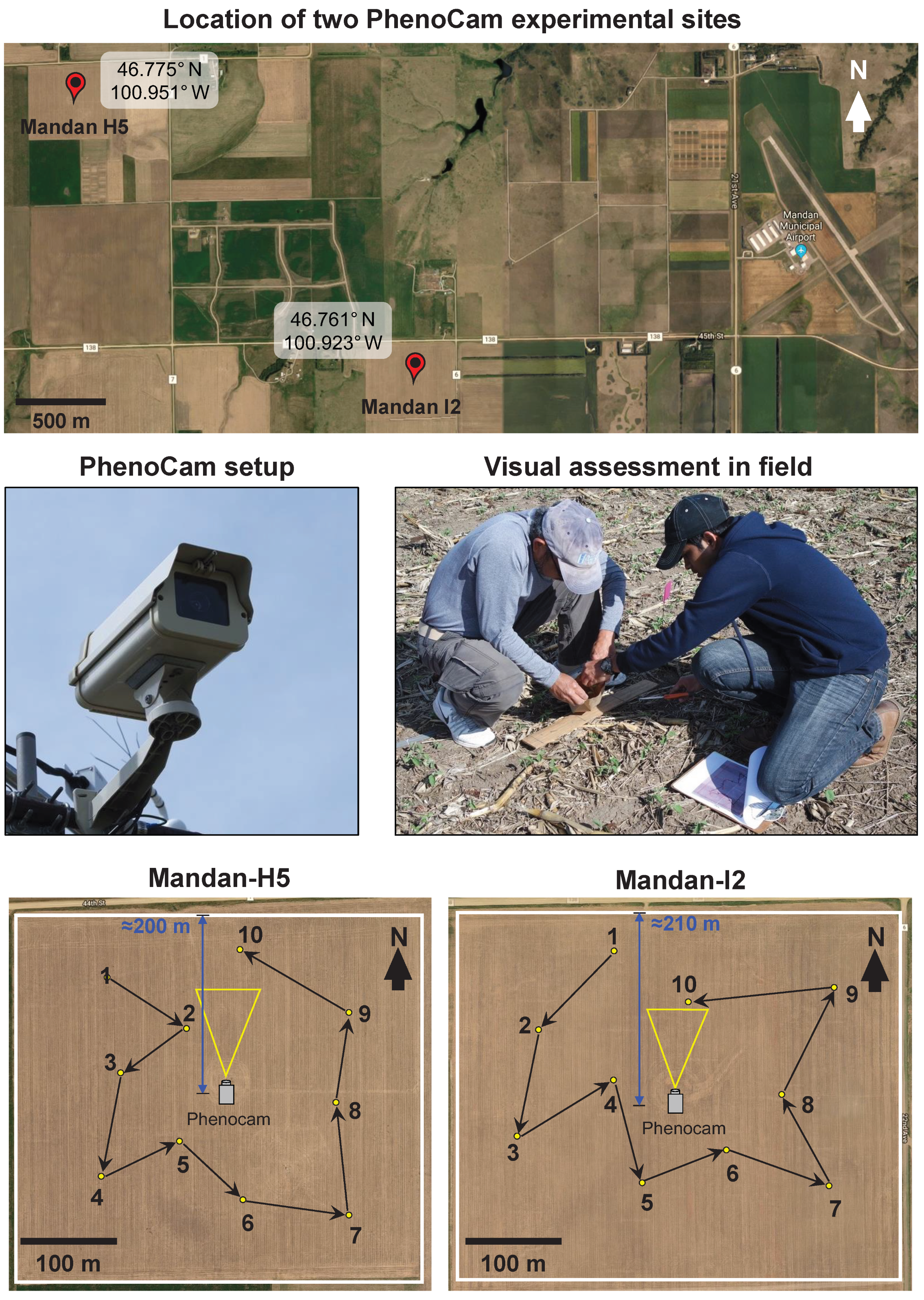
2.1. Site and Management Description
2.2. PhenoCam Setup and Image Analysis for Crop Phenology
2.2.1. PhenoCam Images and Data File Collection
2.2.2. Color Value Extraction from PhenoCam Images
2.2.3. Color Value Extraction from Multiple ROIs
2.3. Visual Assessment of Phenological Stages
2.4. CVIs for Phenological Analysis
2.4.1. CVIs from RGB Color Space
2.4.2. CVIs from Other Color Spaces
2.5. CVIs Analysis and Selection
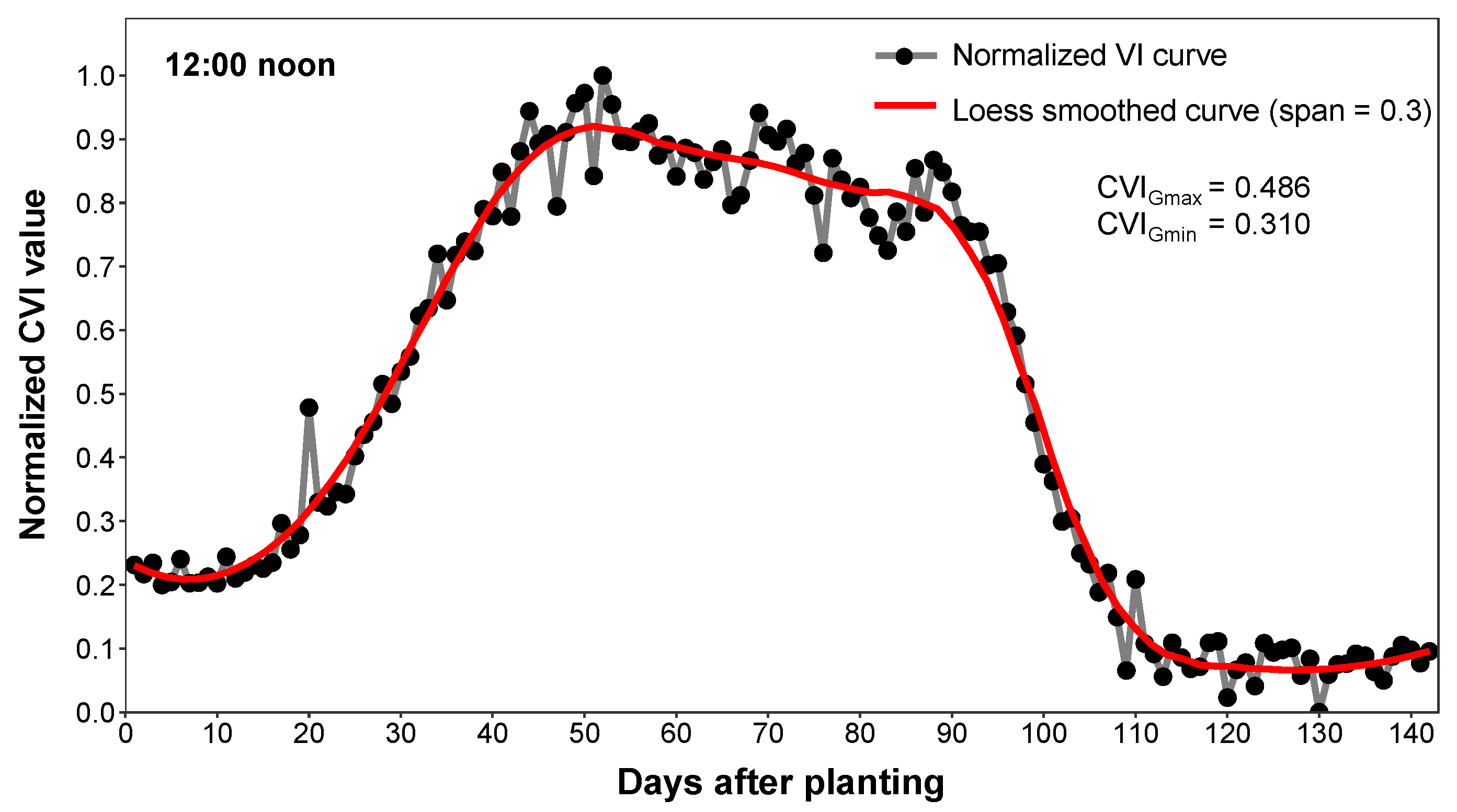
2.5.1. CVI Variation Comparison with Loess Smoothing
2.5.2. Selection of Best Set of CVIs
2.6. Statistical Analysis and Visualizations
3. Results and Discussion
3.1. Phenology CVI Curves and Visual Assessment
3.1.1. Comparison of GCC Curve with Visual Assessment
3.1.2. Phenological Curve Pattern of Other CVIs
3.2. Statistical Comparison of and Selection of the Best CVIs
3.3. Effect of Variables and Comparison of Selected CVIs
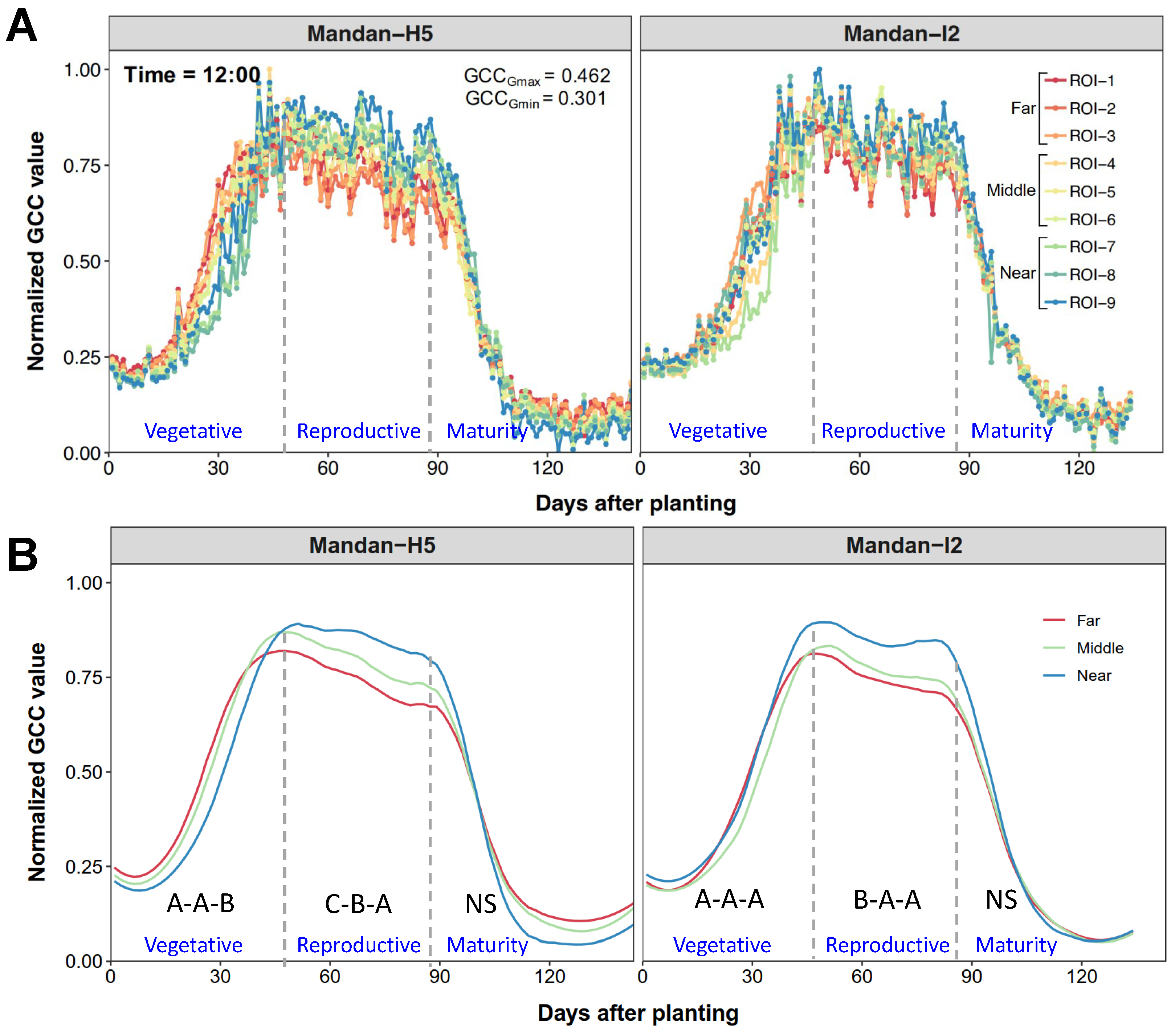
3.3.1. Effect of Image Acquisition Time on Selected CVIs
3.3.2. Effect of Object Position on Selected CVIs
3.3.3. Comparison of Among Selected CVIs Within Time Groups and Position
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Richardson, A.D.; Hufkens, K.; Milliman, T.; Aubrecht, D.M.; Chen, M.; Gray, J.M.; Johnston, M.R.; Keenan, T.F.; Klosterman, S.T.; Kosmala, M.; et al. Tracking vegetation phenology across diverse North American biomes using PhenoCam imagery. Sci. Data 2018, 5, 1–24. [Google Scholar] [CrossRef] [PubMed]
- Richardson, A.D. Tracking seasonal rhythms of plants in diverse ecosystems with digital camera imagery. New Phytol. 2019, 222, 1742–1750. [Google Scholar] [CrossRef] [PubMed]
- Brown, T.B.; Hultine, K.R.; Steltzer, H.; Denny, E.G.; Denslow, M.W.; Granados, J.; Henderson, S.; Moore, D.; Nagai, S.; SanClements, M.; et al. Using phenocams to monitor our changing Earth: Toward a global phenocam network. Front. Ecol. Environ. 2016, 14, 84–93. [Google Scholar] [CrossRef]
- Liu, Y.; Bachofen, C.; Wittwer, R.; Duarte, G.S.; Sun, Q.; Klaus, V.H.; Buchmann, N. Using PhenoCams to track crop phenology and explain the effects of different cropping systems on yield. Agric. Syst. 2022, 195, 103306. [Google Scholar] [CrossRef]
- Aasen, H.; Kirchgessner, N.; Walter, A.; Liebisch, F. PhenoCams for field phenotyping: Using very high temporal resolution digital repeated photography to investigate interactions of growth, phenology, and harvest traits. Front. Plant Sci. 2020, 11, 593. [Google Scholar] [CrossRef]
- Hill, A.C.; Vázquez-Lule, A.; Vargas, R. Linking vegetation spectral reflectance with ecosystem carbon phenology in a temperate salt marsh. Agric. For. Meteorol. 2021, 307, 108481. [Google Scholar] [CrossRef]
- Richardson, A.D. PhenoCam: An evolving, open-source tool to study the temporal and spatial variability of ecosystem-scale phenology. Agric. For. Meteorol. 2023, 342, 109751. [Google Scholar] [CrossRef]
- Guo, Y.; Chen, S.; Fu, Y.H.; Xiao, Y.; Wu, W.; Wang, H.; Beurs, K.d. Comparison of multi-methods for identifying maize phenology using phenocams. Remote Sens. 2022, 14, 244. [Google Scholar] [CrossRef]
- Perez, M. Utilizing Phenocam Imagery and Convolutional Neural Network for Plant Phenological State Prediction. Master’s Thesis, University of Twente, Enschede, The Netherlands, 2024. [Google Scholar]
- Cao, M.; Sun, Y.; Jiang, X.; Li, Z.; Xin, Q. Identifying leaf phenology of deciduous broadleaf forests from phenocam images using a convolutional neural network regression method. Remote Sens. 2021, 13, 2331. [Google Scholar] [CrossRef]
- Zhang, S.; Buttò, V.; Khare, S.; Deslauriers, A.; Morin, H.; Huang, J.G.; Ren, H.; Rossi, S. Calibrating PhenoCam data with phenological observations of a black spruce stand. Can. J. Remote Sens. 2020, 46, 154–165. [Google Scholar] [CrossRef]
- Tran, K.H.; Zhang, X.; Ketchpaw, A.R.; Wang, J.; Ye, Y.; Shen, Y. A novel algorithm for the generation of gap-free time series by fusing harmonized Landsat 8 and Sentinel-2 observations with PhenoCam time series for detecting land surface phenology. Remote Sens. Environ. 2022, 282, 113275. [Google Scholar] [CrossRef]
- Browning, D.; Karl, J.; Morin, D.; Richardson, A.; Tweedie, C. Phenocams bridge the gap between field and satellite observations in an arid grassland ecosystem. Remote Sens. 2017, 9, 1071. [Google Scholar] [CrossRef]
- Watson, C.J.; Restrepo-Coupe, N.; Huete, A.R. Multi-scale phenology of temperate grasslands: Improving monitoring and management with near-surface phenocams. Front. Environ. Sci. 2019, 7, 14. [Google Scholar] [CrossRef]
- Browning, D.M.; Snyder, K.A.; Herrick, J.E. Plant Phenology: Taking the Pulse of Rangelands. Rangelands 2019, 41, 129–134. [Google Scholar] [CrossRef]
- Toda, M.; Richardson, A.D. Estimation of plant area index and phenological transition dates from digital repeat photography and radiometric approaches in a hardwood forest in the Northeastern United States. Agric. For. Meteorol. 2018, 249, 457–466. [Google Scholar] [CrossRef]
- PhenoCam-Ag. PhenoCam Gallery—Agriculture Sites. 2024. Available online: https://phenocam.nau.edu/webcam/network/search/?sitename=&type=&primary_vegtype=AG&dominant_species=&active=unknown&fluxdata=unknown&group= (accessed on 8 February 2025).
- Richardson, A.D.; Hufkens, K.; Milliman, T.; Frolking, S. Intercomparison of phenological transition dates derived from the PhenoCam Dataset V1. 0 and MODIS satellite remote sensing. Sci. Rep. 2018, 8, 5679. [Google Scholar] [CrossRef]
- Zhang, X.; Jayavelu, S.; Liu, L.; Friedl, M.A.; Henebry, G.M.; Liu, Y.; Schaaf, C.B.; Richardson, A.D.; Gray, J. Evaluation of land surface phenology from VIIRS data using time series of PhenoCam imagery. Agric. For. Meteorol. 2018, 256, 137–149. [Google Scholar] [CrossRef]
- Enquist, C.A.; Kellermann, J.L.; Gerst, K.L.; Miller-Rushing, A.J. Phenology research for natural resource management in the United States. Int. J. Biometeorol. 2014, 58, 579–589. [Google Scholar] [CrossRef]
- Sun, Q. Plant Water Relations in Response to Drought and Different Cropping Systems. Ph.D. Thesis, ETH Zurich, Zurich, Switzerland, 2021. [Google Scholar]
- Mendicino, R.; Tritini, S.; Mejia-Aguilar, A.; Monsorno, R. Development of a Smart Irrigation System for Apple Fields using a LoRaWAN Network. In Proceedings of the 9th International Conference on Sensors and Electronic Instrumentation Advances (SEIA’ 2023), Funchal, Portugal, 20–22 September 2023; pp. 70–73. [Google Scholar]
- Richardson, A.D.; Jenkins, J.P.; Braswell, B.H.; Hollinger, D.Y.; Ollinger, S.V.; Smith, M.L. Use of digital webcam images to track spring green-up in a deciduous broadleaf forest. Oecologia 2007, 152, 323–334. [Google Scholar] [CrossRef]
- Xue, J.; Su, B. Significant remote sensing vegetation indices: A review of developments and applications. J. Sens. 2017, 2017, 1353691. [Google Scholar] [CrossRef]
- Torres-Sánchez, J.; Peña, J.M.; de Castro, A.I.; López-Granados, F. Multi-temporal mapping of the vegetation fraction in early-season wheat fields using images from UAV. Comput. Electron. Agric. 2014, 103, 104–113. [Google Scholar] [CrossRef]
- Meyer, G.; Neto, J.C. Verification of color vegetation indices for automated crop imaging applications. Comput. Electron. Agric. 2008, 63, 282–293. [Google Scholar] [CrossRef]
- Hatfield, J.L.; Prueger, J.H.; Sauer, T.J.; Dold, C.; O’brien, P.; Wacha, K. Applications of vegetative indices from remote sensing to agriculture: Past and future. Inventions 2019, 4, 71. [Google Scholar] [CrossRef]
- Radočaj, D.; Šiljeg, A.; Marinović, R.; Jurišić, M. State of major vegetation indices in precision agriculture studies indexed in web of science: A review. Agriculture 2023, 13, 707. [Google Scholar] [CrossRef]
- Barbedo, J.G.A. A review on the main challenges in automatic plant disease identification based on visible range images. Biosyst. Eng. 2016, 144, 52–60. [Google Scholar] [CrossRef]
- Sunoj, S.; Igathinathane, C.; Hendrickson, J. Monitoring Plant Phenology using Phenocam: A Review; ASABE Paper No: 162461829; ASABE: St. Joseph, MI, USA, 2016. [Google Scholar] [CrossRef]
- Andresen, C.G.; Tweedie, C.E.; Lougheed, V.L. Climate and nutrient effects on Arctic wetland plant phenology observed from phenocams. Remote Sens. Environ. 2018, 205, 46–55. [Google Scholar] [CrossRef]
- Sonnentag, O.; Hufkens, K.; Teshera-Sterne, C.; Young, A.; Friedl, M.; Braswell, B.H.; Milliman, T.; O’Keefe, J.; Richardson, A.D. Digital repeat photography for phenological research in forest ecosystems. Agric. For. Meteorol. 2012, 152, 159–177. [Google Scholar] [CrossRef]
- Linkosalmi, M.; Aurela, M.; Tuovinen, J.P.; Peltoniemi, M.; Tanis, C.M.; Arslan, A.N.; Kolari, P.; Böttcher, K.; Aalto, T.; Rainne, J.; et al. Digital photography for assessing the link between vegetation phenology and CO2 exchange in two contrasting northern ecosystems. Geosci. Instrum. Method. Data Syst. 2016, 5, 417–426. [Google Scholar] [CrossRef]
- Koebsch, F.; Sonnentag, O.; Järveoja, J.; Peltoniemi, M.; Alekseychik, P.; Aurela, M.; Arslan, A.N.; Dinsmore, K.; Gianelle, D.; Helfter, C.; et al. Refining the role of phenology in regulating gross ecosystem productivity across European peatlands. Glob. Chang. Biol. 2020, 26, 876–887. [Google Scholar] [CrossRef]
- Richardson, A.D.; Braswell, B.H.; Hollinger, D.Y.; Jenkins, J.P.; Ollinger, S.V. Near-surface remote sensing of spatial and temporal variation in canopy phenology. Ecol. Appl. 2009, 19, 1417–1428. [Google Scholar] [CrossRef]
- Richardson, A.D.; Seyednasrollah, B.; Hufkens, K.; Milliman, T. PhenoCam Installation Instructions (Revised August 2022). 2022. Available online: https://phenocam.nau.edu/pdf/PhenoCam_Install_Instructions.pdf (accessed on 8 February 2025).
- Seyednasrollah, B.; Young, A.M.; Hufkens, K.; Milliman, T.; Friedl, M.A.; Frolking, S.; Richardson, A.D. Tracking vegetation phenology across diverse biomes using Version 2.0 of the PhenoCam Dataset. Sci. Data 2019, 6, 222. [Google Scholar] [CrossRef] [PubMed]
- R Core Team. R: A Language and Environment for Statistical Computing. 2019. Available online: http://www.R-project.org/ (accessed on 8 February 2025).
- RStudio Team. RStudio: Integrated Development Environment for R. Ver. 1.2.1335; RStudio, Inc.: Boston, MA, USA, 2018. [Google Scholar]
- Seyednasrollah, B.; Young, A.M.; Hufkens, K.; Milliman, T.; Friedl, M.A.; Frolking, S.; Richardson, A.D.; Abraha, M.; Allen, D.W.; Apple, M.; et al. PhenoCam Dataset v2.0: Vegetation Phenology from Digital Camera Imagery, 2000–2018. 2019. Available online: https://daac.ornl.gov/cgi-bin/dsviewer.pl?ds_id=1674 (accessed on 8 February 2025).
- Seyednasrollah, B.; Milliman, T.; Richardson, A.D. Data extraction from digital repeat photography using xROI: An interactive framework to facilitate the process. ISPRS J. Photogramm. Remote Sens. 2019, 152, 132–144. [Google Scholar] [CrossRef]
- Rasband, W. ImageJ. US National Institutes of Health: Bethesda, MD, USA, 2018. Available online: https://imagej.net/ij/ (accessed on 8 February 2025).
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An open-source platform for biological-image analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef] [PubMed]
- Endres, G.; Kandel, H. Soybean Growth and Management Quick Guide. 2021. Available online: https://www.ndsu.edu/agriculture/sites/default/files/2021-11/a1174.pdf (accessed on 8 February 2025).
- Hassanijalilian, O.; Igathinathane, C.; Doetkott, C.; Bajwa, S.; Nowatzki, J.; Esmaeili, S.A.H. Chlorophyll estimation in soybean leaves infield with smartphone digital imaging and machine learning. Comput. Electron. Agric. 2020, 174, 105433. [Google Scholar] [CrossRef]
- Evstatiev, B.; Mladenova, T.; Valov, N.; Zhelyazkova, T.; Gerdzhikova, M.; Todorova, M.; Grozeva, N.; Sevov, A.; Stanchev, G. Fast Pasture Classification Method using Ground-based Camera and the Modified Green Red Vegetation Index (MGRVI). Int. J. Adv. Comput. Sci. Appl. 2023, 14, 45–51. [Google Scholar] [CrossRef]
- Roth, R.T.; Chen, K.; Scott, J.R.; Jung, J.; Yang, Y.; Camberato, J.J.; Armstrong, S.D. Prediction of Cereal Rye Cover Crop Biomass and Nutrient Accumulation Using Multi-Temporal Unmanned Aerial Vehicle Based Visible-Spectrum Vegetation Indices. Remote Sens. 2023, 15, 580. [Google Scholar] [CrossRef]
- Ribeiro, A.L.A.; Maciel, G.M.; Siquieroli, A.C.S.; Luz, J.M.Q.; Gallis, R.B.d.A.; Assis, P.H.d.S.; Catão, H.C.R.M.; Yada, R.Y. Vegetation Indices for Predicting the Growth and Harvest Rate of Lettuce. Agriculture 2023, 13, 1091. [Google Scholar] [CrossRef]
- Sarkar, S.; Ramsey, A.F.; Cazenave, A.B.; Balota, M. Peanut leaf wilting estimation from RGB color indices and logistic models. Front. Plant Sci. 2021, 12, 658621. [Google Scholar] [CrossRef]
- Woebbecke, D.M.; Meyer, G.E.; Von Bargen, K.; Mortensen, D. Color indices for weed identification under various soil, residue, and lighting conditions. Trans. ASAE 1995, 38, 259–269. [Google Scholar] [CrossRef]
- Kataoka, T.; Kaneko, T.; Okamoto, H.; Hata, S. Crop growth estimation system using machine vision. In Proceedings of the 2003 IEEE/ASME International Conference on Advanced Intelligent Mechatronics (AIM 2003), Kobe, Japan, 20–24 July 2003; IEEE: Piscataway, NJ, USA, 2003; Volume 2, pp. b1079–b1083. [Google Scholar]
- Hague, T.; Tillett, N.D.; Wheeler, H. Automated crop and weed monitoring in widely spaced cereals. Precis. Agric. 2006, 7, 21–32. [Google Scholar] [CrossRef]
- Crimmins, M.A.; Crimmins, T.M. Monitoring plant phenology using digital repeat photography. Environ. Manag. 2008, 41, 949–958. [Google Scholar] [CrossRef] [PubMed]
- Shajahan, S. Agricultural Field Applications of Digital Image Processing Using an Open Source ImageJ Platform. Ph.D. Thesis, North Dakota State University, Fargo, ND, USA, 2019. [Google Scholar]
- Karcher, D.E.; Richardson, M.D. Quantifying turfgrass color using digital image analysis. Crop Sci. 2003, 43, 943–951. [Google Scholar] [CrossRef]
- Raper, T.B.; Oosterhuis, D.M.; Siddons, U.; Purcell, L.C.; Mozaffari, M. Effectiveness of the dark green color index in determining cotton nitrogen status from multiple camera angles. Int. J. Appl. Sci. Technol. 2012, 2, 71–74. [Google Scholar]
- Cleveland, W.S. Robust locally weighted regression and smoothing scatterplots. J. Am. Stat. Assoc. 1979, 74, 829–836. [Google Scholar] [CrossRef]
- Sunoj, S.; Igathinathane, C.; Jenicka, S. Cashews whole and splits classification using a novel machine vision approach. Postharvest Biol. Technol. 2018, 138, 19–30. [Google Scholar] [CrossRef]
- de Mendiburu, F. Agricolae: Statistical Procedures for Agricultural Research (Version 1.3-1). 2019. Available online: http://CRAN.R-project.org/package=agricolae (accessed on 8 February 2025).
- Hufkens, K.; Friedl, M.; Sonnentag, O.; Braswell, B.H.; Milliman, T.; Richardson, A.D. Linking near-surface and satellite remote sensing measurements of deciduous broadleaf forest phenology. Remote Sens. Environ. 2012, 117, 307–321. [Google Scholar] [CrossRef]
- Toomey, M.; Friedl, M.A.; Frolking, S.; Hufkens, K.; Klosterman, S.; Sonnentag, O.; Baldocchi, D.D.; Bernacchi, C.J.; Biraud, S.C.; Bohrer, G.; et al. Greenness indices from digital cameras predict the timing and seasonal dynamics of canopy-scale photosynthesis. Ecol. Appl. 2015, 25, 99–115. [Google Scholar] [CrossRef]
- Keenan, T.; Darby, B.; Felts, E.; Sonnentag, O.; Friedl, M.; Hufkens, K.; O’Keefe, J.; Klosterman, S.; Munger, J.W.; Toomey, M.; et al. Tracking forest phenology and seasonal physiology using digital repeat photography: A critical assessment. Ecol. Appl. 2014, 24, 1478–1489. [Google Scholar] [CrossRef]
- Wickham, H. ggplot2: Elegant Graphics for Data Analysis; Springer: New York, NY, USA, 2016; Available online: https://ggplot2.tidyverse.org (accessed on 8 February 2025).
- PhenoCam-Closeup. PhenoCam Close-Up Site—Willamettepoplar. 2019. Available online: https://phenocam.nau.edu/webcam/sites/willamettepoplar/ (accessed on 8 February 2025).
- Kyaw, T.; Ferguson, R.B.; Adamchuk, V.I.; Marx, D.; Tarkalson, D.; McCallister, D. Delineating site-specific management zones for pH-induced iron chlorosis. Precis. Agric. 2008, 9, 71–84. [Google Scholar] [CrossRef]
- Bai, G.; Jenkins, S.; Yuan, W.; Graef, G.L.; Ge, Y. Field-based scoring of soybean iron deficiency chlorosis using RGB imaging and statistical learning. Front. Plant Sci. 2018, 9, 1002. [Google Scholar] [CrossRef]
- Dobbels, A.A.; Lorenz, A.J. Soybean iron deficiency chlorosis high throughput phenotyping using an unmanned aircraft system. Plant Methods 2019, 15, 97. [Google Scholar] [CrossRef] [PubMed]
- Klosterman, S.; Hufkens, K.; Gray, J.; Melaas, E.; Sonnentag, O.; Lavine, I.; Mitchell, L.; Norman, R.; Friedl, M.; Richardson, A. Evaluating remote sensing of deciduous forest phenology at multiple spatial scales using PhenoCam imagery. Biogeosci. Discuss. 2014, 11, 4305–4320. [Google Scholar] [CrossRef]
- Kazmi, W.; Garcia-Ruiz, F.J.; Nielsen, J.; Rasmussen, J.; Andersen, H.J. Detecting creeping thistle in sugar beet fields using vegetation indices. Comput. Electron. Agric. 2015, 112, 10–19. [Google Scholar] [CrossRef]
- Sunoj, S.; Igathinathane, C.; Saliendra, N.; Hendrickson, J.; Archer, D. Color calibration of digital images for agriculture and other applications. ISPRS J. Photogramm. Remote Sens. 2018, 146, 221–234. [Google Scholar] [CrossRef]
- Richardson, A.D.; Klosterman, S.; Toomey, M. Near-surface sensor-derived phenology. In Phenology: An Integrative Environmental Science; Springer: Berlin/Heidelberg, Germany, 2013; pp. 413–430. [Google Scholar] [CrossRef]
- Yan, D.; Scott, R.L.; Moore, D.J.P.; Biederman, J.A.; Smith, W.K. Understanding the relationship between vegetation greenness and productivity across dryland ecosystems through the integration of PhenoCam, satellite, and eddy covariance data. Remote Sens. Environ. 2019, 223, 50–62. [Google Scholar] [CrossRef]
- Snyder, K.A.; Huntington, J.L.; Wehan, B.L.; Morton, C.G.; Stringham, T.K. Comparison of Landsat and Land-Based Phenology Camera Normalized Difference Vegetation Index (NDVI) for Dominant Plant Communities in the Great Basin. Sensors 2019, 19, 1139. [Google Scholar] [CrossRef]




| CVI | Physiological Meaning | References |
|---|---|---|
| GCC | Represents the relative proportion of green reflectance in total visible reflectance, directly correlating with chlorophyll content and photosynthetic activity. It is widely used in PhenoCam networks for its simplicity and robustness across illumination conditions. | [18,32] |
| NGRDI | Normalizes the difference between green and red reflectance, making it sensitive to chlorophyll content and leaf structure changes. It is particularly effective in detecting early-season growth and senescence phases in crops. | [25,26] |
| NGBDI | Captures the relationship between green pigments and structural components represented by blue reflectance. It is used to distinguish vegetation from soil background, especially in the early growth stages. | [27] |
| MGRVI | A modified index that uses squared values to enhance sensitivity to green vegetation changes while reducing atmospheric effects. It is more responsive to subtle changes in canopy greenness than simple ratios. | [46] |
| RGBVI | Combines all three color channels to enhance vegetation detection, particularly effective in discriminating between vegetation and non-vegetation features under varying light conditions. | [47] |
| NDI | A scaled version of NGRDI that maintains the same physiological sensitivity while providing values in a more intuitive range (0–255), facilitating comparison across different imaging conditions. | [26] |
| GLI | Emphasizes green vegetation by incorporating all three channels with double weighting for green reflectance. It is effective in detecting subtle changes in canopy greenness during key growth stages. | [48] |
| RGRI | Simple ratio highlighting the inverse relationship between red and green reflectance, sensitive to chlorophyll degradation during senescence. | [49] |
| EXG | Emphasizes excess green coloration by double weighting for the green component, making it particularly suitable for detecting healthy vegetation against soil backgrounds. | [50] |
| CIVE | A linear combination optimized for vegetation extraction, with coefficients derived from a statistical analysis of vegetation spectra. Effective in separating vegetation from the background under various illumination conditions. | [25,51] |
| VEG | A non-linear index developed to be less sensitive to illumination changes while maintaining sensitivity to vegetation changes. The parameter “a” helps optimize the index for specific vegetation types. | [52] |
| EXGR | Combines excess green and red information to improve vegetation detection, particularly useful in distinguishing vegetation during different phenological stages. | [26] |
| Represents pure color information independent of brightness and saturation, making it robust against illumination changes. Particularly useful for tracking seasonal color changes. | [53] | |
| Utilizes the green–red opponent color axis from the Lab color space, providing a perceptually uniform measure of vegetation greenness that correlates well with human visual assessment. | [54] | |
| DGCI | Combines hue, saturation, and brightness information to provide a comprehensive measure of “greenness” that correlates well with nitrogen status and overall plant health. | [45] |
| Vegetation Index | Curve Length Deviation from Smoothed Curve, () | Cumulative Rank | Overall Rank | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 10:00 | 10:30 | 11:00 | 11:30 | 12:00 | 12:30 | 13:00 | 13:30 | 14:00 | |||
| Mandan-H5 | |||||||||||
| EXGR | (2) | (3) | (3) | (1) | (1) | (1) | (1) | (1) | (3) | 16 | 1 |
| CIVE | (1) | (1) | (5) | (4) | (3) | (2) | (5) | (7) | (8) | 36 | 2 |
| GLI | (2) | (1) | (6) | (3) | (2) | (3) | (5) | (8) | (9) | 39 | 3 |
| (7) | (7) | (4) | (6) | (8) | (8) | (4) | (4) | (4) | 52 | 4 | |
| RGRI | (6) | (13) | (8) | (8) | (6) | (6) | (2) | (4) | (2) | 55 | 5 |
| (4) | (4) | (7) | (5) | (5) | (4) | (9) | (9) | (10) | 57 | 6 | |
| NGBDI | (10) | (12) | (10) | (10) | (4) | (7) | (3) | (3) | (1) | 60 | 7 |
| MGRVI | (9) | (6) | (2) | (7) | (9) | (9) | (7) | (6) | (5) | 60 | 7 |
| VEG | (5) | (5) | (10) | (9) | (11) | (5) | (11) | (11) | (11) | 78 | 10 |
| RGBVI | (11) | (8) | (12) | (12) | (12) | (11) | (12) | (12) | (13) | 103 | 12 |
| (12) | (9) | (1) | (1) | (9) | (12) | (10) | (2) | (6) | 62 | 9 | |
| (8) | (10) | (9) | (11) | (7) | (10) | (8) | (10) | (7) | 80 | 11 | |
| DGCI | (13) | (11) | (13) | (13) | (13) | (13) | (13) | (13) | (12) | 114 | 13 |
| Mandan-I2 | |||||||||||
| EXGR | (1) | (4) | (4) | (2) | (4) | (3) | (4) | (3) | (3) | 28 | 2 |
| CIVE | (4) | (2) | (6) | (4) | (8) | (2) | (7) | (4) | (2) | 39 | 3 |
| GLI | (2) | (1) | (5) | (2) | (7) | (1) | (6) | (2) | (1) | 27 | 1 |
| (5) | (6) | (2) | (7) | (3) | (6) | (2) | (5) | (5) | 41 | 4 | |
| RGRI | (3) | (8) | (8) | (8) | (5) | (8) | (3) | (8) | (6) | 57 | 8 |
| (6) | (3) | (7) | (6) | (9) | (4) | (8) | (7) | (3) | 53 | 6 | |
| NGBDI | (8) | (12) | (11) | (9) | (10) | (10) | (5) | (9) | (8) | 82 | 10 |
| MGRVI | (7) | (5) | (4) | (5) | (2) | (7) | (1) | (6) | (7) | 44 | 5 |
| VEG | (10) | (7) | (10) | (10) | (12) | (11) | (13) | (11) | (11) | 95 | 11 |
| RGBVI | (11) | (11) | (13) | (13) | (13) | (12) | (12) | (12) | (12) | 109 | 12 |
| (12) | (9) | (1) | (1) | (1) | (9) | (9) | (1) | (10) | 53 | 6 | |
| (9) | (10) | (9) | (11) | (7) | (5) | (10) | (10) | (9) | 80 | 9 | |
| DGCI | (13) | (13) | (11) | (12) | (11) | (13) | (11) | (13) | (13) | 110 | 13 |
| Image Acquisition Time | Object Position | ||||||
|---|---|---|---|---|---|---|---|
| Vegetation Index | Growth Phases | Start | Midday | End | Far | Middle | Near |
| (10:00–11:00) | (11:30–12:30) | (13:00–14:00) | (ROI-1–ROI-3) | (ROI-4–ROI-6) | (ROI-7–ROI-9) | ||
| Mandan-H5 | |||||||
| EXGR | Vegetative | a | a | a | A | A | B |
| Reproductive | a | a | a | C | B | A | |
| Maturity | a | a | a | C | B | A | |
| Overall | a | a | a | C | B | A | |
| CIVE | Vegetative | a | a | a | C | B | A |
| Reproductive | a | a | a | A | B | A | |
| Maturity | a | a | a | C | B | A | |
| Overall | a | a | a | B | B | A | |
| GLI | Vegetative | a | a | a | A | A | B |
| Reproductive | a | a | a | C | B | A | |
| Maturity | a | a | a | A | A | A | |
| Overall | a | a | a | A | A | A | |
| Vegetative | a | a | a | A | B | C | |
| Reproductive | a | a | a | B | A | A | |
| Maturity | a | a | a | B | B | A | |
| Overall | a | a | a | B | B | A | |
| Vegetative | a | a | a | A | A | B | |
| Reproductive | a | a | a | C | B | A | |
| Maturity | a | a | a | A | A | A | |
| Overall | a | a | a | A | A | A | |
| Mandan-I2 | |||||||
| EXGR | Vegetative | a | a | a | A | A | B |
| Reproductive | a | a | a | B | B | A | |
| Maturity | a | a | a | C | B | A | |
| Overall | a | a | a | B | B | A | |
| CIVE | Vegetative | a | a | a | B | AB | A |
| Reproductive | a | a | a | A | B | A | |
| Maturity | a | a | a | B | AB | A | |
| Overall | a | a | a | B | B | A | |
| GLI | Vegetative | a | a | a | A | A | A |
| Reproductive | a | a | a | B | A | A | |
| Maturity | a | a | a | A | A | A | |
| Overall | a | a | a | A | A | A | |
| Vegetative | a | a | a | A | A | A | |
| Reproductive | a | a | a | A | B | B | |
| Maturity | a | a | a | A | AB | B | |
| Overall | a | a | a | B | B | A | |
| Vegetative | a | a | a | A | A | A | |
| Reproductive | a | a | a | B | A | A | |
| Maturity | a | a | a | A | A | A | |
| Overall | a | a | a | A | A | A | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sunoj, S.; Igathinathane, C.; Saliendra, N.; Hendrickson, J.; Archer, D.; Liebig, M. PhenoCam Guidelines for Phenological Measurement and Analysis in an Agricultural Cropping Environment: A Case Study of Soybean. Remote Sens. 2025, 17, 724. https://doi.org/10.3390/rs17040724
Sunoj S, Igathinathane C, Saliendra N, Hendrickson J, Archer D, Liebig M. PhenoCam Guidelines for Phenological Measurement and Analysis in an Agricultural Cropping Environment: A Case Study of Soybean. Remote Sensing. 2025; 17(4):724. https://doi.org/10.3390/rs17040724
Chicago/Turabian StyleSunoj, S., C. Igathinathane, Nicanor Saliendra, John Hendrickson, David Archer, and Mark Liebig. 2025. "PhenoCam Guidelines for Phenological Measurement and Analysis in an Agricultural Cropping Environment: A Case Study of Soybean" Remote Sensing 17, no. 4: 724. https://doi.org/10.3390/rs17040724
APA StyleSunoj, S., Igathinathane, C., Saliendra, N., Hendrickson, J., Archer, D., & Liebig, M. (2025). PhenoCam Guidelines for Phenological Measurement and Analysis in an Agricultural Cropping Environment: A Case Study of Soybean. Remote Sensing, 17(4), 724. https://doi.org/10.3390/rs17040724










