Modeling Temporal Resistance Assessment of Cotton to Verticillium Wilt Using Airborne Hyperspectral Data and Disease Progression Rates
Highlights
- A method for resistance assessment that integrates the dynamic progression rate of the disease was proposed.
- The model demonstrated good predictive performance in evaluating resistance across cotton genotypes using single-time-point data.
- Incorporating dynamic disease development rates enables effective resistance assessment during early infection stages.
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Hyperspectral Image Acquisition Using UAV
2.3. Ground Data Collection
2.3.1. Resistance Assessment Ground Survey
2.3.2. Acquisition of Ground-Based Canopy Leaf Area Index
2.3.3. Acquisition of Ground-Based Destructive Sampling Data
2.4. Extraction of Canopy Spectral Features
2.5. Statistical Analysis Methods
2.6. Model Construction Methods
3. Results
3.1. Temporal Variation Patterns of Physiological and Biochemical Parameters in Cotton Varieties with Different Resistance Levels During VW Progression
3.2. Temporal Hyperspectral Response Patterns of Cotton Varieties with Different Resistance Levels During VW Progression
3.2.1. Spectral Feature Response Based on Single Time-Phase
3.2.2. Spectral Feature Response Based on Temporal Dynamic Development Rates
3.3. Results of Resistance Evaluation Models Based on Single-Time-Phase Spectral Features
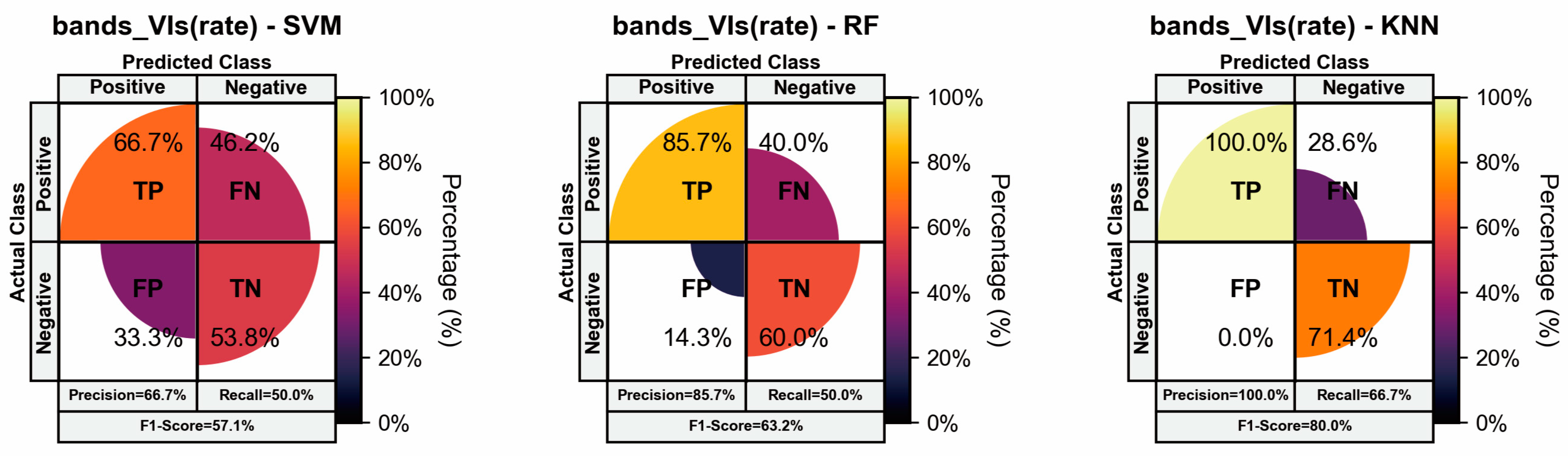
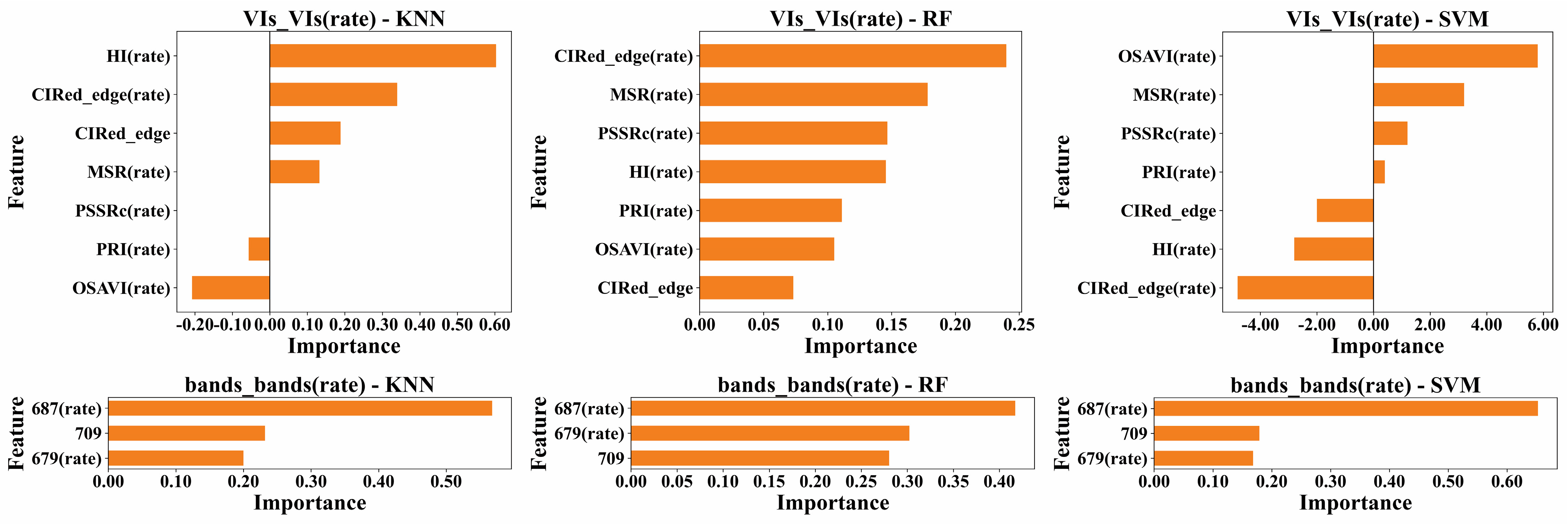
3.4. Results of Resistance Evaluation Models Incorporating Temporal Dynamic Development Rates
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| DI | Disease index |
| ARDI | Average relative disease index |
| DCNN | Deep convolutional neural networks |
| DN | Digital number |
| DTSM | Decision Tree-based Segmentation Model |
| DW | Dry weight |
| KNN | K-Nearest Neighbors |
| LAI | Leaf area index |
| LWC | Leaf water content |
| OA | Overall accuracy |
| RF | Random Forest |
| ROI | Regions of interest |
| SBS | Sequential Backward Selection |
| SEM | Standard errors |
| SVM | Support Vector Machine |
| UAV | Unmanned aerial vehicle |
| VW | Verticillium wilt |
References
- Fradin, E.F.; Thomma, B.P.H.J. Physiology and Molecular Aspects of Verticillium Wilt Diseases Caused by V. dahliae and V. albo-atrum. Mol. Plant Pathol. 2006, 7, 71–86. [Google Scholar] [CrossRef]
- Zhang, D.; Wang, J.; Wang, D.; Kong, Z.; Zhou, L.; Zhang, G.; Gui, Y.; Li, J.; Huang, J.; Wang, B.; et al. Population Genomics Demystifies the Defoliation Phenotype in the Plant Pathogen Verticillium dahliae. New Phytol. 2019, 222, 1012–1029. [Google Scholar] [CrossRef]
- Zhang, Y.; Yuan, Y.; Xi, H.; Zhang, Y.; Gao, C.; Ma, M.; Huang, Q.; Li, F.; Yang, Z. Promotion of Apoplastic Oxidative Burst by Artificially Selected GhCBSX3A Enhances Verticillium dahliae Resistance in Upland Cotton. Plant J. 2024, 118, 2154–2168. [Google Scholar] [CrossRef] [PubMed]
- Shaban, M.; Miao, Y.; Ullah, A.; Khan, A.Q.; Menghwar, H.; Khan, A.H.; Ahmed, M.M.; Tabassum, M.A.; Zhu, L. Physiological and Molecular Mechanism of Defense in Cotton against Verticillium dahliae. Plant Physiol. Biochem. 2018, 125, 193–204. [Google Scholar] [CrossRef] [PubMed]
- Kowalska, B. Management of the Soil-Borne Fungal Pathogen— Verticillium dahliae Kleb. Causing Vascular Wilt Diseases. J. Plant Pathol. 2021, 103, 1185–1194. [Google Scholar] [CrossRef]
- Yin, X.; Zou, B.; Hong, X.; Gao, M.; Yang, W.; Zhong, X.; He, Y.; Kuai, P.; Lou, Y.; Huang, J.; et al. Rice Copine Genes Os BON 1 and Os BON 3 Function as Suppressors of Broad-spectrum Disease Resistance. Plant Biotechnol. J. 2018, 16, 1476–1487. [Google Scholar] [CrossRef]
- Da Graça, J.V.; Douhan, G.W.; Halbert, S.E.; Keremane, M.L.; Lee, R.F.; Vidalakis, G.; Zhao, H. Huanglongbing: An Overview of a Complex Pathosystem Ravaging the World’s Citrus. J. Integr. Plant Biol. 2016, 58, 373–387. [Google Scholar] [CrossRef]
- Bai, Y.; Zarco-Tejada, P.J.; Peñuelas, J.; McCabe, M.F.; Hawkesford, M.J.; Atzberger, C.; Poblete, T.; Kumar, L.; Reynolds, M.P.; Nie, C.; et al. Hyperspectral Remote Sensing for Monitoring Crop Disease: Applications, Challenges, and Perspectives. IEEE Geosci. Remote Sens. Mag. 2025, 2–26. [Google Scholar] [CrossRef]
- Gui, Y.; Huang, C.; Zhou, J.; Yang, M.; Qiu, X.; Zhang, Z.; Liu, Y.; Gao, Y.; Shen, W.; Huang, W.; et al. A Practical Approach for Grading Cotton Verticillium Wilt Severity for Remote Sensing Monitoring. Agric. For. Meteorol. 2025, 368, 110559. [Google Scholar] [CrossRef]
- Mishra, P.; Polder, G.; Vilfan, N. Close Range Spectral Imaging for Disease Detection in Plants Using Autonomous Platforms: A Review on Recent Studies. Curr. Robot. Rep. 2020, 1, 43–48. [Google Scholar] [CrossRef]
- Chen, B.; Han, H.-Y.; Wang, F.-Y.; Liu, Z.; Deng, F.-J.; Lin, H.; Yu, Y.; Li, S.-K.; Wang, K.-R.; Xiao, C.-H. Monitoring Chlorophyll and Nitrogen Contents in Cotton Leaf Infected by Verticillium wilt with Spectra Red Edge Parameters. Acta Agron. Sin. 2013, 39, 319. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, B.; Sun, Z.; Liu, Z.; Cui, Y.; Ke, H.; Wang, Z.; Wu, L.; Zhang, G.; Wang, G.; et al. A Large-scale Genomic Association Analysis Identifies a Fragment in Dt11 Chromosome Conferring Cotton Verticillium Wilt Resistance. Plant Biotechnol. J. 2021, 19, 2126–2138. [Google Scholar] [CrossRef]
- Kang, X. Assessing the Severity of Cotton Verticillium Wilt Disease from in Situ Canopy Images and Spectra Using Convolutional Neural Networks. Crop J. 2023, 11, 933–940. [Google Scholar] [CrossRef]
- Zhao, L. Development of an Improved Standard for Identifying and Evaluating Verticillium Wilt Resistance in Cotton. Cotton Sci. 2017, 29, 50–58. [Google Scholar] [CrossRef]
- Calderón, R.; Navas-Cortés, J.A.; Lucena, C.; Zarco-Tejada, P.J. High-Resolution Airborne Hyperspectral and Thermal Imagery for Early Detection of Verticillium Wilt of Olive Using Fluorescence, Temperature and Narrow-Band Spectral Indices. Remote Sens. Environ. 2013, 139, 231–245. [Google Scholar] [CrossRef]
- Yang, M.; Huang, C.; Kang, X.; Qin, S.; Ma, L.; Wang, J.; Zhou, X.; Lv, X.; Zhang, Z. Early Monitoring of Cotton Verticillium Wilt by Leaf Multiple “Symptom” Characteristics. Remote Sens. 2022, 14, 5241. [Google Scholar] [CrossRef]
- Weng, H.; Yao, Y.; Huang, D. Rapid Evaluation of Rice Neck Blast Resistance Using Low Altitude Remote Sensing of UAV Combined with YOLOv7. Trans. Chin. Soc. Agric. Eng. 2024, 40, 110–118. [Google Scholar]
- Liu, T.; Qi, Y.; Yang, F.; Yi, X.; Guo, S.; Wu, P.; Yuan, Q.; Xu, T. Early Detection of Rice Blast Using UAV Hyperspectral Imagery and Multi-Scale Integrator Selection Attention Transformer Network (MS-STNet). Comput. Electron. Agric. 2025, 231, 110007. [Google Scholar] [CrossRef]
- Hu, J.; Huang, J.; Xu, H.; Wang, Y.; Li, C.; Wen, P.; You, X.; Zhang, X.; Pan, G.; Li, Q.; et al. Rice Stripe Virus Suppresses Jasmonic Acid-Mediated Resistance by Hijacking Brassinosteroid Signaling Pathway in Rice. PLoS Pathog. 2020, 16, e1008801. [Google Scholar] [CrossRef]
- Xia, Z.; Achar, P.N.; Gu, B. Vegetative Compatibility Groupings of Verticillium dahliae from Cotton in Mainland China. Eur. J. Plant Pathol. 1998, 104, 871–876. [Google Scholar] [CrossRef]
- Jiang, Z.; Tu, H.; Bai, B.; Yang, C.; Zhao, B.; Guo, Z.; Liu, Q.; Zhao, H.; Yang, W.; Xiong, L.; et al. Combining UAV-RGB High-throughput Field Phenotyping and Genome-wide Association Study to Reveal Genetic Variation of Rice Germplasms in Dynamic Response to Drought Stress. New Phytol. 2021, 232, 440–455. [Google Scholar] [CrossRef]
- Han, L.; Yang, G.; Yang, H.; Xu, B.; Li, Z.; Yang, X. Clustering Field-Based Maize Phenotyping of Plant-Height Growth and Canopy Spectral Dynamics Using a UAV Remote-Sensing Approach. Front. Plant Sci. 2018, 9, 1638. [Google Scholar] [CrossRef]
- Xie, Z.; Chen, S.; Gao, G.; Li, H.; Wu, X.; Meng, L.; Ma, Y. Evaluation of Rapeseed Flowering Dynamics for Different Genotypes with UAV Platform and Machine Learning Algorithm. Precis. Agric. 2022, 23, 1688–1706. [Google Scholar] [CrossRef]
- Zhou, J.; Huang, C.; Gui, Y.; Yang, M.; Zhang, Z.; Huang, W.; Zhang, L.; Tong, Q. Roles of Physiological and Nonphysiological Information in Sun-Induced Chlorophyll Fluorescence Variations for Detecting Cotton Verticillium Wilt. IEEE J. Sel. Top. Appl. Earth Obs. Remote Sens. 2024, 17, 8835–8850. [Google Scholar] [CrossRef]
- NY/T 2952-2016; Technical Specification for Identification of Cotton Verticillium Wilt Resistance. China Agriculture Press: Beijing, China, 2016.
- Pearse, G.D.; Watt, M.S.; Morgenroth, J. Comparison of Optical LAI Measurements under Diffuse and Clear Skies after Correcting for Scattered Radiation. Agric. For. Meteorol. 2016, 221, 61–70. [Google Scholar] [CrossRef]
- Murray, J.R.; Hackett, W.P. Dihydroflavonol Reductase Activity in Relation to Differential Anthocyanin Accumulation in Juvenile and Mature Phase Hedera helix L. Plant Physiol. 1991, 97, 343–351. [Google Scholar] [CrossRef] [PubMed]
- Hornero, A.; Zarco-Tejada, P.J.; Quero, J.L.; North, P.R.J.; Ruiz-Gómez, F.J.; Sánchez-Cuesta, R.; Hernandez-Clemente, R. Modelling Hyperspectral- and Thermal-Based Plant Traits for the Early Detection of Phytophthora-Induced Symptoms in Oak Decline. Remote Sens. Environ. 2021, 263, 112570. [Google Scholar] [CrossRef]
- Ishiyama, T.; Nakajima, Y.; Kajiwara, K.; Tsuchiya, K. Extraction of Vegetation Cover in an Arid Area Based on Satellite Data. Adv. Space Res. 1997, 19, 1375–1378. [Google Scholar] [CrossRef]
- Roujean, J.-L.; Breon, F.-M. Estimating PAR Absorbed by Vegetation from Bidirectional Reflectance Measurements. Remote Sens. Environ. 1995, 51, 375–384. [Google Scholar] [CrossRef]
- Chen, J.M. Evaluation of Vegetation Indices and a Modified Simple Ratio for Boreal Applications. Can. J. Remote Sens. 1996, 22, 229–242. [Google Scholar] [CrossRef]
- Rondeaux, G.; Steven, M.; Baret, F. Optimization of Soil-Adjusted Vegetation Indices. Remote Sens. Environ. 1996, 55, 95–107. [Google Scholar] [CrossRef]
- Haboudane, D.; Miller, J.R.; Tremblay, N.; Zarco-Tejada, P.J.; Dextraze, L. Integrated Narrow-Band Vegetation Indices for Prediction of Crop Chlorophyll Content for Application to Precision Agriculture. Remote Sens. Environ. 2002, 81, 416–426. [Google Scholar] [CrossRef]
- Haboudane, D. Hyperspectral Vegetation Indices and Novel Algorithms for Predicting Green LAI of Crop Canopies: Modeling and Validation in the Context of Precision Agriculture. Remote Sens. Environ. 2004, 90, 337–352. [Google Scholar] [CrossRef]
- Blackburn, G.A. Spectral Indices for Estimating Photosynthetic Pigment Concentrations: A Test Using Senescent Tree Leaves. Int. J. Remote Sens. 1998, 19, 657–675. [Google Scholar] [CrossRef]
- Penuelas, J.; Pinol, J.; Ogaya, R.; Filella, I. Estimation of Plant Water Concentration by the Reflectance Water Index WI (R900/R970). Int. J. Remote Sens. 1997, 18, 2869–2875. [Google Scholar] [CrossRef]
- Mahlein, A.-K.; Rumpf, T.; Welke, P.; Dehne, H.-W.; Plümer, L.; Steiner, U.; Oerke, E.-C. Development of Spectral Indices for Detecting and Identifying Plant Diseases. Remote Sens. Environ. 2013, 128, 21–30. [Google Scholar] [CrossRef]
- Gitelson, A.A.; Gritz, Y.; Merzlyak, M.N. Relationships between Leaf Chlorophyll Content and Spectral Reflectance and Algorithms for Non-Destructive Chlorophyll Assessment in Higher Plant Leaves. J. Plant Physiol. 2003, 160, 271–282. [Google Scholar] [CrossRef]
- Gamon, J.A.; Peñuelas, J.; Field, C.B. A Narrow-Waveband Spectral Index That Tracks Diurnal Changes in Photosynthetic Efficiency. Remote Sens. Environ. 1992, 41, 35–44. [Google Scholar] [CrossRef]
- Ma, Y.; Zhang, Q.; Yi, X.; Ma, L.; Zhang, L.; Huang, C.; Zhang, Z.; Lv, X. Estimation of Cotton Leaf Area Index (LAI) Based on Spectral Transformation and Vegetation Index. Remote Sens. 2021, 14, 136. [Google Scholar] [CrossRef]
- Yang, P.; van der Tol, C.; Liu, J.; Liu, Z. Separation of the Direct Reflection of Soil from Canopy Spectral Reflectance. Remote Sens. Environ. 2025, 316, 114500. [Google Scholar] [CrossRef]
- Shahi, T.B.; Xu, C.-Y.; Neupane, A.; Guo, W. Recent Advances in Crop Disease Detection Using UAV and Deep Learning Techniques. Remote Sens. 2023, 15, 2450. [Google Scholar] [CrossRef]
- Yuan, L.; Yu, Q.; Xiang, L.; Zeng, F.; Dong, J.; Xu, O.; Zhang, J. Integrating UAV and High-Resolution Satellite Remote Sensing for Multi-Scale Rice Disease Monitoring. Comput. Electron. Agric. 2025, 234, 110287. [Google Scholar] [CrossRef]
- Cai, A.; Wang, L.; Zhang, Y.; Wu, H.; Zhang, H.; Guo, R.; Wu, J. Uncovering the Multiple Socio-Economic Driving Factors of Carbon Emissions in Nine Urban Agglomerations of China Based on Machine Learning. Energy 2025, 319, 134859. [Google Scholar] [CrossRef]
- Smith, K.L.; Steven, M.D.; Colls, J.J. Use of Hyperspectral Derivative Ratios in the Red-Edge Region to Identify Plant Stress Responses to Gas Leaks. Remote Sens. Environ. 2004, 92, 207–217. [Google Scholar] [CrossRef]
- Chen, B.; Li, S.; Wang, K.; Zhou, G.; Bai, J. Evaluating the Severity Level of Cotton Verticillium Using Spectral Signature Analysis. Int. J. Remote Sens. 2012, 33, 2706–2724. [Google Scholar] [CrossRef]
- Li, W.; Li, D.; Warner, T.A.; Liu, S.; Baret, F.; Yang, P.; Jiang, J.; Dong, M.; Cheng, T.; Zhu, Y.; et al. Improved Generality of Wheat Green LAI Models through Mitigation of the Effect of Leaf Chlorophyll Content Variation with Red Edge Vegetation Indices. Remote Sens. Environ. 2025, 318, 114589. [Google Scholar] [CrossRef]
- Zhang, L.; Qiao, N.; Huang, C.; Wang, S. Monitoring Drought Effects on Vegetation Productivity Using Satellite Solar-Induced Chlorophyll Fluorescence. Remote Sens. 2019, 11, 378. [Google Scholar] [CrossRef]
- Ortiz, J.C.M.; Carvajal, L.M.H.; Fernandez, V.B. Detection of Significant Wavelengths for Identifying and Classifying Fusarium Oxysporum during the Incubation Period and Water Stress in Solanum Lycopersicum Plants Using Reflectance Spectroscopy. J. Plant Prot. Res. 2019, 59. [Google Scholar] [CrossRef]
- Tzeng, D.D.; De Vay, J.E. Physiological Responses of Gossypium Hirsutum L. to Infection by Defoliating and Nondefoliating Pathotypes of Verticillium dahliae Kleb. Physiol. Plant Pathol. 1985, 26, 57–72. [Google Scholar] [CrossRef]
- Croft, H.; Chen, J.M.; Luo, X.; Bartlett, P.; Chen, B.; Staebler, R.M. Leaf Chlorophyll Content as a Proxy for Leaf Photosynthetic Capacity. Glob. Change Biol. 2017, 23, 3513–3524. [Google Scholar] [CrossRef]
- Anderson, M.C. Photon Flux, Chlorophyll Content, and Photosynthesis Under Natural Conditions. Ecology 1967, 48, 1050–1053. [Google Scholar] [CrossRef]
- Buttery, B.R.; Buzzell, R.I. The Relationship Between Chlorophyll Content and Rate of Photosynthesis in Soybeans. Can. J. Plant Sci. 1977, 57, 1–5. [Google Scholar] [CrossRef]
- Qiu, P.; Li, J.; Zhang, L.; Chen, K.; Shao, J.; Zheng, B.; Yuan, H.; Qi, J.; Yue, L.; Hu, Q.; et al. Polyethyleneimine-Coated MXene Quantum Dots Improve Cotton Tolerance to Verticillium dahliae by Maintaining ROS Homeostasis. Nat. Commun. 2023, 14, 7392. [Google Scholar] [CrossRef] [PubMed]
- Yuan, L.; Dang, J.; Zhang, J.; Wang, L.; Zheng, H.; Li, G.; Li, J.; Zhou, F.; Khan, A.; Zhang, Z.; et al. A Glutathione S-Transferase Regulates Lignin Biosynthesis and Enhances Salt Tolerance in Tomato. Plant Physiol. 2024, 196, 2989–3006. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.-P.; Su, L.-Y.; Huang, T.; Liu, H.; Tan, S.-S.; Deng, Y.-J.; Wang, Y.-H.; Xiong, A.-S. The Telomere-to-Telomere Genome of Pucai (Typha angustifolia L.), a Distinctive Semi-Aquatic Vegetable with Lignin and Chlorophyll as Quality Characteristics. Hortic. Res. 2025, 12, uhaf079. [Google Scholar] [CrossRef]
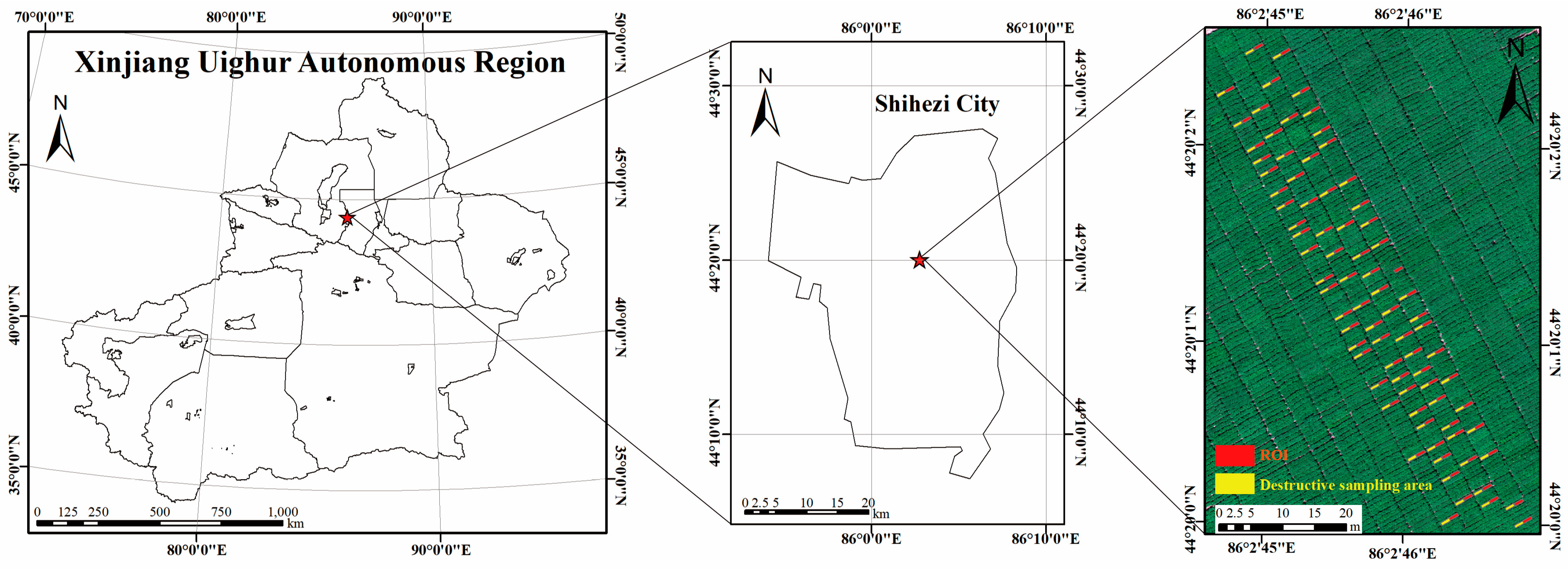
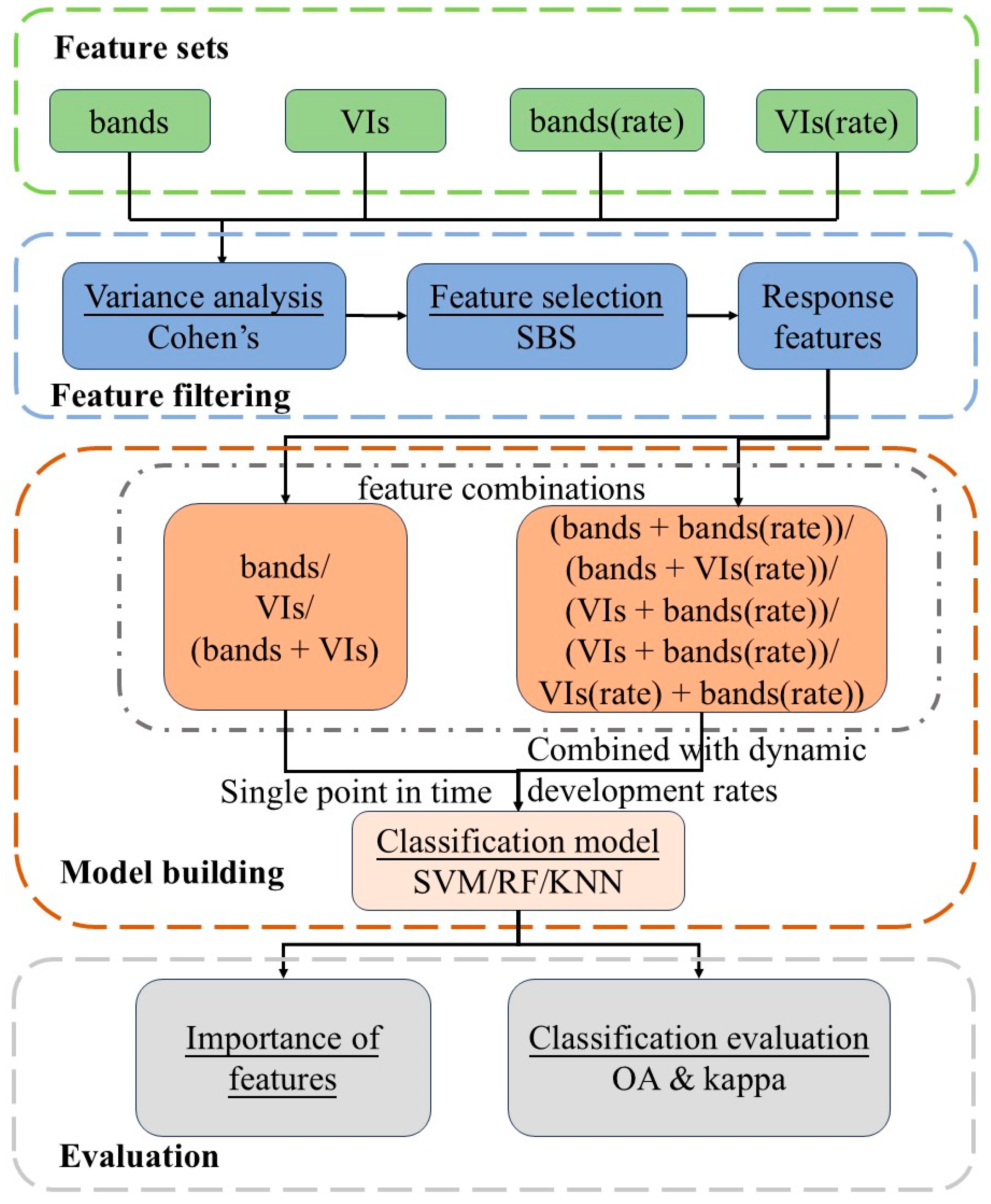
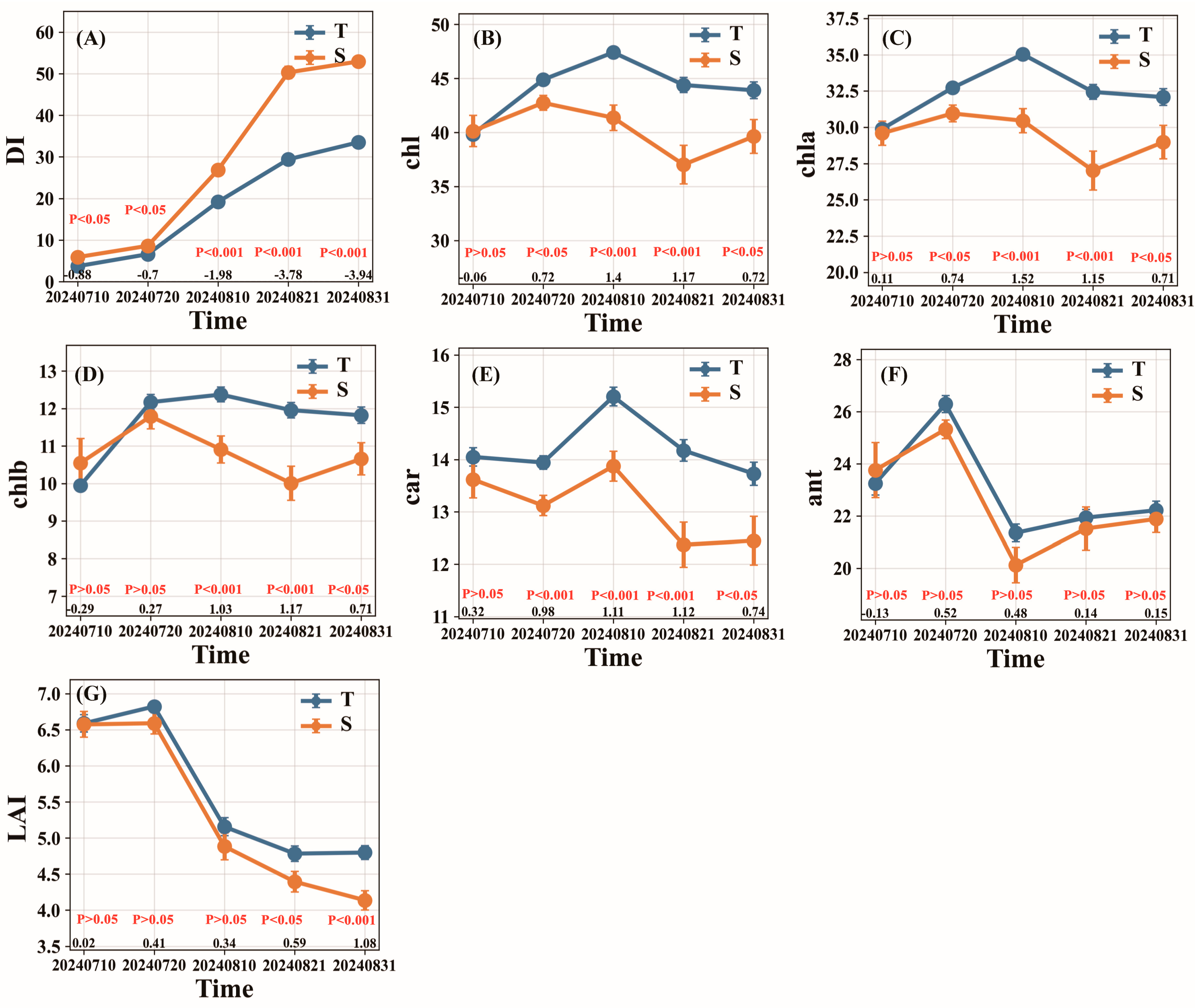




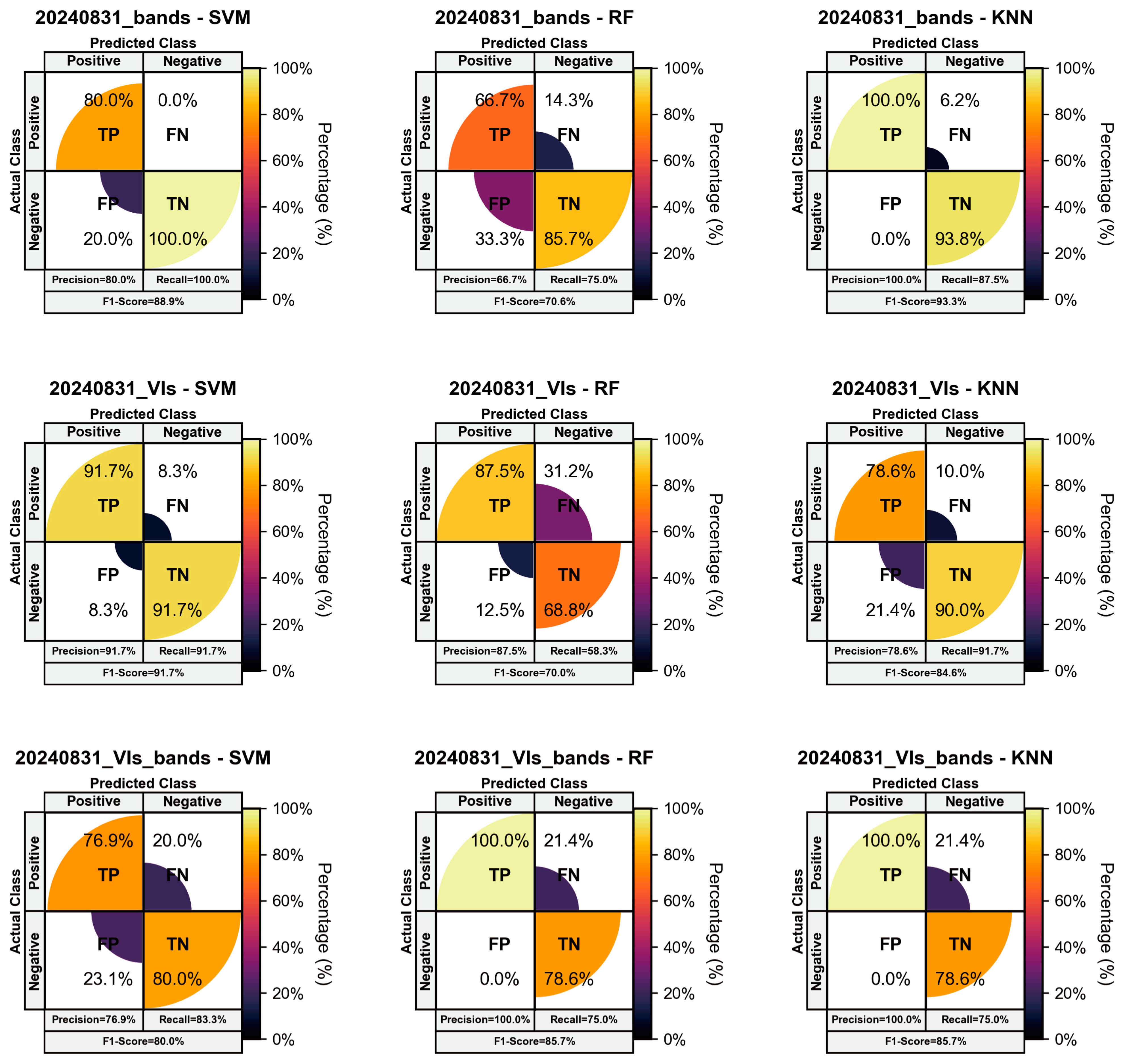
| Level | Resistance Type | Abbreviation | The Average Relative Disease Index (ARDI) |
|---|---|---|---|
| 1 | Resistant | R | 0 ≤ ADRI ≤ 20.0 |
| 2 | Tolerant | T | 20.0 < ADRI ≤ 35.0 |
| 3 | Susceptible | S | ADRI > 35.0 |
| Vegetation Indices | Calculation Formula | References |
|---|---|---|
| Pigment and structural indices | ||
| ()/( + ) | [29] | |
| ()/ | [30] | |
| MSR | [31] | |
| OSAVI | (1 + 0.16)()/( + + 0.16) | [32] |
| Greenness Index | [15] | |
| [33] | ||
| MTVI | 1.2 × | [34] |
| PSSRc | [35] | |
| Water index | ||
| WI | [36] | |
| Red edge and photosynthetic physiological indices | ||
| Healthy Index | [37] | |
| CIRed_edge | / | [38] |
| PRI | ()/( + ) | [39] |
| 20240710–20240720 | 20240720–20240810 | 20240810–20240821 | 20240821–20240831 | |
|---|---|---|---|---|
| DI | 0.15 | −1.21 | −2.72 | 0.55 |
| chla | 0.51 | 0.74 | 0.29 | −0.47 |
| chlb | 0.31 | 0.62 | 0.18 | −0.35 |
| chl | 0.49 | 0.79 | 0.27 | −0.45 |
| car | 0.34 | 0.31 | 0.36 | −0.27 |
| ant | 0.35 | 0.15 | −0.18 | −0.02 |
| LAI | 0.30 | −0.01 | 0.17 | 0.24 |
| Time Period | Features with Significant Differences in Dynamic Development Rates | Features of Dynamic Development Rates Selected Based on the SBS Method |
|---|---|---|
| Significantly different wavelengths | ||
| 20240710–20240720 | 422(rate), 507(rate), 511(rate), 516(rate), 520(rate), 524(rate), 679(rate), 683(rate), 687(rate), 692(rate), 696(rate), 700(rate) | 679(rate), 687(rate) |
| Significantly different vegetation indices | ||
| 20240710–20240720 | NDVIs(rate), MSR(rate), OSAVIs(rate), Greenness Index(rate), PSSRc(rate), HI(rate), CIRed_edge(rate), PRI(rate) | OSAVIs(rate), MSR(rate), PSSRc(rate), HI(rate), CIRed_edge(rate), PRI(rate) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, J.; Yang, M.; Zheng, Z.; Gui, Y.; Zhou, J.; Zhang, C.; Zhao, L.; Gong, M.; Huang, C.; Zhang, Z. Modeling Temporal Resistance Assessment of Cotton to Verticillium Wilt Using Airborne Hyperspectral Data and Disease Progression Rates. Remote Sens. 2025, 17, 3701. https://doi.org/10.3390/rs17223701
Wang J, Yang M, Zheng Z, Gui Y, Zhou J, Zhang C, Zhao L, Gong M, Huang C, Zhang Z. Modeling Temporal Resistance Assessment of Cotton to Verticillium Wilt Using Airborne Hyperspectral Data and Disease Progression Rates. Remote Sensing. 2025; 17(22):3701. https://doi.org/10.3390/rs17223701
Chicago/Turabian StyleWang, Jin, Mi Yang, Zhihong Zheng, Yaohui Gui, Junru Zhou, Cheng Zhang, Lihaopeng Zhao, Mingpan Gong, Changping Huang, and Ze Zhang. 2025. "Modeling Temporal Resistance Assessment of Cotton to Verticillium Wilt Using Airborne Hyperspectral Data and Disease Progression Rates" Remote Sensing 17, no. 22: 3701. https://doi.org/10.3390/rs17223701
APA StyleWang, J., Yang, M., Zheng, Z., Gui, Y., Zhou, J., Zhang, C., Zhao, L., Gong, M., Huang, C., & Zhang, Z. (2025). Modeling Temporal Resistance Assessment of Cotton to Verticillium Wilt Using Airborne Hyperspectral Data and Disease Progression Rates. Remote Sensing, 17(22), 3701. https://doi.org/10.3390/rs17223701







