Development of a Quantitative Survey Method for Pelagic Fish Aggregations Around an Offshore Wind Farm Using Multibeam Sonar
Highlights
- A novel method using multibeam sonar was developed to detect and quantify pelagic fish aggregations around underwater structures of an offshore wind turbine and an observation tower.
- Fish were found to consistently cluster on the leeward side of turbine and observation tower structures, with biomass estimations derived from sonar backscatter data.
- The method enables 3D visualization and biomass estimation of pelagic fish aggregations near a wind farm, supporting ecological assessments of artificial reef effects.
- It provides a practical tool for fostering collaboration between offshore wind energy developers and fisheries stakeholders through shared ecological data.
Abstract
1. Introduction
2. Materials and Methods
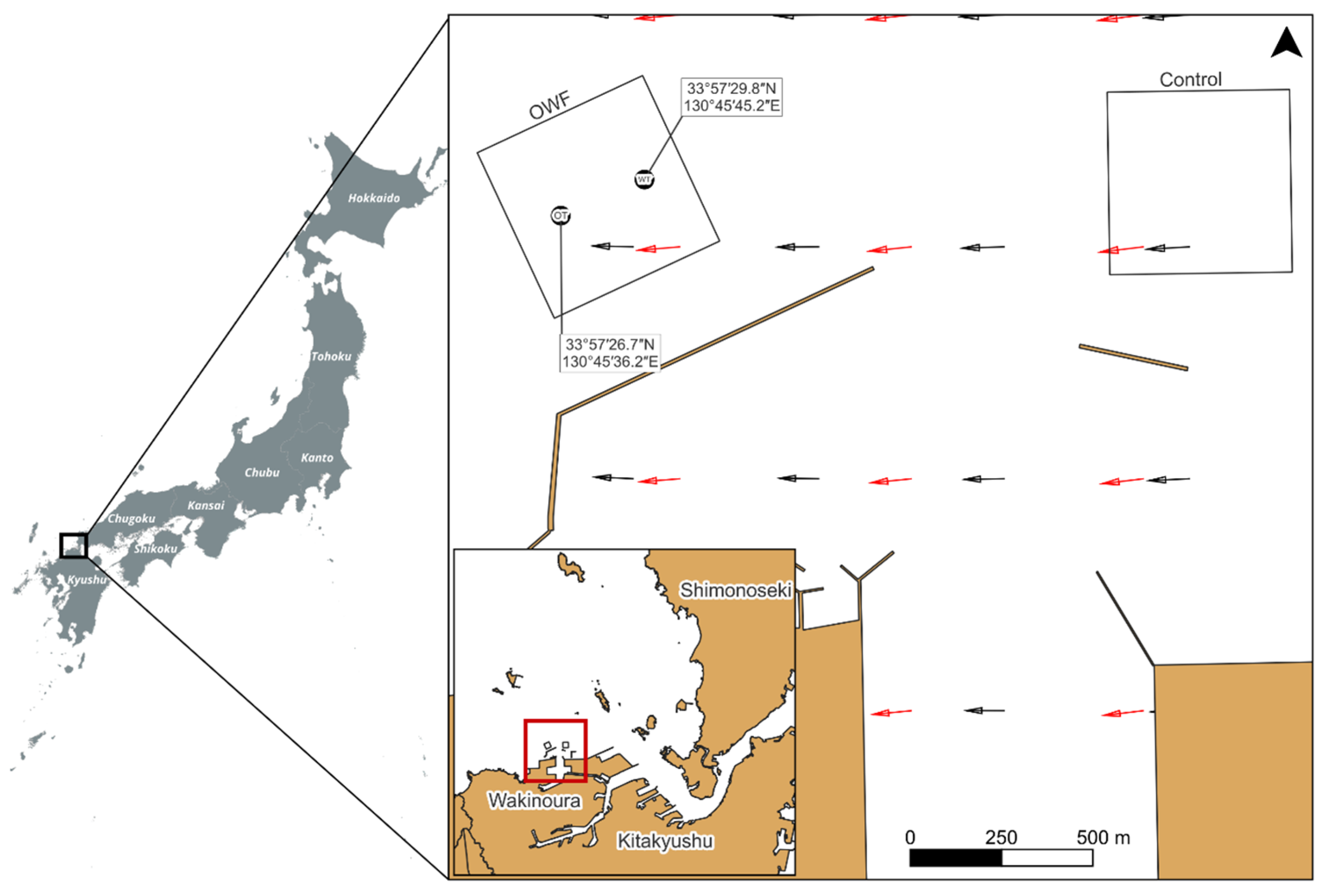
2.1. Survey Area
2.2. Acoustic Survey
2.3. Fish Sampling
2.4. Bathymetric Data Processing
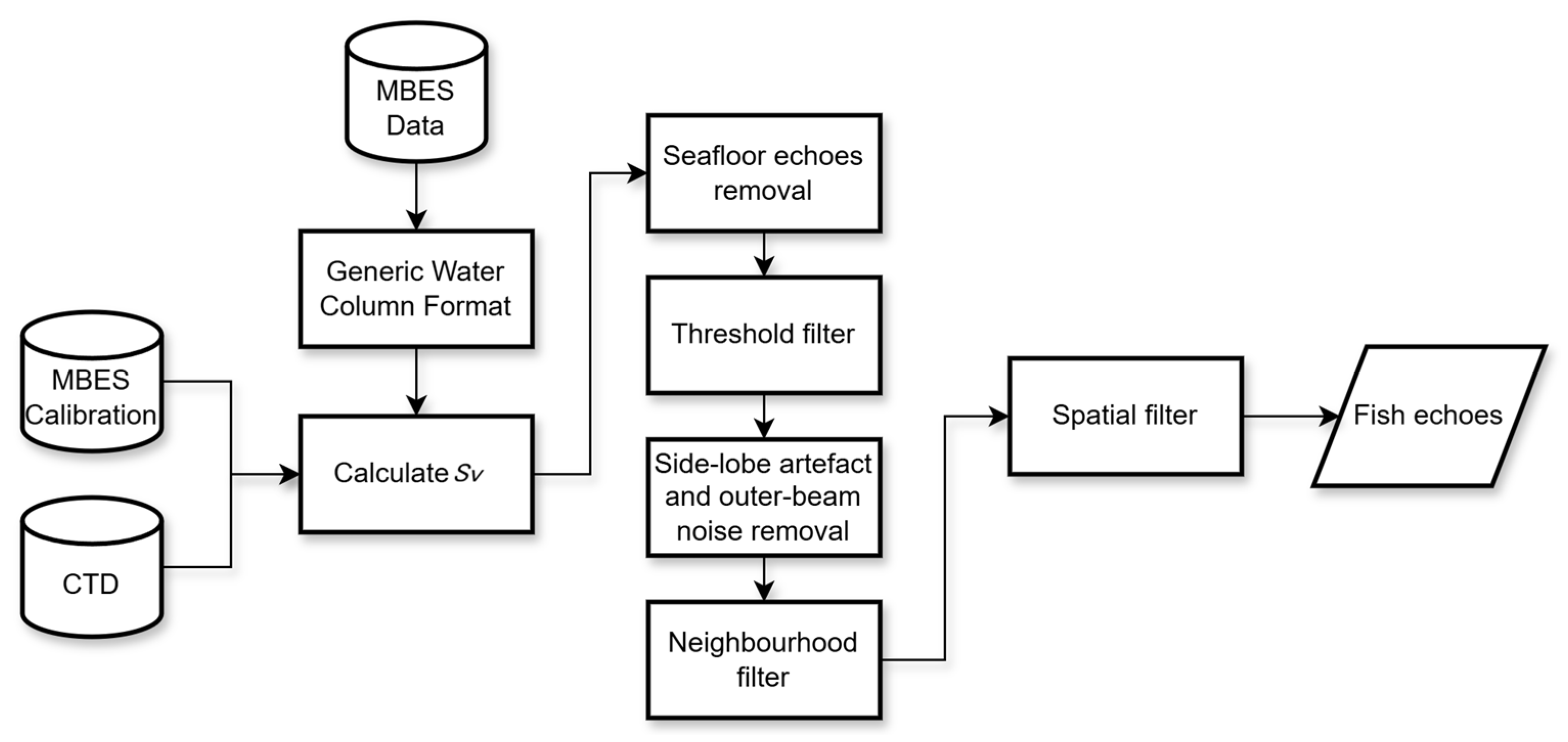
2.5. Water Column Image (WCI) Processing
2.5.1. Calibrated Sv Values
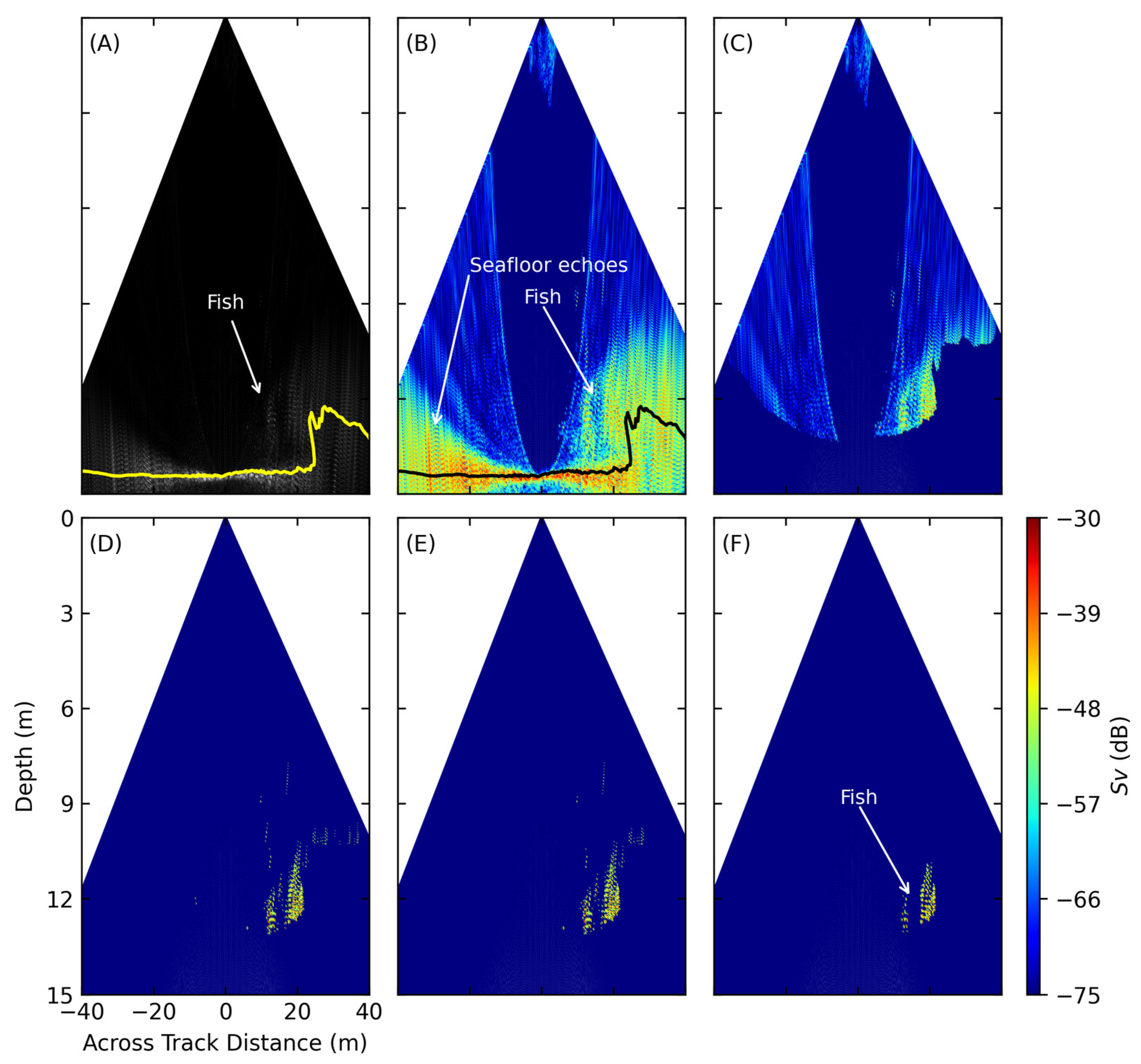
2.5.2. Seafloor Echo Removal
2.5.3. Threshold Filter
2.5.4. Side-Lobe Artifacts and Outer-Beam Noise Removal
2.5.5. Majority Filter (Neighborhood Filter)
2.5.6. Spatial Filter
2.6. Fish Density
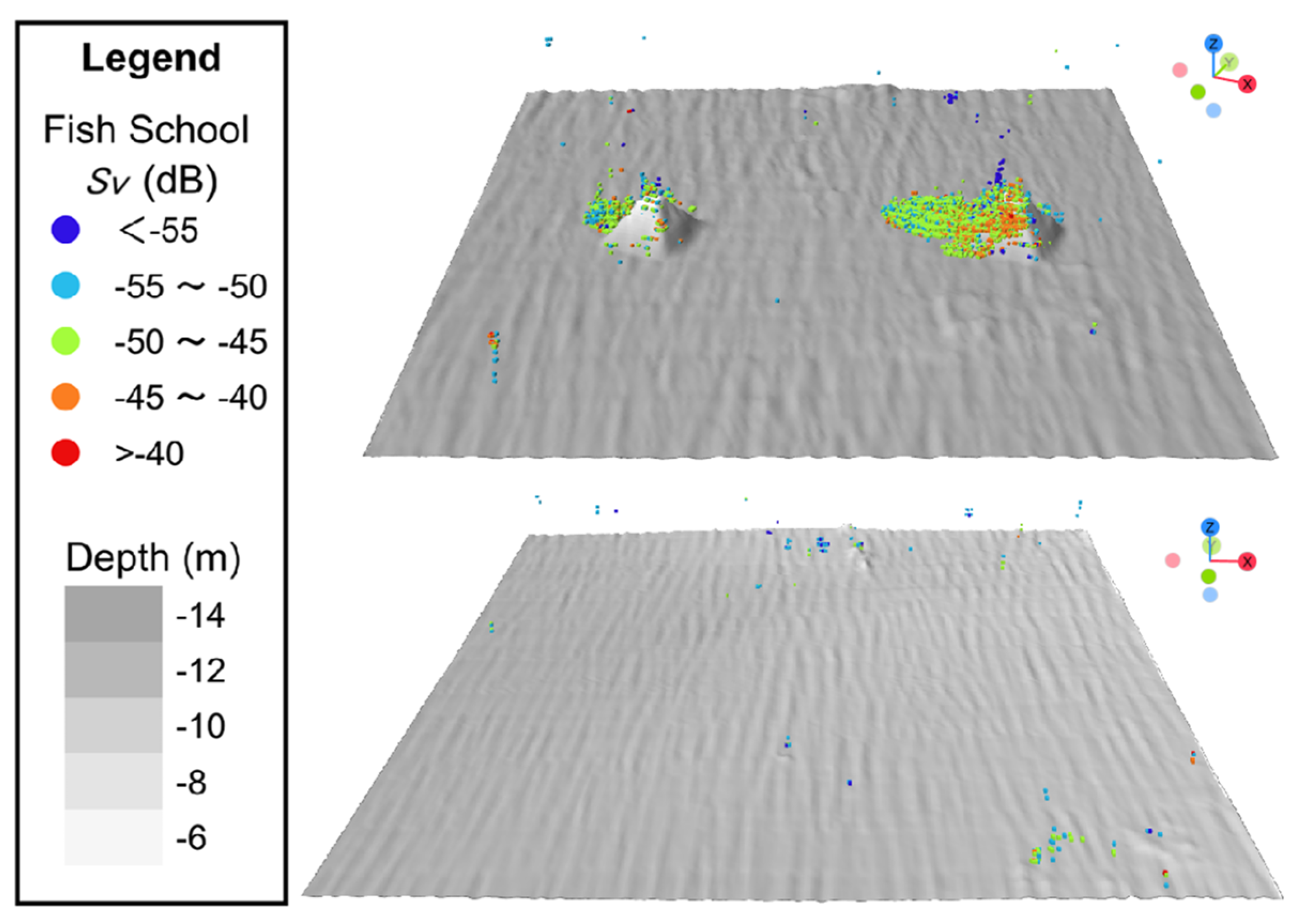
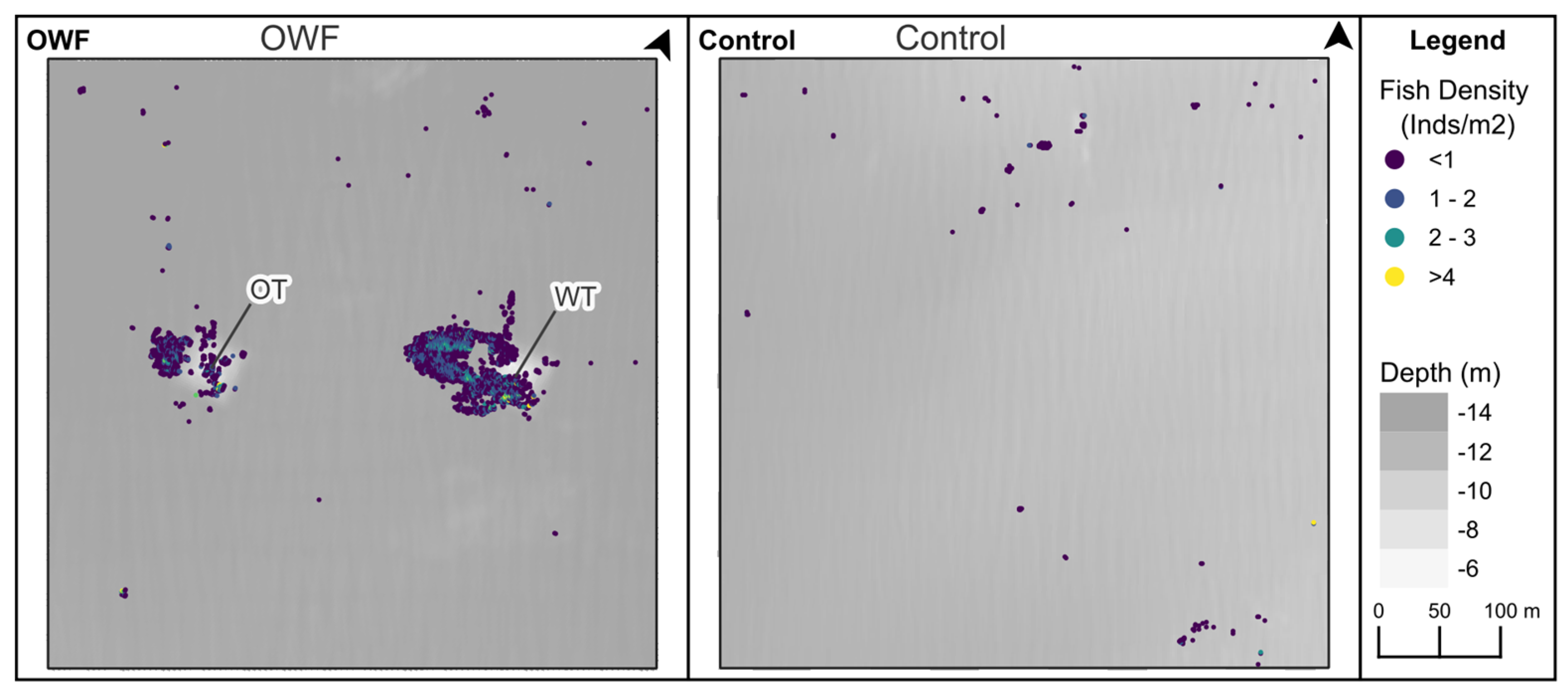
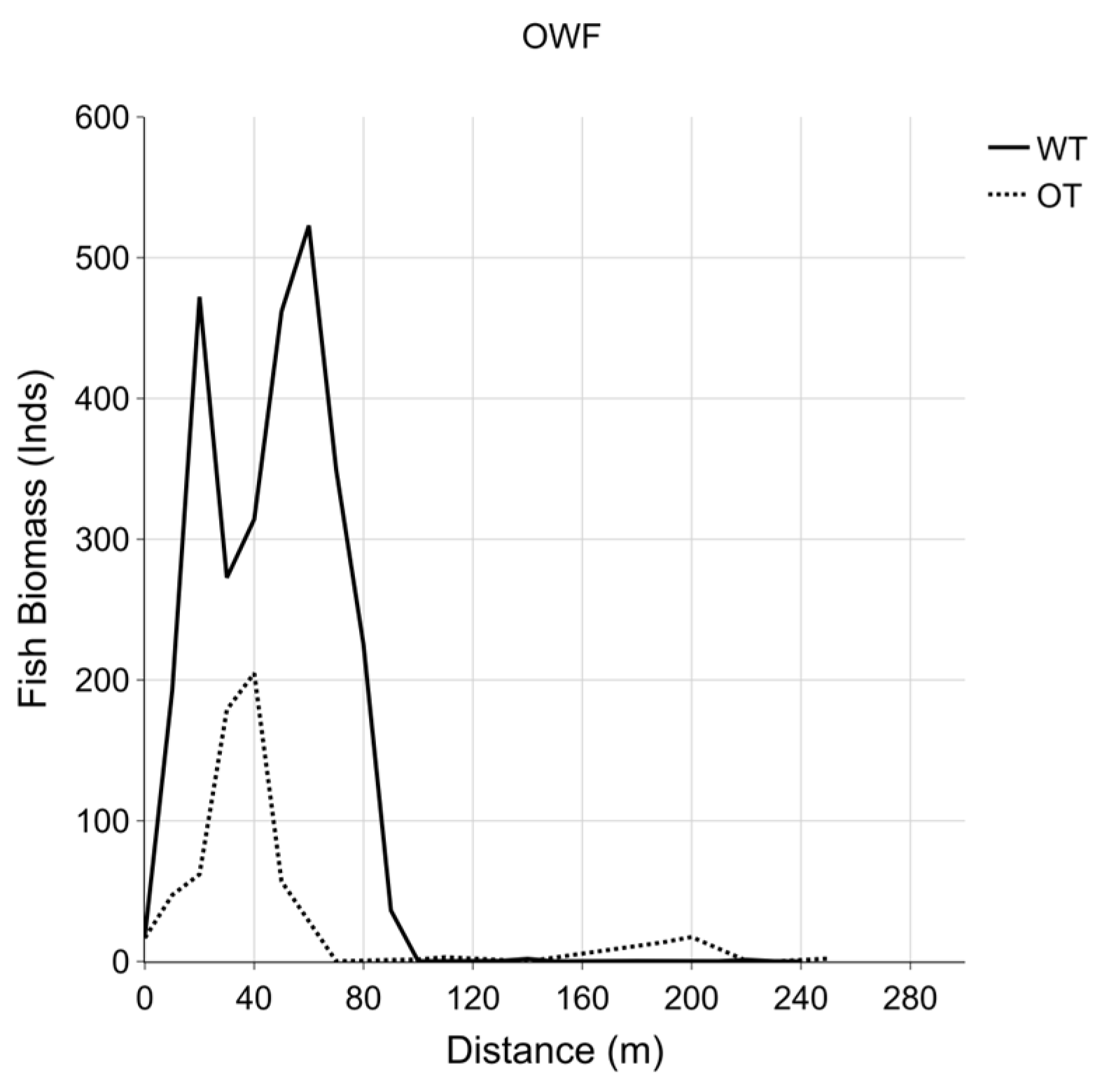
3. Results
3.1. Dominant Fish Species
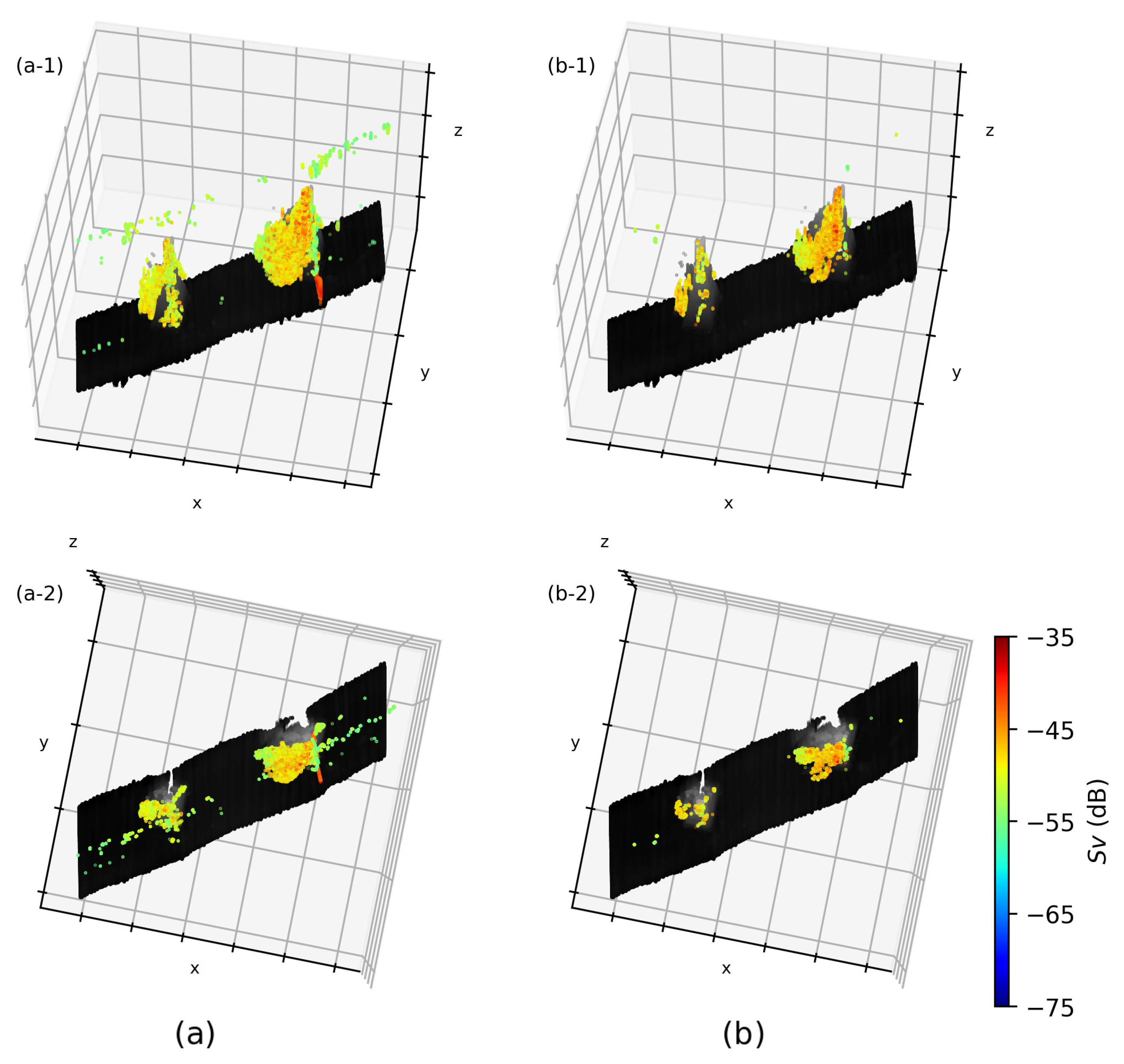
3.2. Fish Cells
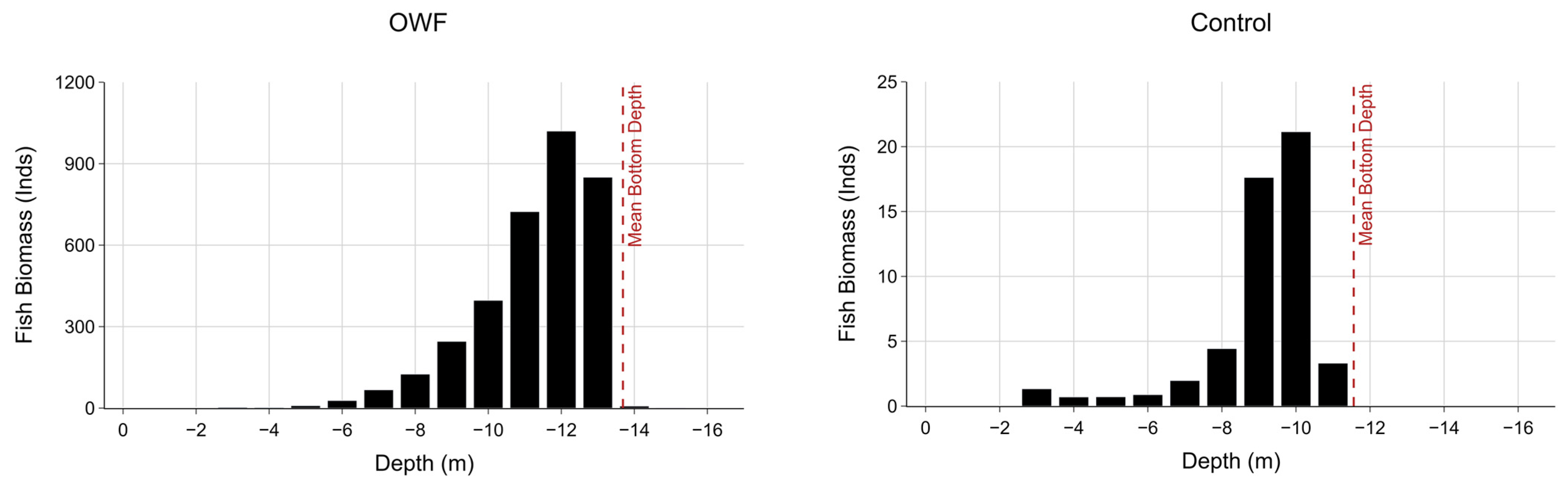
3.3. Fish Biomass
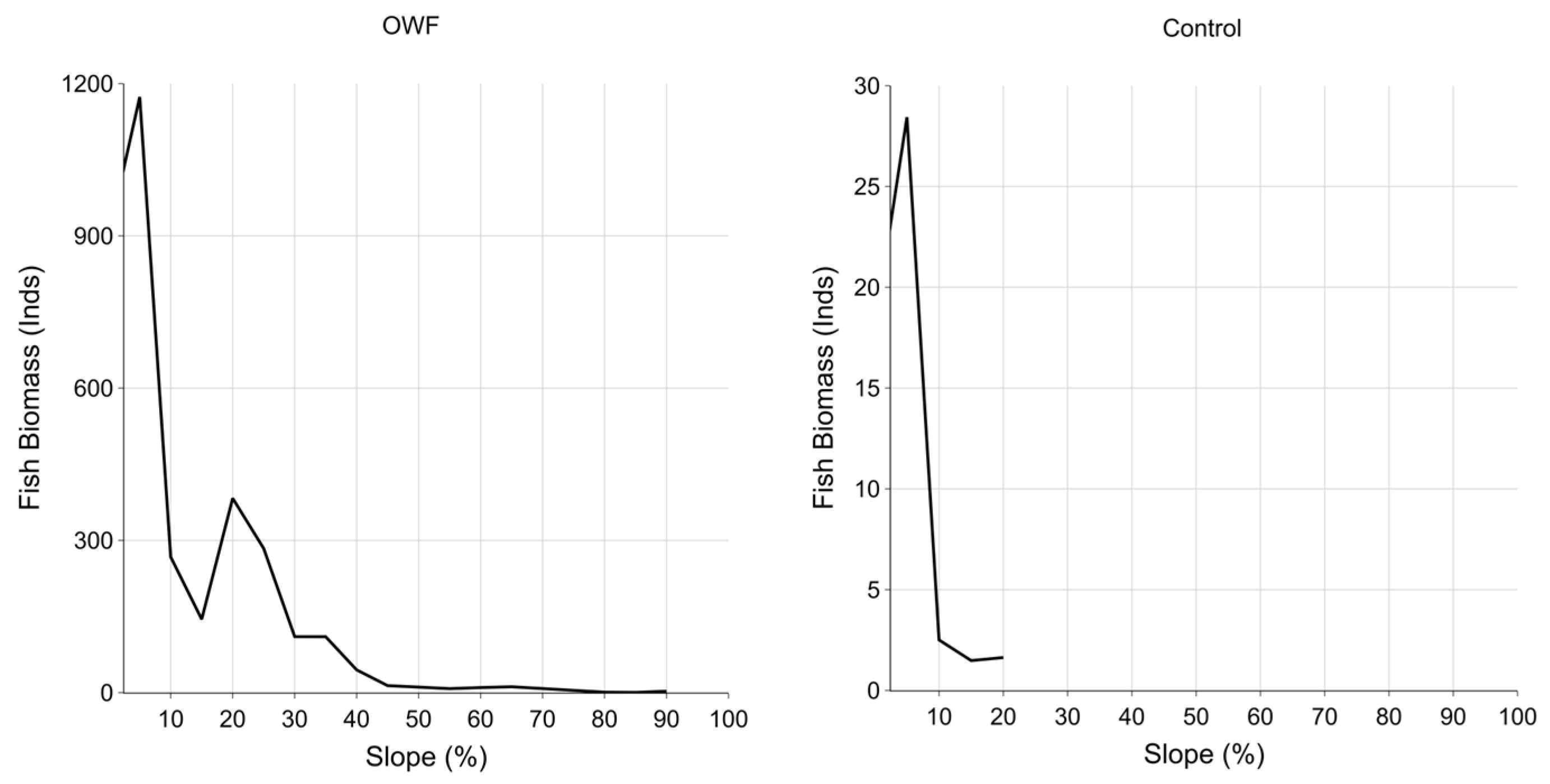
3.4. Seafloor Slope and Fish Distribution
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Perveen, R.; Kishor, N.; Mohanty, S.R. Off-shore wind farm development: Present status and challenges. Renew. Sustain. Energy Rev. 2014, 29, 780–792. [Google Scholar] [CrossRef]
- GWEC. Global Wind Energy Council. Global Wind Report 2025; GWEC: Brussels, Belgium, 2025; pp. 1–108. Available online: https://www.gwec.net/reports/globalwindreport (accessed on 11 September 2025).
- Díaz, H.; Soares, C.G. Review of the current status, technology and future trends of offshore wind farms. Ocean Eng. 2020, 209, 107381. [Google Scholar] [CrossRef]
- Gkeka-Serpetsidaki, P.; Skiniti, G.; Tournaki, S.; Tsoutsos, T. A Review of the Sustainable Siting of Offshore Wind Farms. Sustainability 2024, 16, 6036. [Google Scholar] [CrossRef]
- Shimada, H.; Asano, K.; Nagai, Y.; Ozawa, A. Assessing the Impact of Offshore Wind Power Deployment on Fishery: A Synthetic Control Approach. Environ. Resour. Econ. 2022, 83, 791–829. [Google Scholar] [CrossRef]
- Tajima, S.; Yamaguchi, K.; Shiroyama, H. Consensus building with fishermen on offshore wind farms in Japan: Current status and policy recommendations. Mar. Policy 2024, 160, 105975. [Google Scholar] [CrossRef]
- Methratta, E.T.; Dardick, W.R. Meta-analysis of finfish abundance at offshore wind farms. Rev. Fish. Sci. Aquac. 2019, 27, 242–260. [Google Scholar] [CrossRef]
- Degraer, S.; Carey, D.A.; Coolen, J.W.; Hutchison, Z.L.; Kerckhof, F.; Rumes, B.; Vanaverbeke, J. Offshore wind farm artificial reefs affect ecosystem structure and functioning. Oceanography 2020, 33, 48–57. [Google Scholar] [CrossRef]
- Lemasson, A.J.; Somerfield, P.J.; Schratzberger, M.; Thompson, M.S.; Firth, L.B.; Couce, E.; McNeil, C.L.; Nunes, J.; Pascoe, C.; Watson, S.C.L.; et al. A global meta-analysis of ecological effects from offshore marine artificial structures. Nat. Sustain. 2024, 7, 485–495. [Google Scholar] [CrossRef]
- Bergström, L.; Kautsky, L.; Malm, T.; Rosenberg, R.; Wahlberg, M.; Capetillo, N.Å.; Wilhelmsson, D. Effects of offshore wind farms on marine wildlife—A generalized impact assessment. Environ. Res. Lett. 2014, 9, 034012. [Google Scholar] [CrossRef]
- Hammar, L.; Wikström, A.; Molander, S. Assessing ecological risks of offshore wind power on Kattegat cod. Renew. Energy 2014, 66, 414–424. [Google Scholar] [CrossRef]
- Tsuchida, S.; Kato, R.; Nishitsuji, S.; Anzai, S.; Azmi, S.S.B.; Tanaka, S.; Masumi, S.; Uchida, J.; Aoshima, T.; Hirasaka, K.; et al. Floating Offshore Wind Farms Attract Japanese Horse Mackerel. Aquat. Conserv. Mar. Freshw. Ecosyst. 2025, 35, e70189. [Google Scholar] [CrossRef]
- Nagano, A. Fishing ground creation and restoration, and artificial reefs. In Fisheries Handbook Completely Revised; Shima, K., Seki, F., Maeda, M., Kimura, S., Saeki, H., Sakuramoto, K., Suenaga, Y., Nagano, A., Morinaga, T., Yagi, N., et al., Eds.; Kodansha: Tokyo, Japan, 2012; pp. 72–74. (In Japanese) [Google Scholar]
- Claudet, J.; Pelletier, D. Marine protected areas and artificial reefs: A review of the interactions between management and scientific studies. Aquat. Living Resour. 2004, 17, 129–138. [Google Scholar] [CrossRef]
- Grossman, G.D.; Jones, G.P.; Seaman, W.J. Do Artificial reefs increase regional fish production? A review of existing data. Fisheries 1997, 22, 17–23. [Google Scholar] [CrossRef]
- Hamano, A. studies of acoustic method for estimating fishery resources and fishing reef grounds. Fish. Eng. 2019, 55, 175–185, (In Japanese with English abstract and figure captions). [Google Scholar] [CrossRef]
- Andersson, M.H.; Öhman, M.C. Fish and sessile assemblages associated with wind-turbine constructions in the Baltic Sea. Mar. Freshw. Res. 2010, 61, 642–650. [Google Scholar] [CrossRef]
- Kang, M.; Nakamura, T.; Hamano, A. A methodology for acoustic and geospatial analysis of diverse artificial-reef datasets. ICES J. Mar. Sci. 2011, 68, 2210–2221. [Google Scholar] [CrossRef]
- Sato, M.; Inoue, N.; Nambu, R.; Furuichi, N.; Imaizumi, T.; Ushio, M. Quantitative assessment of multiple fish species around artificial reefs combining environmental DNA metabarcoding and acoustic survey. Sci. Rep. 2021, 11, 19477. [Google Scholar] [CrossRef]
- Lu, H.J.; Lin, J.R.; Huang, T.C.; Sinaga, S. Comparison of fish assemblages aggregated by artificial reefs using scuba diving and acoustic surveys. J. Mar. Sci. Technol. 2021, 29, 8. [Google Scholar] [CrossRef]
- Nakamura, T.; Hamano, A. Seasonal differences in the vertical distribution pattern of Japanese jack mackerel, Trachurus japonicus: Changes according to age? ICES J. Mar. Sci. 2009, 66, 1289–1295. [Google Scholar] [CrossRef]
- Tassetti, A.N.; Malaspina, S.; Fabi, G. Using a multibeam echosounder to monitor an artificial reef. Int. Arch. Photogramm. Remote Sens. Spat. Inf. Sci. 2015, 40, 207–213. [Google Scholar] [CrossRef]
- Zhu, K.; Xiao, B. Research of three-dimensional imaging sonar technology in the detection of underwater engineering project. In Advances in Civil Engineering and Environmental Engineering Volume 1; Yahya, W., Daud, Z., Eds.; CRC Press: London, UK, 2023; pp. 437–444. [Google Scholar] [CrossRef]
- Lurton, X. An Introduction to Underwater Acoustics: Principles and Applications, 2nd ed.; Praxis Publishing: Chichester, UK, 2010; pp. 1–724. [Google Scholar]
- Komatsu, T.; Igarashi, C.; Tatsukawa, K.; Sultana, S.; Matsuoka, Y.; Harada, S. Use of multi-beam sonar to map seagrass beds in Otsuchi Bay on the Sanriku Coast of Japan. Aquat. Living Resour. 2003, 16, 223–230. [Google Scholar] [CrossRef]
- Hamana, M.; Komatsu, T. Mapping 3D structure of a Sargassum forest with high-resolution sounding data obtained by multibeam echosounder. ICES J. Mari. Sci. 2021, 78, 1458–1469. [Google Scholar] [CrossRef]
- Weber, T.C. A CFAR detection approach for identifying gas bubble seeps with multibeam echo sounders. IEEE J. Ocean. Eng. 2021, 46, 1346–1355. [Google Scholar] [CrossRef]
- Zhang, W.; Zhou, T.; Li, J.; Xu, C. An efficient method for detection and quantitation of underwater gas leakage based on a 300-kHz multibeam sonar. Remote Sens. 2022, 14, 4301. [Google Scholar] [CrossRef]
- Brehmer, P.; Vercelli, C.; Gerlotto, F.; Sanguinède, F.; Pichot, Y.; Guennégan, Y.; Buestel, D. Multibeam sonar detection of suspended mussel culture grounds in the open sea: Direct observation methods for management purposes. Aquaculture 2006, 252, 234–241. [Google Scholar] [CrossRef]
- Gerlotto, F.; Soria, M.; Fréon, P. From two dimensions to three: The use of multibeam sonar for a new approach in fisheries acoustics. Can. J. Fish. Aquat. Sci. 1999, 56, 6–12. [Google Scholar] [CrossRef]
- Colbo, K.; Ross, T.; Brown, C.; Weber, T. A review of oceanographic applications of water column data from multibeam echosounders. Estuar. Coast. Shelf Sci. 2014, 145, 41–56. [Google Scholar] [CrossRef]
- Innangi, S.; Bonanno, A.; Tonielli, R.; Gerlotto, F.; Innangi, M.; Mazzola, S. High resolution 3-D shapes of fish schools: A new method to use the water column backscatter from hydrographic MultiBeam Echo Sounders. Appl. Acoust. 2016, 111, 148–160. [Google Scholar] [CrossRef]
- Misund, O.A.; Aglen, A. Swimming behaviour of fish schools in the North Sea during acoustic surveying and pelagic trawl sampling. ICES J. Mar. Sci. 1992, 49, 325–334. [Google Scholar] [CrossRef]
- Misund, O.A. Dynamics of moving masses: Variability in packing density, shape, and size among herring, sprat, and saithe schools. ICES J. Mar. Sci. 1993, 50, 145–160. [Google Scholar] [CrossRef]
- Hafsteinsson, M.T.; Misund, O.A. Recording the migration behaviour of fish schools by multi-beam sonar during conventional acoustic surveys. ICES J. Mar. Sci. 1995, 52, 915–924. [Google Scholar] [CrossRef]
- Nagasawa, R.; Horinouchi, R. Initial study on the visualization of physical structures in the ocean utilizing the water column imaging of multibeam echo sounders. Rep. Hydrogr. Oceanogr. Res. 2023, 61, 1–18, (In Japanese with English abstract and figure captions). [Google Scholar]
- Schimel, A.C.G.; Brown, C.J.; Ierodiaconou, D. Automated Filtering of multibeam water-column data to detect relative abundance of giant kelp (Macrocystis pyrifera). Remote Sens. 2020, 12, 1371. [Google Scholar] [CrossRef]
- Clarke, J.H. Applications of multibeam water column imaging for hydrographic survey. Hydrogr. J. 2006, 120, 1–33. [Google Scholar]
- Tanoue, H.; Komatsu, T.; Hamano, A. Determination of upper boundary of an acoustic blind zone produced by the rugged bottom during a survey using a quantitative echosounder. Bull. Jpn. Soc. Fish. Oceanogr. 2013, 77, 53–58. (In Japanese) [Google Scholar]
- Rasmuson, L. Susceptibility of Five Species of Rockfish (Sebastes spp.) to Different Survey Gears Inferred from High Resolution Behavioral Data; Science Bulletin, 2021–05; Oregon Department of Fish and Wildlife: Salem, OR, USA, 2021. [Google Scholar]
- Hamana, M. Development of Quantitative 3-Dimensional Mapping of Seagrass and Seaweed Beds Using Multibeam Sonar. Ph.D. Thesis, The University of Tokyo, Tokyo, Japan, 2016; pp. 1–82. (In Japanese). [Google Scholar]
- New Energy and Industrial Technology Development Organization. NEDO Offshore Wind Energy Progress Edition II; New Energy Technology Department, New Energy and Industrial Technology Development Organization: Kawasaki, Japan, 2013; pp. 1–22. Available online: https://www.nedo.go.jp/library/pamphlets/FF_201306_yojofuryoku_e.html (accessed on 5 May 2024).
- Copernicus Marine Service. Global Ocean Physics Analysis and Forecast; E.U. Copernicus Marine Service Information (CMEMS), Marine Data Store (MDS): Toulouse, France, 2025. [Google Scholar] [CrossRef]
- François, R.E.; Garrison, G.R. Sound absorption based on ocean measurements. Part II: Boric acid contribution and equation for total absorption. J. Acoust. Soc. Am. 1982, 72, 1879–1890. [Google Scholar] [CrossRef]
- Quality Positioning Services, B.V. Fledermaus 7.8 Documentation FMGT 7.10/FMMidwater 7.9; QPS BV: Zeist, The Netherlands, 2022; pp. 1–539. Available online: https://publicdownload.qps.nl/Fledermaus/Fledermaus-7.8-Documentation_v3.pdf (accessed on 1 May 2024).
- Urban, P.; Köser, K.; Greinert, J. Processing of multibeam water column image data for automated bubble/seep detection and repeated mapping. Limnol. Oceanogr. Methods 2017, 15, 1–21. [Google Scholar] [CrossRef]
- Nau, A.W.; Scoulding, B.; Kloser, R.J.; Ladroit, Y.; Lucieer, V. Extended detection of shallow water gas seeps from multibeam echosounder water column data. Front. Remote Sens. 2022, 3, 839417. [Google Scholar] [CrossRef]
- R2SONIC, Inc. Sonic 2026/2024/2022 Broadband Multibeam Echosounders, Operation Manual V6.3; R2SONIC: Austin, TX, USA, 2022; pp. 1–254. Available online: https://www.manualslib.com/manual/2991159/R2sonic-2026.html#manual (accessed on 17 February 2023).
- Foote, K.G.; Chu, D.; Hammar, T.R.; Baldwin, K.C.; Mayer, L.A.; Hufnagle, L.C., Jr.; Jech, J.M. Protocols for calibrating multibeam sonar. J. Acoust. Soc. Am. 2005, 117, 2013–2027. [Google Scholar] [CrossRef]
- Noguchi, S. Developments of hydroacoustic measurement technology. Oki Tech. Rev. 2005, 72, 38–41. [Google Scholar]
- Aoki, I.; Inagaki, T.; Long, L.V. Measurements of the three-dimensional structure of free-swimming pelagic fish schools in a natural environment. Bull. Jpn. Soc. Sci. Fish. 1986, 52, 2069–2077. [Google Scholar] [CrossRef][Green Version]
- Pavlov, D.S.; Kasumyan, A.O. Patterns and mechanisms of schooling behavior in fish: A review. J. Ichthyol. 2000, 40, S163. [Google Scholar]
- Nakamura, T.; Hamano, A.; Abe, K.; Yasuma, H.; Miyashita, K. Acoustic scattering properties of juvenile jack mackerel Trachurus japonicus based on a scattering model and ex situ target strength measurements. Nippon Suisan Gakkaishi 2013, 79, 345–354, (In Japanese with English abstract and figure captions). [Google Scholar] [CrossRef][Green Version]
- Genin, A. Bio-physical coupling in the formation of zooplankton and fish aggregations over abrupt topographies. J. Mar. Syst. 2004, 50, 3–20. [Google Scholar] [CrossRef]
- Van Hal, R.; Griffioen, A.B.; van Keeken, O.A. Changes in fish communities on a small spatial scale, an effect of increased habitat complexity by an offshore wind farm. Mar. Environ. Res. 2017, 126, 26–36. [Google Scholar] [CrossRef]
- Washio, T.; Sakamoto, N.; Nakashima, S.; Aoki, I.; Kawaguchi, K.; Nagai, T.; Nakai, K. Construction of ocean observation tower system off the Kita-Kyushu city for offshore wind farm design. J. Jpn. Soc. Civ. Eng. Ser. B3 Ocean. Eng. 2013, 69, I_1–I_6. (In Japanese) [Google Scholar]
- Yoshimura, Y. Present situation of offshore wind power generation system proving research in Kitakyushu city. Proc. Japan Wind Energy Sympo. 2013, 35, 478–481. (In Japanese) [Google Scholar]
- Shimaya, M.; Chikarashi, M.; Sasaki, M.; Nakai, T.; Yamaguchi, Y. Construction Management for Offshore Wind Power Facilities. J. Jpn. Soc. Civ. Eng. Ser. B3 Ocean. Eng. 2013, 69, I_114–I_119. (In Japanese) [Google Scholar]
- Sheng, Y.P. Chapter 3 Physical Characteristics and Engineering at Reef Sites. In Artificial Reef Evaluation: With Application to Natural Marine Habitats; Seaman, W., Ed.; CRC Press: Boca Raton, FL, USA, 2000; pp. 51–94. [Google Scholar]
- Liao, J.C.; Beal, D.N.; Lauder, G.V.; Triantafyllou, M.S. Fish exploiting vortices decrease muscle activity. Science 2003, 302, 1566–1569. [Google Scholar] [CrossRef]
- Takeuchi, T. Design of artificial reefs in consideration of environmental characteristics. In Japan–US Symposium on Artificial Habitats for Fisheries Proceedings; Nakamura, M., Grove, R.S., Sonu, C.J., Eds.; Southern California Edison Co.: Rosemead, CA, USA, 1991; pp. 195–203. [Google Scholar]
- Seto, M. Studies on development of wake flow and generation of unsteady hydrodynamic force. Fish. Eng. 2001, 38, 127–132. (In Japanese) [Google Scholar]
- Rasmuson, L.K.; Marion, S.R.; Fields, S.A.; Blume, M.T.O.; Lawrence, K.A.; Rankin, P.S. Influence of near bottom fish distribution on the efficacy of a combined hydroacoustic video survey. ICES J. Mar. Sci. 2022, 79, 2069–2083. [Google Scholar] [CrossRef]
- Bazigos, G.P. A Manual on Acoustic Surveys: Sampling Methods for Acoustic Surveys; Food and Agriculture Organization: Rome, Italy, 1981; 203p. [Google Scholar]
- Kloser, R.J. Improved precision of acoustic surveys of benthopelagic fish by means of a deep-towed transducer. ICES J. Mar. Sci. 1996, 53, 407–413. [Google Scholar] [CrossRef]
- Sawada, K.; Takahashi, H.; Takao, Y.; Watanabe, K.; Horne, J.K.; McClatchie, S.; Abe, K. (2004, November). Development of an acoustic-optical system to estimate target-strengths and tilt angles from fish aggregations. In Proceedings of the Oceans’ 04 MTS/IEEE Techno-Ocean’04 (IEEE Cat. No. 04CH37600), Kobe, Japan, 9–12 November 2004; Volume 1, pp. 395–400. [Google Scholar]
- Sawada, K.; Takahashi, H.; Abe, K.; Takao, Y. In situ measurement of target strength, tilt angle, and swimming speed of Boreopacific gonate squid (Gonatopsis borealis). J. Acoust. Soc. Am. 2006, 120 (Suppl. S5), 3107. [Google Scholar] [CrossRef]
- Scoulding, B.; Gastauer, S.; Taylor, J.C.; Boswell, K.M.; Fairclough, D.V.; Jackson, G.; Sullivan, P.; Shertzer, K.; Campanella, F.; Bacheler, N.; et al. Estimating abundance of fish associated with structured habitats by combining acoustics and optics. J. Appl. Ecol. 2023, 60, 1274–1285. [Google Scholar] [CrossRef]

| Parameter | Value |
|---|---|
| Frequency (kHz) | 200 |
| Swath Coverage (degrees) | 150 |
| Number of beams | 256 |
| Pulse length (μs) | 100 |
| Power (dB) | 215 |
| Gain (dB) | 10 |
| Depth (m) | Fish Cell Count | Biomass (Inds) | ||
|---|---|---|---|---|
| OWF | Control | OWF | Control | |
| −3 | 19 | 9 | 2.8 | 1.3 |
| −4 | 6 | 6 | 2.3 | 0.7 |
| −5 | 13 | 7 | 8.9 | 0.7 |
| −6 | 46 | 6 | 27.6 | 0.9 |
| −7 | 146 | 20 | 67.1 | 2.0 |
| −8 | 280 | 35 | 124.9 | 4.4 |
| −9 | 422 | 54 | 245.5 | 17.6 |
| −10 | 814 | 44 | 396.6 | 21.1 |
| −11 | 1450 | 13 | 723.2 | 3.3 |
| −12 | 1976 | 1019.8 | ||
| −13 | 1660 | 850.0 | ||
| −14 | 5 | 7.3 | ||
| −15 | 4 | 0.5 | ||
| Total | 6841 | 194 | 3476.5 | 52.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hamana, M.; Gonzalvo, S.; Otaki, T.; Komatsu, T. Development of a Quantitative Survey Method for Pelagic Fish Aggregations Around an Offshore Wind Farm Using Multibeam Sonar. Remote Sens. 2025, 17, 3255. https://doi.org/10.3390/rs17183255
Hamana M, Gonzalvo S, Otaki T, Komatsu T. Development of a Quantitative Survey Method for Pelagic Fish Aggregations Around an Offshore Wind Farm Using Multibeam Sonar. Remote Sensing. 2025; 17(18):3255. https://doi.org/10.3390/rs17183255
Chicago/Turabian StyleHamana, Masahiro, Sara Gonzalvo, Takayoshi Otaki, and Teruhisa Komatsu. 2025. "Development of a Quantitative Survey Method for Pelagic Fish Aggregations Around an Offshore Wind Farm Using Multibeam Sonar" Remote Sensing 17, no. 18: 3255. https://doi.org/10.3390/rs17183255
APA StyleHamana, M., Gonzalvo, S., Otaki, T., & Komatsu, T. (2025). Development of a Quantitative Survey Method for Pelagic Fish Aggregations Around an Offshore Wind Farm Using Multibeam Sonar. Remote Sensing, 17(18), 3255. https://doi.org/10.3390/rs17183255








