Towards a General Monitoring System for Terrestrial Primary Production: A Test Spanning the European Drought of 2018
Abstract
1. Introduction
2. Materials and Methods
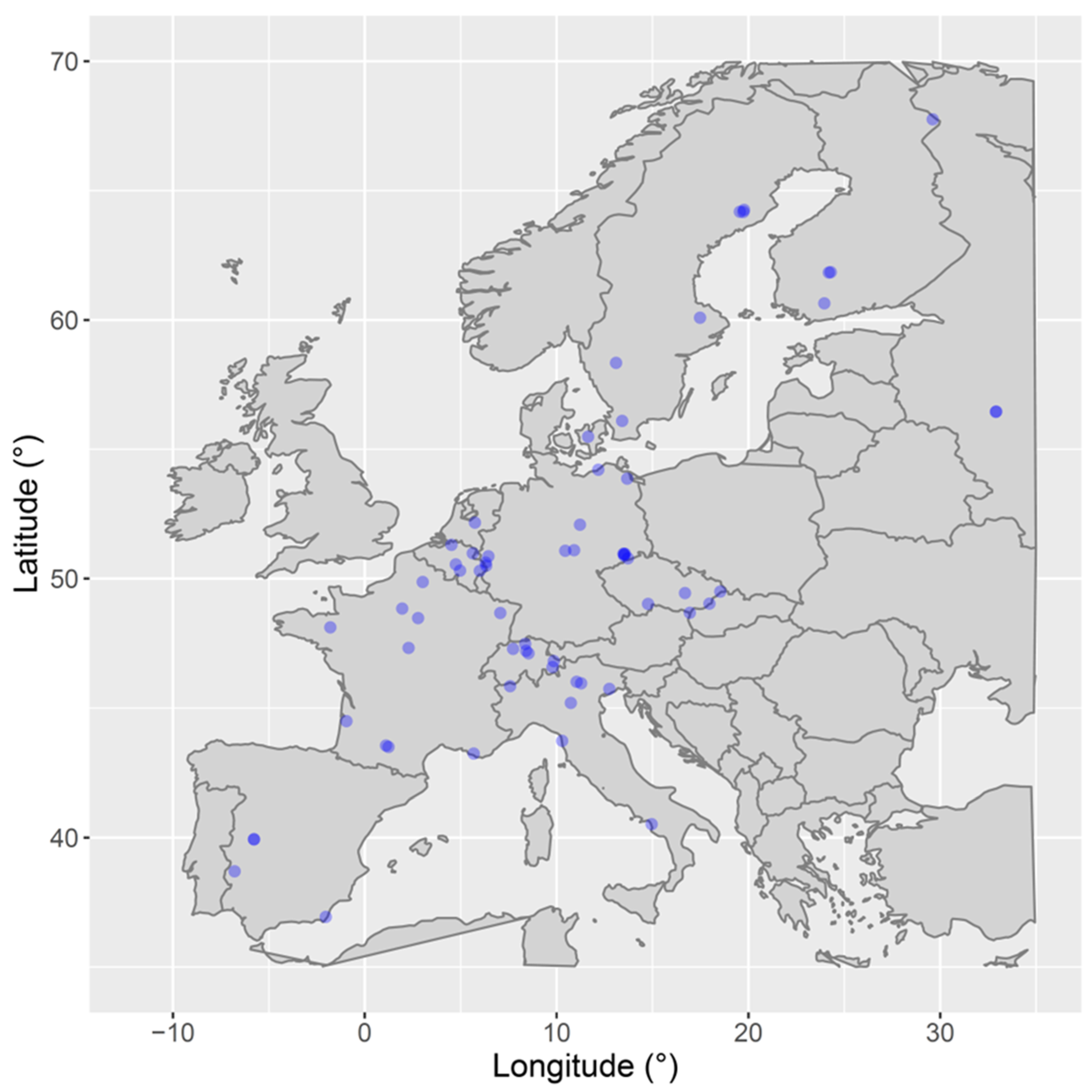
2.1. Validation Data
2.2. Drought Index
2.3. Driving Variables for the P Model Simulations
2.4. C3 versus C4 Photosynthesis
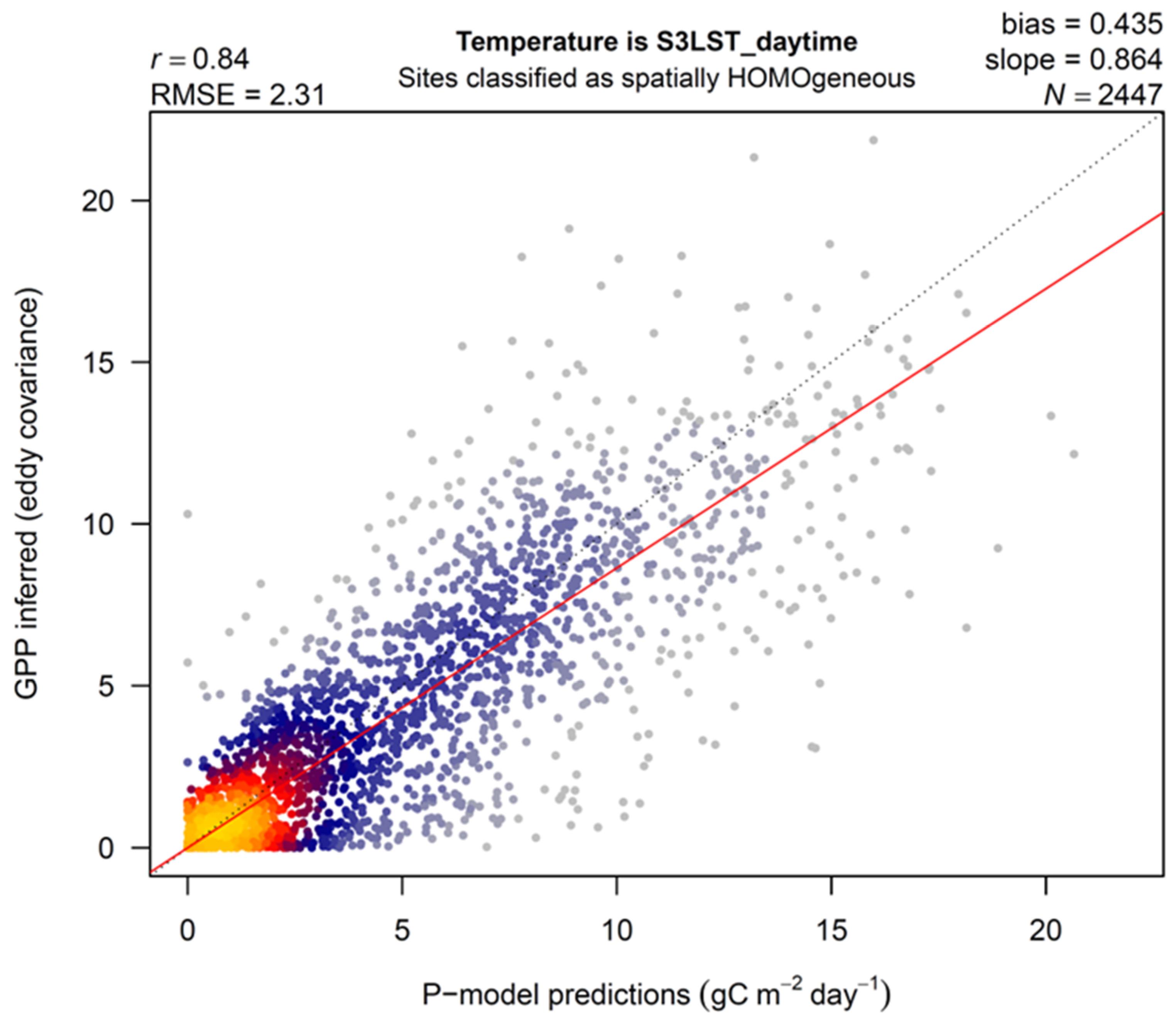
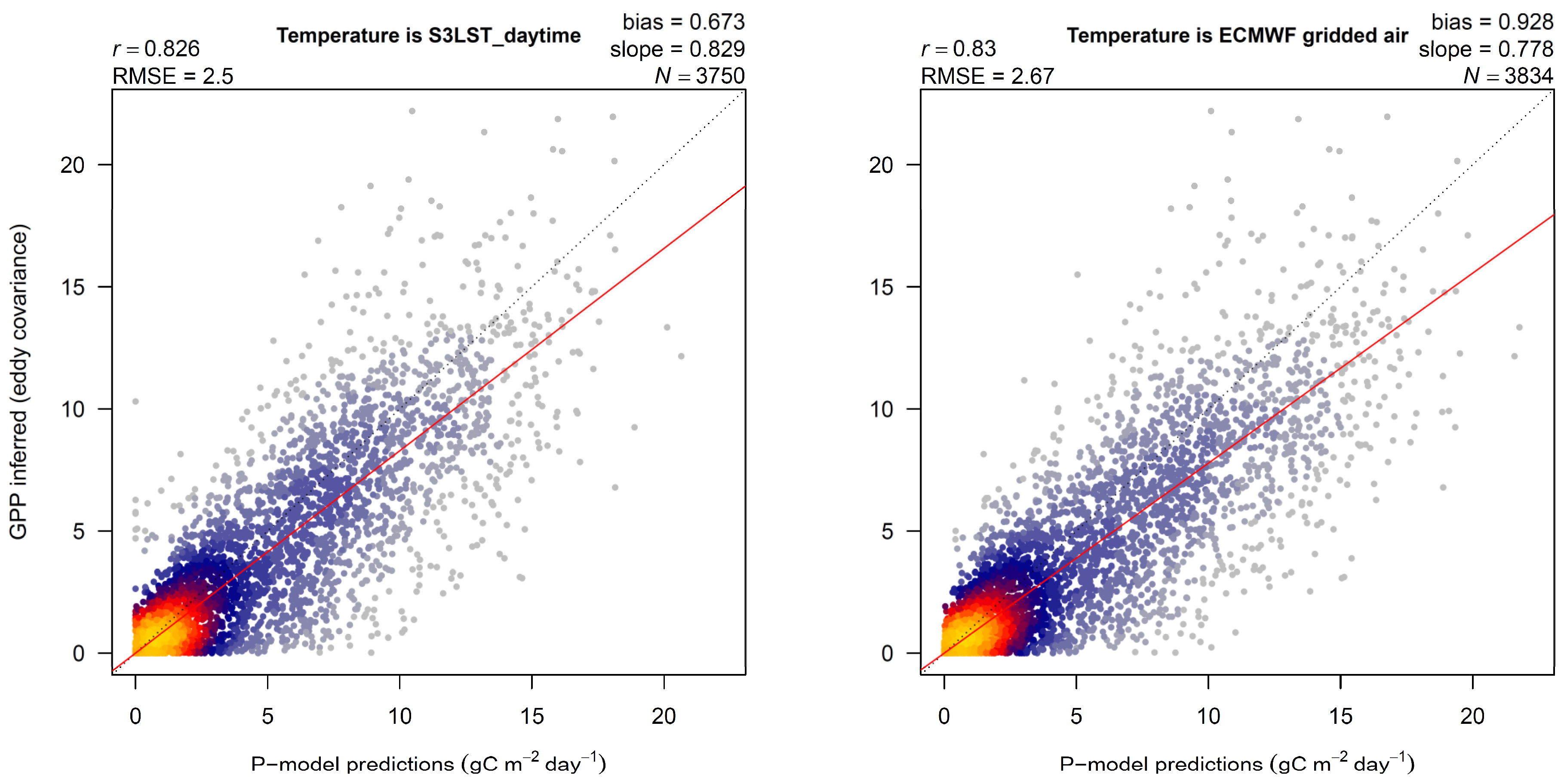
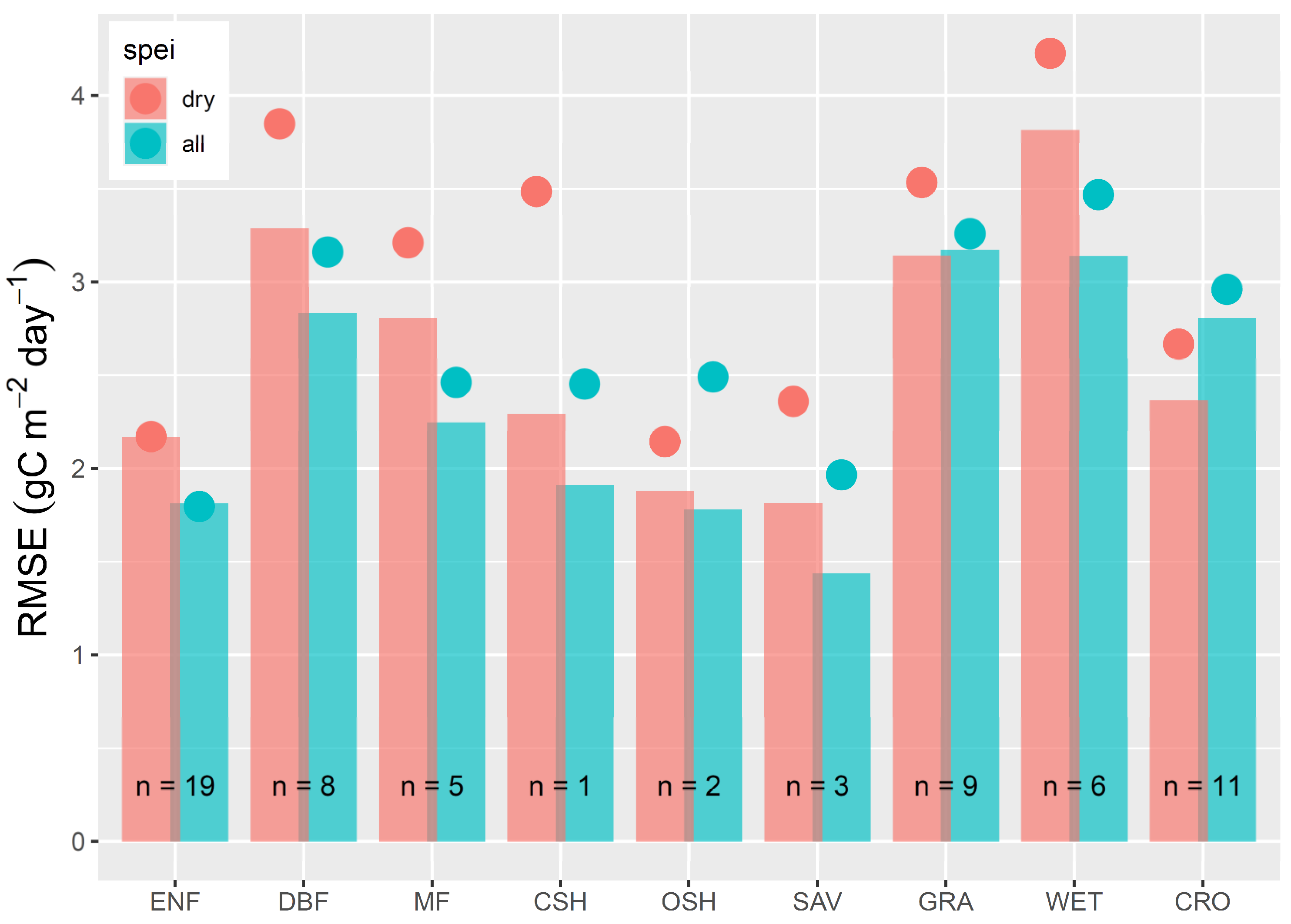
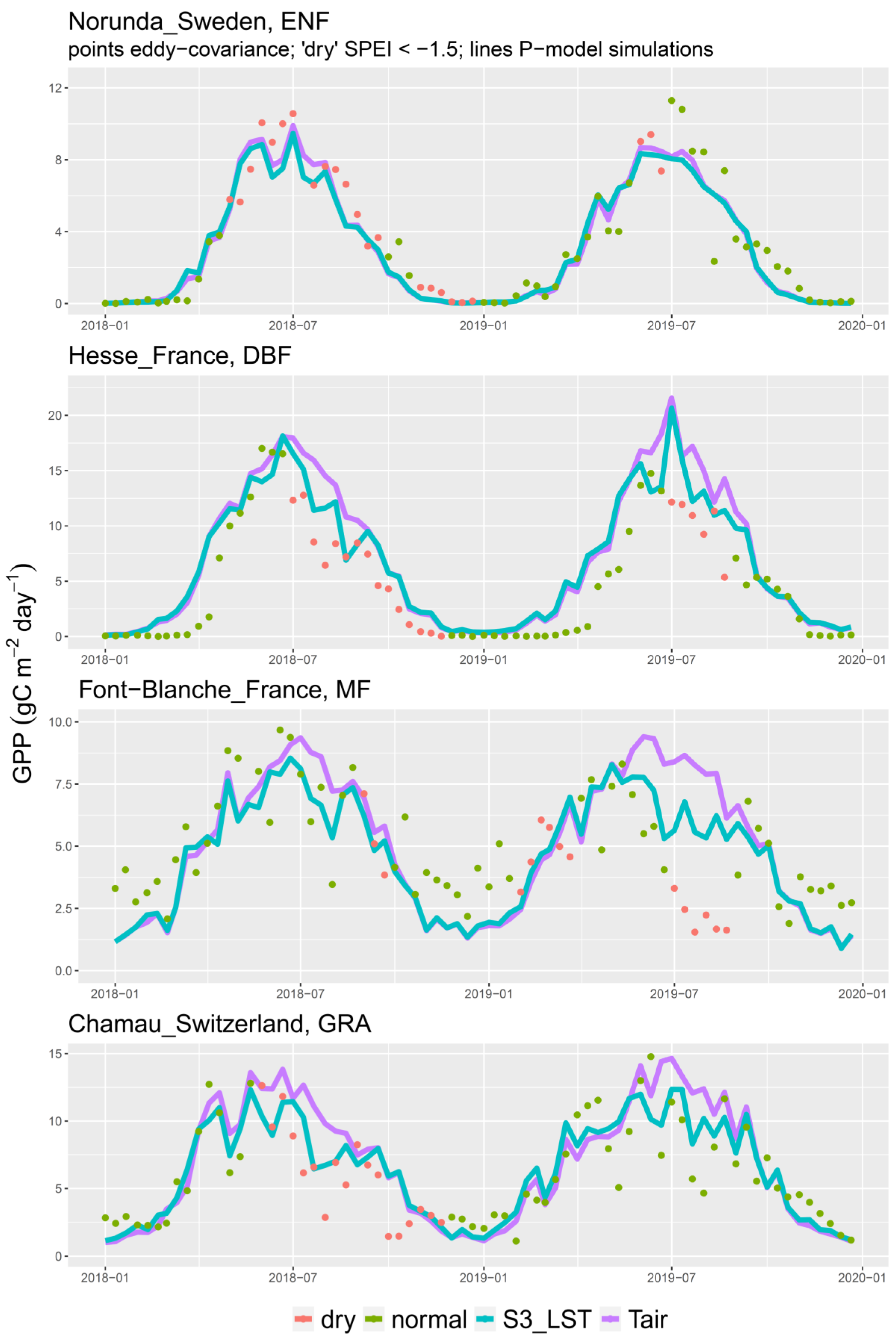
3. Results
4. Discussion
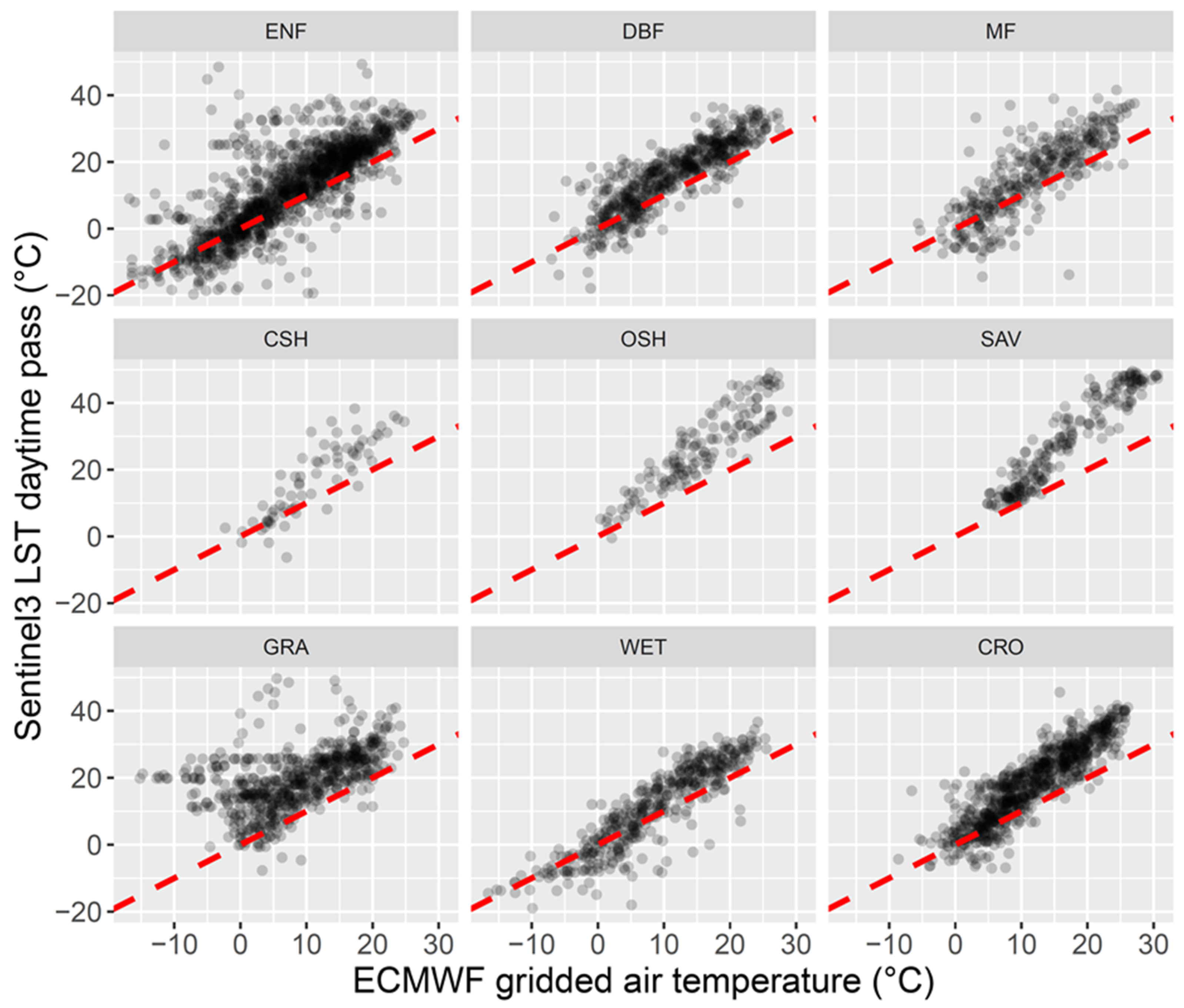
4.1. LST versus Tair
4.2. Possible Data Imprecisions
4.3. The 2018 Drought
4.4. Next Steps for the Application of LST
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Code | Sitename | Lat | Lon | Elevation (masl) | IGBP Code | Metabolism | Homogeneity |
|---|---|---|---|---|---|---|---|
| BE-Bra | Brasschaat | 51.308 | 4.520 | 30 | MF | C3 | Homo |
| BE-Dor | Dorinne | 50.312 | 4.968 | 248 | GRA | C3 | Hetero |
| BE-Lon | Lonzee | 50.552 | 4.746 | 169 | CRO | C3 | Homo |
| BE-Maa | Maasmechelen | 50.980 | 5.632 | 86 | CSH | C3 | Homo |
| BE-Vie | Vielsalm | 50.305 | 5.998 | 495 | MF | C3 | Homo |
| CH-Aws | Alp Weissenstein | 46.583 | 9.790 | 1969 | GRA | C3 | Hetero |
| CH-Cha | Chamau | 47.210 | 8.410 | 391 | GRA | C3 | Hetero |
| CH-Dav | Davos | 46.815 | 9.856 | 1652 | ENF | C3 | Hetero |
| CH-Fru | Früebüel | 47.116 | 8.538 | 980 | GRA | C3 | Hetero |
| CH-Lae | Laegern | 47.478 | 8.364 | 685 | MF | C3 | Homo |
| CH-Oe2 | Oensingen crop | 47.286 | 7.734 | 452 | CRO | C3 | Hetero |
| CZ-BK1 | Bily Kriz forest | 49.502 | 18.537 | 876 | ENF | C3 | Homo |
| CZ-Lnz | Lanzhot | 48.682 | 16.946 | 181 | MF | C3 | Homo |
| CZ-RAJ | Rajec | 49.444 | 16.697 | 653 | ENF | C3 | Homo |
| CZ-Stn | Stitna | 49.036 | 17.970 | 580 | DBF | C3 | Homo |
| CZ-wet | Trebon (CZECHWET) | 49.025 | 14.770 | 426 | WET | C3 | Hetero |
| DE-Akm | Anklam | 53.866 | 13.683 | - | WET | C3 | Hetero |
| DE-Geb | Gebesee | 51.100 | 10.915 | 161 | CRO | C3 | Homo |
| DE-Gri | Grillenburg | 50.950 | 13.513 | 377 | GRA | C3 | Hetero |
| DE-Hai | Hainich | 51.079 | 10.453 | 467 | DBF | C3 | Homo |
| DE-HoH | Hohes Holz | 52.085 | 11.219 | 220 | DBF | C3 | Hetero |
| DE-Hte | Huetelmoor | 54.210 | 12.176 | 2 | WET | C3 | Homo |
| DE-Hzd | Hetzdorf | 50.964 | 13.490 | 385 | DBF | C3 | Hetero |
| DE-Kli | Klingenberg | 50.893 | 13.522 | 481 | CRO | Rotation: C3 2018, C4 2019 | Homo |
| DE-Obe | Oberbärenburg | 50.787 | 13.721 | 755 | ENF | C3 | Homo |
| DE-RuR | Rollesbroich | 50.622 | 6.304 | 515 | GRA | C3 | Hetero |
| DE-RuS | Selhausen Juelich | 50.866 | 6.447 | 103 | CRO | C3 | Homo |
| DE-RuW | Wustebach | 50.505 | 6.331 | 624 | ENF | C3 | Homo |
| DE-Tha | Tharandt | 50.963 | 13.565 | 403 | ENF | C3 | Homo |
| DK-Sor | Soroe | 55.486 | 11.645 | 52 | DBF | C3 | Hetero |
| ES-Abr | Albuera | 38.702 | −6.786 | 280 | SAV | C3 | Homo |
| ES-Agu | Aguamarga | 36.940 | −2.033 | 203 | OSH | C3 | Hetero |
| ES-LM1 | Majadas del Tietar North | 39.943 | −5.779 | 264 | SAV | C3 | Homo |
| ES-LM2 | Majadas del Tietar South | 39.935 | −5.776 | 269 | SAV | C3 | Homo |
| FI-Hyy | Hyytiala | 61.847 | 24.295 | 190 | ENF | C3 | Homo |
| FI-Let | Lettosuo | 60.642 | 23.960 | 124 | ENF | C3 | Homo |
| FI-Sii | Siikaneva | 61.833 | 24.193 | 166 | WET | C3 | Hetero |
| FI-Var | Varrio | 67.755 | 29.610 | 395 | ENF | C3 | Homo |
| FR-Aur | Aurade | 43.550 | 1.106 | 244 | CRO | C3 | Homo |
| FR-Bil | Bilos | 44.494 | −0.956 | 39 | ENF | C3 | Hetero |
| FR-EM2 | Estrees-Mons A28 | 49.872 | 3.021 | 85 | CRO | C3 | Homo |
| FR-FBn | Font-Blanche | 43.241 | 5.679 | 434 | MF | C3 | Homo |
| FR-Fon | Fontainebleau-Barbeau | 48.476 | 2.780 | 112 | DBF | C3 | Homo |
| FR-Gri | Grignon | 48.844 | 1.952 | 123 | CRO | C4 | Homo |
| FR-Hes | Hesse | 48.674 | 7.065 | 323 | DBF | C3 | Homo |
| FR-Lam | Lamasquere | 43.496 | 1.238 | 179 | CRO | Rotation: C3 2018, C4 2019 | Hetero |
| FR-LGt | La Guette | 47.323 | 2.284 | 157 | WET | C3 | Hetero |
| FR-Mej | Mejusseaume | 48.118 | −1.796 | 39 | GRA | C4 | Homo |
| IT-BFt | Bosco Fontana | 45.198 | 10.742 | 42 | DBF | C3 | Homo |
| IT-BCi | Borgo Cioffi | 40.524 | 14.957 | 9 | CRO | C4 | Hetero |
| IT-Lav | Lavarone | 45.956 | 11.281 | 1371 | ENF | C3 | Homo |
| IT-Lsn | Lison | 45.740 | 12.750 | - | OSH | C3 | Hetero |
| IT-MBo | Monte Bondone | 46.015 | 11.046 | 1557 | GRA | C3 | Homo |
| IT-SR2 | San Rossore 2 | 43.732 | 10.291 | 10 | ENF | C3 | Homo |
| IT-Tor | Torgnon | 45.844 | 7.578 | 2158 | GRA | C3 | Hetero |
| NL-Loo | Loobos | 52.167 | 5.744 | 37 | ENF | C3 | Homo |
| RU-Fy2 | Fyodorovskoye, dry spruce stand | 56.448 | 32.902 | 275 | ENF | C3 | Homo |
| RU-Fyo | Fyodorovskoye | 56.462 | 32.922 | 278 | ENF | C3 | Homo |
| SE-Deg | Degero | 64.182 | 19.557 | 266 | WET | C3 | Homo |
| SE-Htm | Hyltemossa | 56.098 | 13.419 | 123 | ENF | C3 | Homo |
| SE-Lnn | Lanna | 58.341 | 13.102 | 71 | CRO | C3 | Homo |
| SE-Nor | Norunda | 60.086 | 17.480 | 89 | ENF | C3 | Homo |
| SE-Ros | Rosinedal-3 | 64.173 | 19.738 | 168 | ENF | C3 | Homo |
| SE-Svb | Svartberget | 64.256 | 19.775 | 277 | ENF | C3 | Homo |
References
- Beer, C.; Reichstein, M.; Tomelleri, E.; Ciais, P.; Jung, M.; Carvalhais, N.; Rödenbeck, C.; Arain, M.A.; Baldocchi, D.; Bonan, G.B.; et al. Terrestrial gross carbon dioxide uptake: Global distribution and covariation with climate. Science 2010, 329, 834–838. [Google Scholar] [CrossRef] [PubMed]
- Friedlingstein, P.; Meinshausen, M.; Arora, V.K.; Jones, C.D.; Anav, A.; Liddicoat, S.K.; Knutti, R. Uncertainties in CMIP5 Climate Projections due to Carbon Cycle Feedbacks. J. Clim. 2014, 27, 511–526. [Google Scholar] [CrossRef]
- Baldocchi, D.D. How eddy covariance flux measurements have contributed to our understanding of Global Change Biology. Glob. Chang. Biol. 2020, 26, 242–260. [Google Scholar] [CrossRef] [PubMed]
- Pastorello, G.; Trotta, C.; Canfora, E.; Chu, H.; Christianson, D.; Cheah, Y.-W.; Poindexter, C.; Chen, J.; Elbashandy, A.; Humphrey, M.; et al. The FLUXNET2015 dataset and the ONEFlux processing pipeline for eddy covariance data. Sci. Data 2020, 7, 225. [Google Scholar] [CrossRef]
- Baldocchi, D.D. Assessing the eddy covariance technique for evaluating carbon dioxide exchange rates of ecosystems: Past, present and future. Glob. Chang. Biol. 2003, 9, 479–492. [Google Scholar] [CrossRef]
- Chu, H.; Luo, X.; Ouyang, Z.; Chan, W.S.; Dengel, S.; Biraud, S.C.; Torn, M.S.; Metzger, S.; Kumar, J.; Arain, M.A.; et al. Representativeness of Eddy-Covariance flux footprints for areas surrounding AmeriFlux sites. Agric. For. Meteorol. 2021, 301, 108350. [Google Scholar] [CrossRef]
- Farquhar, G.D.; Von Caemmerer, S.; Berry, J.A. A biochemical model of photosynthetic CO2 assimilation in leaves of C3 species. Planta 1980, 149, 78–90. [Google Scholar] [CrossRef]
- Medlyn, B. Physiological basis of the light use efficiency model. Tree Physiol. 1998, 18, 167–176. [Google Scholar] [CrossRef]
- Dewar, R.C.; Medlyn, B.; McMurtrie, R.E. A mechanistic analysis of light and carbon use efficiencies. Plant Cell Environ. 1998, 21, 573–588. [Google Scholar] [CrossRef]
- Monteith, J.L. Solar Radiation and Productivity in Tropical Ecosystems. J. Appl. Ecol. 1972, 9, 747–766. [Google Scholar] [CrossRef]
- Monteith, J.L. Climate and the efficiency of crop production in Britain. Philos. Trans. R. Soc. B 1977, 281, 277–294. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, X.; Ren, J. Simulation of Gross Primary Productivity Using Multiple Light Use Efficiency Models. Land 2021, 10, 329. [Google Scholar] [CrossRef]
- Prentice, I.C.; Liang, X.; Medlyn, B.E.; Wang, Y.-P. Reliable, robust and realistic: The three R’s of next-generation land-surface modelling. Atmospheric Meas. Tech. 2015, 15, 5987–6005. [Google Scholar] [CrossRef]
- Wang, H.; Prentice, I.C.; Keenan, T.F.; Davis, T.W.; Wright, I.J.; Cornwell, W.K.; Evans, B.J.; Peng, C. Towards a universal model for carbon dioxide uptake by plants. Nat. Plants 2017, 3, 734–741. [Google Scholar] [CrossRef]
- Stocker, B.D.; Wang, H.; Smith, N.G.; Harrison, S.P.; Keenan, T.F.; Sandoval, D.; Davis, T.; Prentice, I.C. P-model v1.0: An optimality-based light use efficiency model for simulating ecosystem gross primary production. Geosci. Model Dev. 2020, 13, 1545–1581. [Google Scholar] [CrossRef]
- Cai, W.; Prentice, I.C. Recent trends in gross primary production and their drivers: Analysis and modelling at flux-site and global scales. Environ. Res. Lett. 2020, 15, 124050. [Google Scholar] [CrossRef]
- Bernacchi, C.J.; Pimentel, C.; Long, S.P. In vivo temperature response functions of parameters required to model RuBP-limited photosynthesis. Plant Cell Environ. 2003, 26, 1419–1430. [Google Scholar] [CrossRef]
- Rogers, A.; Medlyn, B.E.; Dukes, J.S.; Bonan, G.; von Caemmerer, S.; Dietze, M.C.; Kattge, J.; Leakey, A.D.B.; Mercado, L.M.; Niinemets, Ü.; et al. A roadmap for improving the representation of photosynthesis in Earth system models. New Phytol. 2017, 213, 22–42. [Google Scholar] [CrossRef]
- Bernacchi, C.J.; Singsaas, E.L.; Pimentel, C.; Portis, A.R., Jr.; Long, S.P. Improved temperature response functions for models of Rubisco-limited photosynthesis. Plant Cell Environ. 2001, 24, 253–259. [Google Scholar] [CrossRef]
- Berry, J.A.; Bjorkman, O. Photosynthetic Response and Adaptation to Temperature in Higher Plants. Annu. Rev. Plant Physiol. 1980, 31, 491–543. [Google Scholar] [CrossRef]
- Kumarathunge, D.P.; Medlyn, B.E.; Drake, J.; Tjoelker, M.G.; Aspinwall, M.J.; Battaglia, M.; Cano, F.J.; Carter, K.R.; Cavaleri, M.A.; Cernusak, L.A.; et al. Acclimation and adaptation components of the temperature dependence of plant photosynthesis at the global scale. New Phytol. 2019, 222, 768–784. [Google Scholar] [CrossRef] [PubMed]
- Still, C.J.; Rastogi, B.; Page, G.F.M.; Griffith, D.M.; Sibley, A.; Schulze, M.; Hawkins, L.; Pau, S.; Detto, M.; Helliker, B.R. Imaging canopy temperature: Shedding (thermal) light on ecosystem processes. New Phytol. 2021, 230, 1746–1753. [Google Scholar] [CrossRef] [PubMed]
- Dong, N.; Prentice, I.C.; Harrison, S.P.; Song, Q.H.; Zhang, Y.P.; Sykes, M. Biophysical homoeostasis of leaf temperature: A neglected process for vegetation and land-surface modelling. Glob. Ecol. Biogeogr. 2017, 26, 998–1007. [Google Scholar] [CrossRef]
- Fauset, S.; Freitas, H.C.; Galbraith, D.R.; Sullivan, M.J.; Aidar, M.P.; Joly, C.A.; Phillips, O.L.; Vieira, S.A.; Gloor, M.U. Differences in leaf thermoregulation and water use strategies between three co-occurring Atlantic forest tree species. Plant Cell Environ. 2018, 41, 1618–1631. [Google Scholar] [CrossRef]
- Cowan, I.; Farquhar, G. Stomatal function in relation to leaf metabolism and environment. In Integration of Activity in Higher Plants; Jennings, D., Ed.; Cambridge University Press: Cambridge, UK, 1977; pp. 471–505. [Google Scholar]
- Medlyn, B.E.; Duursma, R.A.; Eamus, D.; Ellsworth, D.S.; Prentice, I.C.; Barton, C.V.M.; Crous, K.Y.; De Angelis, P.; Freeman, M.; Wingate, L. Reconciling the optimal and empirical approaches to modelling stomatal conductance. Glob. Chang. Biol. 2011, 17, 2134–2144. [Google Scholar] [CrossRef]
- Buras, A.; Rammig, A.; Zang, C.S. Quantifying impacts of the 2018 drought on European ecosystems in comparison to 2003. Biogeosciences 2020, 17, 1655–1672. [Google Scholar] [CrossRef]
- Ciais, P.; Reichstein, M.; Viovy, N.; Granier, A.; Ogée, J.; Allard, V.; Aubinet, M.; Buchmann, N.; Bernhofer, C.; Carrara, A.; et al. Europe-wide reduction in primary productivity caused by the heat and drought in 2003. Nature 2005, 437, 529–533. [Google Scholar] [CrossRef]
- Vereecken, H.; Schnepf, A.; Hopmans, J.; Javaux, M.; Or, D.; Roose, T.; Vanderborght, J.; Young, M.; Amelung, W.; Aitkenhead, M.; et al. Modeling Soil Processes: Review, Key Challenges, and New Perspectives. Vadose Zone J. 2016, 15, 1–57. [Google Scholar] [CrossRef]
- Fu, Z.; Ciais, P.; Prentice, I.C.; Gentine, P.; Makowski, D.; Bastos, A.; Luo, X.; Green, J.K.; Stoy, P.C.; Yang, H.; et al. Atmospheric dryness reduces photosynthesis along a large range of soil water deficits. Nat. Commun. 2022, 13, 989. [Google Scholar] [CrossRef]
- Vicca, S.; Balzarolo, M.; Filella, I.; Granier, A.; Herbst, M.; Knohl, A.; Longdoz, B.; Mund, M.; Nagy, Z.; Pintér, K.; et al. Remotely-sensed detection of effects of extreme droughts on gross primary production. Sci. Rep. 2016, 6, 28269. [Google Scholar] [CrossRef]
- Stocker, B.D.; Zscheischler, J.; Keenan, T.F.; Prentice, I.C.; Peñuelas, J.; Seneviratne, S.I. Quantifying soil moisture impacts on light use efficiency across biomes. New Phytol. 2018, 218, 1430–1449. [Google Scholar] [CrossRef]
- Zhou, S.; Duursma, R.A.; Medlyn, B.E.; Kelly, J.W.; Prentice, I.C. How should we model plant responses to drought? An analysis of stomatal and non-stomatal responses to water stress. Agric. For. Meteorol. 2013, 182, 204–214. [Google Scholar] [CrossRef]
- Stocker, B.D.; Zscheischler, J.; Keenan, T.F.; Prentice, I.C.; Seneviratne, S.I.; Peñuelas, J. Drought impacts on terrestrial primary production underestimated by satellite monitoring. Nat. Geosci. 2019, 12, 264–270. [Google Scholar] [CrossRef]
- Slette, I.J.; Post, A.K.; Awad, M.; Even, T.; Punzalan, A.; Williams, S.; Smith, M.D.; Knapp, A.K. How ecologists define drought, and why we should do better. Glob. Chang. Biol. 2019, 25, 3193–3200. [Google Scholar] [CrossRef]
- Vicente-Serrano, S.M.; Beguería, S.; López-Moreno, J.I. A Multiscalar Drought Index Sensitive to Global Warming: The Standardized Precipitation Evapotranspiration Index. J. Clim. 2010, 23, 1696–1718. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2020; Available online: http://www.R-project.org (accessed on 15 February 2023).
- Stocker, B.D. rpmodel v1.0.4. Available online: https://zenodo.org/record/3560169#.ZBl05HZBxD8 (accessed on 15 February 2023).
- Meek, D.W.; Hatfield, J.L.; Howell, T.A.; Idso, S.B.; Reginato, R.J. A Generalized Relationship between Photosynthetically Active Radiation and Solar Radiation. Agron. J. 1984, 76, 939–945. [Google Scholar] [CrossRef]
- Allen, R.; Pereira, L.; Raes, D.; Smith, M. Crop Evapotranspiration-Guidelines for Computing Crop Water Requirements; FAO: Rome, Italy, 1998; p. 56. Available online: http://www.fao.org/docrep/x0490e/x0490e00.htm (accessed on 26 October 2022).
- Ghent, D.; Dodd, E.; Veal, K.; Perry, M.; Jimenez, C.; Ermida, S. CCI Land Surface Temperature Algorithm Theoretical Basis Document. LST-CCI-D2.2-ATBD. 2021. Available online: https://admin.climate.esa.int/media/documents/LST-CCI-D2.2-ATBD_-_i3r0_-_Algorithm_Theoretical_Basis_Document.pdf (accessed on 15 February 2023).
- Pabon-Moreno, D.E.; Migliavacca, M.; Reichstein, M.; Mahecha, M.D. On the Potential of Sentinel-2 for Estimating Gross Primary Production. IEEE Trans. Geosci. Remote Sens. 2022, 60, 4409412. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhao, L.; Lin, A. Evaluating the Performance of Sentinel-3A OLCI Land Products for Gross Primary Productivity Estimation Using AmeriFlux Data. Remote Sens. 2020, 12, 1927. [Google Scholar] [CrossRef]
- Grossiord, C.; Buckley, T.N.; Cernusak, L.A.; Novick, K.A.; Poulter, B.; Siegwolf, R.T.W.; Sperry, J.S.; McDowell, N.G. Plant responses to rising vapor pressure deficit. New Phytol. 2020, 226, 1550–1566. [Google Scholar] [CrossRef]
- Lasslop, G.; Reichstein, M.; Papale, D.; Richardson, A.D.; Arneth, A.; Barr, A.; Stoy, P.; Wohlfahrt, G. Separation of net ecosystem exchange into assimilation and respiration using a light response curve approach: Critical issues and global evaluation. Glob. Chang. Biol. 2010, 16, 187–208. [Google Scholar] [CrossRef]
- Moffat, A.M.; Beckstein, C.; Churkina, G.; Mund, M.; Heimann, M. Characterization of ecosystem responses to climatic controls using artificial neural networks. Glob. Chang. Biol. 2010, 16, 2737–2749. [Google Scholar] [CrossRef]
- Bloomfield, K.J.; Stocker, B.D.; Keenan, T.F.; Prentice, I.C. Environmental controls on the light use efficiency of terrestrial gross primary production. Glob. Chang. Biol. 2023, 29, 1037–1053. [Google Scholar] [CrossRef] [PubMed]
- Miller, B.D.; Carter, K.R.; Reed, S.C.; Wood, T.E.; Cavaleri, M.A. Only sun-lit leaves of the uppermost canopy exceed both air temperature and photosynthetic thermal optima in a wet tropical forest. Agric. For. Meteorol. 2021, 301, 108347. [Google Scholar] [CrossRef]
- Maleki, M.; Arriga, N.; Barrios, J.M.; Wieneke, S.; Liu, Q.; Peñuelas, J.; Janssens, I.A.; Balzarolo, M. Estimation of Gross Primary Productivity (GPP) Phenology of a Short-Rotation Plantation Using Remotely Sensed Indices Derived from Sentinel-2 Images. Remote Sens. 2020, 12, 2104. [Google Scholar] [CrossRef]
- Barr, A.G.; Black, T.A.; Hogg, E.H.; Griffis, T.J.; Morgenstern, K.; Kljun, N.; Theede, A.; Nesic, Z. Climatic controls on the carbon and water balances of a boreal aspen forest, 1994–2003. Glob. Chang. Biol. 2007, 13, 561–576. [Google Scholar] [CrossRef]
- Balzarolo, M.; Peñuelas, J.; Veroustraete, F. Influence of Landscape Heterogeneity and Spatial Resolution in Multi-Temporal In Situ and MODIS NDVI Data Proxies for Seasonal GPP Dynamics. Remote Sens. 2019, 11, 1656. [Google Scholar] [CrossRef]
- Liu, J.; Rambal, S.; Mouillot, F. Soil Drought Anomalies in MODIS GPP of a Mediterranean Broadleaved Evergreen Forest. Remote Sens. 2015, 7, 1154–1180. [Google Scholar] [CrossRef]
- Novick, K.A.; Ficklin, D.L.; Stoy, P.C.; Williams, C.A.; Bohrer, G.; Oishi, A.C.; Papuga, S.A.; Blanken, P.D.; Noormets, A.; Sulman, B.N.; et al. The increasing importance of atmospheric demand for ecosystem water and carbon fluxes. Nat. Clim. Chang. 2016, 6, 1023–1027. [Google Scholar] [CrossRef]
- Sims, D.A.; Rahman, A.F.; Cordova, V.D.; El-Masri, B.Z.; Baldocchi, D.D.; Bolstad, P.V.; Flanagan, L.B.; Goldstein, A.H.; Hollinger, D.Y.; Misson, L.; et al. A new model of gross primary productivity for North American ecosystems based solely on the enhanced vegetation index and land surface temperature from MODIS. Remote Sens. Environ. 2008, 112, 1633–1646. [Google Scholar] [CrossRef]
- Guzinski, R.; Nieto, H.; Sandholt, I.; Karamitilios, G. Modelling High-Resolution Actual Evapotranspiration through Sentinel-2 and Sentinel-3 Data Fusion. Remote Sens. 2020, 12, 1433. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bloomfield, K.J.; van Hoolst, R.; Balzarolo, M.; Janssens, I.A.; Vicca, S.; Ghent, D.; Prentice, I.C. Towards a General Monitoring System for Terrestrial Primary Production: A Test Spanning the European Drought of 2018. Remote Sens. 2023, 15, 1693. https://doi.org/10.3390/rs15061693
Bloomfield KJ, van Hoolst R, Balzarolo M, Janssens IA, Vicca S, Ghent D, Prentice IC. Towards a General Monitoring System for Terrestrial Primary Production: A Test Spanning the European Drought of 2018. Remote Sensing. 2023; 15(6):1693. https://doi.org/10.3390/rs15061693
Chicago/Turabian StyleBloomfield, Keith J., Roel van Hoolst, Manuela Balzarolo, Ivan A. Janssens, Sara Vicca, Darren Ghent, and I. Colin Prentice. 2023. "Towards a General Monitoring System for Terrestrial Primary Production: A Test Spanning the European Drought of 2018" Remote Sensing 15, no. 6: 1693. https://doi.org/10.3390/rs15061693
APA StyleBloomfield, K. J., van Hoolst, R., Balzarolo, M., Janssens, I. A., Vicca, S., Ghent, D., & Prentice, I. C. (2023). Towards a General Monitoring System for Terrestrial Primary Production: A Test Spanning the European Drought of 2018. Remote Sensing, 15(6), 1693. https://doi.org/10.3390/rs15061693






