Impact of a Tropical Cyclone on Terrestrial Inputs and Bio-Optical Properties in Princess Charlotte Bay (Great Barrier Reef Lagoon)
Abstract
1. Introduction
2. Materials and Methods
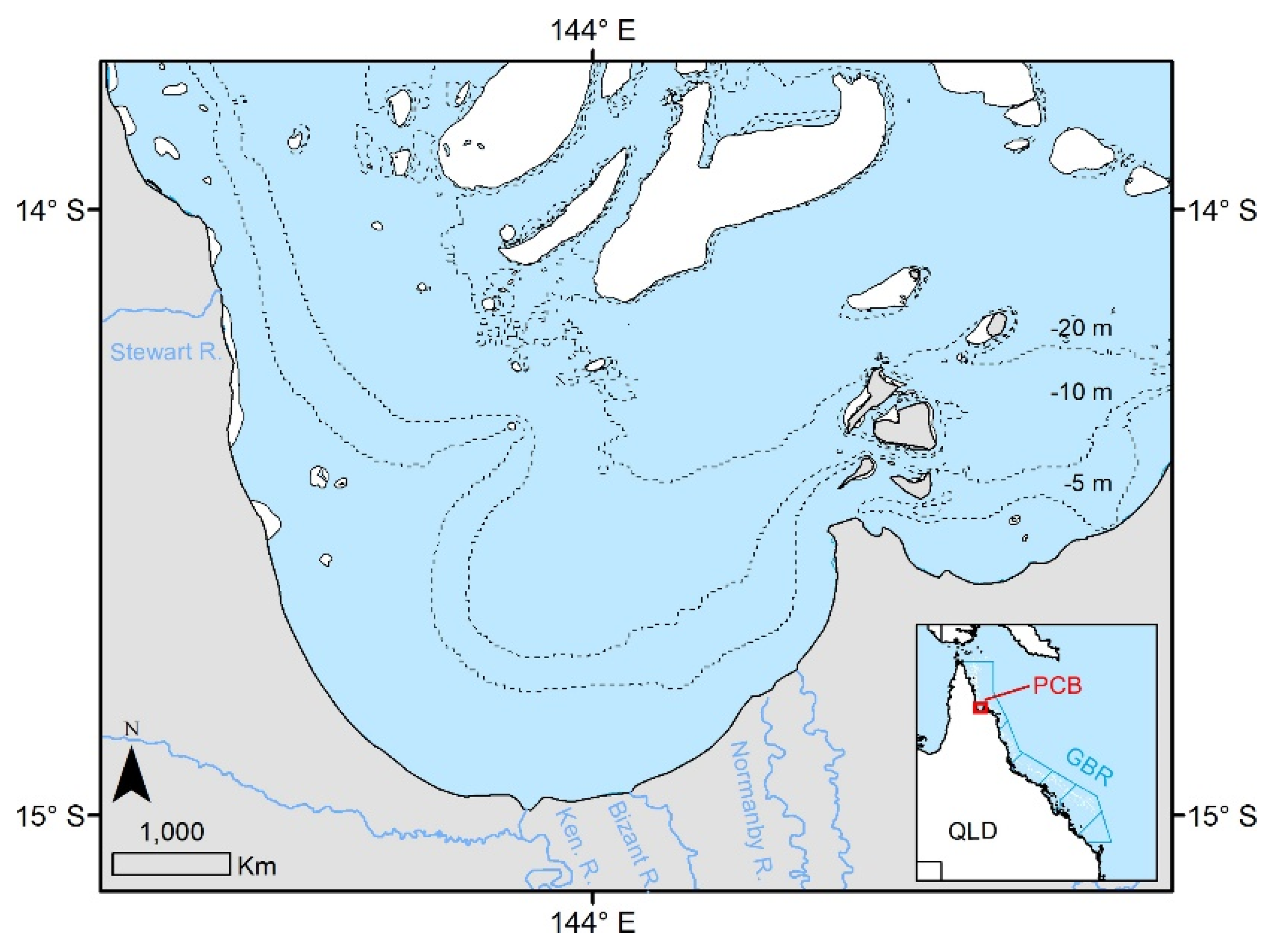
2.1. Study Area and Field Sampling
2.2. Discrete Biogeochemical Determinations
2.3. In Situ Instrumentation
3. Results
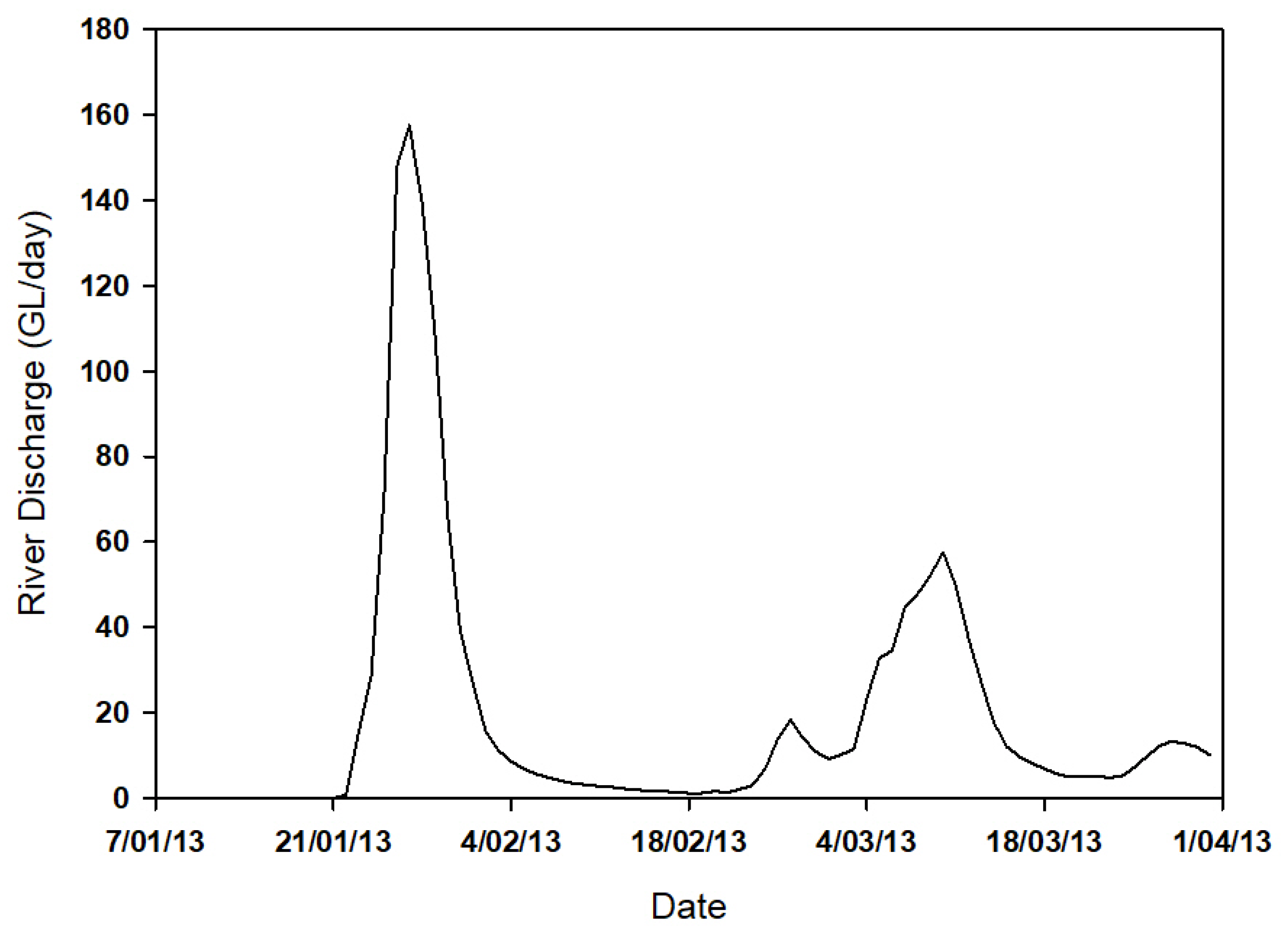
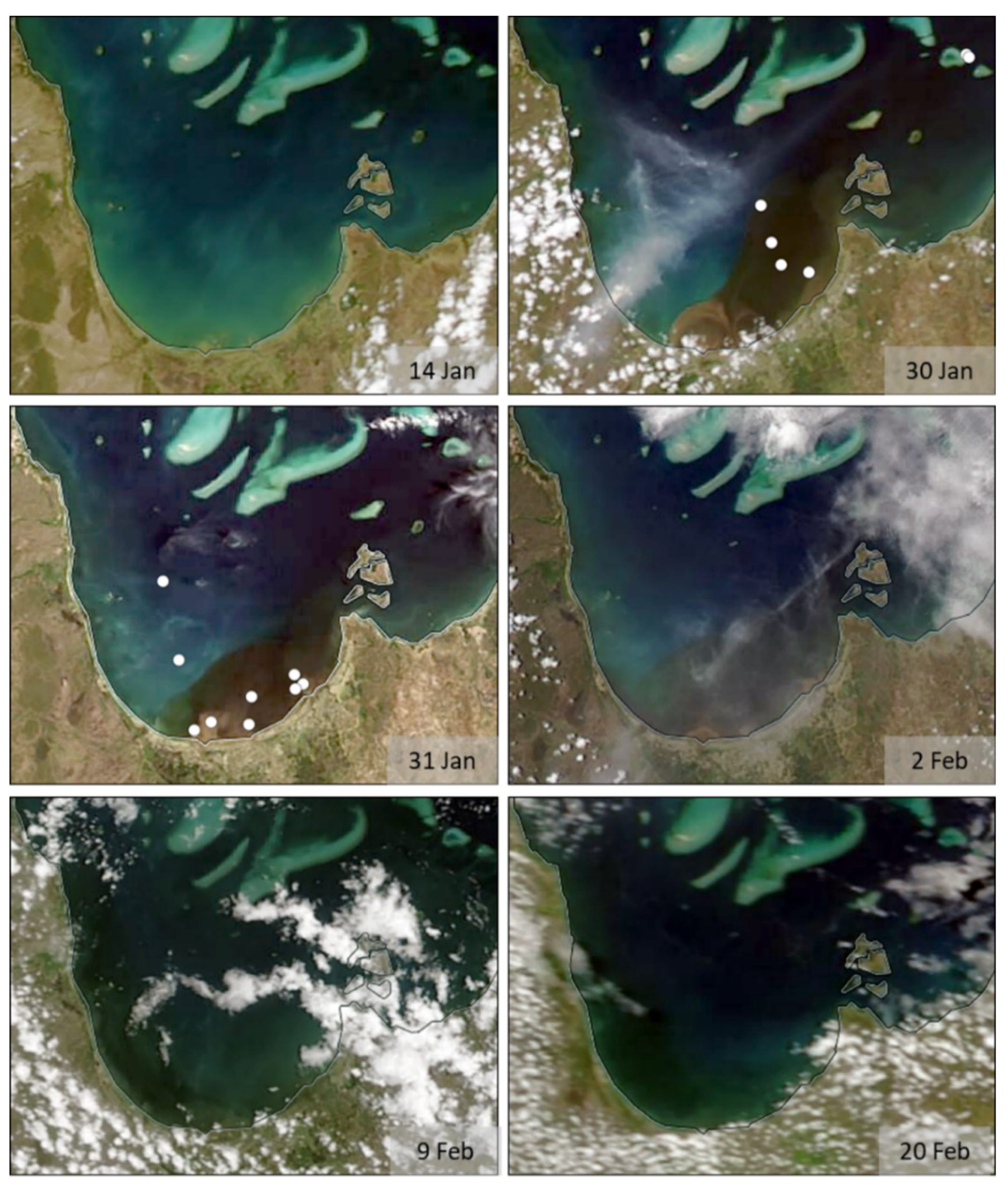
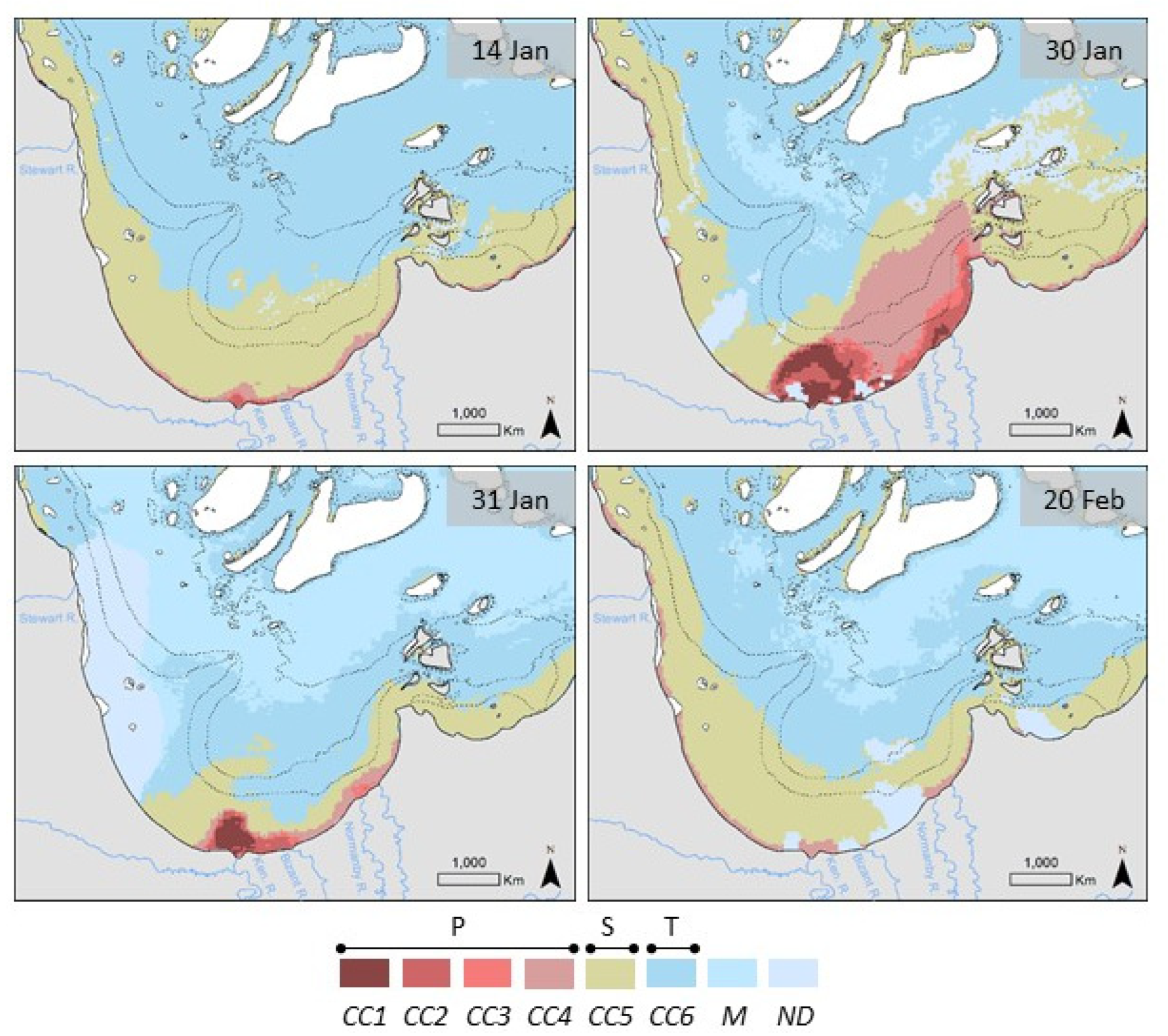
3.1. MODIS Ocean Color Maps
3.2. Physical Properties, Photosynthetically Active Radiation and Nutrients
3.3. Biogeochemical Properties and High Frequency Bio-Optical Proxies
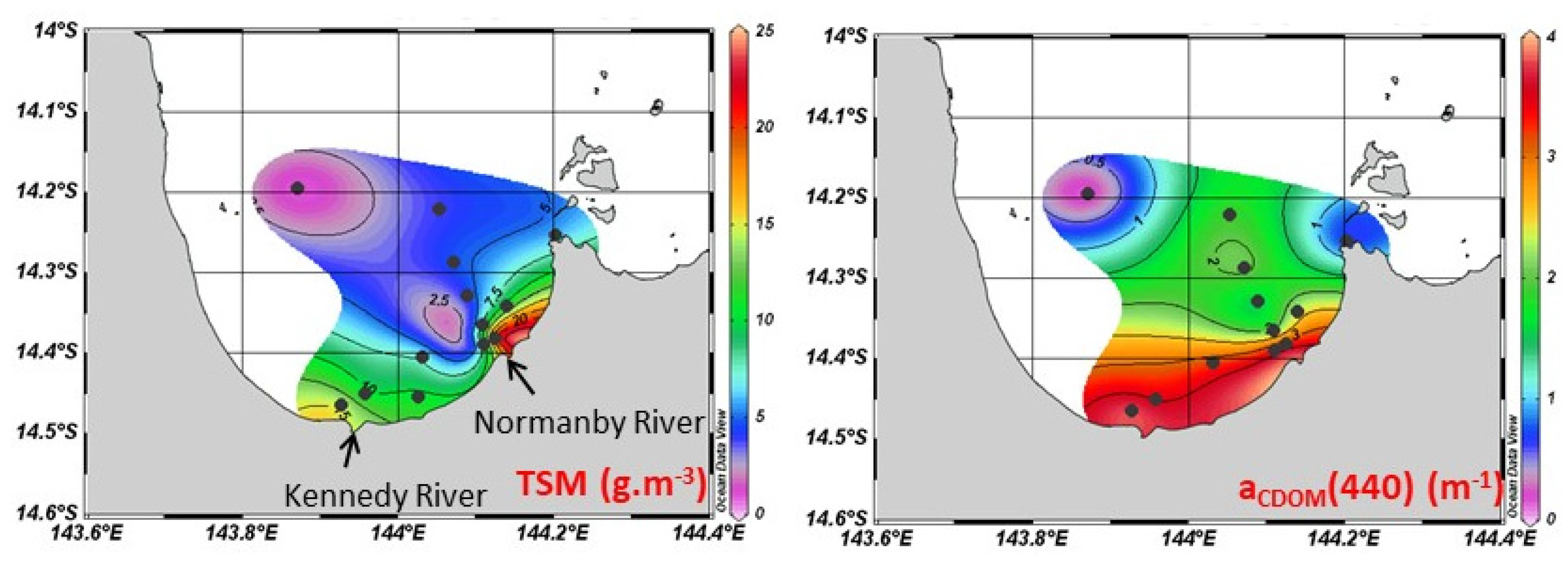
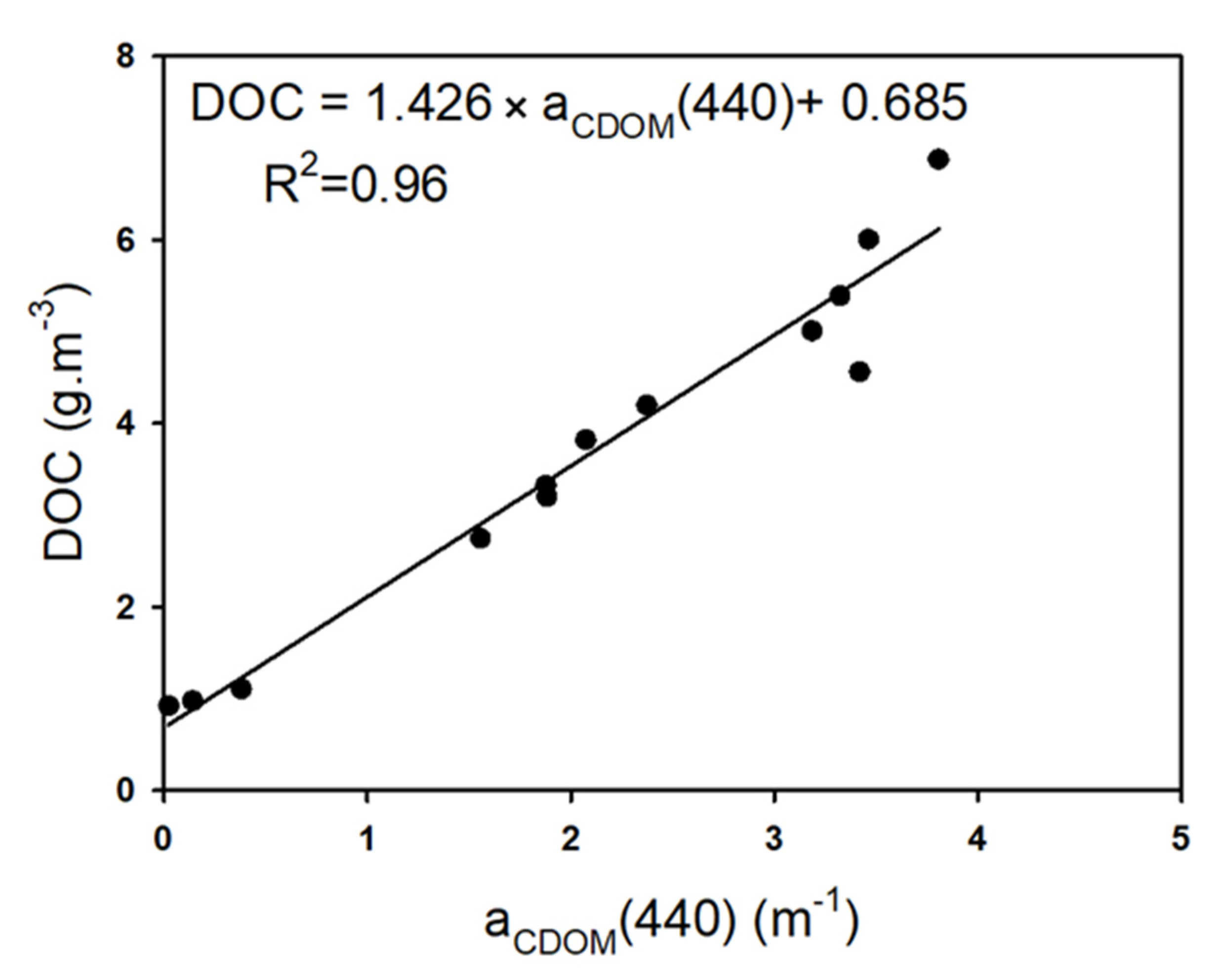
3.3.1. Suspended Solids and Dissolved Organic Matter
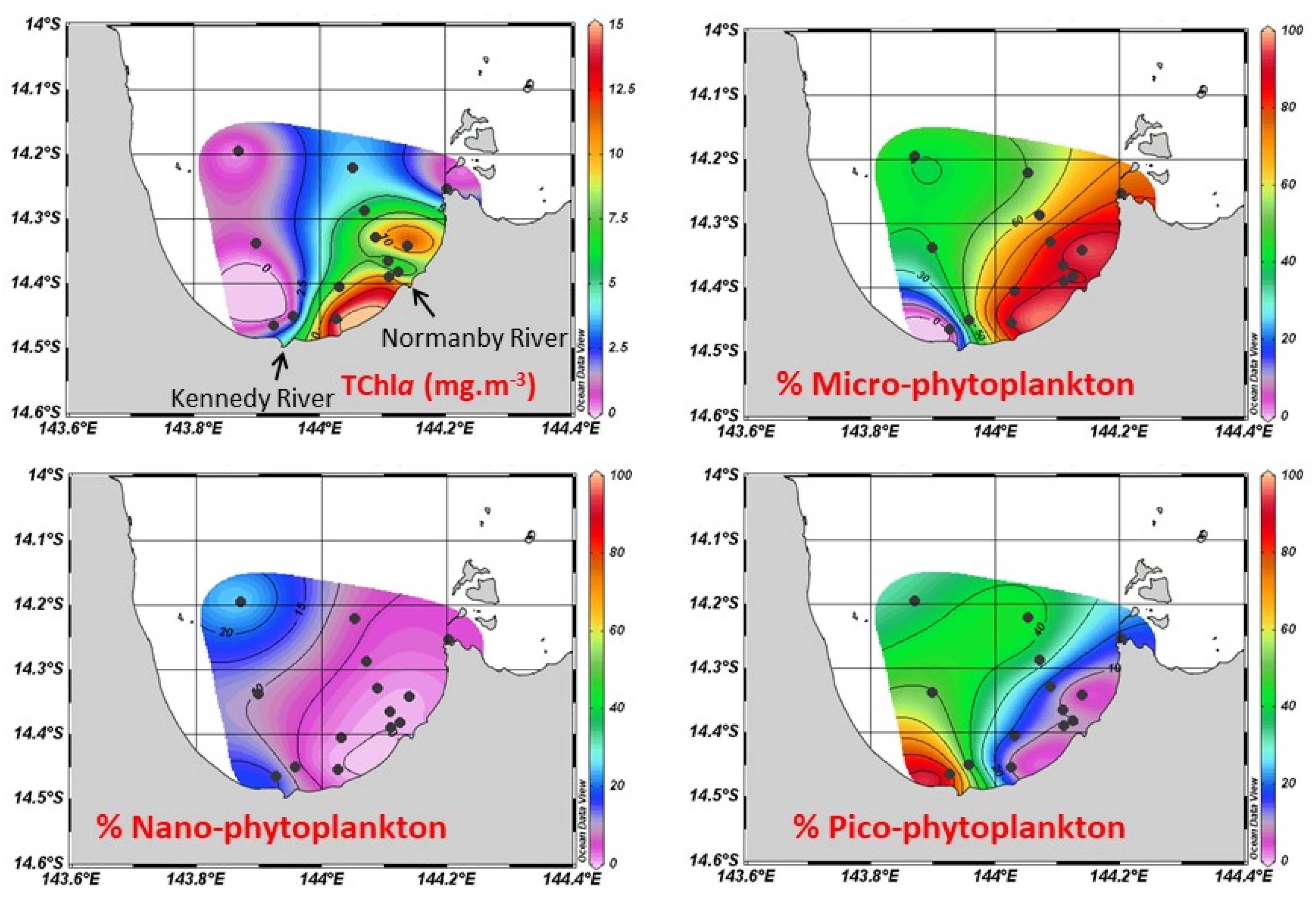
3.3.2. Phytoplankton Biomass and Composition
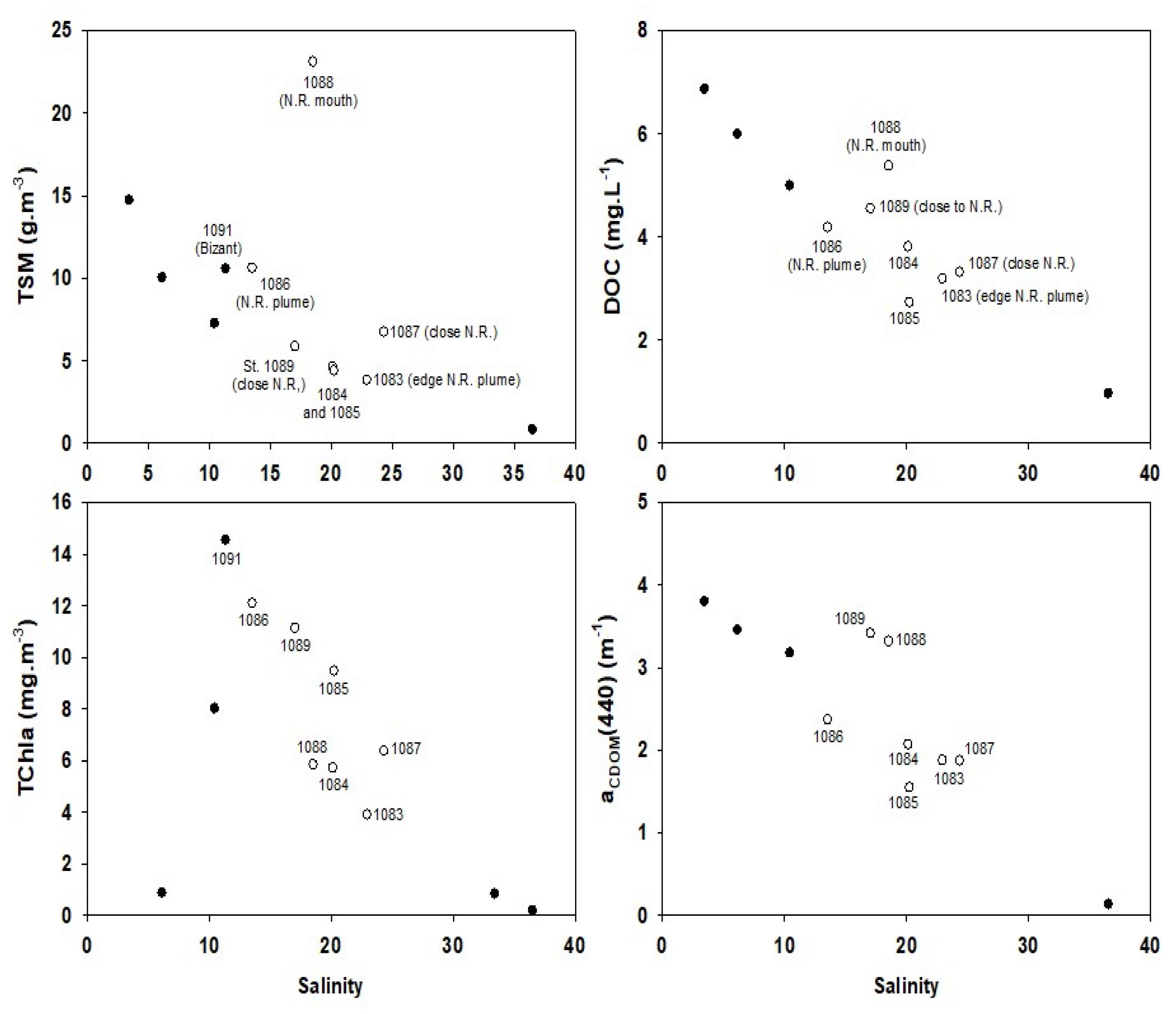
3.4. Biogeochemical Quantities versus Salinity
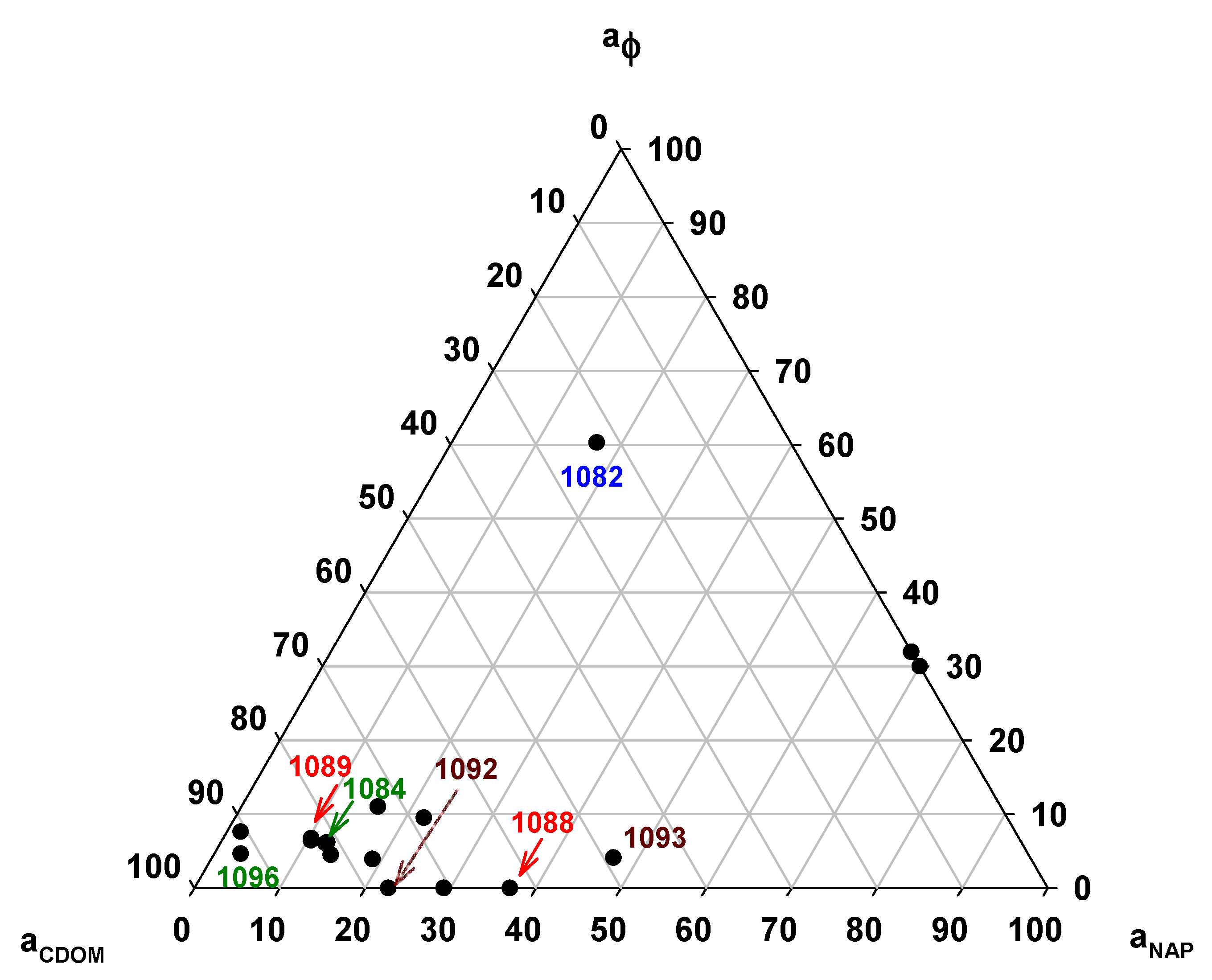
3.5. Absorption Budget
4. Discussion
4.1. From Catchment to Coast: Drivers of Riverine Inputs and Impact on Optical Properties
4.2. Transformation Processes and Critical Time Scales
4.3. Phytoplankton Response
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bauer, J.E.; Cai, W.J.; Raymond, P.A.; Bianchi, T.S.; Hopkinson, C.S.; Regnier, P.A. The changing carbon cycle of the coastal ocean. Nature 2013, 504, 61–70. [Google Scholar] [CrossRef] [PubMed]
- Van Vliet, M.T.; Franssen, W.H.; Yearsley, J.R.; Ludwig, F.; Haddeland, I.; Lettenmaier, D.P.; Kabat, P. Global river discharge and water temperature under climate change. Glob. Environ. Chang. 2013, 23, 450–464. [Google Scholar] [CrossRef]
- Kundzewicz, Z.W.; Szwed, M.; Pinskwar, I. Climate Variability and Floods—A Global Review. Water 2019, 11, 1399. [Google Scholar] [CrossRef]
- Liu, B.Q.; D’Sa, E.J.; Joshi, I.D. Floodwater impact on Galveston Bay phytoplankton taxonomy, pigment composition and photo-physiological state following Hurricane Harvey from field and ocean color (Sentinel-3A OLCI) observations. Biogeosciences 2019, 16, 1975–2001. [Google Scholar] [CrossRef]
- Steichen, J.L.; Labonte, J.M.; Windham, R.; Hala, D.; Kaiser, K.; Setta, S.; Faulkner, P.C.; Bacosa, H.; Yan, G.; Kamalanathan, M.; et al. Microbial, Physical, and Chemical Changes in Galveston Bay Following an Extreme Flooding Event, Hurricane Harvey. Front. Mar. Sci. 2020, 7, 186. [Google Scholar] [CrossRef]
- D’Sa, E.J.; Joshi, I.D.; Liu, B.Q.; Ko, D.S.; Osburn, C.L.; Bianchi, T.S. Biogeochemical Response of Apalachicola Bay and the Shelf Waters to Hurricane Michael Using Ocean Color Semi-Analytic/Inversion and Hydrodynamic Models. Front. Mar. Sci. 2019, 6, 523. [Google Scholar] [CrossRef]
- D’Sa, E.J.; Joshi, I.; Liu, B.Q. Galveston Bay and Coastal Ocean Optical-Geochemical Response to Hurricane Harvey from VIIRS Ocean Color. Geophys. Res. Lett. 2018, 45, 10579–10589. [Google Scholar] [CrossRef]
- Cherukuru, N.; Brando, V.E.; Schroeder, T.; Clementson, L.A.; Dekker, A.G. Influence of river discharge and ocean currents on coastal optical properties. Cont. Shelf Res. 2014, 84, 188–203. [Google Scholar] [CrossRef]
- Eccles, R.; Zhang, H.; Hamilton, D. A review of the effects of climate change on riverine flooding in subtropical and tropical regions. J. Water Clim. Chang. 2019, 10, 687–707. [Google Scholar] [CrossRef]
- Paerl, H.W.; Crosswell, J.R.; Van Dam, B.; Hall, N.S.; Rossignol, K.L.; Osburn, C.L.; Hounshell, A.G.; Sloup, R.S.; Harding, L.W. Two decades of tropical cyclone impacts on North Carolina’s estuarine carbon, nutrient and phytoplankton dynamics: Implications for biogeochemical cycling and water quality in a stormier world. Biogeochemistry 2018, 141, 307–332. [Google Scholar] [CrossRef]
- Baird, M.E.; Mongin, M.; Rizwi, F.; Bay, L.K.; Cantin, N.E.; Morris, L.A.; Skerratt, J. The effect of natural and anthropogenic nutrient and sediment loads on coral oxidative stress on runoff-exposed reefs. Mar. Pollut. Bull. 2021, 168, 112409. [Google Scholar] [CrossRef] [PubMed]
- Baird, M.E.; Mongin, M.; Skerratt, J.; Margvelashvili, N.; Tickell, S.; Steven, A.D.L.; Robillot, C.; Ellis, R.; Waters, D.; Kaniewska, P.; et al. Impact of catchment-derived nutrients and sediments on marine water quality on the Great Barrier Reef: An application of the eReefs marine modelling system. Mar. Pollut. Bull. 2021, 167, 112297. [Google Scholar] [CrossRef] [PubMed]
- Lewis, S.E.; Bartley, R.; Wilkinson, S.N.; Bainbridge, Z.T.; Henderson, A.E.; James, C.S.; Irvine, S.A.; Brodie, J.E. Land use change in the river basins of the Great Barrier Reef, 1860 to 2019: A foundation for understanding environmental history across the catchment to reef continuum. Mar. Pollut. Bull. 2021, 166, 112193. [Google Scholar] [CrossRef] [PubMed]
- Wolff, N.H.; Mumby, P.J.; Devlin, M.; Anthony, K.R.N. Vulnerability of the Great Barrier Reef to climate change and local pressures. Glob. Chang. Biol. 2018, 24, 1978–1991. [Google Scholar] [CrossRef]
- Devlin, M.J.; Petus, C.; da Silva, E.; Tracey, D.; Wolff, N.H.; Waterhouse, J.; Brodie, J. Water Quality and River Plume Monitoring in the Great Barrier Reef: An Overview of Methods Based on Ocean Colour Satellite Data. Remote Sens. 2015, 7, 12909–12941. [Google Scholar] [CrossRef]
- Brodie, J.E.; Kroon, F.J.; Schaffelke, B.; Wolanski, E.C.; Lewis, S.E.; Devlin, M.J.; Bohnet, I.C.; Bainbridge, Z.T.; Waterhouse, J.; Davis, A.M. Terrestrial pollutant runoff to the Great Barrier Reef: An update of issues, priorities and management responses. Mar. Pollut. Bull. 2012, 65, 81–100. [Google Scholar] [CrossRef]
- Petus, C.; Waterhouse, J.; Lewis, S.; Vacher, M.; Tracey, D.; Devlin, M. A flood of information: Using Sentinel-3 water colour products to assure continuity in the monitoring of water quality trends in the Great Barrier Reef (Australia). J. Environ. Manag. 2019, 248, 109255. [Google Scholar] [CrossRef]
- Hughes, T.P.; Barnes, M.L.; Bellwood, D.R.; Cinner, J.E.; Cumming, G.S.; Jackson, J.B.C.; Kleypas, J.; van de Leemput, I.A.; Lough, J.M.; Morrison, T.H.; et al. Coral reefs in the Anthropocene. Nature 2017, 546, 82–90. [Google Scholar] [CrossRef] [PubMed]
- McCulloch, M.; Fallon, S.; Wyndham, T.; Hendy, E.; Lough, J.; Barnes, D. Coral record of increased sediment flux to the inner Great Barrier Reef since European settlement. Nature 2003, 421, 727–730. [Google Scholar] [CrossRef] [PubMed]
- Kroon, F.J.; Kuhnert, P.M.; Henderson, B.L.; Wilkinson, S.N.; Kinsey-Henderson, A.; Abbott, B.; Brodie, J.E.; Turner, R.D.R. River loads of suspended solids, nitrogen, phosphorus and herbicides delivered to the Great Barrier Reef lagoon. Mar. Pollut. Bull. 2012, 65, 167–181. [Google Scholar] [CrossRef]
- Carlson, R.R.; Foo, S.A.; Asner, G.P. Land Use Impacts on Coral Reef Health: A Ridge-to-Reef Perspective. Front. Mar. Sci. 2019, 6, 562. [Google Scholar] [CrossRef]
- Schaffelke, B.; Carleton, J.; Skuza, M.; Zagorskis, I.; Furnas, M.J. Water quality in the inshore Great Barrier Reef lagoon: Implications for long-term monitoring and management. Mar. Pollut. Bull. 2012, 65, 249–260. [Google Scholar] [CrossRef] [PubMed]
- Waterhouse, J.; Gruber, R.; Logan, M.; Petus, C.; Howley, C.; Lewis, S.; Tracey, D.; James, C.; Mellors, J.; Tonin, H. Marine Monitoring Program: Annual Report for Inshore Water Quality Monitoring 2019–2020; Great Barrier Reef Marine Park Authority: Townsville, QLD, Australia, 2021. [Google Scholar]
- Steven, A.D.L.; Baird, M.E.; Brinkman, R.; Car, N.J.; Cox, S.J.; Herzfeld, M.; Hodge, J.; Jones, E.; King, E.; Margvelashvili, N.; et al. eReefs: An operational information system for managing the Great Barrier Reef. J. Oper. Oceanogr. 2019, 12, S12–S28. [Google Scholar] [CrossRef]
- Cherukuru, N.; Anstee, J.; Oubelkheir, K.; Ford, P.; Clementson, L.; Brando, V.; Schroeder, T.; King, E.; Steven, A. eReefs Wet Season Bio-Optics Field Sampling and Remote Sensing Algorithm Evaluation Report; Commonwealth Scientific and Industrial Research Organisation: Canberra, NSW, Australia, 2015. [Google Scholar]
- Brando, V.E.; Braga, F.; Zaggia, L.; Giardino, C.; Bresciani, M.; Matta, E.; Bellafiore, D.; Ferrarin, C.; Maicu, F.; Benetazzo, A.; et al. High-resolution satellite turbidity and sea surface temperature observations of river plume interactions during a significant flood event. Ocean Sci. 2015, 11, 909–920. [Google Scholar] [CrossRef]
- King, E.; Schroeder, T.; Brando, V.; Suber, K. A Pre-Operational System for Satellite Monitoring of Great Barrier Reef Marine Water Quality; CSIRO: Hobart, TAS, Australia, 2014. [Google Scholar] [CrossRef]
- Schroeder, T.; Devlin, M.J.; Brando, V.E.; Dekker, A.G.; Brodie, J.E.; Clementson, L.A.; McKinna, L. Inter-annual variability of wet season freshwater plume extent into the Great Barrier Reef lagoon based on satellite coastal ocean colour observations. Mar. Pollut. Bull. 2012, 65, 210–223. [Google Scholar] [CrossRef]
- Schroeder, T.; Lovell, J.; King, E.; Clementson, L.A.; Scott, R. IMOS Ocean Colour Validation Report 2015–2016; CSIRO: Hobart, TAS, Australia, 2016; p. 33. [Google Scholar]
- Brando, V.; Schroeder, T.; King, E.; Dyce, P. Reef Rescue Marine Monitoring Program: Using Remote Sensing for GBR-Wide Water Quality; Final Report for 2012/13 Activities; Commonwealth Scientific and Industrial Research Organisation: Canberra, NSW, Australia, 2015. [Google Scholar]
- Deng, D.F.; Ritchie, E.A. Rainfall Mechanisms for One of the Wettest Tropical Cyclones on Record in Australia-Oswald (2013). Mon. Weather Rev. 2020, 148, 2503–2525. [Google Scholar] [CrossRef]
- Howley, C.; Devlin, M.; Burford, M. Assessment of water quality from the Normanby River catchment to coastal flood plumes on the northern Great Barrier Reef, Australia. Mar. Freshw. Res. 2018, 69, 859–873. [Google Scholar] [CrossRef]
- Crosswell, J.R.; Carlin, G.; Steven, A. Controls on Carbon, Nutrient, and Sediment Cycling in a Large, Semiarid Estuarine System; Princess Charlotte Bay, Australia. J. Geophys. Res.-Biogeosci. 2020, 125, e2019JG005049. [Google Scholar] [CrossRef]
- Joo, M.; McNeil, V.H.; Carroll, C.; Waters, D.; Choy, S.C. Sediment and Nutrient Load Estimates for Major Great Barrier Reef Catchments (1987–2009) for Source Catchment Model Validation; Department of Science, Information Technology, Innovation and the Arts: Brisbane, QLD, Australia, 2014. [Google Scholar]
- McCloskey, G.L.; Baheerathan, R.; Dougall, C.; Ellis, R.; Bennett, F.R.; Waters, D.; Darr, S.; Fentie, B.; Hateley, L.R.; Askildsen, M. Modelled estimates of fine sediment and particulate nutrients delivered from the Great Barrier Reef catchments. Mar. Pollut. Bull. 2021, 165, 112163. [Google Scholar] [CrossRef]
- Clementson, L.A. The CSIRO Method; NASA Goddard Space Flight Center: Greenbelt, MD, USA, 2013. [Google Scholar]
- Chase, A.P.; Kramer, S.J.; Haëntjens, N.; Boss, E.S.; Karp-Boss, L.; Edmondson, M.; Graff, J.R. Evaluation of diagnostic pigments to estimate phytoplankton size classes. Limnol. Oceanogr. Methods 2020, 18, 570–584. [Google Scholar] [CrossRef]
- Vidussi, F.; Claustre, H.; Manca, B.B.; Luchetta, A.; Marty, J.C. Phytoplankton pigment distribution in relation to upper thermocline circulation in the eastern Mediterranean Sea during winter. J. Geophys. Res.-Ocean. 2001, 106, 19939–19956. [Google Scholar] [CrossRef]
- Uitz, J.; Claustre, H.; Morel, A.; Hooker, S.B. Vertical distribution of phytoplankton communities in open ocean: An assessment based on surface chlorophyll. J. Geophys. Res.-Ocean. 2006, 111, C08005. [Google Scholar] [CrossRef]
- Peltzer, E.T.; Fry, B.; Doering, P.H.; McKenna, J.H.; Norrman, B.; Zweifel, U.L. A comparison of methods for the measurement of dissolved organic carbon in natural waters. Mar. Chem. 1996, 54, 85–96. [Google Scholar] [CrossRef]
- Eaton, A.D.; Clesceri, L.S.; Rice, E.W.; Greenberg, A.E.; Franson, M.A.H. Standard Methods for the Examination of Water and Waste Water; American Public Health Association: Washington, DC, USA, 2005; p. 1368. [Google Scholar]
- Kishino, M.; Takahashi, M.; Okami, N.; Ichimura, S. Estimation of the spectral absorption coefficients of phytoplankton in the sea. Bull. Mar. Sci. 1985, 37, 634–642. [Google Scholar]
- Mitchell, B. Algorithms for Determining the Absorption Coefficient for Aquatic Particulates Using the Quantitative Filter Technique; SPIE: Washington, DC, USA, 1990; Volume 1302. [Google Scholar]
- Fichot, C.G.; Benner, R. A novel method to estimate DOC concentrations from CDOM absorption coefficients in coastal waters. Geophys. Res. Lett. 2011, 38, L03610. [Google Scholar] [CrossRef]
- Helms, J.R.; Stubbins, A.; Ritchie, J.D.; Minor, E.C.; Kieber, D.J.; Mopper, K. Absorption spectral slopes and slope ratios as indicators of molecular weight, source, and photobleaching of chromophoric dissolved organic matter. Limnol. Oceanogr. 2008, 53, 955–969. [Google Scholar] [CrossRef]
- Sullivan, J.M.; Twardowski, M.S.; Zaneveld, J.R.V.; Moore, C.M.; Barnard, A.H.; Donaghay, P.L.; Rhoades, B. Hyperspectral temperature and salt dependencies of absorption by water and heavy water in the 400–750 nm spectral range. Appl. Opt. 2006, 45, 5294–5309. [Google Scholar] [CrossRef]
- Zaneveld, J.R.; Kitchen, J.; Moore, C. Scattering Error Correction of Reflection-Tube Absorption Meters; SPIE: Washington, DC, USA, 1994; Volume 2258. [Google Scholar]
- Maffione, R.A.; Dana, D.R. Instruments and methods for measuring the backward-scattering coefficient of ocean waters. Appl. Opt. 1997, 36, 6057–6067. [Google Scholar] [CrossRef]
- Petus, C.; Devlin, M.; Teixera da Silva, E.; Lewis, S.; Waterhouse, J.; Wenger, A.; Bainbridge, Z.; Tracey, D. Defining wet season water quality target concentrations for ecosystem conservation using empirical light attenuation models: A case study in the Great Barrier Reef (Australia). J. Environ. Manag. 2018, 213, 451–466. [Google Scholar] [CrossRef]
- Kirk, J.T.O. Light and Photosynthesis in Aquatic Ecosystems; Cambridge University Press: Cambridge, UK, 2011. [Google Scholar]
- Oubelkheir, K.; Ford, P.W.; Clementson, L.A.; Cherukuru, N.; Fry, G.; Steven, A.D.L. Impact of an extreme flood event on optical and biogeochemical properties in a subtropical coastal periurban embayment (Eastern Australia). J. Geophys. Res.-Ocean. 2014, 119, 6024–6045. [Google Scholar] [CrossRef]
- Vantrepotte, V.; Danhiez, F.P.; Loisel, H.; Ouillon, S.; Meriaux, X.; Cauvin, A.; Dessailly, D. CDOM-DOC relationship in contrasted coastal waters: Implication for DOC retrieval from ocean color remote sensing observation. Opt. Express 2015, 23, 33–54. [Google Scholar] [CrossRef]
- Valerio, A.D.M.; Kampel, M.; Vantrepotte, V.; Ward, N.D.; Sawakuchi, H.O.; Less, D.F.D.S.; Neu, V.; Cunha, A.; Richey, J. Using CDOM optical properties for estimating DOC concentrations and pCO2 in the Lower Amazon River. Opt. Express 2018, 26, A657–A677. [Google Scholar] [CrossRef]
- Zhang, Q.; Blomquist, J.D. Watershed export of fine sediment, organic carbon, and chlorophyll-a to Chesapeake Bay: Spatial and temporal patterns in 1984–2016. Sci. Total Environ. 2018, 619–620, 1066–1078. [Google Scholar] [CrossRef]
- Yang, H.F.; Yang, S.L.; Xu, K.H.; Milliman, J.D.; Wang, H.; Yang, Z.; Chen, Z.; Zhang, C.Y. Human impacts on sediment in the Yangtze River: A review and new perspectives. Glob. Planet. Chang. 2018, 162, 8–17. [Google Scholar] [CrossRef]
- Bartley, R.; Roth, C.H.; Ludwig, J.; McJannet, D.; Liedloff, A.; Corfield, J.; Hawdon, A.; Abbott, B. Runoff and erosion from Australia’s tropical semi-arid rangelands: Influence of ground cover for differing space and time scales. Hydrol. Process. 2006, 20, 3317–3333. [Google Scholar] [CrossRef]
- Silburn, D.M.; Carroll, C.; Ciesiolka, C.A.A.; deVoil, R.C.; Burger, P. Hillslope runoff and erosion on duplex soils in grazing lands in semi-arid central Queensland. I. Influences of cover, slope, and soil. Soil Res. 2011, 49, 105–117. [Google Scholar] [CrossRef]
- Morehead, M.D.; Syvitski, J.P.; Hutton, E.W.H.; Peckham, S.D. Modeling the temporal variability in the flux of sediment from ungauged river basins. Glob. Planet. Chang. 2003, 39, 95–110. [Google Scholar] [CrossRef]
- Moore, C.E.; Brown, T.; Keenan, T.F.; Duursma, R.A.; van Dijk, A.I.J.M.; Beringer, J.; Culvenor, D.; Evans, B.; Huete, A.; Hutley, L.B.; et al. Reviews and syntheses: Australian vegetation phenology: New insights from satellite remote sensing and digital repeat photography. Biogeosciences 2016, 13, 5085–5102. [Google Scholar] [CrossRef]
- Yamashita, Y.; Nosaka, Y.; Suzuki, K.; Ogawa, H.; Takahashi, K.; Saito, H. Photobleaching as a factor controlling spectral characteristics of chromophoric dissolved organic matter in open ocean. Biogeosciences 2013, 10, 7207–7217. [Google Scholar] [CrossRef]
- Catalá, T.S.; Martínez-Pérez, A.M.; Nieto-Cid, M.; Álvarez, M.; Otero, J.; Emelianov, M.; Reche, I.; Arístegui, J.; Álvarez-Salgado, X.A. Dissolved Organic Matter (DOM) in the open Mediterranean Sea. I. Basin–wide distribution and drivers of chromophoric DOM. Prog. Oceanogr. 2018, 165, 35–51. [Google Scholar] [CrossRef]
- Bainbridge, Z.; Lewis, S.; Bartley, R.; Fabricius, K.; Collier, C.; Waterhouse, J.; Garzon-Garcia, A.; Robson, B.; Burton, J.; Wenger, A. Fine sediment and particulate organic matter: A review and case study on ridge-to-reef transport, transformations, fates, and impacts on marine ecosystems. Mar. Pollut. Bull. 2018, 135, 1205–1220. [Google Scholar] [PubMed]
- Li, P.; Chen, L.; Zhang, W.; Huang, Q. Spatiotemporal distribution, sources, and photobleaching imprint of dissolved organic matter in the Yangtze estuary and its adjacent sea using fluorescence and parallel factor analysis. PLoS ONE 2015, 10, e0130852. [Google Scholar] [CrossRef] [PubMed]
- Oubelkheir, K.; Clementson, L.A.; Webster, I.T.; Ford, P.W.; Dekker, A.G.; Radke, L.C.; Daniel, P. Using inherent optical properties to investigate biogeochemical dynamics in a tropical macrotidal coastal system. J. Geophys. Res.-Ocean. 2006, 111. [Google Scholar] [CrossRef]
- Dutkiewicz, S.; Cermeno, P.; Jahn, O.; Follows, M.J.; Hickman, A.E.; Taniguchi, D.A.A.; Ward, B.A. Dimensions of marine phytoplankton diversity. Biogeosciences 2020, 17, 609–634. [Google Scholar] [CrossRef]
- Nelson, N.B.; Siegel, D.A. The Global Distribution and Dynamics of Chromophoric Dissolved Organic Matter. Annu. Rev. Mar. Sci. 2013, 5, 447–476. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, M.; Fu, X.; Sun, J.; Xie, H. Chromophoric dissolved organic matter (CDOM) release by Dictyocha fibula in the central Bohai Sea. Mar. Chem. 2022, 241, 104107. [Google Scholar] [CrossRef]
- Fasching, C.; Behounek, B.; Singer, G.A.; Battin, T.J. Microbial degradation of terrigenous dissolved organic matter and potential consequences for carbon cycling in brown-water streams. Sci. Rep. 2014, 4, 4981. [Google Scholar] [CrossRef]
- Lønborg, C.; McKinna, L.I.W.; Slivkoff, M.M.; Carreira, C. Coloured dissolved organic matter dynamics in the Great Barrier Reef. Cont. Shelf Res. 2021, 219, 104395. [Google Scholar] [CrossRef]
- Matsuoka, A.; Hooker, S.B.; Bricaud, A.; Gentili, B.; Babin, M. Estimating absorption coefficients of colored dissolved organic matter (CDOM) using a semi-analytical algorithm for southern Beaufort Sea waters: Application to deriving concentrations of dissolved organic carbon from space. Biogeosciences 2013, 10, 917–927. [Google Scholar] [CrossRef]
- Sharples, J.; Middelburg, J.J.; Fennel, K.; Jickells, T.D. What proportion of riverine nutrients reaches the open ocean? Glob. Biogeochem. Cycles 2017, 31, 39–58. [Google Scholar] [CrossRef]
- Davies, P.L.; Eyre, B.D. Nutrient and suspended sediment input to Moreton Bay—The role of episodic events and estuarine processes. In Moreton Bay and Catchment; Tibbets, I.R., Hall, N.J., Dennison, W.C., Eds.; University of Queensland: Brisbane, QLD, Australia, 1998. [Google Scholar]
- Furnas, M.; Alongi, D.; McKinnon, D.; Trott, L.; Skuza, M. Regional-scale nitrogen and phosphorus budgets for the northern (14° S) and central (17° S) Great Barrier Reef shelf ecosystem. Cont. Shelf Res. 2011, 31, 1967–1990. [Google Scholar] [CrossRef]
- Boscolo-Galazzo, F.; Crichton, K.A.; Barker, S.; Pearson, P.N. Temperature dependency of metabolic rates in the upper ocean: A positive feedback to global climate change? Glob. Planet. Chang. 2018, 170, 201–212. [Google Scholar] [CrossRef]
- Clementson, L.A.; Richardson, A.J.; Rochester, W.A.; Oubelkheir, K.; Liu, B.Q.; D’Sa, E.J.; Gusmao, L.F.M.; Ajani, P.; Schroeder, T.; Ford, P.W.; et al. Effect of a Once in 100-Year Flood on a Subtropical Coastal Phytoplankton Community. Front. Mar. Sci. 2021, 8, 580516. [Google Scholar] [CrossRef]
- Cherukuru, N.; Brando, V.E.; Blondeau-Patissier, D.; Ford, P.W.; Clementson, L.A.; Robson, B.J. Impact of wet season river flood discharge on phytoplankton absorption properties in the southern Great Barrier Reef region coastal waters. Estuar. Coast. Shelf Sci. 2017, 196, 379–386. [Google Scholar] [CrossRef]
- Peierls, B.L.; Christian, R.R.; Paerl, H.W. Water quality and phytoplankton as indicators of hurricane impacts on a large estuarine ecosystem. Estuaries 2003, 26, 1329–1343. [Google Scholar] [CrossRef]
- Muylaert, K.; Vyverman, W. Impact of a Flood Event on the Planktonic Food Web of the Schelde Estuary (Belgium) in Spring 1998. Hydrobiologia 2006, 559, 385–394. [Google Scholar] [CrossRef]
- Dorado, S.; Booe, T.; Steichen, J.; McInnes, A.S.; Windham, R.; Shepard, A.; Lucchese, A.E.; Preischel, H.; Pinckney, J.L.; Davis, S.E.; et al. Towards an Understanding of the Interactions between Freshwater Inflows and Phytoplankton Communities in a Subtropical Estuary in the Gulf of Mexico. PLoS ONE 2015, 10, e0130931. [Google Scholar] [CrossRef]
- ODonohue, M.J.H.; Dennison, W.C. Phytoplankton productivity response to nutrient concentrations, light availability and temperature along an Australian estuarine gradient. Estuaries 1997, 20, 521–533. [Google Scholar] [CrossRef]
- Dagg, M.; Benner, R.; Lohrenz, S.; Lawrence, D. Transformation of dissolved and particulate materials on continental shelves influenced by large rivers: Plume processes. Cont. Shelf Res. 2004, 24, 833–858. [Google Scholar] [CrossRef]
- Loos, E.A.; Costa, M. Inherent optical properties and optical mass classification of the waters of the Strait of Georgia, British Columbia, Canada. Prog. Oceanogr. 2010, 87, 144–156. [Google Scholar] [CrossRef]
- Schaeffer, B.A.; Kurtz, J.C.; Hein, M.K. Phytoplankton community composition in nearshore coastal waters of Louisiana. Mar. Pollut. Bull. 2012, 64, 1705–1712. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, S.; Lohrenz, S.E. Phytoplankton community structure in the river-influenced continental margin of the northern Gulf of Mexico. Mar. Ecol. Prog. Ser. 2015, 521, 31–47. [Google Scholar] [CrossRef]
- Paczkowska, J.; Brugel, S.; Rowe, O.; Lefébure, R.; Brutemark, A.; Andersson, A. Response of Coastal Phytoplankton to High Inflows of Terrestrial Matter. Front. Mar. Sci. 2020, 7. [Google Scholar] [CrossRef]
- Blondeau-Patissier, D.; Brando, V.E.; Oubelkheir, K.; Dekker, A.G.; Clementson, L.A.; Daniel, P. Bio-optical variability of the absorption and scattering properties of the Queensland inshore and reef waters, Australia. J. Geophys. Res.-Ocean. 2009, 114, C05003. [Google Scholar] [CrossRef]
- Bricaud, A.; Claustre, H.; Ras, J.; Oubelkheir, K. Natural variability of phytoplanktonic absorption in oceanic waters: Influence of the size structure of algal populations. J. Geophys. Res.-Ocean. 2004, 109, C11010. [Google Scholar] [CrossRef]
- Xi, H.Y.; Losa, S.N.; Mangin, A.; Soppa, M.A.; Garnesson, P.; Demaria, J.; Liu, Y.Y.; D’Andon, O.H.F.; Bracher, A. Global retrieval of phytoplankton functional types based on empirical orthogonal functions using CMEMS GlobColour merged products and further extension to OLCI data. Remote Sens. Environ 2020, 240, 111704. [Google Scholar] [CrossRef]
- Ciotti, A.M.; Bricaud, A. Retrievals of a size parameter for phytoplankton and spectral light absorption by colored detrital matter from water-leaving radiances at SeaWiFS channels in a continental shelf region off Brazil. Limnol. Oceanogr.-Methods 2006, 4, 237–253. [Google Scholar] [CrossRef]
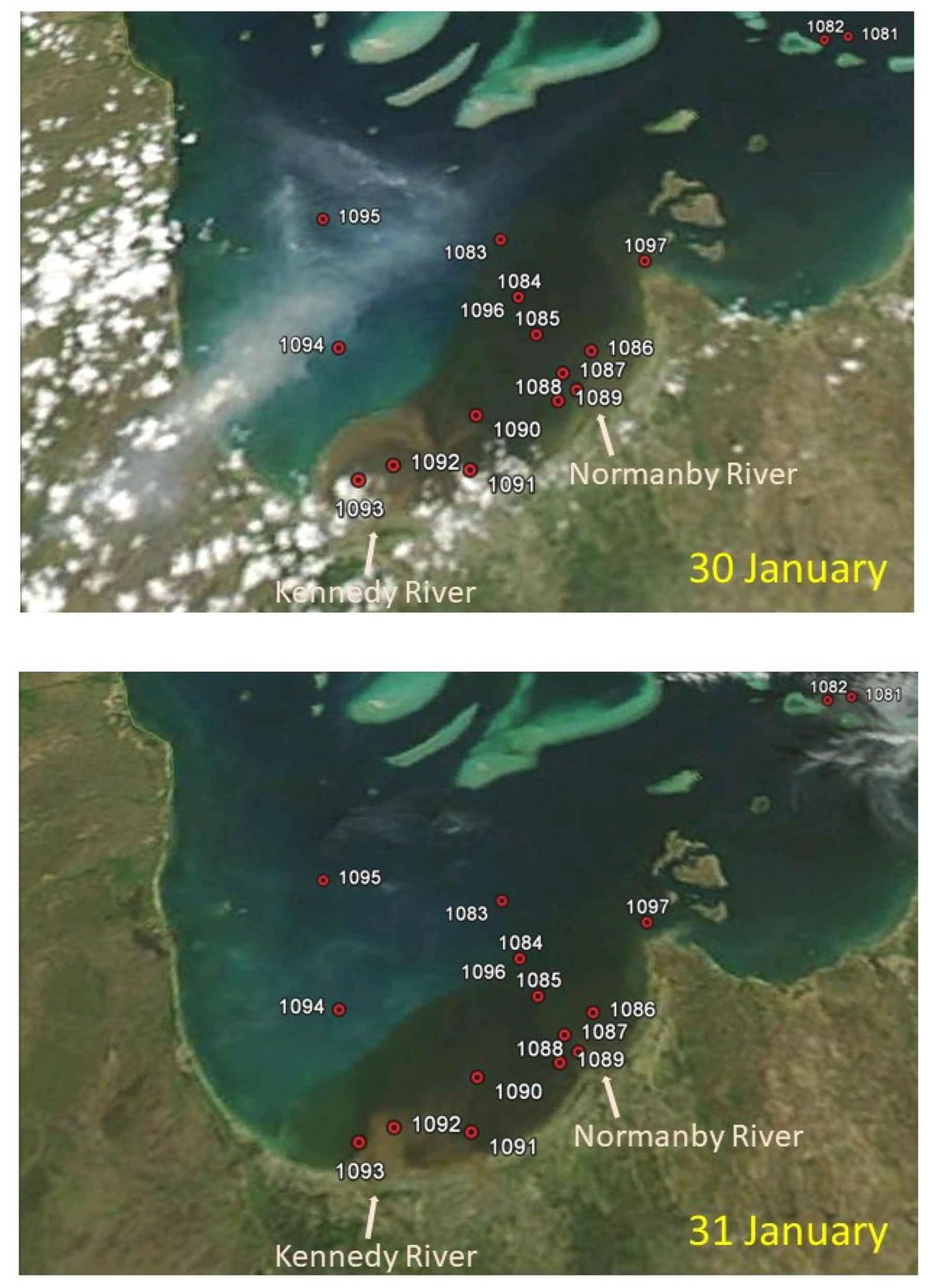

| Station No. Region | 1082 Outer-Reef | 1087–1088–1089 Normanby R. Mouth | 1092–1093 Kennedy R. Mouth | 1095 North PCB | 1084 (1096) Mid-Eastern PCB |
|---|---|---|---|---|---|
| Date | 30 Jan. | 31 Jan. | 31 Jan. | 31 Jan. | 30 Jan. (1 Feb.) |
| Time | 10:20 | 7:30–8:00–8:50 | 12:45–13:32 | 16:40 | 16:00 (10:10) |
| Salinity | n.a. | 24.30–18.50–17.00 | 6.10–3.40 | 36.50 | 20.10 (35.30) |
| DIN (μM) | 1.50 | 1.14–1.57–1.43 | 5.85–5.50 | 1.57 | 2.21–1.36 |
| TSM (g m−3) | 1.05 | 6.77–23.15–5.89 | 10.07–14.76 | 0.87 | 4.67 (0.84) |
| TSM Inorganic (%) | 63.5 | 71.4–81.7–68.8 | 88.1–86.9 | 57.7 | 78.6 (69.7) |
| TChla (mg m−3) | 2.97 | 6.38–5.86–11.15 | 0.90–1.20 | 0.20 | 5.74 (0.29) |
| DOC (g m−3) | 0.92 | 3.32–5.39–4.56 | 6.00–6.88 | 0.98 | 3.82 (1.11) |
| aCDOM(440) (m−1) | 0.02 | 1.88–3.32–3.42 | 3.46–3.81 | 0.14 | 2.07 (0.38) |
| aCDOM(412) (m−1) | 0.04 | 2.95–5.26–5.14 | 5.53–6.09 | 0.19 | 3.20 (0.55) |
| SR (Slope Ratio) | 3.12 | 0.88–0.87–0.95 | 0.86–0.85 | 2.92 | 0.96 (1.61) |
| aCDOM*(440) (m2 g−1) | 0.026 | 0.56–0.62–0.75 | 0.58–0.55 | 0.15 | 0.54 (0.34) |
| bbp/bp(550) (%) | n.a. | 1.9–4.7–1.5 | 3.3–n.a. | 0.5 | 2.7 (n.a.) |
| b*(550) (m2 g−1) | 0.60 | 0.87–0.23–n.a. | 0.43–n.a. | 1.05 | 0.43 (n.a.) |
| aCDOM/a(440) (%) | 22.7 | 81.6–63.0–82.9 | 77.3–48.8 | n.a. | 81.4 (92.3) |
| aNAP/a(440) (%) | 17.0 | 12.4–37.0–10.3 | 22.7–47.1 | n.a. | 12.5 (3.1) |
| aφ/a(440) (%) | 60.3 | 6.0–0.0–6.7 | 0.0–4.1 | n.a. | 6.2 (4.6) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oubelkheir, K.; Ford, P.W.; Cherukuru, N.; Clementson, L.A.; Petus, C.; Devlin, M.; Schroeder, T.; Steven, A.D.L. Impact of a Tropical Cyclone on Terrestrial Inputs and Bio-Optical Properties in Princess Charlotte Bay (Great Barrier Reef Lagoon). Remote Sens. 2023, 15, 652. https://doi.org/10.3390/rs15030652
Oubelkheir K, Ford PW, Cherukuru N, Clementson LA, Petus C, Devlin M, Schroeder T, Steven ADL. Impact of a Tropical Cyclone on Terrestrial Inputs and Bio-Optical Properties in Princess Charlotte Bay (Great Barrier Reef Lagoon). Remote Sensing. 2023; 15(3):652. https://doi.org/10.3390/rs15030652
Chicago/Turabian StyleOubelkheir, Kadija, Phillip W. Ford, Nagur Cherukuru, Lesley A. Clementson, Caroline Petus, Michelle Devlin, Thomas Schroeder, and Andrew D. L. Steven. 2023. "Impact of a Tropical Cyclone on Terrestrial Inputs and Bio-Optical Properties in Princess Charlotte Bay (Great Barrier Reef Lagoon)" Remote Sensing 15, no. 3: 652. https://doi.org/10.3390/rs15030652
APA StyleOubelkheir, K., Ford, P. W., Cherukuru, N., Clementson, L. A., Petus, C., Devlin, M., Schroeder, T., & Steven, A. D. L. (2023). Impact of a Tropical Cyclone on Terrestrial Inputs and Bio-Optical Properties in Princess Charlotte Bay (Great Barrier Reef Lagoon). Remote Sensing, 15(3), 652. https://doi.org/10.3390/rs15030652








