Plugging the Gaps in the Global PhenoCam Monitoring of Forests—The Need for a PhenoCam Network across Indian Forests
Abstract
1. Phenology as an Indicator of the Impact of Climate Change on Forests
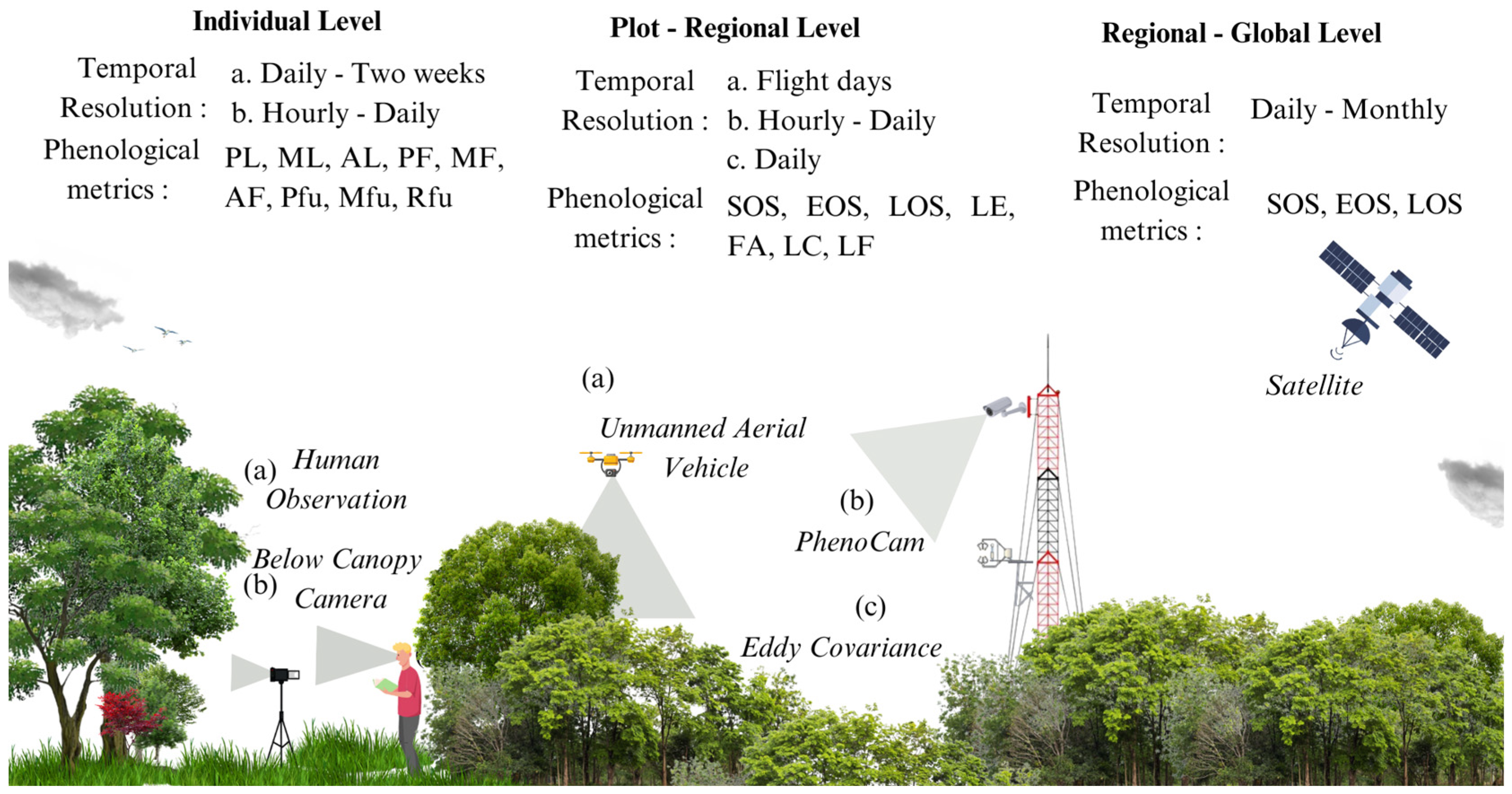
2. Methods for Monitoring Phenology
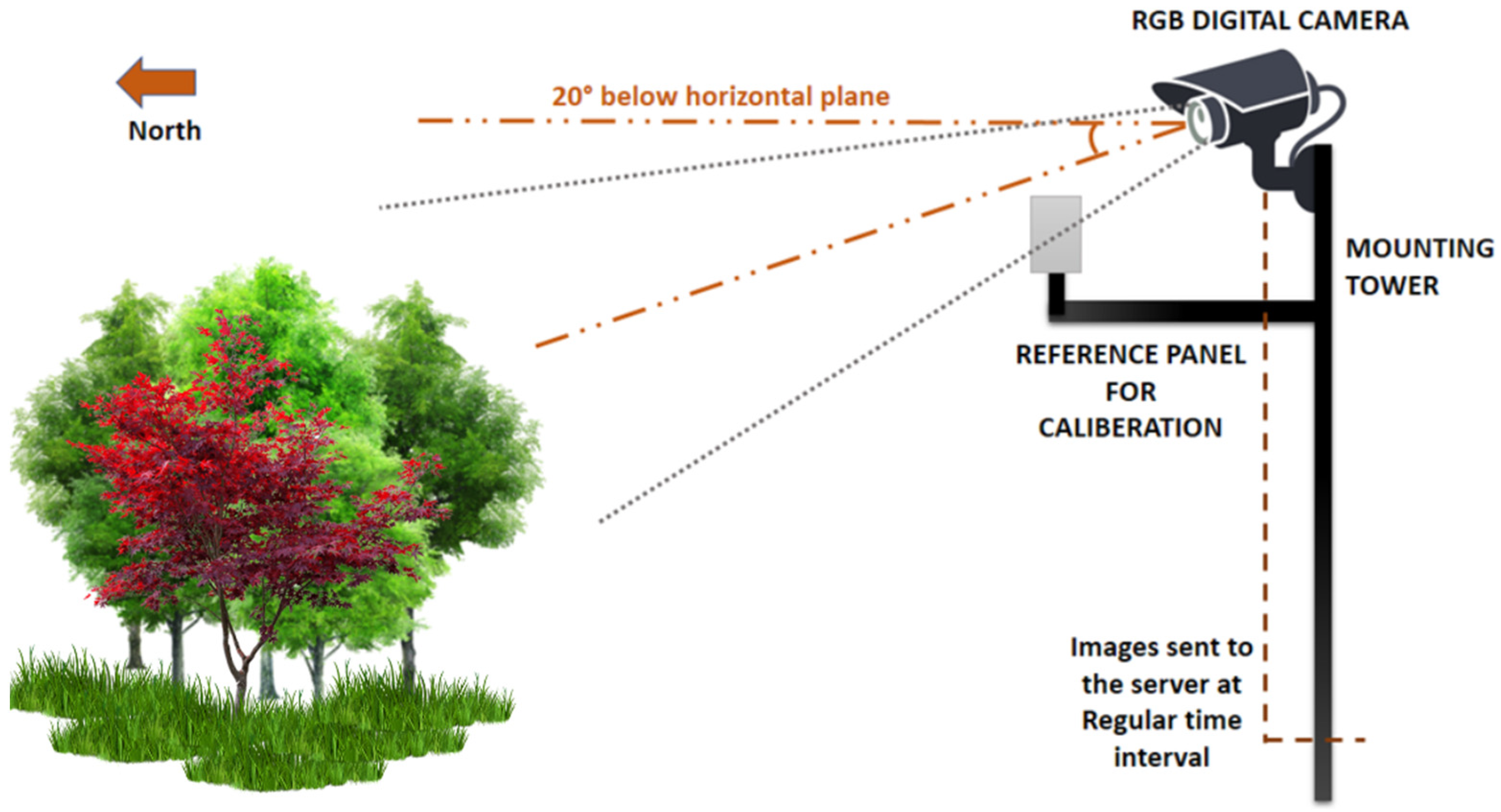
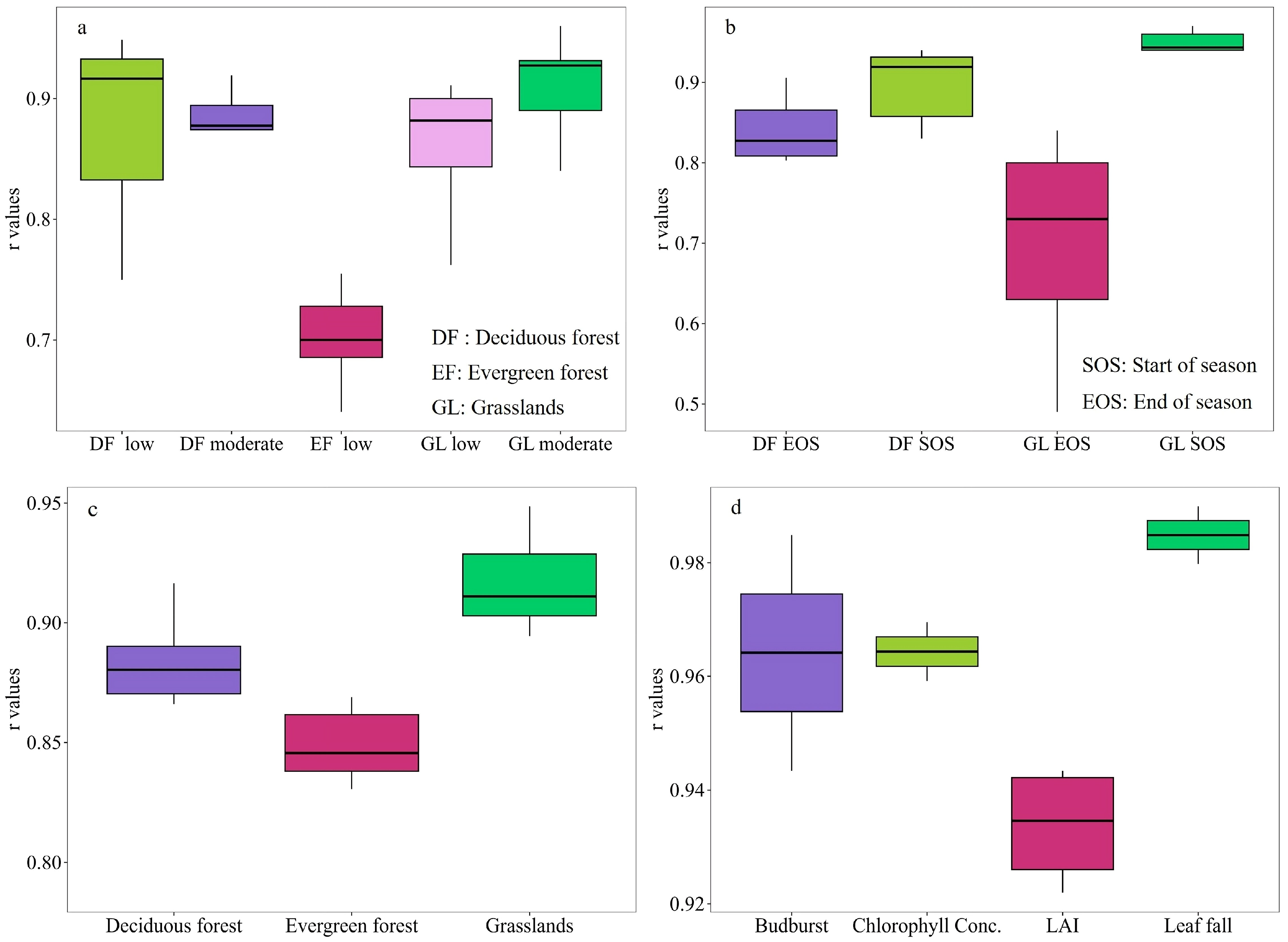
3. PhenoCam as a Promising Technology for Long-Term and Continuous Phenological Monitoring
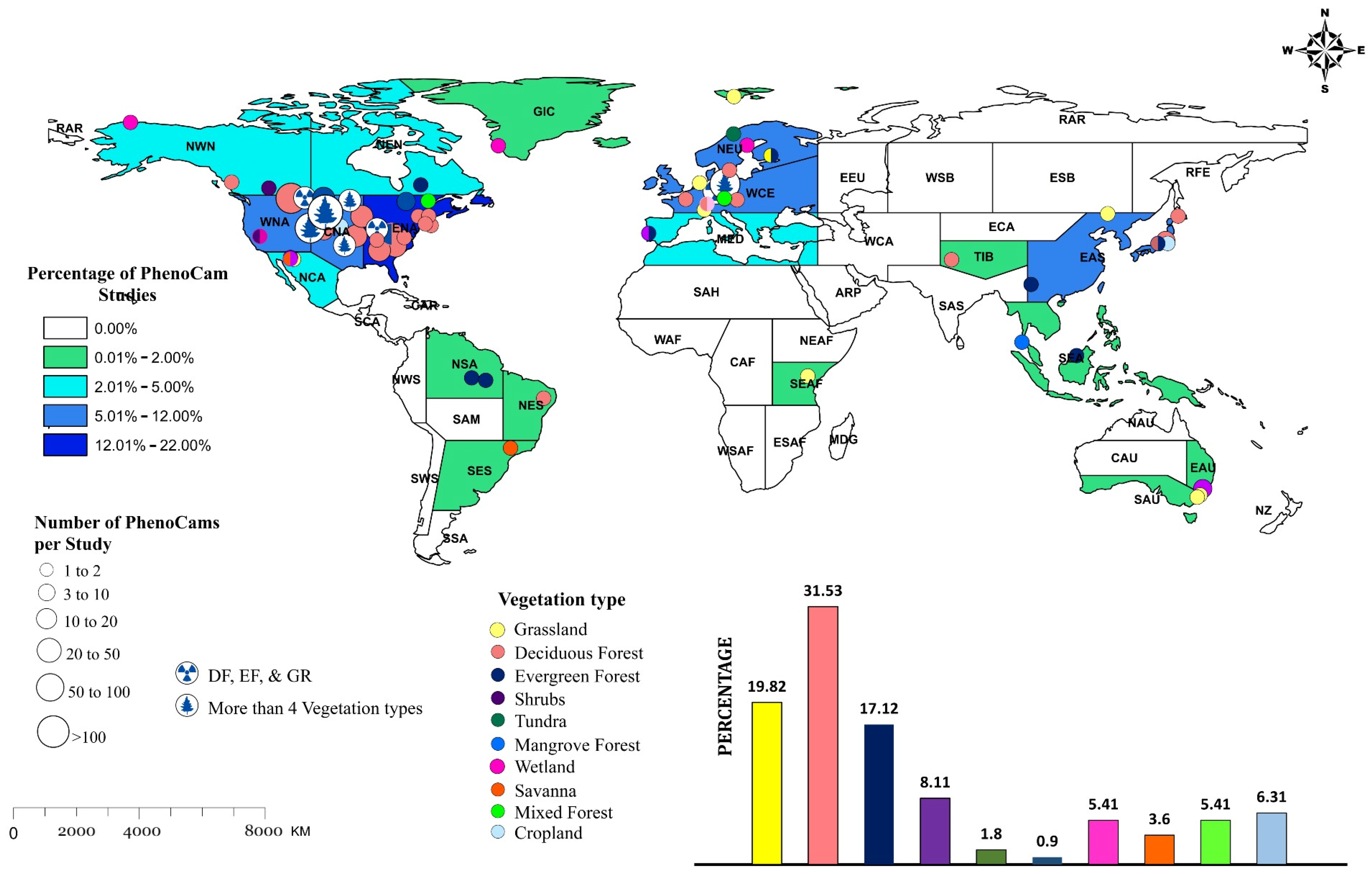
4. Global Distribution of PhenoCams
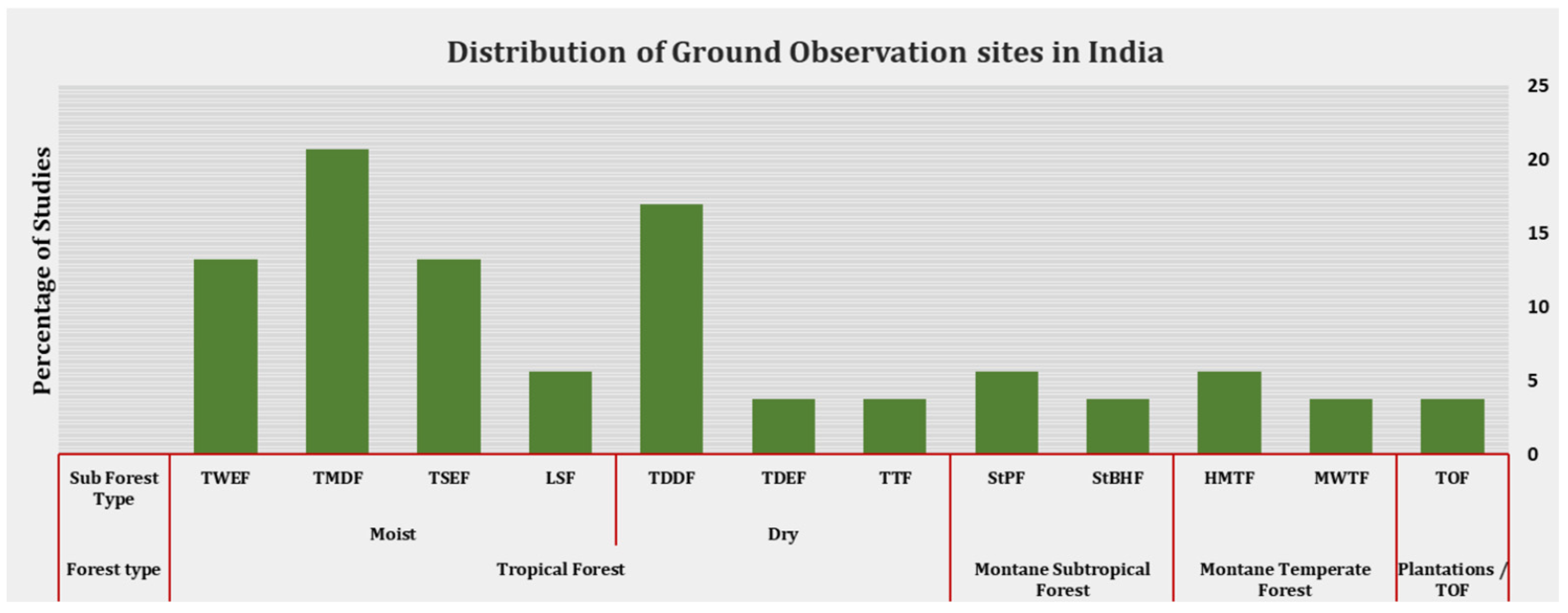
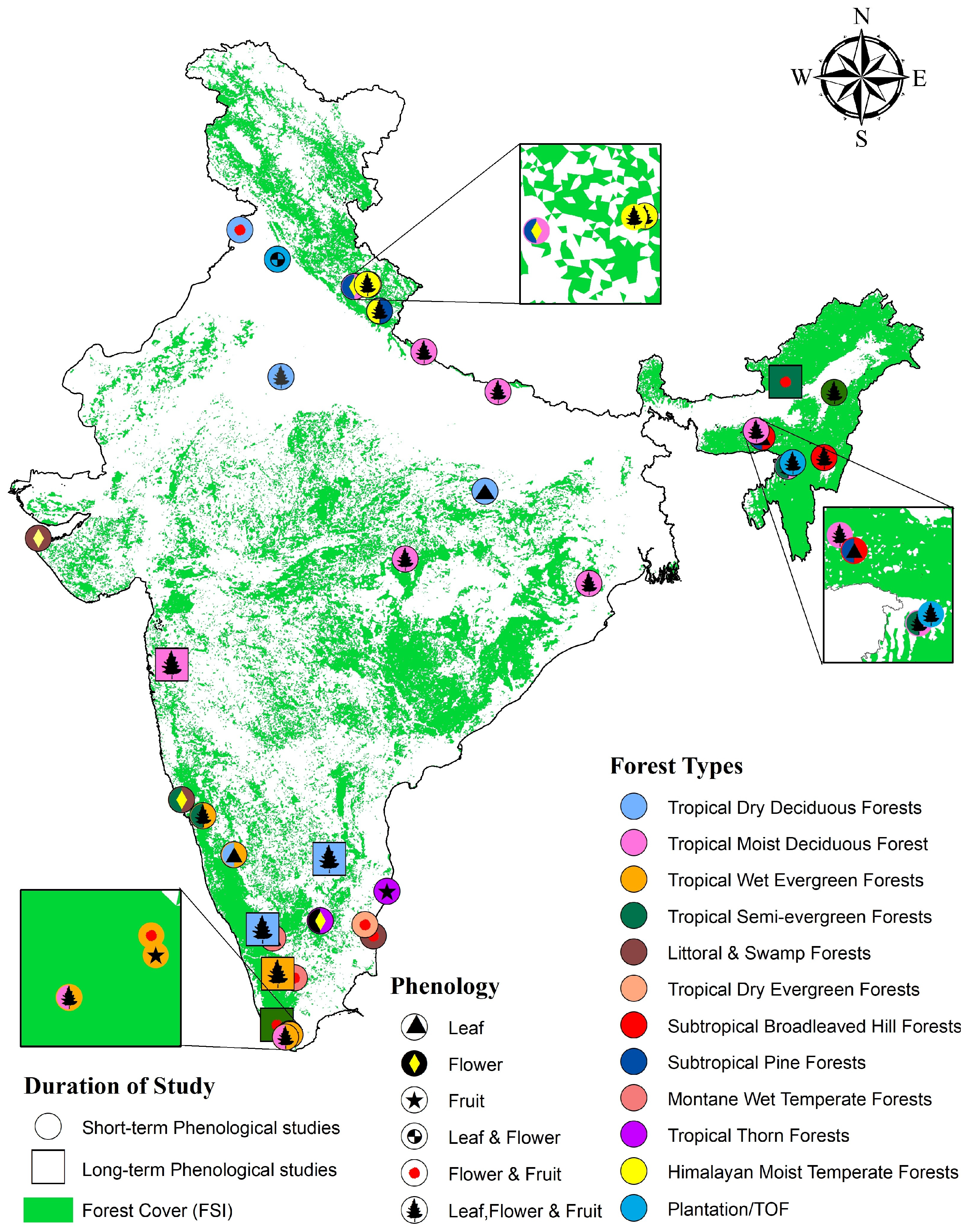
5. The State of Phenological Monitoring in India
6. Role of the PhenoCam Network in Supporting Forest Management in India
7. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Lieth, H.H. Contributions to phenology seasonality research. Int. J. Biometeorol. 1976, 20, 197–199. [Google Scholar] [CrossRef]
- Richardson, A.D.; Keenan, T.F.; Migliavacca, M.; Ryu, Y.; Sonnentag, O.; Toomey, M. Climate change, phenology, and phenological control of vegetation feedbacks to the climate system. Agric. For. Meteorol. 2013, 169, 156–173. [Google Scholar] [CrossRef]
- Rihan, H.Z.; Kareem, F.; El-Mahrouk, M.E.; Fuller, M.P. Artificial seeds (principle, aspects and applications). Agronomy 2017, 7, 71. [Google Scholar] [CrossRef]
- Chaurasia, A.N.; Parmar, R.M.; Dave, M.G.; Mehta, N.; Kallaje, R.; Sahu, A.; Murthy, I.K.; Singh, C.P.; Krishnayya, N.S.R. Syncing phenology phase and canopy spectral reflectance of common tree species of four forest covers in India. Curr. Sci. 2021, 120, 567. [Google Scholar] [CrossRef]
- Nord, E.A.; Lynch, J.P. Plant phenology: A critical controller of soil resource acquisition. J. Exp. Bot. 2009, 60, 1927–1937. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Peng, C.; Xiang, W.; Deng, X.; Tian, D.; Zhou, X.; Yu, G.; He, H.; Zhao, Z. Plant phenological modeling and its application in global climate change research: Overview and future challenges. Environ. Rev. 2013, 21, 1–14. [Google Scholar] [CrossRef]
- Cremonese, E.; Filippa, G.; Galvagno, M.; Siniscalco, C.; Oddi, L.; di Cella, U.M.; Migliavacca, M. Heat wave hinders green wave: The impact of climate extreme on the phenology of a mountain grassland. Agric. For. Meteorol. 2017, 247, 320–330. [Google Scholar] [CrossRef]
- Beil, I.; Kreyling, J.; Meyer, C.; Lemcke, N.; Malyshev, A.V. Late to bed, late to rise—Warmer autumn temperatures delay spring phenology by delaying dormancy. Glob. Chang. Biol. 2021, 27, 5806–5817. [Google Scholar] [CrossRef]
- Meng, L.; Zhou, Y.; Gu, L.; Richardson, A.D.; Peñuelas, J.; Fu, Y.; Wang, Y.; Asrar, G.R.; De Boeck, H.J.; Mao, J.; et al. Photoperiod decelerates the advance of spring phenology of six deciduous tree species under climate warming. Glob. Chang. Biol. 2021, 27, 2914–2927. [Google Scholar] [CrossRef]
- Jeganathan, C.; Nishant, N. Scrutinising MODIS and GIMMS vegetation indices for extracting growth rhythm of natural vegetation in India. J. Indian Soc. Remote Sens. 2014, 42, 397–408. [Google Scholar] [CrossRef]
- Zohner, C.M.; Renner, S.S.; Sebald, V.; Crowther, T.W. How changes in spring and autumn phenology translate into growth-experimental evidence of asymmetric effects. J. Ecol. 2021, 109, 2717–2728. [Google Scholar] [CrossRef]
- Parker, V.T.; Stickrod, M.A. Reproductive phenological shifts and other phylogenetic trait changes in the Arbutoideae (Ericaceae) in the context of drought, seed predation, and fire. Botany 2022, 100, 387–399. [Google Scholar] [CrossRef]
- Caparros-Santiago, J.A.; Rodriguez-Galiano, V.; Dash, J. Land surface phenology as indicator of global terrestrial ecosystem dynamics: A systematic review. ISPRS J. Photogramm. Remote Sens. 2021, 171, 330–347. [Google Scholar] [CrossRef]
- Keenan, T.F.; Gray, J.; Friedl, M.A.; Toomey, M.; Bohrer, G.; Hollinger, D.Y.; Munger, J.W.; O’keefe, J.; Schmid, H.P.; Wing, I.S.; et al. Net carbon uptake has increased through warming-induced changes in temperate forest phenology. Nat. Clim. Chang. 2014, 4, 598–604. [Google Scholar] [CrossRef]
- Piao, S.; Ciais, P.; Friedlingstein, P.; Peylin, P.; Reichstein, M.; Luyssaert, S.; Margolis, H.; Fang, J.; Barr, A.; Chen, A.; et al. Net carbon dioxide losses of northern ecosystems in response to autumn warming. Nature 2008, 451, 49–52. [Google Scholar] [CrossRef] [PubMed]
- Workie, T.G.; Debella, H.J. Climate change and its effects on vegetation phenology across ecoregions of Ethiopia. Glob. Ecol. Conserv. 2018, 13, e00366. [Google Scholar] [CrossRef]
- Brown, M.E.; De Beurs, K.M.; Marshall, M. Global phenological response to climate change in crop areas using satellite remote sensing of vegetation, humidity and temperature over 26 years. Remote Sens. Environ. 2012, 126, 174–183. [Google Scholar] [CrossRef]
- Saderi, S.; Rathgeber, C.B.; Rozenberg, P.; Fournier, M. Phenology of Wood Formation in Larch (Larix decidua Mill.) Trees Growing Along a 1000-m elevation gradient in the French Southern Alps. Ann. For. Sci. 2019, 76, 89. [Google Scholar] [CrossRef]
- Ovaskainen, O.; Meyke, E.; Lo, C.; Tikhonov, G.; Delgado, M.d.M.; Roslin, T.; Gurarie, E.; Abadonova, M.; Abduraimov, O.; Adrianova, O.; et al. Chronicles of nature calendar, a long-term and large-scale multitaxon database on phenology. Sci. Data 2020, 7, 47. [Google Scholar] [CrossRef]
- Suresh, H.S.; Sukumar, R. Influence of climatic variability on tree phenology in the tropical dry forests of Mudumalai, southern India. Trop. For. A Chang. World 2009, 5, 39–61. [Google Scholar]
- Singh, S.L.; Sahoo, U.K. Shift in phenology of some dominant tree species due to climate change in Mizoram, North-East India. Indian J. Ecol. 2019, 46, 132–136. [Google Scholar]
- Bolton, D.K.; Gray, J.M.; Melaas, E.K.; Moon, M.; Eklundh, L.; Friedl, M.A. Continental-scale land surface phenology from harmonized Landsat 8 and Sentinel-2 imagery. Remote Sens. Environ. 2020, 240, 111685. [Google Scholar] [CrossRef]
- Bórnez, K.; Descals, A.; Verger, A.; Peñuelas, J. Land surface phenology from VEGETATION and PROBA-V data. Assess. Over Deciduous For. Int. J. Appl. Earth Obs. Geoinf. 2020, 84, 101974. [Google Scholar]
- Julien, Y.; Sobrino, J.A. Global land surface phenology trends from GIMMS database. Int. J. Remote Sens. 2009, 30, 3495–3513. [Google Scholar] [CrossRef]
- Wang, H.; Jia, G.; Epstein, H.E.; Zhao, H.; Zhang, A. Integrating a PhenoCam-derived vegetation index into a light use efficiency model to estimate daily gross primary production in a semi-arid grassland. Agric. For. Meteorol. 2020, 288, 107983. [Google Scholar] [CrossRef]
- Mohapatra, J.; Singh, C.P.; Tripathi, O.P.; Pandya, H.A. Remote sensing of alpine treeline ecotone dynamics and phenology in Arunachal Pradesh Himalaya. Int. J. Remote Sens. 2019, 40, 7986–8009. [Google Scholar] [CrossRef]
- Prakash Singh, C.; Mohapatra, J.; Pandya, H.A.; Gajmer, B.; Sharma, N.; Shrestha, D.G. Evaluating changes in treeline position and land surface phenology in Sikkim Himalaya. Geocarto Int. 2020, 35, 453–469. [Google Scholar] [CrossRef]
- Seyednasrollah, B.; Young, A.M.; Hufkens, K.; Milliman, T.; Friedl, M.A.; Frolking, S.; Richardson, A.D. Tracking vegetation phenology across diverse biomes using Version 2.0 of the PhenoCam Dataset. Sci. Data 2019, 6, 222. [Google Scholar] [CrossRef]
- Liu, F.; Wang, X.; Wang, C. Measuring vegetation phenology with near-surface remote sensing in a temperate deciduous forest: Effects of sensor type and deployment. Remote Sens. 2019, 11, 1063. [Google Scholar] [CrossRef]
- Gonsamo, A.; Chen, J.M.; Price, D.T.; Kurz, W.A.; Wu, C. Land surface phenology from optical satellite measurement and CO2 eddy covariance technique. J. Geophys. Res. Biogeosci. 2012, 117. [Google Scholar] [CrossRef]
- Berra, E.F.; Gaulton, R.; Barr, S. Assessing spring phenology of a temperate woodland: A multiscale comparison of ground, unmanned aerial vehicle and Landsat satellite observations. Remote Sens. Environ. 2019, 223, 229–242. [Google Scholar] [CrossRef]
- Richardson, A.D.; Jenkins, J.P.; Braswell, B.H.; Hollinger, D.Y.; Ollinger, S.V.; Smith, M.-L. Use of digital webcam images to track spring green-up in a deciduous broadleaf forest. Oecologia 2007, 152, 323–334. [Google Scholar] [CrossRef] [PubMed]
- Watson, C.J.; Restrepo-Coupe, N.; Huete, A.R. Multi-scale phenology of temperate grasslands: Improving monitoring and management with near-surface phenocams. Front. Environ. Sci. 2019, 7, 14. [Google Scholar] [CrossRef]
- Shajahan, S.; Cannayen, I.; Hendrickson, J. Monitoring plant phenology using phenocam: A review. In Proceedings of the 2016 ASABE Annual International Meeting, Orlando, FL, USA, 17–20 July 2016; American Society of Agricultural and Biological Engineers: St. Joseph, MI, USA, 2016; p. 1. [Google Scholar] [CrossRef]
- Hufkens, K.; Friedl, M.; Sonnentag, O.; Braswell, B.H.; Milliman, T.; Richardson, A.D. Linking near-surface and satellite remote sensing measurements of deciduous broadleaf forest phenology. Remote Sens. Environ. 2012, 117, 307–321. [Google Scholar] [CrossRef]
- Westergaard-Nielsen, A.; Lund, M.; Hansen, B.U.; Tamstorf, M.P. Camera derived vegetation greenness index as proxy for gross primary production in a low Arctic wetland area. ISPRS J. Photogramm. Remote Sens. 2013, 86, 89–99. [Google Scholar] [CrossRef]
- Klosterman, S.T.; Hufkens, K.; Gray, J.M.; Melaas, E.; Sonnentag, O.; Lavine, I.; Mitchell, L.; Norman, R.; Friedl, M.A.; Richardson, A.D. Evaluating remote sensing of deciduous forest phenology at multiple spatial scales using PhenoCam imagery. Biogeosciences 2014, 11, 4305–4320. [Google Scholar] [CrossRef]
- Hufkens, K.; Keenan, T.F.; Flanagan, L.B.; Scott, R.L.; Bernacchi, C.J.; Joo, E.; Brunsell, N.A.; Verfaillie, J.; Richardson, A.D. Productivity of North American grasslands is increased under future climate scenarios despite rising aridity. Nat. Clim. Chang. 2016, 6, 710–714. [Google Scholar] [CrossRef]
- Toomey, M.; Friedl, M.A.; Frolking, S.; Hufkens, K.; Klosterman, S.; Sonnentag, O.; Baldocchi, D.D.; Bernacchi, C.J.; Biraud, S.C.; Bohrer, G.; et al. Greenness indices from digital cameras predict the timing and seasonal dynamics of canopy-scale photosynthesis. Ecol. Appl. 2015, 25, 99–115. [Google Scholar] [CrossRef]
- Keenan, T.; Darby, B.A.; Felts, E.; Sonnentag, O.; Friedl, M.A.; Hufkens, K.; O’Keefe, J.; Klosterman, S.; Munger, J.W.; Toomey, M.; et al. Tracking forest phenology and seasonal physiology using digital repeat photography: A critical assessment. Ecol. Appl. 2014, 24, 1478–1489. [Google Scholar] [CrossRef]
- Richardson, A.D.; Hufkens, K.; Milliman, T.; Aubrecht, D.M.; Chen, M.; Gray, J.M.; Johnston, M.R.; Keenan, T.F.; Klosterman, S.T.; Kosmala, M.; et al. Tracking vegetation phenology across diverse North American biomes using PhenoCam imagery. Sci. Data 2018, 5, 180028. [Google Scholar] [CrossRef]
- Chandra, S.; Singh, A.; Mathew, J.R.; Singh, C.P.; Pandya, M.R.; Bhattacharya, B.K.; Solanki, H.; Nautiyal, M.C.; Joshi, R. Phenocam observed flowering anomaly of Rhododendron arboreum Sm. in Himalaya: A climate change impact perspective. Environ. Monit. Assess. 2022, 194, 877. [Google Scholar] [CrossRef] [PubMed]
- Mann, H.M.; Iosifidis, A.; Jepsen, J.U.; Welker, J.M.; Loonen, M.J.; Høye, T.T. Automatic flower detection and phenology monitoring using time-lapse cameras and deep learning. Remote Sens. Ecol. Conserv. 2022, 8, 765–777. [Google Scholar] [CrossRef]
- Delpierre, N.; Soudani, K.; Berveiller, D.; Dufrêne, E.; Hmimina, G.; Vincent, G. “Green pointillism”: Detecting the within-population variability of budburst in temperate deciduous trees with phenological cameras. Int. J. Biometeorol. 2020, 64, 663–670. [Google Scholar] [CrossRef] [PubMed]
- Kurc, S.A.; Benton, L.M. Digital image-derived greenness links deep soil moisture to carbon uptake in a creosotebush-dominated shrubland. J. Arid Environ. 2010, 74, 585–594. [Google Scholar] [CrossRef]
- Liu, Y.; Wu, C.; Sonnentag, O.; Desai, A.R.; Wang, J. Using the red chromatic coordinate to characterize the phenology of forest canopy photosynthesis. Agric. For. Meteorol. 2020, 285, 107910. [Google Scholar] [CrossRef]
- Gutman, G.; Skakun, S.; Gitelson, A. Revisiting the use of red and near-infrared reflectances in vegetation studies and numerical climate models. Sci. Remote Sens. 2021, 4, 100025. [Google Scholar] [CrossRef]
- Moore, C.E.; Beringer, J.; Evans, B.; Hutley, L.B.; Tapper, N.J. Tree–grass phenology information improves light use efficiency modelling of gross primary productivity for an Australian tropical savanna. Biogeosciences 2017, 14, 111–129. [Google Scholar] [CrossRef]
- Menzel, A.; Helm, R.; Zang, C. Patterns of late spring frost leaf damage and recovery in a European beech (Fagus sylvatica L.) stand in south-eastern Germany based on repeated digital photographs. Front. Plant Sci. 2015, 6, 110. [Google Scholar] [CrossRef]
- Anderson, H.B.; Nilsen, L.; Tømmervik, H.; Karlsen, S.R.; Nagai, S.; Cooper, E.J. Using ordinary digital cameras in place of near-infrared sensors to derive vegetation indices for phenology studies of high arctic vegetation. Remote Sens. 2016, 8, 847. [Google Scholar] [CrossRef]
- Cui, T.; Martz, L.; Lamb, E.G.; Zhao, L.; Guo, X. Comparison of grassland phenology derived from MODIS satellite and PhenoCam near-surface remote sensing in North America. Can. J. Remote Sens. 2019, 45, 707–722. [Google Scholar] [CrossRef]
- Alberton, B.; Almeida, J.; Helm, R.; Torres, R.D.S.; Menzel, A.; Morellato, L.P.C. Using phenological cameras to track the green up in a cerrado savanna and its on-the-ground validation. Ecol. Inform. 2014, 19, 62–70. [Google Scholar] [CrossRef]
- Inoue, T.; Nagai, S.; Kobayashi, H.; Koizumi, H. Utilization of ground-based digital photography for the evaluation of seasonal changes in the aboveground green biomass and foliage phenology in a grassland ecosystem. Ecol. Inform. 2015, 25, 1–9. [Google Scholar] [CrossRef]
- Filippa, G.; Cremonese, E.; Migliavacca, M.; Galvagno, M.; Sonnentag, O.; Humphreys, E.; Hufkens, K.; Ryu, Y.; Verfaillie, J.; di Cella, U.M.; et al. NDVI derived from near-infrared-enabled digital cameras: Applicability across different plant functional types. Agric. For. Meteorol. 2018, 249, 275–285. [Google Scholar] [CrossRef]
- Nagai, S.; Ichie, T.; Yoneyama, A.; Kobayashi, H.; Inoue, T.; Ishii, R.; Suzuki, R.; Itioka, T. Usability of time-lapse digital camera images to detect characteristics of tree phenology in a tropical rainforest. Ecol. Inform. 2016, 32, 91–106. [Google Scholar] [CrossRef]
- Sakamoto, T.; Gitelson, A.A.; Nguy-Robertson, A.L.; Arkebauer, T.J.; Wardlow, B.D.; Suyker, A.E.; Verma, S.B.; Shibayama, M. An alternative method using digital cameras for continuous monitoring of crop status. Agric. For. Meteorol. 2012, 154, 113–126. [Google Scholar] [CrossRef]
- Tsuchida, S.; Nishida, K.; Iwao, K.; Kawato, W.; Oguma, H.; Iwasaki, A. Phenological eyes network for validation of remote sensing data. J. Remote Sens. Soc. Jpn. 2005, 25, 282–288. [Google Scholar] [CrossRef]
- Kampe, T.U.; Johnson, B.R.; Kuester, M.A.; Keller, M. NEON: The first continental-scale ecological observatory with airborne remote sensing of vegetation canopy biochemistry and structure. J. Appl. Remote Sens. 2010, 4, 043510. [Google Scholar] [CrossRef]
- Wingate, L.; Ogée, J.; Cremonese, E.; Filippa, G.; Mizunuma, T.; Migliavacca, M.; Moisy, C.; Wilkinson, M.; Moureaux, C.; Wohlfahrt, G.; et al. Interpreting canopy development and physiology using a European phenology camera network at flux sites. Biogeosciences 2015, 12, 5995–6015. [Google Scholar] [CrossRef]
- Moore, C.E.; Brown, T.; Keenan, T.F.; Duursma, R.A.; van Dijk, A.I.J.M.; Beringer, J.; Culvenor, D.; Evans, B.; Huete, A.; Hutley, L.B.; et al. Reviews and syntheses: Australian vegetation phenology: New insights from satellite remote sensing and digital repeat photography. Biogeosciences 2016, 13, 5085–5102. [Google Scholar] [CrossRef]
- Brown, T.B.; Hultine, K.R.; Steltzer, H.; Denny, E.G.; Denslow, M.W.; Granados, J.; Henderson, S.; Moore, D.; Nagai, S.; SanClements, M.; et al. Using phenocams to monitor our changing Earth: Toward a global phenocam network. Front. Ecol. Environ. 2016, 14, 84–93. [Google Scholar] [CrossRef]
- FSI. Chapter 2: Forest Cover. 2021. Available online: https://fsi.nic.in/isfr-2021/chapter-2.pdf (accessed on 28 June 2022).
- Champion, H.G.; Seth, S.K. A Revised Survey of the Forest Types of India; Government of India: New Delhi, India, 1968.
- Das, T.; Das, A.K. Vegetative and reproductive phenology of some multipurpose tree species in the homegardens of Barak Valley, northeast India. Int. J. Biometeorol. 2013, 57, 185–196. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, A.K.; Pandey, P.C.; Sharma, J.K.; Triantakonstantis, D.; Srivastava, P.K. Climate Change and Its Impact on Forest of Indian Himalayan Region: A Review. Clim. Chang. 2022, 207–222. [Google Scholar] [CrossRef]
- Prasad, S.N.; Hegde, M. Phenology and seasonality in the tropical deciduous forest of Bandipur, South India. Proc. Plant Sci. 1986, 96, 121–133. [Google Scholar] [CrossRef]
- Ralhan, P.K.; Khanna, R.; Singh, S.P.; Singh, J.S. Certain phenological characters of the shrub layer of Kumaun Himalayan forests. Vegetatio 1985, 63, 113–119. [Google Scholar] [CrossRef]
- Murali, K.S.; Sukumar, R. Leaf flushing phenology and herbivory in a tropical dry deciduous forest, southern India. Oecologia 1993, 94, 114–119. [Google Scholar] [CrossRef] [PubMed]
- Ganesh, T.; Davidar, P. Flowering phenology and flower predation of Cullenia exarillata (Bombacaceae) by arboreal vertebrates in Western Ghats, India. J. Trop. Ecol. 1997, 13, 459–468. [Google Scholar] [CrossRef]
- Boojh, R.; Ramakrishnan, P.S. Growth strategy of trees related to successional status II. Leaf dynamics. For. Ecol. Manag. 1982, 4, 375–386. [Google Scholar] [CrossRef]
- Shukla, R.P.; Ramakrishnan, P.S. Phenology of trees in a sub-tropical humid forest in north-eastern India. Vegetatio 1982, 49, 103–109. [Google Scholar] [CrossRef]
- Omesh, B.; Anoop, K.; Mishra, A.K.; Nayan, S.; Behera, S.K.; Chaudhary, L.B. Phenological study of two dominant tree species in tropical moist deciduous forest from the northern India. Int. J. Bot. 2012, 8, 66–72. [Google Scholar]
- Datta, A.; Rane, A. Phenology, seed dispersal and regeneration patterns of Horsfieldia kingii, a rare wild nutmeg. Trop. Conserv. Sci. 2013, 6, 674–689. [Google Scholar] [CrossRef]
- Suresh, H.S.; Sukumar, R. Phenology of Ficus spp. in a tropical dry forest, Mudumalai, south India. J. For. Res. 2018, 29, 1129–1138. [Google Scholar] [CrossRef]
- Ramaswami, G.; Datta, A.; Reddy, A.; Quader, S. Tracking phenology in the tropics and in India: The impacts of climate change. Implic. Clim. Chang. Indian Biodivers. Overv. 2019, 16, 45–59. [Google Scholar]
- Prabakaran, C.; Singh, C.P.; Panigrahy, S.; Parihar, J.S. Retrieval of forest phenological parameters from remote sensing-based NDVI time-series data. Curr. Sci. 2013, 105, 795–802. [Google Scholar]
- Jeganathan, C.; Dash, J.; Atkinson, P.M. Remotely sensed trends in the phenology of northern high latitude terrestrial vegetation, controlling for land cover change and vegetation type. Remote Sens. Environ. 2014, 143, 154–170. [Google Scholar] [CrossRef]
- Rajan, H.; Jeganathan, C. Understanding Spatio-temporal Pattern of Grassland Phenology in the western Indian Himalayan State. J. Indian Soc. Remote Sens. 2019, 47, 1137–1151. [Google Scholar] [CrossRef]
- Sharma, R.; Kaur, S.; Uniyal, S.K. Tracking the seasonal dynamics of Himalayan birch using a time-lapse camera. Folia Geobot. 2021, 56, 125–138. [Google Scholar] [CrossRef]
- Parihar, J.S.; Goroshi, S.; Singh, R.P.; Krishnayya, N.S.R.; Sirsayya, M.B.; Kumar, A.; Rawat, L.S.; Sonakia, A. Observation of forest phenology using field-based digital photography and satellite data. Curr. Sci. 2013, 105, 1740–1747. [Google Scholar]
- Liu, Z.; An, S.; Lu, X.; Hu, H.; Tang, J. Using canopy greenness index to identify leaf ecophysiological traits during the foliar senescence in an oak forest. Ecosphere 2018, 9, e02337. [Google Scholar] [CrossRef]
- Graham, E.A.; Riordan, E.C.; Yuen, E.M.; Estrin, D.; Rundel, P.W. Public Internet-connected cameras used as a cross-continental ground-based plant phenology monitoring system. Glob. Chang. Biol. 2010, 16, 3014–3023. [Google Scholar] [CrossRef]
- Negi, V.S.; Pathak, R.; Rawal, R.S.; Bhatt, I.D.; Sharma, S. Long-term ecological monitoring on forest ecosystems in Indian Himalayan Region: Criteria and indicator approach. Ecol. Indic. 2019, 102, 374–381. [Google Scholar] [CrossRef]
- Sarkar, D.P.; Shankar, B.U.; Parida, B.R. Machine learning approach to predict terrestrial gross primary productivity using topographical and remote sensing data. Ecol. Inform. 2022, 70, 101697. [Google Scholar] [CrossRef]
- Reid, A.M.; Chapman, W.K.; Prescott, C.E.; Nijland, W. Using excess greenness and green chromatic coordinate colour indices from aerial images to assess lodgepole pine vigour, mortality and disease occurrence. For. Ecol. Manag. 2016, 374, 146–153. [Google Scholar] [CrossRef]
- Toda, M.; Richardson, A.D. Estimation of plant area index and phenological transition dates from digital repeat photography and radiometric approaches in a hardwood forest in the Northeastern United States. Agric. For. Meteorol. 2018, 249, 457–466. [Google Scholar] [CrossRef]
- Migliavacca, M.; Galvagno, M.; Cremonese, E.; Rossini, M.; Meroni, M.; Sonnentag, O.; Cogliati, S.; Manca, G.; Diotri, F.; Busetto, L.; et al. Using digital repeat photography and eddy covariance data to model grassland phenology and photosynthetic CO2 uptake. Agric. For. Meteorol. 2011, 151, 1325–1337. [Google Scholar] [CrossRef]
- Gonçalves, N.B.; Lopes, A.P.; Dalagnol, R.; Wu, J.; Pinho, D.M.; Nelson, B.W. Both near-surface and satellite remote sensing confirm drought legacy effect on tropical forest leaf phenology after 2015/2016 ENSO drought. Remote Sens. Environ. 2020, 237, 111489. [Google Scholar] [CrossRef]
- Restrepo-Coupe, N.; Huete, A.; Davies, K.; Cleverly, J.; Beringer, J.; Eamus, D.; van Gorsel, E.; Hutley, L.B.; Meyer, W.S. MODIS vegetation products as proxies of photosynthetic potential: A look across meteorological and biologic driven ecosystem productivity. Biogeosci. Discuss. 2015, 12, 19213–19267. [Google Scholar]
- Tian, F.; Cai, Z.; Jin, H.; Hufkens, K.; Scheifinger, H.; Tagesson, T.; Smets, B.; Van Hoolst, R.; Bonte, K.; Ivits, E.; et al. Calibrating vegetation phenology from Sentinel-2 using eddy covariance, PhenoCam, and PEP725 networks across Europe. Remote Sens. Environ. 2021, 260, 112456. [Google Scholar] [CrossRef]
- Foley, J.A.; Levis, S.; Prentice, I.C.; Pollard, D.; Thompson, S.L. Coupling dynamic models of climate and vegetation. Glob. Chang. Biol. 1998, 4, 561–579. [Google Scholar] [CrossRef]
- Nagai, S.; Maeda, T.; Gamo, M.; Muraoka, H.; Suzuki, R.; Nasahara, K.N. Using digital camera images to detect canopy condition of deciduous broad-leaved trees. Plant Ecol. Divers. 2011, 4, 79–89. [Google Scholar] [CrossRef]
- Zhang, X.; Jayavelu, S.; Liu, L.; Friedl, M.A.; Henebry, G.M.; Liu, Y.; Schaaf, C.B.; Richardson, A.D.; Gray, J. Evaluation of land surface phenology from VIIRS data using time series of PhenoCam imagery. Agric. For. Meteorol. 2018, 256–257, 137–149. [Google Scholar] [CrossRef]
- Nagai, S.; Saitoh, T.M.; Noh, N.J.; Yoon, T.K.; Kobayashi, H.; Suzuki, R.; Nasahara, K.N.; Son, Y.; Muraoka, H. Utility of information in photographs taken upwards from the floor of closed-canopy deciduous broadleaved and closed-canopy evergreen coniferous forests for continuous observation of canopy phenology. Ecol. Inform. 2013, 18, 10–19. [Google Scholar] [CrossRef]
- Descals, A.; Verger, A.; Yin, G.; Peñuelas, J. Improved estimates of arctic land surface phenology using sentinel-2 time series. Remote Sens. 2020, 12, 3738. [Google Scholar] [CrossRef]
- Snyder, K.A.; Huntington, J.L.; Wehan, B.L.; Morton, C.G.; Stringham, T.K. Comparison of landsat and land-based phenology camera normalized difference vegetation index (NDVI) for dominant plant communities in the great basin. Sensors 2019, 19, 1139. [Google Scholar] [CrossRef] [PubMed]
- Andresen, C.; Tweedie, C.; Lougheed, V. Climate and nutrient effects on Arctic wetland plant phenology observed from phenocams. Remote Sens. Environ. 2018, 205, 46–55. [Google Scholar] [CrossRef]
- Baumann, M.; Ozdogan, M.; Richardson, A.D.; Radeloff, V.C. Phenology from Landsat when data is scarce: Using MODIS and Dynamic Time-Warping to combine multi-year Landsat imagery to derive annual phenology curves. Int. J. Appl. Earth Obs. Geoinf. 2017, 54, 72–83. [Google Scholar] [CrossRef]
- Zhang, S.; Buttò, V.; Khare, S.; Deslauriers, A.; Morin, H.; Huang, J.-G.; Ren, H.; Rossi, S. Calibrating PhenoCam data with phenological observations of a black spruce stand. Can. J. Remote Sens. 2020, 46, 154–165. [Google Scholar] [CrossRef]
- Marchin, R.M.; McHugh, I.; Simpson, R.R.; Ingram, L.J.; Balas, D.S.; Evans, B.J.; Adams, M.A. Productivity of an Australian mountain grassland is limited by temperature and dryness despite long growing seasons. Agric. For. Meteorol. 2018, 256–257, 116–124. [Google Scholar] [CrossRef]
- Inoue, T.; Nagai, S.; Saitoh, T.M.; Muraoka, H.; Nasahara, K.N.; Koizumi, H. Detection of the different characteristics of year-to-year variation in foliage phenology among deciduous broad-leaved tree species by using daily continuous canopy surface images. Ecol. Inform. 2014, 22, 58–68. [Google Scholar] [CrossRef]
- Ahrends, H.; Etzold, S.; Kutsch, W.; Stoeckli, R.; Bruegger, R.; Jeanneret, F.; Wanner, H.; Buchmann, N.; Eugster, W. Tree phenology and carbon dioxide fluxes: Use of digital photography for process-based interpretation at the ecosystem scale. Clim. Res. 2009, 39, 261–274. [Google Scholar] [CrossRef]
- Crimmins, M.A.; Crimmins, T.M. Monitoring plant phenology using digital repeat photography. Environ. Manag. 2008, 41, 949–958. [Google Scholar] [CrossRef]
- Linkosalmi, M.; Aurela, M.; Tuovinen, J.-P.; Peltoniemi, M.; Tanis, C.M.; Arslan, A.N.; Kolari, P.; Böttcher, K.; Aalto, T.; Rainne, J.; et al. Digital photography for assessing the link between vegetation phenology and CO2 exchange in two contrasting northern ecosystems. Geosci. Instrum. Methods Data Syst. 2016, 5, 417–426. [Google Scholar] [CrossRef]
- Jorge, C.; Silva, J.M.N.; Boavida-Portugal, J.; Soares, C.; Cerasoli, S. Using digital photography to track understory phenology in mediterranean cork oak woodlands. Remote Sens. 2021, 13, 776. [Google Scholar] [CrossRef]
- Nijland, W.; Coops, N.; Coogan, S.; Bater, C.; Wulder, M.; Nielsen, S.; McDermid, G.; Stenhouse, G. Vegetation phenology can be captured with digital repeat photography and linked to variability of root nutrition in Hedysarum alpinum. Appl. Veg. Sci. 2013, 16, 317–324. [Google Scholar] [CrossRef]
- Paloschi, R.A.; Ramos, D.M.; Ventura, D.J.; Souza, R.; Souza, E.; Morellato, L.P.C.; Nóbrega, R.L.B.; Coutinho, Í.A.C.; Verhoef, A.; Körting, T.S.; et al. Environmental drivers of water use for caatinga woody plant species: Combining remote sensing phenology and sap flow measurements. Remote Sens. 2020, 13, 75. [Google Scholar] [CrossRef]
- Koide, D.; Ide, R.; Oguma, H. Detection of autumn leaf phenology and color brightness from repeat photography: Accurate, robust, and sensitive indexes and modeling under unstable field observations. Ecol. Indic. 2019, 106, 105482. [Google Scholar] [CrossRef]
- Wang, J.; Yang, D.; Detto, M.; Nelson, B.W.; Chen, M.; Guan, K.; Wu, S.; Yan, Z.; Wu, J. Multi-scale integration of satellite remote sensing improves characterization of dry-season green-up in an Amazon tropical evergreen forest. Remote Sens. Environ. 2020, 246, 111865. [Google Scholar] [CrossRef]
- Atkins, J.W.; Stovall, A.E.L.; Yang, X. Mapping temperate forest phenology using tower, UAV, and terrestrial-based sensors. Drones 2020, 4, 56. [Google Scholar] [CrossRef]
- Liu, Y.; Hill, M.J.; Zhang, X.; Wang, Z.; Richardson, A.D.; Hufkens, K.; Filippa, G.; Baldocchi, D.D.; Ma, S.; Verfaillie, J.; et al. Using data from Landsat, MODIS, VIIRS and PhenoCams to monitor the phenology of California oak/grass savanna and open grassland across spatial scales. Agric. For. Meteorol. 2017, 237–238, 311–325. [Google Scholar] [CrossRef]
- Julitta, T.; Cremonese, E.; Migliavacca, M.; Colombo, R.; Galvagno, M.; Siniscalco, C.; Rossini, M.; Fava, F.; Cogliati, S.; Di Cella, U.M.; et al. Using digital camera images to analyse snowmelt and phenology of a subalpine grassland. Agric. For. Meteorol. 2014, 198–199, 116–125. [Google Scholar] [CrossRef]
- Xie, Y.; Civco, D.L.; Silander, J.A., Jr. Species-specific spring and autumn leaf phenology captured by time-lapse digital cameras. Ecosphere 2018, 9, e02089. [Google Scholar] [CrossRef]
- Vrieling, A.; Meroni, M.; Darvishzadeh, R.; Skidmore, A.K.; Wang, T.; Zurita-Milla, R.; Oosterbeek, K.; O’Connor, B.; Paganini, M. Vegetation phenology from Sentinel-2 and field cameras for a Dutch barrier island. Remote Sens. Environ. 2018, 215, 517–529. [Google Scholar] [CrossRef]
- Melaas, E.K.; Friedl, M.A.; Richardson, A.D. Multiscale modeling of spring phenology across Deciduous Forests in the Eastern United States. Glob. Chang. Biol. 2016, 22, 792–805. [Google Scholar] [CrossRef] [PubMed]
- Nezval, O.; Krejza, J.; Světlík, J.; Šigut, L.; Horáček, P. Comparison of traditional ground-based observations and digital remote sensing of phenological transitions in a floodplain forest. Agric. For. Meteorol. 2020, 291, 108079. [Google Scholar] [CrossRef]
- Thapa, S.; Millan, V.E.G.; Eklundh, L. Assessing forest phenology: A multi-scale comparison of near-surface (UAV, spectral reflectance sensor, phenocam) and satellite (MODIS, sentinel-2) remote sensing. Remote Sens. 2021, 13, 1597. [Google Scholar] [CrossRef]
- Brown, L.A.; Dash, J.; Ogutu, B.O.; Richardson, A.D. On the relationship between continuous measures of canopy greenness derived using near-surface remote sensing and satellite-derived vegetation products. Agric. For. Meteorol. 2017, 247, 280–292. [Google Scholar] [CrossRef]
- Henneken, R.; Dose, V.; Schleip, C.; Menzel, A. Detecting plant seasonality from webcams using Bayesian multiple change point analysis. Agric. For. Meteorol. 2013, 168, 177–185. [Google Scholar] [CrossRef][Green Version]
- Morris, D.E.; Boyd, D.S.; Crowe, J.A.; Johnson, C.S.; Smith, K.L. Exploring the potential for automatic extraction of vegetation phenological metrics from traffic webcams. Remote Sens. 2013, 5, 2200–2218. [Google Scholar] [CrossRef]
- Melaas, E.K.; Sulla-Menashe, D.; Gray, J.M.; Black, T.A.; Morin, T.H.; Richardson, A.D.; Friedl, M.A. Multisite analysis of land surface phenology in North American temperate and boreal deciduous forests from Landsat. Remote Sens. Environ. 2016, 186, 452–464. [Google Scholar] [CrossRef]
- Peichl, M.; Sonnentag, O.; Nilsson, M.B. Bringing color into the picture: Using digital repeat photography to investigate phenology controls of the carbon dioxide exchange in a boreal mire. Ecosystems 2015, 18, 115–131. [Google Scholar] [CrossRef]
- Cheng, Y.; Vrieling, A.; Fava, F.; Meroni, M.; Marshall, M.; Gachoki, S. Phenology of short vegetation cycles in a Kenyan rangeland from PlanetScope and Sentinel-2. Remote Sens. Environ. 2020, 248, 112004. [Google Scholar] [CrossRef]
- Matiu, M.; Bothmann, L.; Steinbrecher, R.; Menzel, A. Monitoring succession after a non-cleared windthrow in a Norway spruce mountain forest using webcam, satellite vegetation indices and turbulent CO2 exchange. Agric. For. Meteorol. 2017, 244–245, 72–81. [Google Scholar] [CrossRef]
- Zhao, J.; Zhang, Y.; Tan, Z.; Song, Q.; Liang, N.; Yu, L.; Zhao, J. Using digital cameras for comparative phenological monitoring in an evergreen broad-leaved forest and a seasonal rain forest. Ecol. Inform. 2012, 10, 65–72. [Google Scholar] [CrossRef]
- Yan, D.; Scott, R.; Moore, D.; Biederman, J.; Smith, W. Understanding the relationship between vegetation greenness and productivity across dryland ecosystems through the integration of PhenoCam, satellite, and eddy covariance data. Remote Sens. Environ. 2019, 223, 50–62. [Google Scholar] [CrossRef]
- Songsom, V.; Koedsin, W.; Ritchie, R.J.; Huete, A. Mangrove phenology and water influences measured with digital repeat photography. Remote Sens. 2021, 13, 307. [Google Scholar] [CrossRef]
- Seyednasrollah, B.; Bowling, D.R.; Cheng, R.; Logan, B.A.; Magney, T.S.; Frankenberg, C.; Yang, J.C.; Young, A.M.; Hufkens, K.; Arain, M.A.; et al. Seasonal variation in the canopy color of temperate evergreen conifer forests. New Phytol. 2021, 229, 2586–2600. [Google Scholar] [CrossRef] [PubMed]
- Wheeler, K.I.; Dietze, M.C. Improving the monitoring of deciduous broadleaf phenology using the Geostationary Operational Environmental Satellite (GOES) 16 and 17. Biogeosciences 2021, 18, 1971–1985. [Google Scholar] [CrossRef]
- Cao, M.; Sun, Y.; Jiang, X.; Li, Z.; Xin, Q. Identifying leaf phenology of deciduous broadleaf forests from phenocam images using a convolutional neural network regression method. Remote Sens. 2021, 13, 2331. [Google Scholar] [CrossRef]
- Moon, M.; Richardson, A.D.; Friedl, M.A. Multiscale assessment of land surface phenology from harmonized Landsat 8 and Sentinel-2, PlanetScope, and PhenoCam imagery. Remote Sens. Environ. 2021, 266, 112716. [Google Scholar] [CrossRef]
- Khare, S.; Deslauriers, A.; Morin, H.; Latifi, H.; Rossi, S. Comparing time-lapse PhenoCams with satellite observations across the boreal forest of Quebec, Canada. Remote Sens. 2021, 14, 100. [Google Scholar] [CrossRef]
- Yan, D.; Zhang, X.; Nagai, S.; Yu, Y.; Akitsu, T.; Nasahara, K.N.; Ide, R.; Maeda, T. Evaluating land surface phenology from the Advanced Himawari Imager using observations from MODIS and the Phenological Eyes Network. Int. J. Appl. Earth Obs. Geoinf. 2019, 79, 71–83. [Google Scholar] [CrossRef]
- Shukla, R.P.; Ramakrishnan, P.S. Leaf dynamics of tropical trees related to successional status. New Phytol. 1984, 97, 697–706. [Google Scholar] [CrossRef]
- Bhat, D.M. Phenology of tree species of tropical moist forest of Uttara Kannada district, Karnataka, India. J. Biosci. 1992, 17, 325–352. [Google Scholar] [CrossRef]
- Murali, K.S.; Sukumar, R. Reproductive phenology of a tropical dry forest in Mudumalai, southern India. J. Ecol. 1994, 82, 759. [Google Scholar] [CrossRef]
- Ganesh, T.; Davidar, P. Fruiting phenology and pre-dispersal seed predation in a rainforest in southern Western Ghats, India. Trop. Fruits Frugivores Search Strong Interactors 2005, 139–154. [Google Scholar]
- Krishnan, R.M. Reproductive phenology of a wet forest understorey in the Western Ghats, South India. Glob. Ecol. Biogeogr. 2002, 11, 179–182. [Google Scholar] [CrossRef]
- Sundarapandian, S.M.; Chandrasekaran, S.; Swamy, P.S. Phenological behaviour of selected tree species in tropical forests at Kodayar in the Western Ghats, Tamil Nadu, India. Curr. Sci. 2005, 88, 805–810. [Google Scholar]
- Selwyn, M.A.; Parthasarathy, N. Reproductive traits and phenology of plants in tropical dry evergreen forest on the Coromandel coast of India. Biodivers. Conserv. 2006, 15, 3207–3234. [Google Scholar] [CrossRef]
- Sellamuthu, S.; Lalitha, V. Plant diversity and phenological pattern in the montane wet temperate forests of the southern Western Ghats, India. For. Stud. China 2010, 12, 116–125. [Google Scholar] [CrossRef]
- Suresh, H.S.; Sukumar, R. Vegetative phenology of tropical montane forests in the Nilgiris, South India. J. Natl. Sci. Found. Sri Lanka 2011, 39, 333. [Google Scholar] [CrossRef]
- Nanda, A.; Krishnamurthy, Y.L.; Suresh, H.S. Canopy trees leaf phenology in tropical dry deciduous and evergreen forests of Bhadra Wildlife Sanctuary Karnataka, India. Afr. J. Plant Sci. 2013, 7, 170–175. [Google Scholar] [CrossRef][Green Version]
- Nanda, A.; Suresh, H.S.; Krishna Murthy, Y.L. Leafing phenology of tropical forests of Bhadra wildlife sanctuary, Karnataka, India. Appl. Sci. Rep. 2015, 12, 33–40. [Google Scholar]
- Kushwaha, C.P.; Singh, K.P. Diversity of leaf phenology in a tropical deciduous forest in India. J. Trop. Ecol. 2005, 21, 47–56. [Google Scholar] [CrossRef]
- Bajpai, O.; Pandey, J.; Chaudhary, L.B. Periodicity of different phenophases in selected trees from Himalayan Terai of India. Agrofor. Syst. 2017, 91, 363–374. [Google Scholar] [CrossRef]
- Chaurasia, B.; Shukla, R.P. Changes in reproductive phenology and sex ratio of Trewia nudiflora Linn. Growing in sal Forest of North-eastern Uttar Pradesh, India. Trop. Ecol. 2016, 57, 89–99. [Google Scholar]
- Mishra, R.K.; Upadhyay, V.P.; Bal, S.; Mohapatra, P.K.; Mohanty, R.C. Phenology of species of moist deciduous forest sites of Similipal biosphere reserve. Lyonia 2006, 11, 5–17. [Google Scholar]
- Yadav, R.K.; Yadav, A.S. Phenology of selected woody species in a tropical dry deciduous forest in Rajasthan, India. Trop. Ecol. 2008, 49, 25–34. [Google Scholar]
- Nautiyal, M.C.; Nautiyal, B.P.; Prakash, V. Phenology and growth form distribution in an alpine pasture at Tungnath, Garhwal, Himalaya. Mt. Res. Dev. 2001, 21, 168–174. [Google Scholar] [CrossRef]
- Joshi, V.C.; Janarthanam, M.K. The diversity of life-form type, habitat preference and phenology of the endemics in the Goa region of the Western Ghats, India. J. Biogeogr. 2004, 31, 1227–1237. [Google Scholar] [CrossRef]
- Borah, M.; Devi, A. Phenology, growth and survival of Vatica lanceaefolia Bl.: A critically endangered tree species in moist tropical forest of Northeast India. Trop. Plant Res. 2014, 1, 1–12. [Google Scholar]
- Nath, S.; Nath, A.J.; Das, A.K. Vegetative and reproductive phenology of a floodplain tree species Barringtonia acutangula from North East India. J. Environ. Biol. 2016, 37, 215–220. [Google Scholar]
- Aruna, R.; Balasubramanian, P. Fruiting phenology of a scrub forest in thiruporur, Eastern Ghats, India. Int. Lett. Nat. Sci. 2015, 44, 25–30. [Google Scholar] [CrossRef]
- Arunparasath, A.; Gomathinayagam, M. Reproductive phenology of true mangrove species in Pichavaram mangrove forests, Tamilnadu, India–A comparative account. J. Environ. Treat. Tech. 2015, 15, 17–21. [Google Scholar]
- Kaur, G.; Singh, B.P.; Nagpal, A.K. Phenology of Some Phanerogams (Trees and Shrubs) of Northwestern Punjab, India. J. Bot. 2013, 2013, 712405. [Google Scholar] [CrossRef][Green Version]
- Kikim, A.; Yadava, P.S. Phenology of tree species in subtropical forests of Manipur in north eastern India. Trop. Ecol. 2001, 42, 269–276. [Google Scholar]
- Pandey, R.; Pandey, C.N. Reproductive Strategy of Aegiceras corniculatum L. (Blanco.)—A Mangrove Species, in MNP&S, Gujarat, India. J. Plant Stud. 2014, 3, 35. [Google Scholar] [CrossRef]
- Singh, K.P.; Kushwaha, C.P. Diversity of flowering and fruiting phenology of trees in a tropical deciduous forest in India. Ann. Bot. 2006, 97, 265–276. [Google Scholar] [CrossRef] [PubMed]
- Newton, P.N. The structure and phenology of a moist deciduous forest in the Central Indian Highlands. Vegetatio 1988, 75, 3–16. [Google Scholar] [CrossRef]
- Thakur, P.S.; Dutt, V.; Thakur, A. Impact of inter-annual climate variability on the phenology of eleven multipurpose tree species. Curr. Sci. 2008, 94, 1053–1058. [Google Scholar]
- Sivaraj, N.; Krishnamurthy, K.V. Flowering phenology in the vegetation of Shervaroys, South India. Vegetatio 1988, 79, 85–88. [Google Scholar] [CrossRef]
- Sundriyal, R.C. Phenology of some temperate woody species of the Garhwal Himalaya. Int. J. Ecol. Environ. Sci. 1990, 16, 107–117. [Google Scholar]
- Sundriyal, R.C.; Joshi, A.P.; Dhasmana, R. Phenology of high altitude plants at Tungnath in the Garhwal Himalaya. Trop. Ecol. 1987, 28, 289–299. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jose, K.; Chaturvedi, R.K.; Jeganathan, C.; Behera, M.D.; Singh, C.P. Plugging the Gaps in the Global PhenoCam Monitoring of Forests—The Need for a PhenoCam Network across Indian Forests. Remote Sens. 2023, 15, 5642. https://doi.org/10.3390/rs15245642
Jose K, Chaturvedi RK, Jeganathan C, Behera MD, Singh CP. Plugging the Gaps in the Global PhenoCam Monitoring of Forests—The Need for a PhenoCam Network across Indian Forests. Remote Sensing. 2023; 15(24):5642. https://doi.org/10.3390/rs15245642
Chicago/Turabian StyleJose, Karun, Rajiv Kumar Chaturvedi, Chockalingam Jeganathan, Mukunda Dev Behera, and Chandra Prakash Singh. 2023. "Plugging the Gaps in the Global PhenoCam Monitoring of Forests—The Need for a PhenoCam Network across Indian Forests" Remote Sensing 15, no. 24: 5642. https://doi.org/10.3390/rs15245642
APA StyleJose, K., Chaturvedi, R. K., Jeganathan, C., Behera, M. D., & Singh, C. P. (2023). Plugging the Gaps in the Global PhenoCam Monitoring of Forests—The Need for a PhenoCam Network across Indian Forests. Remote Sensing, 15(24), 5642. https://doi.org/10.3390/rs15245642











