Hyperspectral Indices for Predicting Nitrogen Use Efficiency in Maize Hybrids
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Design and Site Description
2.2. Plant Measurements and Physiological N Parameters Measured
2.3. Remote Sensing Data
2.4. Feature Extraction with Hyperspectral Indices (HSI)
2.5. Statistical Analysis
2.5.1. Treatment Effects
2.5.2. Hybrid Rankings
2.6. Relationship of HSI to N Parameters
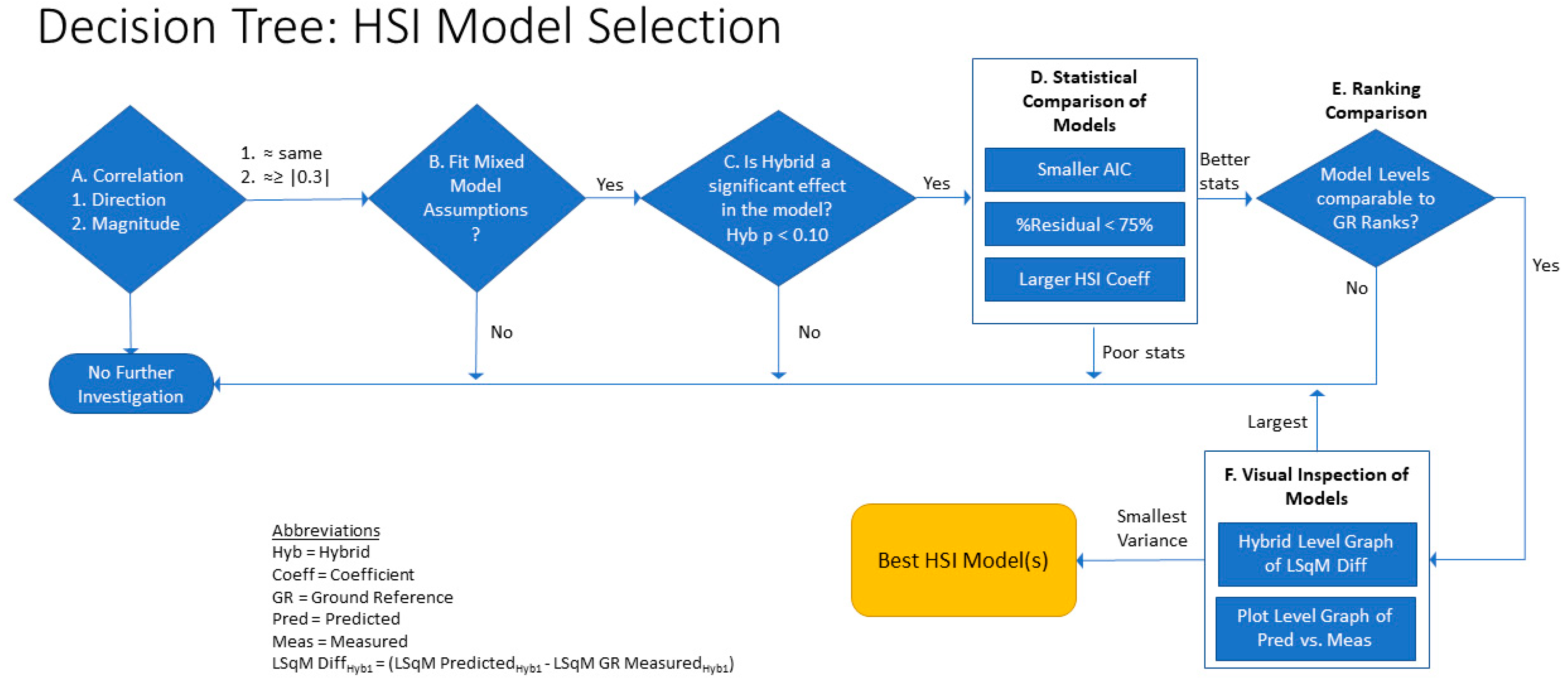
2.6.1. HSI Selection
- A.
- Survey correlation statistics based on sign and magnitude as described in Methods;
- B.
- Examine residual diagnostics, selecting HSI models where statistical assumptions are satisfied;
- C.
- Select models where hybrid is a significant effect at α = 0.10;
- D.
- Compare statistical values of each model with preference for models with smaller Akaike’s Information Criterion (AIC), % residual <75%, and larger coefficient estimate for the standardized (z-score) HSI effect;
- E.
- Compare hybrid ranking level letters (obtained from a Tukey–Kramer’s HSD means comparison) of the HSI model to the ground reference hybrid rankings;
- F.
- Visually inspect charts of the difference in the hybrid least square mean values of the HSI and ground reference models as well as plots of the predicted by measured values at the per-plot level; preference is given to the models with smallest variance.
2.6.2. Impact of Downsampling
3. Results and Discussion
3.1. Treatment Effect on Physiological Parameters
3.2. Treatment and Time Effect on N Parameters
3.2.1. Effect on pN at R6: Ground Reference Model
3.2.2. Effect on NCE: Ground Reference Model
3.2.3. Effect on NIE: Ground Reference Model
3.2.4. Hybrid Rankings by NCE or NIE at R6
3.3. Evaluating the Relationship between N Parameters and Hyperspectral Indices
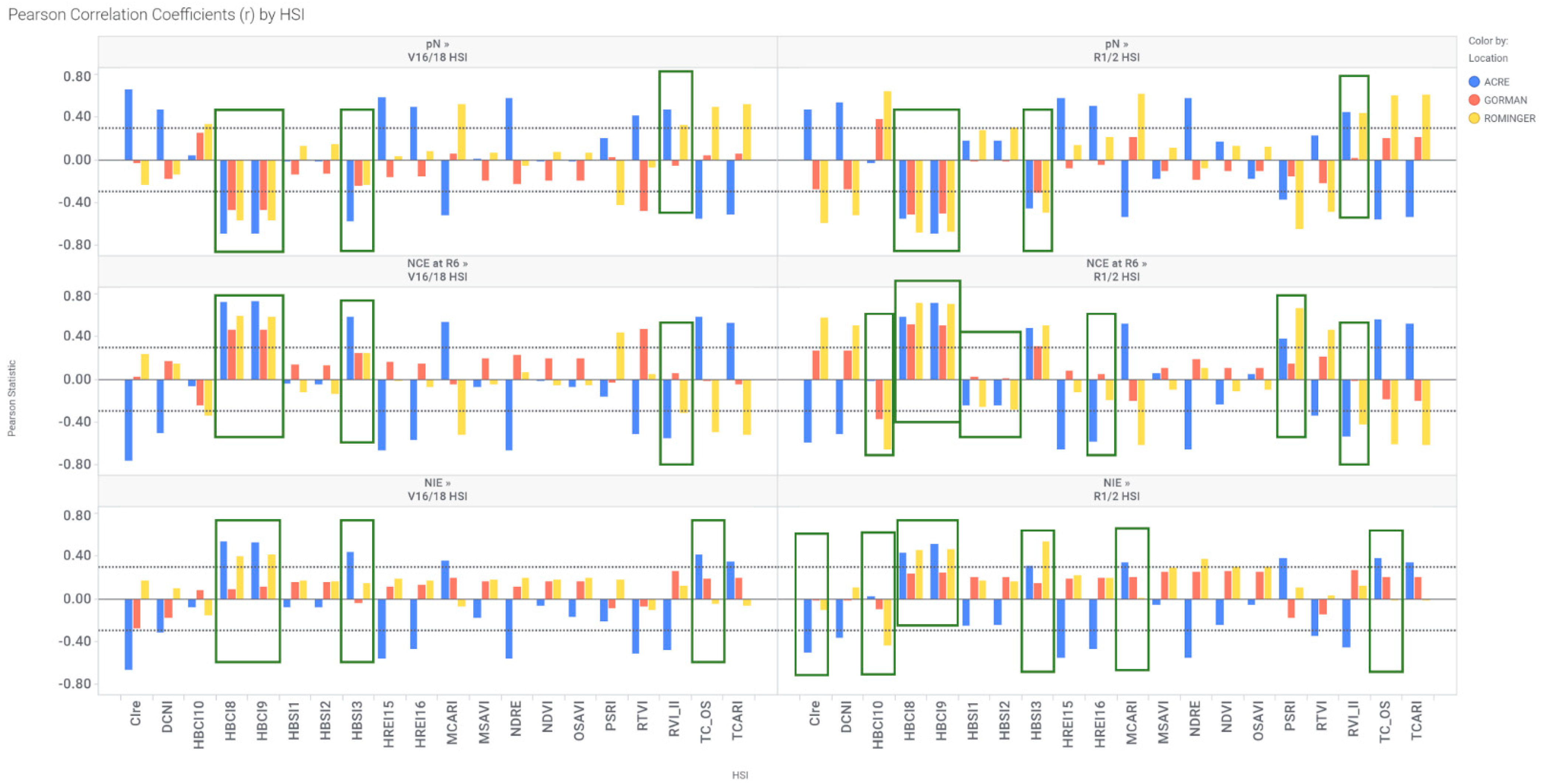
3.4. Correlation between N Parameter (pN, NCE, NIE) and HSI
3.5. N Parameter Estimation by HSI
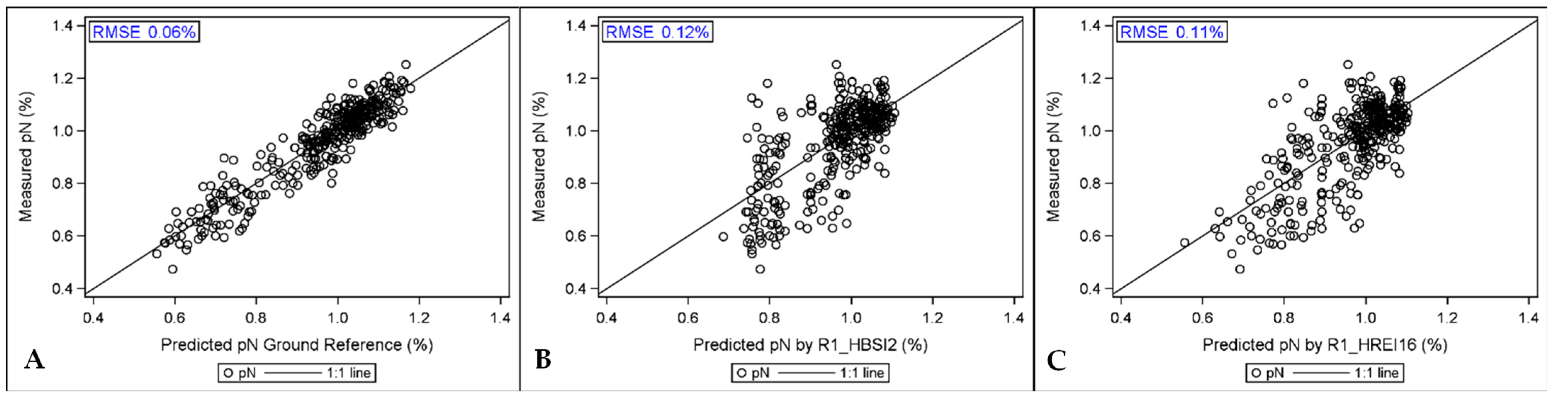
3.5.1. Predicting pN with Hyperspectral Indices
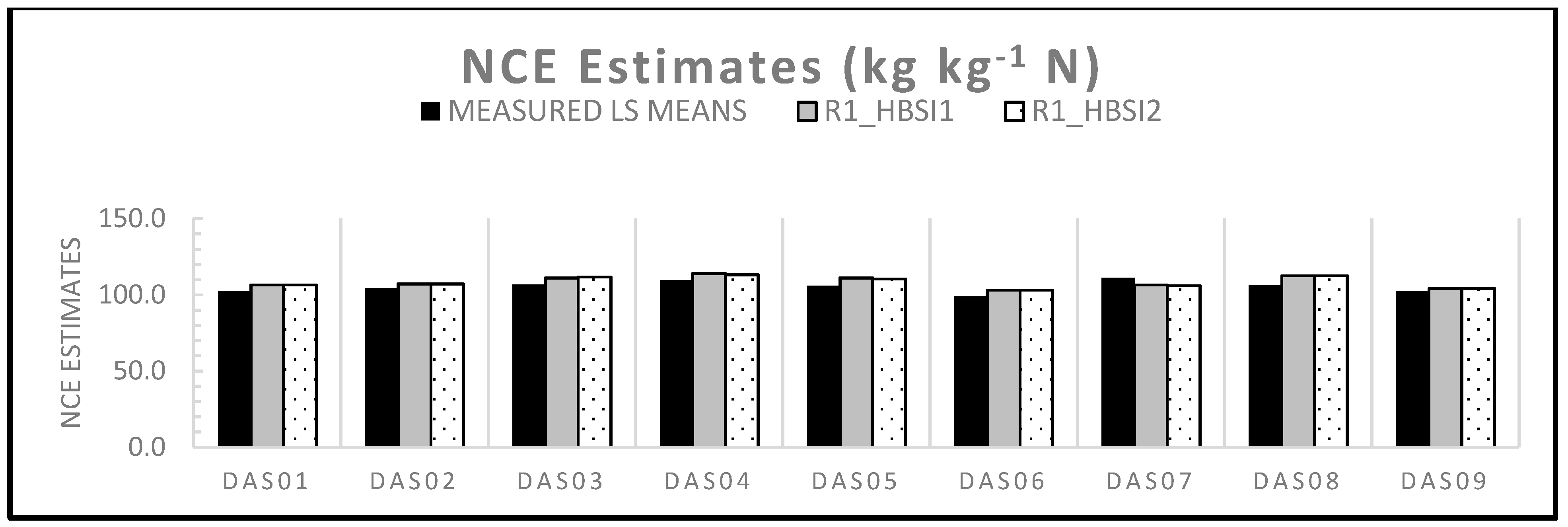
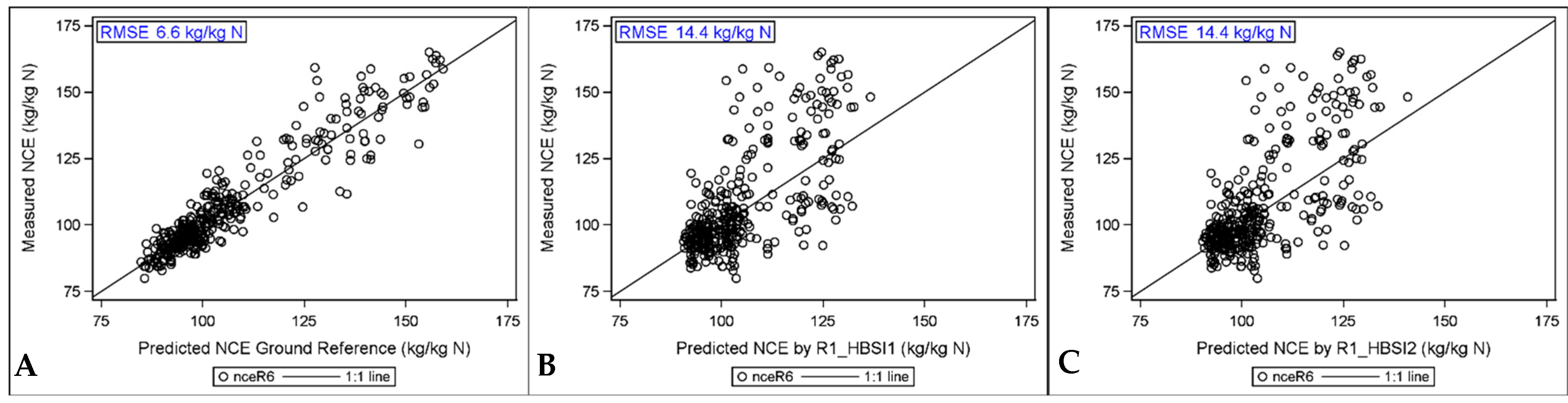
3.5.2. Predicting NCE with Hyperspectral Indices
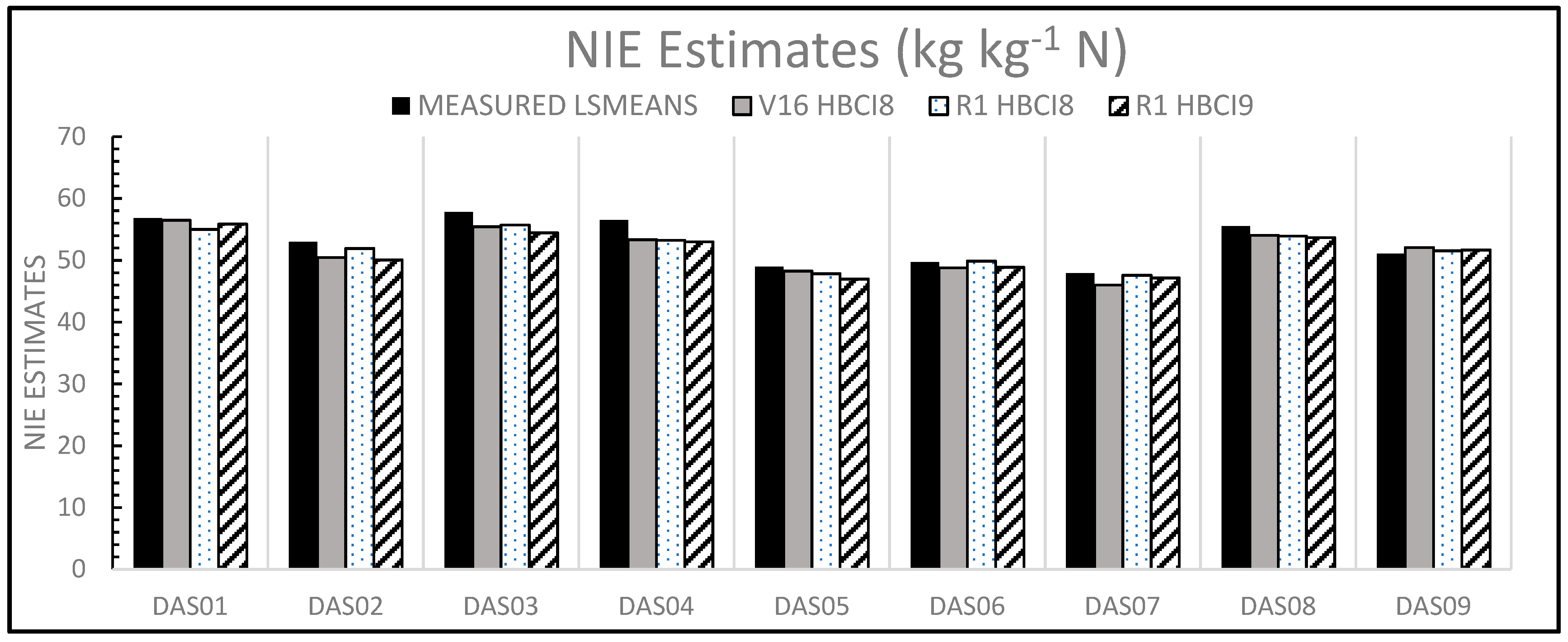
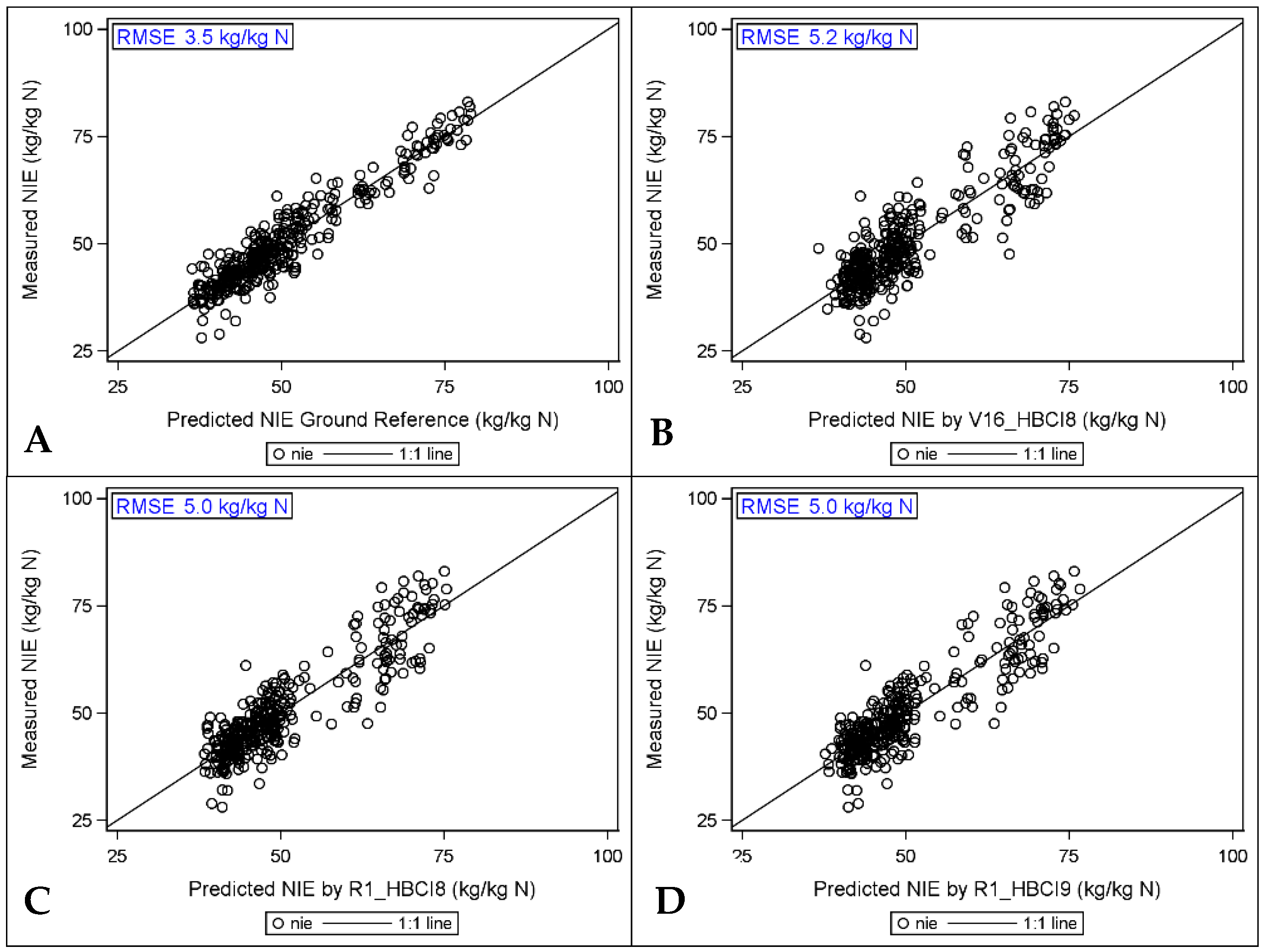
3.5.3. Predicting NIE with Hyperspectral Indices
3.6. Comparison of High to Low Spatial Resolution HSI
Impact of Resolution Difference on Rankings
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ribaudo, M.; Hansen, L.; Livingston, M.; Mosheim, R.; Williamson, J.; Delgado, J. Nitrogen in agricultural systems: Implications for conservation policy. USDA-ERS Econ. Res. Rep. 2011, 127, 89. [Google Scholar] [CrossRef]
- Mueller, S.M.; Messina, C.D.; Vyn, T.J. Simultaneous gains in grain yield and nitrogen efficiency over 70 years of maize genetic improvement. Sci. Rep. 2019, 9, 9095. [Google Scholar] [CrossRef] [PubMed]
- Doering, O.; Galloway, J.; Theis, T.; Aneja, V.; Boyer, E.; Cassman, K.; Cowling, E.; Dickerson, R.; Herz, W.; Hey, D. Reactive Nitrogen in the United States: An Analysis of Inputs, Flows, Consequences, and Management Options; Board, E.S.A., Ed.; United States Environmental Protection Agency: Washington, DC, USA, 2011.
- Chen, K.; Vyn, T.J. Post-silking factor consequences for N efficiency changes over 38 years of commercial maize hybrids. Front. Plant Sci. 2017, 8, 1737. [Google Scholar] [CrossRef] [PubMed]
- Ciampitti, I.A.; Vyn, T.J. Physiological perspectives of changes over time in maize yield dependency on nitrogen uptake and associated nitrogen efficiencies: A review. Field Crops Res. 2012, 133, 48–67. [Google Scholar] [CrossRef]
- DeBruin, J.L.; Schussler, J.R.; Mo, H.; Cooper, M. Grain yield and nitrogen accumulation in maize hybrids released during 1934 to 2013 in the US Midwest. Crop Sci. 2017, 57, 1431–1446. [Google Scholar] [CrossRef]
- Moose, S.; Below, F.E. Biotechnology approaches to improving maize nitrogen use efficiency. In Molecular Genetic Approaches to Maize Improvement; Kriz, A.L., Larkins, B.A., Eds.; Springer: Berlin/Heidelberg, Germany, 2009; pp. 65–77. [Google Scholar]
- Moll, R.H.; Kamprath, E.J.; Jackson, W.A. Analysis and interpretation of factors which contribute to efficiency of nitrogen utilization. Agron. J. 1982, 74, 562–564. [Google Scholar] [CrossRef]
- Shrawat, A.; Zayed, A.; Lightfoot, D.A. (Eds.) Engineering Nitrogen Utilization in Crop Plants; Springer International Publishing: Berlin/Heidelberg, Germany, 2018. [Google Scholar]
- Salvagiotti, F.; Castellarín, J.M.; Miralles, D.J.; Pedrol, H.M. Sulfur fertilization improves nitrogen use efficiency in wheat by increasing nitrogen uptake. Field Crops Res. 2009, 113, 170–177. [Google Scholar] [CrossRef]
- Ciampitti, I.A.; Vyn, T.J. A comprehensive study of plant density consequences on nitrogen uptake dynamics of maize plants from vegetative to reproductive stages. Field Crops Res. 2011, 121, 2–18. [Google Scholar] [CrossRef]
- Gastal, F.; Lemaire, G.; Durand, J.-L.; Louarn, G. Quantifying crop responses to nitrogen and avenues to improve nitrogen-use efficiency. In Crop Physiology, 2nd ed.; Academic Press: Cambridge, MA, USA, 2015; pp. 161–206. [Google Scholar]
- Muñoz-Huerta, R.F.; Guevara-Gonzalez, R.G.; Contreras-Medina, L.M.; Torres-Pacheco, I.; Prado-Olivarez, J.; Ocampo-Velazquez, R.V. A review of methods for sensing the nitrogen status in plants: Advantages, disadvantages and recent advances. Sensors 2013, 13, 10823–10843. [Google Scholar] [CrossRef] [PubMed]
- Unkovich, M.; Herridge, D.; Peoples, M.; Cadisch, G.; Boddey, B.; Giller, K.; Alves, B.; Chalk, P. Measuring Plant-Associated Nitrogen Fixation in Agricultural Systems; Australian Centre for International Agricultural Research (ACIAR): Canberra, Australia, 2008.
- Zhao, D.; Raja Reddy, K.; Kakani, V.G.; Read, J.J.; Carter, G.A. Corn (Zea mays L.) growth, leaf pigment concentration, photosynthesis and leaf hyperspectral reflectance properties as affected by nitrogen supply. Plant Soil 2003, 257, 205–218. [Google Scholar] [CrossRef]
- Araus, J.L.; Cairns, J.E. Field high-throughput phenotyping: The new crop breeding frontier. Trends Plant Sci. 2014, 19, 52–61. [Google Scholar] [CrossRef] [PubMed]
- Cobb, J.N.; DeClerck, G.; Greenberg, A.; Clark, R.; McCouch, S. Next-generation phenotyping: Requirements and strategies for enhancing our understanding of genotype–phenotype relationships and its relevance to crop improvement. Theor. Appl. Genet. 2013, 126, 867–887. [Google Scholar] [CrossRef]
- Jin, X.; Zarco-Tejada, P.; Schmidhalter, U.; Reynolds, M.P.; Hawkesford, M.J.; Varshney, R.K.; Yang, T.; Nie, C.; Li, Z.; Ming, B. High-throughput estimation of crop traits: A review of ground and aerial phenotyping platforms. IEEE Geosci. Remote Sens. Mag. 2020, 9, 200–231. [Google Scholar] [CrossRef]
- Nguyen, G.N.; Kant, S. Improving nitrogen use efficiency in plants: Effective phenotyping in conjunction with agronomic and genetic approaches. Funct. Plant Biol. 2018, 45, 606. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues Junior, F.A.; Ortiz-Monasterio, I.; Zarco-Tejada, P.J.; Ammar, K.; Gérard, B. Using precision agriculture and remote sensing techniques to improve genotype selection in a breeding program. In Proceedings of the 12th International Conference on Precision Agriculture (ICPA), Sacramento, CA, USA, 20–23 July 2014. [Google Scholar]
- White, J.W.; Andrade-Sanchez, P.; Gore, M.A.; Bronson, K.F.; Coffelt, T.A.; Conley, M.M.; Feldmann, K.A.; French, A.N.; Heun, J.T.; Hunsaker, D.J.; et al. Field-based phenomics for plant genetics research. Field Crops Res. 2012, 133, 101–112. [Google Scholar] [CrossRef]
- Lillesand, T.; Kiefer, R.W.; Chipman, J. Remote Sensing and Image Interpretation; John Wiley & Sons: Hoboken, NJ, USA, 2014. [Google Scholar]
- Campbell, J.B.; Wynne, R.H. Introduction to Remote Sensing; Guilford Press: New York, NY, USA, 2011. [Google Scholar]
- Mulla, D.J. Twenty five years of remote sensing in precision agriculture: Key advances and remaining knowledge gaps. Biosyst. Eng. 2013, 114, 358–371. [Google Scholar] [CrossRef]
- Goel, P.K.; Prasher, S.O.; Landry, J.A.; Patel, R.M.; Viau, A.A.; Miller, J.R. Estimation of crop biophysical parameters through airborne and field hyperspectral remote sensing. Trans. ASAE 2003, 46, 1235–1246. [Google Scholar]
- Osborne, S.L.; Schepers, J.S.; Francis, D.D.; Schlemmer, M.R. Use of spectral radiance to estimate in-season biomass and grain yield in nitrogen- and water- stressed corn. Crop Sci. 2002, 42, 165–171. [Google Scholar]
- Thenkabail, P.S.; Gumma, M.K.; Teluguntla, P.; Mohammed, I.A. Hyperspectral remote sensing of vegetation and agricultural crops. Photogramm. Eng. Remote Sens. 2014, 80, 697–723. [Google Scholar]
- Thenkabail, P.S.; Smith, R.B.; De Pauw, E. Hyperspectral vegetation indices and their relationships with agricultural crop characteristics. Remote Sens. Environ. 2000, 71, 158–182. [Google Scholar] [CrossRef]
- Blackmer, T.M.; Schepers, J.S.; Varvel, G.E.; Walter-Shea, E.A. Nitrogen deficiency detection using reflected shortwave radiation from irrigated corn canopies. Agron. J. 1996, 88, 1–5. [Google Scholar] [CrossRef]
- Elsayed, S.; Darwish, W. Hyperspectral remote sensing to assess the water status, biomass, and yield of maize cultivars under salinity and water stress. Bragantia 2017, 76, 62–72. [Google Scholar] [CrossRef]
- Campbell, P.; Middleton, E.; McMurtrey, J.; Chappelle, E. Assessment of vegetation stress using reflectance or fluorescence measurements. J. Environ. Qual. 2007, 36, 832–845. [Google Scholar] [CrossRef]
- Osborne, S.L.; Schepers, J.S.; Francis, D.D.; Schlemmer, M.R. Detection of phosphorus and nitrogen deficiencies in corn using spectral radiance measurements. Agron. J. 2002, 94, 1215–1221. [Google Scholar] [CrossRef]
- Schlemmer, M.R.; Francis, D.D.; Shanahan, J.; Schepers, J.S. Remotely measuring chlorophyll content in corn leaves with differing nitrogen levels and relative water content. Agron. J. 2005, 97, 106–112. [Google Scholar] [CrossRef]
- Thenkabail, P.S.; Lyon, J.G.; Huete, A. Hyperspectral Remote Sensing of Vegetation; CRC Press: Boca Raton, FL, USA, 2012. [Google Scholar]
- Midwestern Regional Climate Center. Available online: https://mrcc.illinois.edu/CLIMATE/ (accessed on 8 June 2019).
- Indiana State Climate Office. Available online: www.iclimate.org (accessed on 2 December 2017).
- Miller, R.O.; Gavlak, R.; Horneck, D. Soil, Plant and Water Reference Methods for the Western Region; WCC-103 Publication: Fort Collins, CO, USA, 2013; p. 156. [Google Scholar]
- NCERA-13; Eliason, R.; Goos, R.J.; Hoskins, B. Recommended Chemical Soil Test Procedures for the North Central Region; NCERA-13, Ed.; Missouri Agricultural Experiment Station: Columbia, MO, USA, 2015; p. 76. [Google Scholar]
- US EPA. Standard method 350.1: Nitrogen, ammonia (colorimetric, automated phenate). In Methods for the Determination of Inorganic Substances in Environmental Samples; Office of Research and Development, US EPA: Cincinnati, OH, USA, 1993. [Google Scholar]
- US EPA. Method 353.2: Determination of Nitrate—Nitrite Nitrogen by Automated Colorimetry, Revision 2.0; O’Dell, J., Ed.; Environmental Monitoring Systems Laboratory: Cincinnati, OH, USA, 1993.
- Dellavalle Laboratory Inc. Soil Interpretation Report; Kasapligil, D., Ed.; Dellavalle Laboratory, Inc.: Davis, CA, USA, 2014. [Google Scholar]
- Vitosh, M.; Johnson, J.; Mengel, D. Tri-State Fertilizer Recommendations for Corn, Soybeans, Wheat and Alfalfa; Michigan State University Extension: East Lansing, MI, USA, 1995. [Google Scholar]
- Rouse, J.W.J.; Haas, R.H.; Schell, J.A.; Deering, D.W.; Harlan, J.C. Monitoring the Vernal Advancment and Retrogradation (Greenwave Effect) of Natural Vegetation; Texas A&M University Remote Sensing Center: College Station, TX, USA, 1974. [Google Scholar]
- Qi, J.; Chehbouni, A.; Huete, A.; Kerr, Y.; Sorooshian, S. A modified soil adjusted vegetation index. Remote Sens. Environ. 1994, 48, 119–126. [Google Scholar] [CrossRef]
- Chen, P.-F.; Nicolas, T.; Wang, J.-H.; Philippe, V.; Huang, W.-J.; Li, B.-G. New index for crop canopy fresh biomass estimation. Spectrosc. Spectr. Anal. 2010, 30, 512–517. [Google Scholar]
- Merzlyak, M.N.; Gitelson, A.A.; Chivkunova, O.B.; Rakitin, V.Y. Non-destructive optical detection of pigment changes during leaf senescence and fruit ripening. Physiol. Plant. 1999, 106, 135–141. [Google Scholar] [CrossRef]
- Daughtry, C.S.T.; Walthall, C.L.; Kim, M.S.; Brown de Colstoun, E.; McMurtrey, J.E., III. Estimating corn leaf chlorophyll concentration from leaf and canopy reflectance. Remote Sens. Environ. 2000, 74, 229–239. [Google Scholar] [CrossRef]
- Chen, P.; Haboudane, D.; Tremblay, N.; Wang, J.; Vigneault, P.; Li, B. New spectral indicator assessing the efficiency of crop nitrogen treatment in corn and wheat. Remote Sens. Environ. 2010, 114, 1987–1997. [Google Scholar] [CrossRef]
- Xue, L.; Cao, W.; Luo, W.; Dai, T.; Zhu, Y. Monitoring leaf nitrogen status in rice with canopy spectral reflectance. Agron. J. 2004, 96, 135–142. [Google Scholar] [CrossRef]
- Kim, M.S.; Daughtry, C.; Chappelle, E.; McMurtrey, J.; Walthall, C. The use of high spectral resolution bands for estimating absorbed photosynthetically active radiation (A par). In Proceedings of the 6th Symposium on Physical Measurements and Signatures in Remote Sensing, Val D’Isere, France, 17–24 January 1994; pp. 299–306. [Google Scholar]
- Kim, M.S. The Use of Narrow Spectral Bands for Improving Remote Sensing Estimations of Fractionally Absorbed Photosynthetically Active Radiation. In Department of Geography; University of Maryland: College Park, MD, USA, 1994. [Google Scholar]
- Rondeaux, G.; Steven, M.; Baret, F. Optimization of soil-adjusted vegetation indices. Remote Sens. Environ. 1996, 55, 95–107. [Google Scholar] [CrossRef]
- Haboudane, D.; Miller, J.R.; Tremblay, N.; Zarco-Tejada, P.J.; Dextraze, L. Integrated narrow-band vegetation indices for prediction of crop chlorophyll content for application to precision agriculture. Remote Sens. Environ. 2002, 81, 416–426. [Google Scholar] [CrossRef]
- Barnes, E.; Clarke, T.; Richards, S.; Colaizzi, P.; Haberland, J.; Kostrzewski, M.; Waller, P.; Choi, C.; Riley, E.; Thompson, T. Coincident detection of crop water stress, nitrogen status and canopy density using ground based multispectral data. In Proceedings of the Fifth International Conference on Precision Agriculture, Bloomington, MN, USA, 16–19 July 2000. [Google Scholar]
- Gitelson, A.A.; Gritz, Y.; Merzlyak, M.N. Relationships between leaf chlorophyll content and spectral reflectance and algorithms for non-destructive chlorophyll assessment in higher plant leaves. J. Plant Physiol. 2003, 160, 271–282. [Google Scholar] [CrossRef] [PubMed]
- Montgomery, D.C.; Peck, E.A.; Vining, G.G. Introduction to Linear Regression Analysis, 5th ed.; John Wiley & Sons: Hoboken, NJ, USA, 2012; Volume 821. [Google Scholar]
- Bundy, L.; Carter, P. Corn hybrid response to nitrogen fertilization in the northern corn belt. J. Prod. Agric. 1988, 1, 99–104. [Google Scholar] [CrossRef][Green Version]
- Jeschke, M.; DeBruin, J. Corn hybrid response to nitrogen fertilizer. In Crop Insights; DuPont Pioneer Agronomy Sciences: Johnston, Hungary, 2016; pp. 1–7. [Google Scholar]
- Chevalier, P.; Schrader, L. Genotypic differences in nitrate absorption and partitioning of N among plant parts in maize. Crop Sci. 1977, 17, 897–901. [Google Scholar] [CrossRef]
- Sadras, V.O.; Calderini, D.F. Crop Physiology: Applications for Genetic Improvement and Agronomy, 2nd ed.; Academic Press: Cambridge, MA, USA, 2015. [Google Scholar]
- Plénet, D.; Lemaire, G. Relationships between dynamics of nitrogen uptake and dry matter accumulation in maize crops. Determination of critical N concentration. Plant Soil 2000, 216, 65–82. [Google Scholar] [CrossRef]
- Haegele, J.W.; Cook, K.A.; Nichols, D.M.; Below, F.E. Changes in nitrogen use traits associated with genetic improvement for grain yield of maize hybrids released in different decades. Crop Sci. 2013, 53, 1256. [Google Scholar] [CrossRef]
- D’Andrea, K.E.; Otegui, M.E.; Cirilo, A.G.; Eyhérabide, G.H. Ecophysiological traits in maize hybrids and their parental inbred lines: Phenotyping of responses to contrasting nitrogen supply levels. Field Crops Res. 2009, 114, 147–158. [Google Scholar] [CrossRef]
- Schlemmer, M.; Gitelson, A.; Schepers, J.; Ferguson, R.; Peng, Y.; Shanahan, J.; Rundquist, D. Remote estimation of nitrogen and chlorophyll contents in maize at leaf and canopy levels. Int. J. Appl. Earth Obs. Geoinf. 2013, 25, 47–54. [Google Scholar] [CrossRef]
- Zhao, B.; Duan, A.; Ata-Ul-Karim, S.T.; Liu, Z.; Chen, Z.; Gong, Z.; Zhang, J.; Xiao, J.; Liu, Z.; Qin, A.; et al. Exploring new spectral bands and vegetation indices for estimating nitrogen nutrition index of summer maize. Eur. J. Agron. 2018, 93, 113–125. [Google Scholar] [CrossRef]
- Haboudane, D.; Tremblay, N.; Miller, J.R.; Vigneault, P. Remote estimation of crop chlorophyll content using spectral indices derived From hyperspectral data. IEEE Trans. Geosci. Remote Sens. 2008, 46, 423–437. [Google Scholar] [CrossRef]
- Hunt Jr, E.R.; Doraiswamy, P.C.; McMurtrey, J.E.; Daughtry, C.S.; Perry, E.M.; Akhmedov, B. A visible band index for remote sensing leaf chlorophyll content at the canopy scale. Int. J. Appl. Earth Obs. Geoinf. 2013, 21, 103–112. [Google Scholar] [CrossRef]
- Zhou, K.; Cheng, T.; Zhu, Y.; Cao, W.; Ustin, S.L.; Zheng, H.; Yao, X.; Tian, Y. Assessing the impact of spatial resolution on the estimation of leaf nitrogen concentration over the full season of paddy rice using near-surface imaging spectroscopy data. Front. Plant Sci. 2018, 9, 964. [Google Scholar] [CrossRef]
- Boomsma, C.R.; Santini, J.B.; Tollenaar, M.; Vyn, T.J. Maize morphophysiological responses to intense crowding and low nitrogen availability: An analysis and review. Agron. J. 2009, 101, 1426. [Google Scholar] [CrossRef]
- Buschmann, C.; Nagel, E. In vivo spectroscopy and internal optics of leaves as basis for remote sensing of vegetation. Int. J. Remote Sens. 1993, 14, 711–722. [Google Scholar] [CrossRef]
- Thenkabail, P.S.; Smith, R.B.; De Pauw, E. Evaluation of narrowband and broadband vegetation indices for determining optimal hyperspectral wavebands for agricultural crop characterization. Photogramm. Eng. Remote Sens. 2002, 68, 607–622. [Google Scholar]
- Blackburn, G.A. Quantifying chlorophylls and carotenoids at leaf and canopy scales: An evaluation of some hyperspectral approaches. Remote Sens. Environ. 1998, 66, 273–285. [Google Scholar] [CrossRef]
- Goel, N.S.; Qin, W. Influences of canopy architecture on relationships between various vegetation indices and LAI and Fpar: A computer simulation. Remote Sens. Rev. 1994, 10, 309–347. [Google Scholar] [CrossRef]
- Jiang, Z.; Chen, Y.; Li, J.; Dou, W. The impact of spatial resolution on NDVI over heterogeneous surface. In Proceedings of the 2005 IEEE International Geoscience and Remote Sensing Symposium, 2005, IGARSS’05, Seoul, Korea, 29 July 2005; pp. 1310–1313. [Google Scholar]
- Google Data SIO, NOAA, U.S. Navy, NGA, GEBCO Landsat/Copernicus INEGI Data LDEO-Columbia, NSF, and N. IBCAO. Google Earth 2022. Available online: earth.google.com (accessed on 11 March 2022).
- Chenu, K. Characterizing the crop environment–nature, significance and applications. In Crop Physiology; Elsevier: Amsterdam, The Netherlands, 2015; pp. 321–348. [Google Scholar]
- Connor, D.J.; Loomis, R.S.; Cassman, K.G. Crop Ecology: Productivity and Management in Agricultural Systems; Cambridge University Press: Cambridge, UK, 2011. [Google Scholar]
- Schepers, J.S.; Raun, W. (Eds.) Nitrogen in Agricultural Systems; ASA-CSSA-SSSA: Madison, WI, USA, 2008. [Google Scholar]

| Year | Location | Planting Date | Harvest Date | Final Plant Density (Plants ha−1) | Plot Size (w × l) | N Trt (kg N ha−1) (Appl Time) | BM Sampling Stages and Dates | Hybrids | RS Dates |
|---|---|---|---|---|---|---|---|---|---|
| 2014 | Woodland, CA (Gorman) | 5/14 | 9/17 | 65,000 95,000 | 4.6 m × 12 m (6 rows) | 0 56 (V4) 224 (pre, V4 and V8) | V12 (6/25) V18 (7/14) R2 (7/22) R6 (9/17) | DAS01 DAS02 DAS03 DAS04 DAS05 DAS06 DAS07 DAS08 DAS09 | V18 (7/11) R1 (7/24) |
| Woodland, CA (Rominger) | 5/27 | 10/2 | V12 (7/7) R1 (7/25) R6 (10/2) | V18 (7/11) R2 (7/24) | |||||
| 2017 | West Lafayette, IN (ACRE) | 5/17 | 10/17 | 69,100 99,600 | 3 m × 15 m (4 rows) | 0 56 (V5) 224 (V5) | V12 (7/12) R1 (7/24) R6 (10/5) | DAS02 DAS03 DAS04 DAS05 DAS09 | V5 (6/16) V8 (6/27) V16/17 (7/18) R1 (7/25) R3/R4 (8/20) R5 (9/8) R5/R6 (9/22) |
| N Levels (mg kg−1) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Sampling Time | Pre-Plant | V3 | V12 | |||||||
| Sampling Depth (cm) | Gor NO3− N LN | Gor NO3− N HN | Rom NO3− N LN | Rom NO3− N HN | ACRE NO3− N LN | ACRE NH4+ N LN | ACRE NO3− N LN | ACRE NO3− N HN | ACRE NH4+ N LN | ACRE NH4+ N HN |
| 0–15 | 19 | 24 | 9 | 10 | 14 | 4 | 2 | 3 | 6 | 6 |
| 15–30 | 29 | 25 | 9 | 12 | ||||||
| 30–60 | na | na | na | na | 11 | 3 | 2 | 4 | 4 | 4 |
| Category | Spectral Index | Equation | Reference |
|---|---|---|---|
| Biomass | NDVI | (R800 − R670)/(R800 + R670) | [43] |
| MSAVI | 0.5 × (2 × R800 + 1 − (sqrt((2 × R800 +1)2 − 8 × (R800 − R670))) | [44] | |
| RTVI | (100 × (R750 − R730) − (10 × (R750 − R550)) × sqrt(R700/R670)) | [45] | |
| Grain Yield/Structural | PSRI | (R678 − R500)/R750 | [46] |
| HBSI1 | (R855 − R682)/(R855 + R682) | [27,34] | |
| HBSI2 | (R910 − R682)/(R910 + R682) | ||
| HBSI3 | (R550 − R682)/(R550 + R682) | ||
| Chlorophyll or N Concentration | HBCI8 | (R550 − R515)/(R550 + R515) | [27,34] |
| HBCI9 | (R550 − R490)/(R550 + R490) | ||
| HBCI10 | (R720 − R550)/(R720 + R550) | ||
| MCARI | ((R700 − R670) − 0.2 × (R700 − R550)) × (R700/R670) | [47] | |
| DCNI | (R720 − R700)/(R700 − R670)/(R720 − R670 + 0.03) | [48] | |
| RVI II | R810/R560 | [49] | |
| TCARI | 3 × ((R700 − R670) − 0.2 × (R700 − R550) × (R700/R670)) | [50,51] | |
| OSAVI | ((1 + 0.16) × (R800 − R670))/(R800 + R670 + 0.16) | [52] | |
| TCARI/OSAVI | TCARI/OSAVI | [53] | |
| Plant Stress | HREI15 | (R855 − R720)/(R855 + R720) | [27,34] |
| HREI16 | (R910 − R705)/(R910 + R705) | [27,34] | |
| NDRE | (R790 − R720)/(R790 + R720) | [54] | |
| CIRE | (R750 − R800)/(R695 − R740) − 1 | [55] |
| Trt Class | Main Fixed Effects | R6 pN Estimate (%) | GY Estimate (Mg ha−1) | |||||
|---|---|---|---|---|---|---|---|---|
| Means β | LCL | UCL | Means β | SE | ||||
| H | DAS01 | 0.97 | ABC | 0.94 | 1.00 | 13.82 | AB | 1.13 |
| DAS02 | 0.96 | ABC | 0.93 | 0.98 | 12.76 | ABC | 1.07 | |
| DAS03 | 0.92 | BC | 0.90 | 0.94 | 14.32 | A | 1.07 | |
| DAS04 | 0.91 | BC | 0.89 | 0.94 | 13.33 | AB | 1.07 | |
| DAS05 | 0.93 | ABC | 0.91 | 0.95 | 12.79 | ABC | 1.07 | |
| DAS06 | 1.01 | A | 0.98 | 1.03 | 12.19 | ABC | 1.13 | |
| DAS07 | 0.89 | C | 0.86 | 0.92 | 10.48 | C | 1.13 | |
| DAS08 | 0.94 | ABC | 0.90 | 0.97 | 14.35 | A | 1.13 | |
| DAS09 | 0.97 | AB | 0.95 | 0.99 | 11.59 | BC | 1.07 | |
| N | High_N | 1.04 | A | 1.03 | 1.05 | 14.22 | ns | 1.14 |
| Med_N | 0.92 | AB | 0.90 | 0.94 | 12.96 | ns | 1.14 | |
| Low_N | 0.86 | B | 0.84 | 0.88 | 11.36 | ns | 1.14 | |
| PD | High | 0.92 | B | 0.91 | 0.93 | 12.93 | ns | 0.97 |
| Low | 0.96 | A | 0.95 | 0.98 | 12.76 | ns | 0.97 | |
| Var | Summary of Fit Model Statistics | Random Effects | Type 3 Tests of Fixed Effects | |||||
|---|---|---|---|---|---|---|---|---|
| REML Var Comp Est | ||||||||
| (% of Total) | Effect | DF | Den DF | Pr > F | ||||
| pN at R6 | N Obs | 363 | Loc | 58.1 | H | 8 | 12 | 0.015 |
| N | 2 | 4 | 0.038 | |||||
| AIC | −394.4 | N*Loc | 22.9 | PD | 1 | 299 | <0.0001 | |
| H*N | 16 | 299 | 0.080 | |||||
| Res(%) | 17.2 | H*Loc | 1.9 | H*PD | 8 | 299 | 0.069 | |
| N*PD | 2 | 299 | ns | |||||
| NCE at R6 | N Obs | 363 | Loc | 58.3 | H | 8 | 311 | <0.0001 |
| N | 2 | 4 | 0.040 | |||||
| AIC | −6400.2 | PD | 1 | 311 | <0.0001 | |||
| N*Loc | 24.9 | H*N | 16 | 311 | 0.060 | |||
| Res(%) | 16.8 | H*PD | 8 | 311 | 0.019 | |||
| N*PD | 2 | 311 | ns | |||||
| NIE at R6 | N Obs | 363 | Loc | 84 | H | 8 | 12 | 0.002 |
| N | 2 | 4 | 0.040 | |||||
| AIC | 1947.2 | N*Loc | 4.7 | PD | 1 | 299 | ns | |
| H*N | 16 | 299 | ns | |||||
| Res(%) | 8.8 | H*Loc | 2.5 | H*PD | 8 | 299 | <0.0001 | |
| N*PD | 2 | 299 | ns | |||||
| Analysis | Hybrid | NCE (kg kgN−1) | NIE (kg kgN−1) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Means | LCL | UCL | Means | LCL | UCL | ||||
| Global | DAS01 θ | 102.7 | DEF | 100.0 | 105.5 | 56.8 | AB | 44.3 | 69.3 |
| DAS02 | 104.4 | CDE | 102.5 | 106.6 | 53.0 | ABC | 40.6 | 65.4 | |
| DAS03 | 106.7 | BC | 104.6 | 108.9 | 57.8 | A | 45.4 | 70.2 | |
| DAS04 | 109.5 | AB | 107.0 | 112.3 | 56.5 | AB | 44.1 | 68.9 | |
| DAS05 | 105.9 | BCD | 103.9 | 108.3 | 49.0 | C | 36.6 | 61.4 | |
| DAS06 θ | 98.8 | F | 96.4 | 101.2 | 49.7 | BC | 37.2 | 62.2 | |
| DAS07 θ | 111.1 | A | 108.0 | 114.6 | 47.9 | C | 35.4 | 60.4 | |
| DAS08 θ | 106.6 | ABCD | 103.5 | 109.9 | 55.5 | AB | 43.0 | 68.0 | |
| DAS09 | 102.2 | EF | 100.2 | 104.2 | 51.1 | BC | 38.7 | 63.4 | |
| ACRE only | DAS02 | 122.7 | AB | 120.7 | 124.7 | 67.2 | B | 65.3 | 69.1 |
| DAS03 | 128.7 | A | 126.5 | 131.1 | 71.4 | A | 69.8 | 73.0 | |
| DAS04 | 123.4 | AB | 121.3 | 125.5 | 68.8 | AB | 66.7 | 71.0 | |
| DAS05 | Not-est | Not-est | |||||||
| DAS09 | 120.2 | B | 118.2 | 122.2 | 66.6 | B | 65.0 | 68.3 | |
| Hybrid | pN Ground Ref Means (%) | pN Est from R1 HSI (%) | ||||
|---|---|---|---|---|---|---|
| HBSI2 | HREI16 | |||||
| DAS01 | 0.97 | ABC | 0.93 | AB | 1.00 | AB |
| DAS02 | 0.96 | ABC | 0.94 | AB | 0.97 | AB |
| DAS03 | 0.92 | BC | 0.89 | AB | 0.92 | B |
| DAS04 | 0.91 | BC | 0.88 | B | 0.91 | B |
| DAS05 | 0.93 | ABC | 0.89 | AB | 0.93 | B |
| DAS06 | 1.01 | A | 0.97 | AB | 1.03 | A |
| DAS07 | 0.89 | C | 0.94 | AB | 0.94 | AB |
| DAS08 | 0.94 | ABC | 0.88 | AB | 0.98 | AB |
| DAS09 | 0.97 | AB | 0.96 | A | 1.01 | A |
| AIC | −349.4 | −9.2 | −39.2 | |||
| N | 363 | 345 | 345 | |||
| %Res | 17.2 | 33.9 | 64.3 | |||
| HYB P-VAL | 0.015 | 0.016 | 0.001 | |||
| HSI P-VAL | NA | 0.002 | <0.0001 | |||
| HSI Coeff | NA | 0.054 | 0.263 | |||
| Hybrid | NCE Ground Ref Means | NCE Estimates from R1 HSI | ||||
|---|---|---|---|---|---|---|
| HBSI1 | HBSI2 | |||||
| DAS01 | 102.7 | DEF | 106.6 | AB | 106.6 | AB |
| DAS02 | 104.4 | CDE | 107.2 | AB | 107.2 | AB |
| DAS03 | 106.7 | BC | 111.1 | AB | 111.8 | AB |
| DAS04 | 109.5 | AB | 114.0 | A | 113.2 | A |
| DAS05 | 105.9 | BCD | 111.1 | AB | 110.4 | AB |
| DAS06 | 98.8 | F | 103.1 | B | 103.1 | B |
| DAS07 | 111.1 | A | 106.6 | AB | 106.0 | AB |
| DAS08 | 106.6 | ABCD | 112.5 | AB | 112.5 | AB |
| DAS09 | 102.2 | EF | 104.3 | B | 104.3 | B |
| AIC | −6400 | −6074 | −6076 | |||
| N | 363 | 345 | 345 | |||
| %Res | 16.8 | 31.8 | 33.3 | |||
| HYB P-VAL | <0.0001 | 0.009 | 0.007 | |||
| HSI P-VAL | NA | 0.004 | 0.001 | |||
| HSI Coeff | NA | 5.56E-06 | 5.17E-06 | |||
| Hybrid | NIE Ground Ref Means | NIE Estimates from | ||||||
|---|---|---|---|---|---|---|---|---|
| V16 HSI | R1 HSI | |||||||
| HBCI8 | HBCI8 | HBCI9 | ||||||
| DAS01 | 56.8 | AB | 56.5 | A | 55.0 | AB | 55.8 | A |
| DAS02 | 53.0 | ABC | 50.5 | BCD | 51.9 | BC | 50.1 | CDE |
| DAS03 | 57.8 | A | 55.4 | A | 55.7 | A | 54.5 | AB |
| DAS04 | 56.5 | AB | 53.3 | ABC | 53.3 | ABC | 53.0 | AD |
| DAS05 | 49.0 | C | 48.3 | D | 47.8 | E | 47.0 | E |
| DAS06 | 49.7 | BC | 48.8 | CD | 49.9 | CDE | 48.9 | DE |
| DAS07 | 47.9 | C | 46.0 | D | 47.6 | DE | 47.2 | BCDE |
| DAS08 | 55.5 | AB | 54.1 | AB | 53.9 | AB | 53.7 | AC |
| DAS09 | 51.1 | BC | 52.1 | BC | 51.5 | BCD | 51.7 | AD |
| AIC | 1947.2 | 2209.9 | 2076.1 | 2067.7 | ||||
| N | 363 | 363 | 345 | 345 | ||||
| %Res | 8.8 | 28.3 | 15.8 | 32.7 | ||||
| HYB P-VAL | 0.0018 | <0.0001 | <0.0001 | <0.0001 | ||||
| HSI P-VAL | NA | <0.0001 | <0.0001 | <0.0001 | ||||
| HSI Coeff | NA | 2.64 | 1.76 | 4.28 | ||||
| Hybrid | NCE GROUND REF | R1 HBSI1 | R1 HBSI2 | ||
|---|---|---|---|---|---|
| LOW | HIGH | LOW | HIGH | ||
| DAS02 | AB | A | A | A | A |
| DAS03 | A | A | A | A | A |
| DAS04 | AB | A | A | A | A |
| DAS05 | not est | A | A | A | A |
| DAS09 | B | A | A | A | A |
| Hybrid | NIE GROUND REF | V16 HBCI8 | R1 HBCI8 | R1 HBCI9 | |||
|---|---|---|---|---|---|---|---|
| LOW | HIGH | LOW | HIGH | LOW | HIGH | ||
| DAS02 | B | ABC | A | A | A | AB | AB |
| DAS03 | A | A | A | A | A | A | A |
| DAS04 | AB | AB | A | A | A | A | A |
| DAS05 | not est | C | B | B | B | B | B |
| DAS09 | B | BC | AB | AB | AB | AB | AB |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Olson, M.B.; Crawford, M.M.; Vyn, T.J. Hyperspectral Indices for Predicting Nitrogen Use Efficiency in Maize Hybrids. Remote Sens. 2022, 14, 1721. https://doi.org/10.3390/rs14071721
Olson MB, Crawford MM, Vyn TJ. Hyperspectral Indices for Predicting Nitrogen Use Efficiency in Maize Hybrids. Remote Sensing. 2022; 14(7):1721. https://doi.org/10.3390/rs14071721
Chicago/Turabian StyleOlson, Monica B., Melba M. Crawford, and Tony J. Vyn. 2022. "Hyperspectral Indices for Predicting Nitrogen Use Efficiency in Maize Hybrids" Remote Sensing 14, no. 7: 1721. https://doi.org/10.3390/rs14071721
APA StyleOlson, M. B., Crawford, M. M., & Vyn, T. J. (2022). Hyperspectral Indices for Predicting Nitrogen Use Efficiency in Maize Hybrids. Remote Sensing, 14(7), 1721. https://doi.org/10.3390/rs14071721






