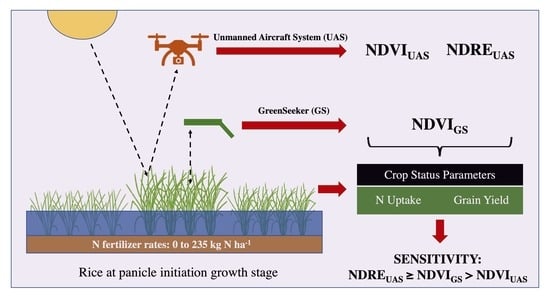
Comparative Sensitivity of Vegetation Indices Measured via Proximal and Aerial Sensors for Assessing N Status and Predicting Grain Yield in Rice Cropping Systems
Abstract
:1. Introduction
2. Materials and Methods
2.1. Site Description
2.2. Experimental Design
2.3. Plant Sampling and Analysis
2.4. Measuring Canopy Reflectance
2.4.1. Sensors Used for Measuring NDVI and NDRE
2.4.2. Normalizing the Raw Vegetation Indices Using Sufficiency-Index
2.5. Data Analysis
3. Results
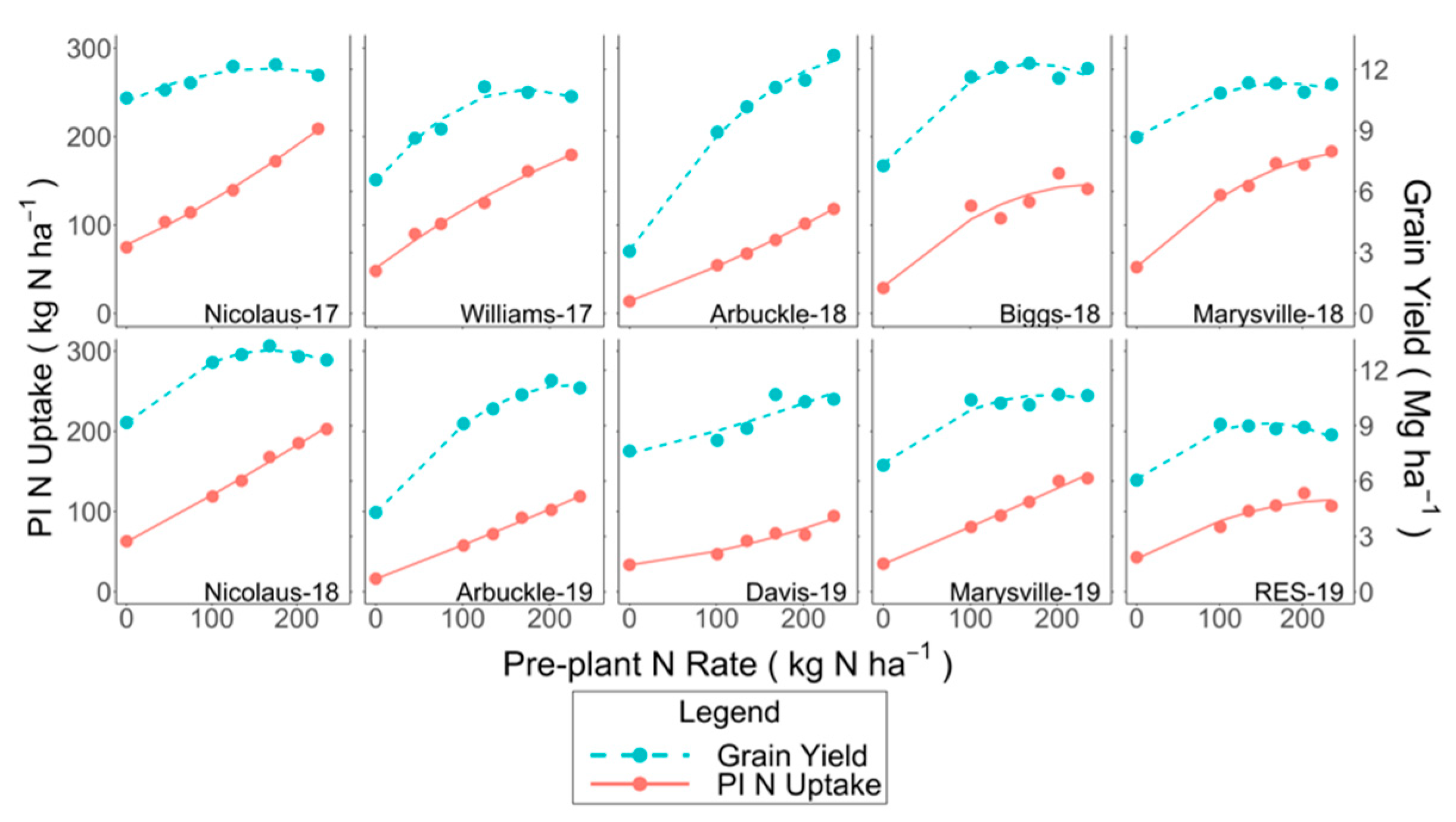
3.1. PI Total N Uptake and Grain Yield
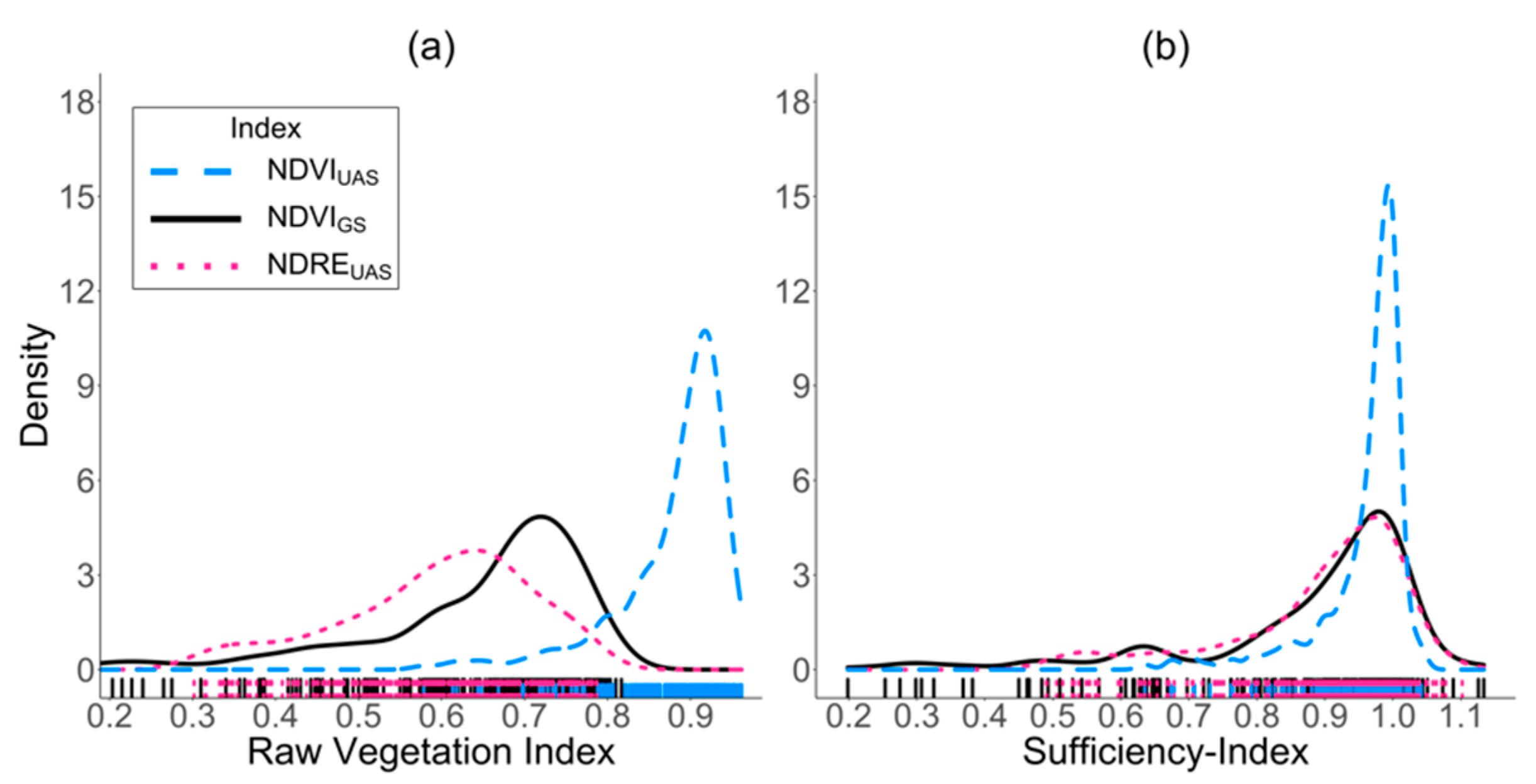
3.2. Canopy Reflectance Data
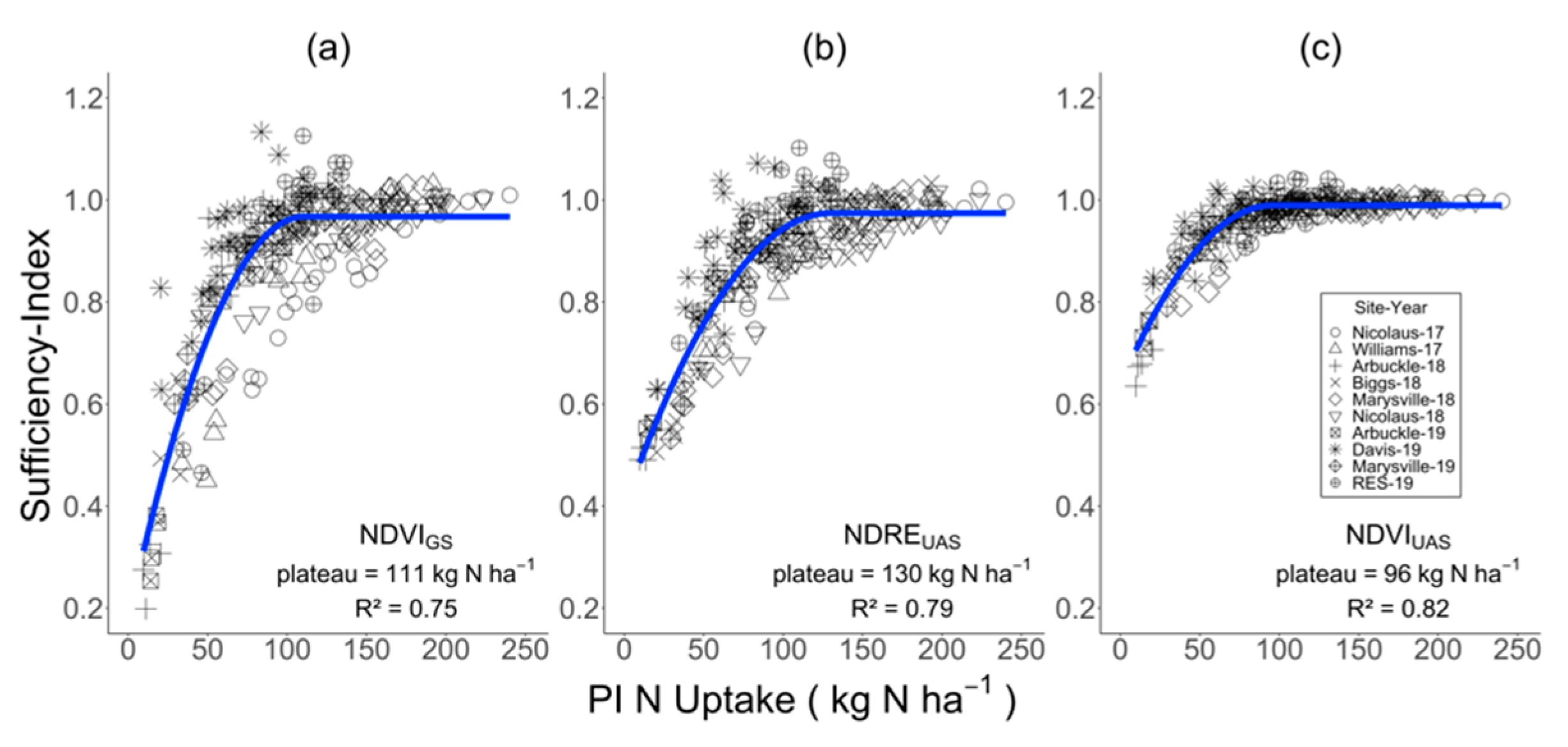
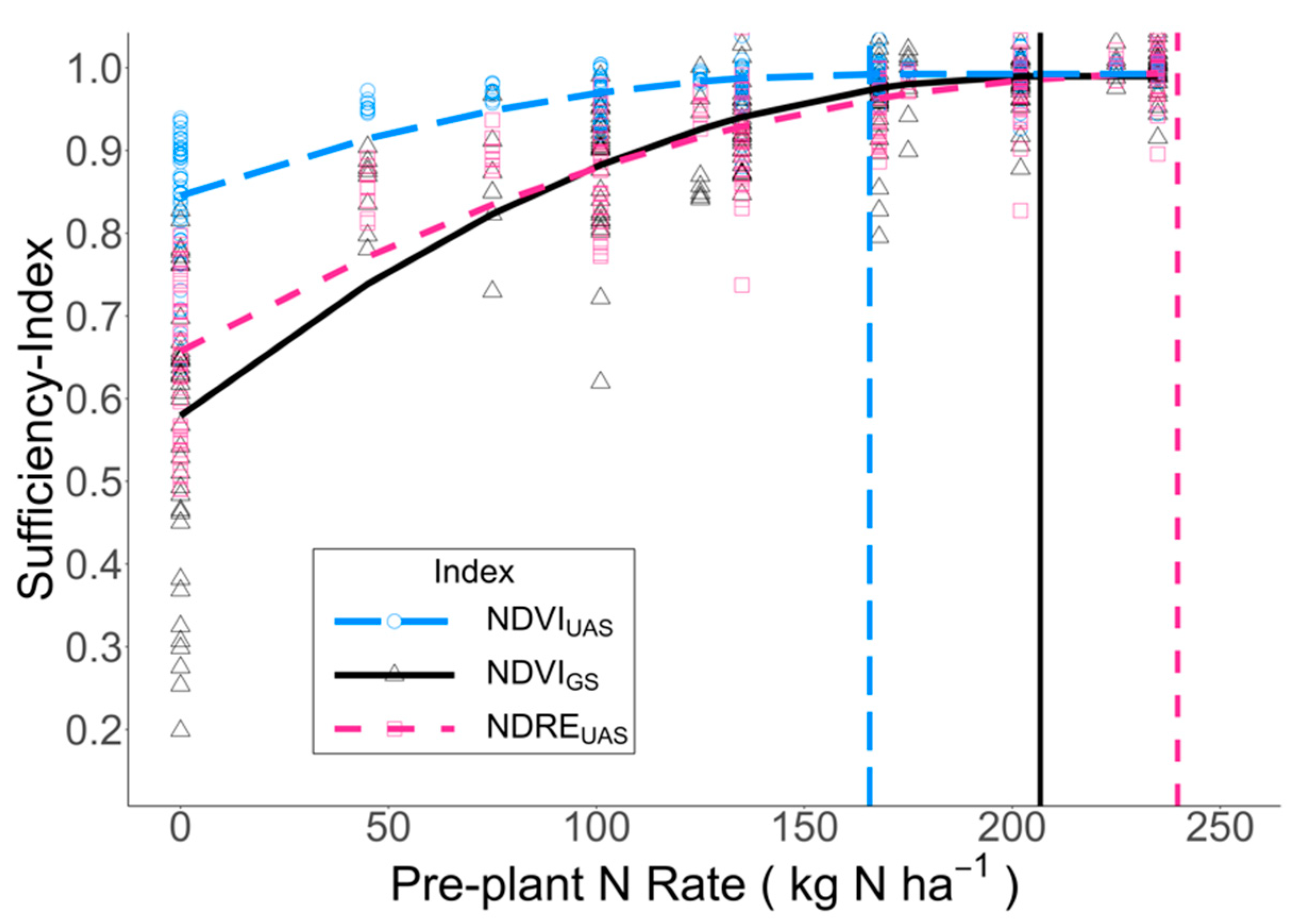
3.3. Relationship between N Rate and PI-NUP and Sufficiency-Index
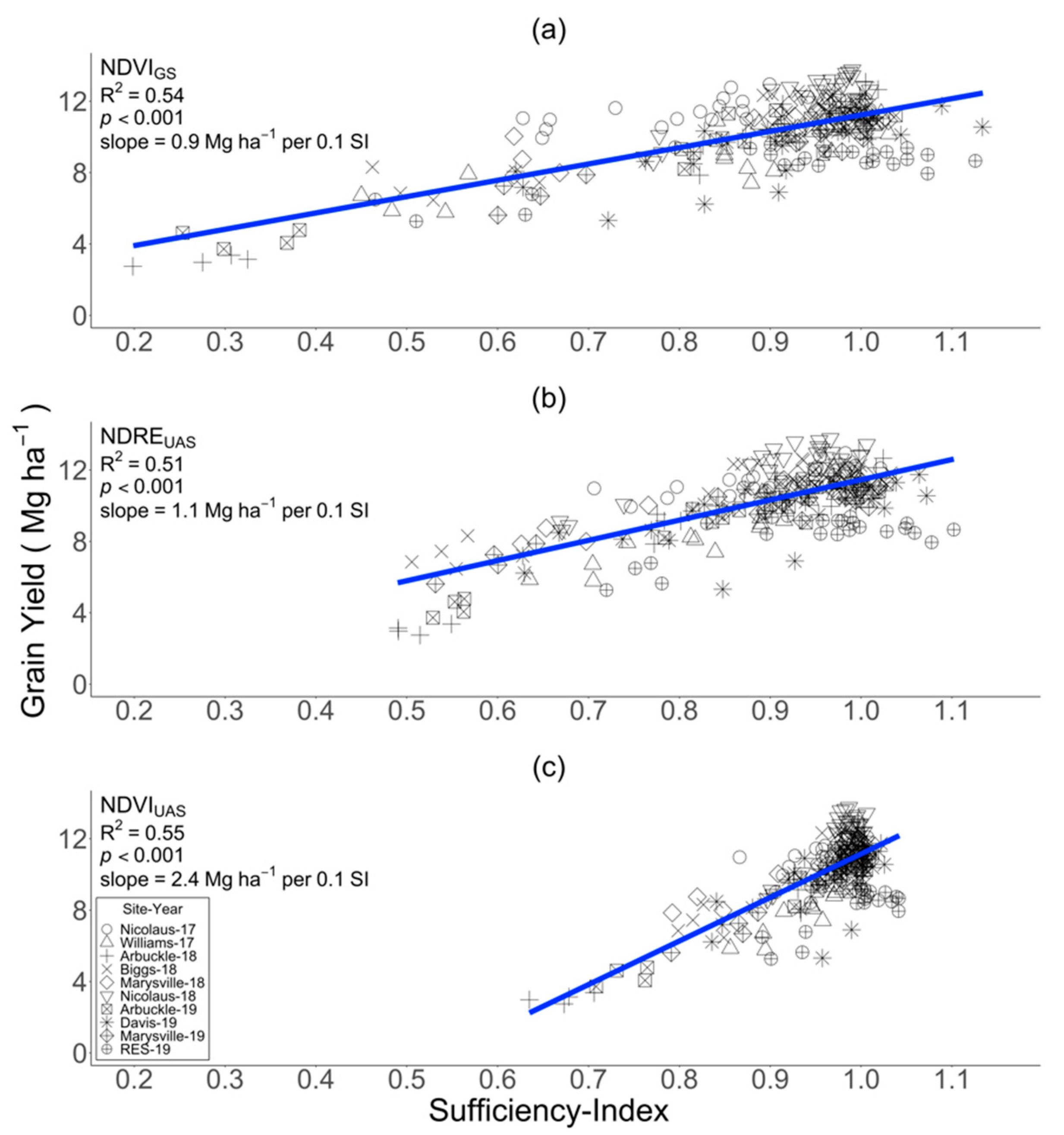
3.4. Relationship between SI Measured at PI and Grain Yield
4. Discussion
4.1. Crop Response to N Fertilizer
4.2. Index Saturation
4.3. Practical Implications of Index Saturation
4.3.1. Approaches for Comparing Indices
4.3.2. Assessing Crop N Status and Predicting Grain Yield at PI
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hatfield, J.L.; Gitelson, A.A.; Schepers, J.S.; Walthall, C.L. Application of Spectral Remote Sensing for Agronomic Decisions. Agron. J. 2008, 100, 117–131. [Google Scholar] [CrossRef] [Green Version]
- Xue, J.; Su, B. Significant Remote Sensing Vegetation Indices: A Review of Developments and Applications. J. Sens. 2017, 2017, 1353691. [Google Scholar] [CrossRef] [Green Version]
- Hatfield, J.L.; Prueger, J.H.; Sauer, T.J.; Dold, C.; O’Brien, P.; Wacha, K. Applications of Vegetative Indices from Remote Sensing to Agriculture: Past and Future. Inventions 2019, 4, 71. [Google Scholar] [CrossRef] [Green Version]
- Toth, C.; Jóźków, G. Remote Sensing Platforms and Sensors: A Survey. ISPRS J. Photogramm. 2016, 115, 22–36. [Google Scholar] [CrossRef]
- De Datta, S.K. Principles and Practices of Rice Production; International Rice Research Institute: Los Baños, Philippines, 1981. [Google Scholar]
- Williams, J.F. Rice Nutrient Management in California; University of California Agriculture and Natural Resources Publication: Davis, CA, USA, 2010; Volume 3516. [Google Scholar]
- Tamagno, S.; Eagle, A.J.; McLellan, E.L.; van Kessel, C.; Linquist, B.A.; Ladha, J.K.; Pittelkow, C.M. Quantifying N Leaching Losses as a Function of N Balance: A Path to Sustainable Food Supply Chains. Agric. Ecosyst. Environ. 2022, 324, 107714. [Google Scholar] [CrossRef]
- Pittelkow, C.M.; Adviento-Borbe, M.A.; van Kessel, C.; Hill, J.E.; Linquist, B.A. Optimizing rice yields while minimizing yield-scaled global warming potential. Global Change Biol. 2014, 20, 1382–1393. [Google Scholar] [CrossRef]
- Smith, J.; Sutula, M.; Bouma-Gregson, K.; Van Dyke, M. California Water Boards’ Framework and Strategy for Freshwater Harmful Algal Bloom Monitoring: Executive Synthesis; Southern California Coastal Water Research Project Technical Report for California State Water Resources Control Board: Sacramento, CA, USA, 2021; pp. 1141.A:1–1141.A:220. [Google Scholar]
- Daughtry, C.S.T.; Walthall, C.L.; Kim, M.S.; Brown de Colstoun, E.; McMurtrey, J.E., III. Estimating Corn Leaf Chlorophyll Concentration from Leaf and Canopy Reflectance. Remote Sens. Environ. 2000, 74, 229–239. [Google Scholar] [CrossRef]
- Balasubramanian, V.; Morales, A.C.; Cruz, R.T.; Abdulrachman, S. On-Farm Adaptation of Knowledge-Intensive Nitrogen Management Technologies for Rice Systems. Nutr. Cycl. Agroecosys. 1999, 53, 59–69. [Google Scholar] [CrossRef]
- Witt, C.; Pasuquin, J.M.C.A.; Mutters, R.; Buresh, R.J. New Leaf Color Chart for Effective Nitrogen Management in Rice. Better Crop. 2005, 89, 36–39. [Google Scholar]
- Saberioon, M.M.; Amin, M.S.M.; Gholizadeh, A.; Ezri, M.H. A Review of Optical Methods for Assessing Nitrogen Contents during Rice Growth. Appl. Eng. Agric. 2014, 30, 657–669. [Google Scholar] [CrossRef]
- Colomina, I.; Molina, P. Unmanned Aerial Systems for Photogrammetry and Remote Sensing: A Review. ISPRS J. Photogramm. 2014, 92, 79–97. [Google Scholar] [CrossRef] [Green Version]
- Mulla, D.J. Twenty Five Years of Remote Sensing in Precision Agriculture: Key Advances and Remaining Knowledge Gaps. Biosyst. Eng. 2013, 114, 358–371. [Google Scholar] [CrossRef]
- Tucker, C.J. Red and Photographic Infrared Linear Combinations for Monitoring Vegetation. Remote Sens. Environ. 1979, 8, 127–150. [Google Scholar] [CrossRef] [Green Version]
- Huang, S.; Tang, L.; Hupy, J.P.; Wang, Y.; Shao, G. A Commentary Review on the Use of Normalized Difference Vegetation Index (NDVI) in the Era of Popular Remote Sensing. J. For. Res. 2021, 32, 1–6. [Google Scholar] [CrossRef]
- Harrell, D.L.; Tubana, B.S.; Walker, T.W.; Phillips, S.B. Estimating Rice Grain Yield Potential Using Normalized Difference Vegetation Index. Agron. J. 2011, 103, 1717–1723. [Google Scholar] [CrossRef]
- Rehman, T.R.; Reis, A.F.B.; Akbar, N.; Linquist, B.A. Use of Normalized Difference Vegetation Index to Assess N Status and Predict Grain Yield in Rice. Agron. J. 2019, 111, 2889–2898. [Google Scholar] [CrossRef]
- Yao, Y.; Miao, Y.; Huang, S.; Gao, L.; Ma, X.; Zha, G.; Jiang, R.; Chen, X.; Zhang, F.; Yu, K.; et al. Active Canopy Sensor-Based Precision N Management Strategy for Rice. Agron. Sustain. Dev. 2012, 32, 925–933. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Chen, D.; Walker, C.N.; Angus, J.F. Estimating the Nitrogen Status of Crops Using a Digital Camera. Field Crop. Res. 2010, 118, 221–227. [Google Scholar] [CrossRef]
- Teal, R.K.; Tubana, B.; Girma, K.; Freeman, K.W.; Arnall, D.B.; Walsh, O.; Raun, W.R. In-Season Prediction of Corn Grain Yield Potential Using Normalized Difference Vegetation Index. Agron. J. 2006, 98, 1488–1494. [Google Scholar] [CrossRef] [Green Version]
- Xia, T.; Miao, Y.; Wu, D.; Shao, H.; Khosla, R.; Mi, G. Active Optical Sensing of Spring Maize for In-Season Diagnosis of Nitrogen Status Based on Nitrogen Nutrition Index. Remote Sens. 2016, 8, 605. [Google Scholar] [CrossRef] [Green Version]
- Tsouros, D.C.; Bibi, S.; Sarigiannidis, P.G. A Review on UAV-Based Applications for Precision Agriculture. Information 2019, 10, 349. [Google Scholar] [CrossRef] [Green Version]
- Delavarpour, N.; Koparan, C.; Nowatzki, J.; Bajwa, S.; Sun, X. A Technical Study on UAV Characteristics for Precision Agriculture Applications and Associated Practical Challenges. Remote Sens. 2021, 13, 1204. [Google Scholar] [CrossRef]
- Fu, Y.; Yang, G.; Song, X.; Li, Z.; Xu, X.; Feng, H.; Zhao, C. Improved Estimation of Winter Wheat Aboveground Biomass using Multiscale Textures Extracted from UAV-Based Digital Images and Hyperspectral Feature Analysis. Remote Sens. 2021, 13, 581. [Google Scholar] [CrossRef]
- Esposito, M.; Crimaldi, M.; Cirillo, V.; Sarghini, F.; Maggio, A. Drone and Sensor Technology for Sustainable Weed Management: A Review. Chem. Biol. Technol. Agric. 2021, 8, 1–11. [Google Scholar] [CrossRef]
- Dunn, B.W.; Dunn, T.S.; Hume, I.; Orchard, B.A.; Dehaan, R.; Robson, A. Remote Sensing PI Nitrogen Uptake in Rice. IREC Newsl. 2016, 195, 48–50. [Google Scholar]
- Li, F.; Miao, Y.; Feng, G.; Yuan, F.; Yue, S.; Gao, X.; Liu, Y.; Liu, B.; Ustin, S.L.; Chen, X. Improving Estimation of Summer Maize Nitrogen Status with Red Edge-Based Spectral Vegetation Indices. Field Crop. Res. 2014, 157, 111–123. [Google Scholar] [CrossRef]
- Dunn, B.W.; Dehaan, R.; Schmidtke, L.M.; Dunn, T.S.; Meder, R. Using Field-Derived Hyperspectral Reflectance Measurement to Identify the Essential Wavelengths for Predicting Nitrogen Uptake of Rice at Panicle Initiation. J. Near Infrared Spec. 2016, 24, 473–483. [Google Scholar] [CrossRef]
- Small, C.; Milesi, C. Multi-Scale Standardized Spectral Mixture Models. Remote Sens. Environ. 2013, 136, 442–454. [Google Scholar] [CrossRef] [Green Version]
- He, D.C.; Wang, L. Texture Unit, Texture Spectrum, and Texture Analysis. IEEE Trans. Geosci. Remote Sens. 1990, 28, 509–512. [Google Scholar]
- Maxwell, A.E.; Warner, T.A.; Fang, F. Implementation of Machine-Learning Classification in Remote Sensing: An Applied Review. Int. J. Remote Sens. 2018, 39, 2784–2817. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.; Chen, S.; Li, D.; Wang, C.; Jiang, H.; Zheng, Q.; Peng, Z. Estimation of Paddy Rice Nitrogen Content and Accumulation both at Leaf and Plant Levels from UAV Hyperspectral Imagery. Remote Sens. 2021, 13, 2956. [Google Scholar] [CrossRef]
- Zha, H.; Miao, Y.; Wang, T.; Li, Y.; Zhang, J.; Sun, W.; Feng, Z.; Kusnierek, K. Improving Unmanned Aerial Vehicle Remote Sensing-Based Rice Nitrogen Nutrition Index Prediction with Machine Learning. Remote Sens. 2020, 12, 215. [Google Scholar] [CrossRef] [Green Version]
- Hardin, P.J.; Jensen, R.R. Small-Scale Unmanned Aerial Vehicles in Environmental Remote Sensing: Challenges and Opportunities. GISci. Remote Sens. 2011, 48, 99–111. [Google Scholar] [CrossRef]
- Zhang, C.; Kovacs, J.M. The Application of Small Unmanned Aerial Systems for Precision Agriculture: A Review. Precis. Agric. 2012, 13, 693–712. [Google Scholar] [CrossRef]
- Zheng, H.; Cheng, T.; Zhou, M.; Li, D.; Yao, X.; Tian, Y.; Cao, W.; Zhu, Y. Improved Estimation of Rice Aboveground Biomass Combining Textural and Spectral Analysis of UAV Imagery. Precis. Agric. 2019, 20, 611–629. [Google Scholar] [CrossRef]
- Walsh, O.S.; Shafian, S.; Marshall, J.M.; Jackson, C.; McClintick-Chess, J.R.; Blanscet, S.M.; Swoboda, K.; Thompson, C.; Belmont, K.M.; Walsh, W.L. Assessment of UAV Based Vegetation Indices for Nitrogen Concentration Estimation in Spring Wheat. Adv. Remote Sens. 2018, 7, 71–90. [Google Scholar] [CrossRef] [Green Version]
- Becker, T.; Nelsen, T.S.; Leinfelder-Miles, M.; Lundy, M.E. Differentiating Between Nitrogen and Water Deficiency in Irrigated Maize Using a UAV-Based Multi-Spectral Camera. Agronomy 2020, 10, 1671. [Google Scholar] [CrossRef]
- Zheng, H.; Cheng, T.; Li, D.; Yao, X.; Tian, Y.; Cao, W.; Zhu, Y. Combining Unmanned Aerial Vehicle (UAV)-Based Multispectral Imagery and Ground-Based Hyperspectral Data for Plant Nitrogen Concentration Estimation in Rice. Front. Plant Sci. 2018, 9, 936. [Google Scholar] [CrossRef]
- Sumner, Z.; Varco, J.J.; Dhillon, J.S.; Fox, A.A.; Czarnecki, J.; Henry, W.B. Ground Versus Aerial Canopy Reflectance of Corn: Red-Edge and Non-Red Edge Vegetation Indices. Agron. J. 2021, 113, 2782–2797. [Google Scholar] [CrossRef]
- Hassan, M.A.; Yang, M.; Rasheed, A.; Yang, G.; Reynolds, M.; Xia, X.; Xiao, Y.; He, Z. A Rapid Monitoring of NDVI across the Wheat Growth Cycle for Grain Yield Prediction Using a Multi-Spectral UAV Platform. Plant Sci. 2019, 282, 95–103. [Google Scholar] [CrossRef]
- Duan, T.; Chapman, S.C.; Guo, Y.; Zheng, B. Dynamic Monitoring of NDVI in Wheat Agronomy and Breeding Trials Using an Unmanned Aerial Vehicle. Field Crop. Res. 2017, 210, 71–80. [Google Scholar] [CrossRef]
- CIMIS. California Irrigation Management Information System. Internet Resource. 2020. Available online: http://www.cimis.water.ca.gov/WSNReportCriteria.aspx) (accessed on 1 September 2020).
- Hill, J.E.; Williams, J.F.; Mutters, R.G.; Greer, C.A. The California Rice Cropping System: Agronomic Resource Issues for Long-Term Sustainability. Paddy Water Environ. 2006, 4, 13–19. [Google Scholar] [CrossRef]
- Sharp, Z. Principles of Stable Isotope Geochemistry, 2nd ed.; University of New Mexico Press: Albuquerque, NM, USA, 2017. [Google Scholar]
- Rouse, J.W., Jr.; Haas, R.H.; Schell, J.A.; Deering, D.W. Monitoring Vegetation Systems in the Great Plains with ERTS. In Proceedings of the Third Earth Resources Technology Satellite-1 Symposium: Section AB. Technical Presentations, Washington, DC, USA, 10–14 December 1973. [Google Scholar]
- Gitelson, A.A.; Merzlyak, M.N. Quantitative Estimation of Chlorophyll-A Using Reflectance Spectra: Experiments with Autumn Chestnut and Maple Leaves. J. Photochem. Photobiol. 1994, 22, 247–252. [Google Scholar] [CrossRef]
- Haghighattalab, A.; Pérez, L.G.; Mondal, S.; Singh, D.; Schinstock, D.; Rutkoski, J.; Ortiz-Monasterio, I.; Singh, R.P.; Goodin, D.; Poland, J. Application of Unmanned Aerial Systems for High Throughput Phenotyping of Large Wheat Breeding Nurseries. Plant Methods 2016, 12, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Nelsen, T.; Lundy, M.; Drone Data in Agricultural Research. GitHub Repository. 2021. Available online: https://github.com/Grain-Cropping-Systems-Lab/Drone-Data-in-Agricultural-Research (accessed on 1 August 2019).
- Colaço, A.F.; Bramley, R.G. Do Crop Sensors Promote Improved Nitrogen Management in Grain Crops? Field Crop. Res. 2018, 218, 126–140. [Google Scholar] [CrossRef]
- Bijay-Singh; Ali, A.M. Using Hand-Held Chlorophyll Meters and Canopy Reflectance Sensors for Fertilizer Nitrogen Management in Cereals in Small Farms in Developing Countries. Sensors 2020, 20, 1127. [Google Scholar] [CrossRef] [Green Version]
- Holland, K.H.; Schepers, J.S. Derivation of a Variable Rate Nitrogen Application Model for In-Season Fertilization of Corn. Agron. J. 2010, 102, 1415–1424. [Google Scholar] [CrossRef]
- Nelsen, T.S.; Lundy, M.E. Canopy Reflectance Informs In-Season Malting Barley Nitrogen Management: An Ex-Ante Classification Approach. Agron. J. 2020, 112, 4705–4722. [Google Scholar] [CrossRef]
- Lu, J.; Miao, Y.; Shi, W.; Li, J.; Yuan, F. Evaluating Different Approaches to Non-Destructive Nitrogen Status Diagnosis of Rice Using Portable Rapidscan Active Canopy Sensor. Sci. Rep. 2017, 7, 1–10. [Google Scholar] [CrossRef]
- Chen, Z.; Miao, Y.; Lu, J.; Zhou, L.; Li, Y.; Zhang, H.; Lou, W.; Zhang, Z.; Kusnierek, K.; Liu, C. In-Season Diagnosis of Winter Wheat Nitrogen Status in Smallholder Farmer Fields Across a Village Using Unmanned Aerial Vehicle-Based Remote Sensing. Agronomy 2019, 9, 619. [Google Scholar] [CrossRef] [Green Version]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2021; Available online: https://www.R-project.org/ (accessed on 1 October 2016).
- Wickham, H. ggplot2: Elegant Graphics for Data Analysis; Springer: New York, NY, USA, 2016; Available online: https://ggplot2.tidyverse.org (accessed on 1 October 2016).
- Mangiafico, S.S. Summary and Analysis of Extension Program Evaluation in R. 2016. Available online: https://rcompanion.org/handbook (accessed on 1 April 2022).
- Pinheiro, J.; Bates, D.; DebRoy, S.; Sarkar, D.; R Core Team. nlme: Linear and Nonlinear Mixed Effects Models. 2021. Available online: https://CRAN.R-project.org/package=nlme (accessed on 15 December 2017).
- Mangiafico, S.S. rcompanion: Functions to Support Extension Education Program Evaluation. Cran Repos. 2022, 20, 1–71. [Google Scholar]
- Cox, D.R.; Snell, E.J. Analysis of Binary Data; Chapman & Hall: Boca Raton, FL, USA, 2018. [Google Scholar]
- Bartoń, K. MuMIn: Multi-Model Inference. 2020. Available online: https://CRAN.R-project.org/package=MuMIn (accessed on 1 August 2020).
- Espe, M.B.; Yang, H.; Cassman, K.G.; Guilpart, N.; Sharifi, H.; Linquist, B.A. Estimating Yield Potential in Temperate High-Yielding, Direct-Seeded US Rice Production Systems. Field Crop. Res. 2016, 193, 123–132. [Google Scholar] [CrossRef] [Green Version]
- Linquist, B.A.; Hill, J.E.; Mutters, R.G.; Greer, C.A.; Hartley, C.; Ruark, M.; van Kessel, C. Assessing the Necessity of Surface Applied Preplant Nitrogen Fertilizer in Rice Systems. Agron. J. 2009, 101, 906–915. [Google Scholar] [CrossRef] [Green Version]
- Cassman, K.G.; Gines, G.C.; Dizon, M.A.; Samson, M.I.; Alcantara, J.M. Nitrogen-Use Efficiency in Tropical Lowland Rice Systems: Contributions from Indigenous and Applied Nitrogen. Field Crop. Res. 1996, 47, 1–12. [Google Scholar] [CrossRef]
- Peng, S.; Cassman, K.G. Upper Thresholds of Nitrogen Uptake Rates and Associated Nitrogen Fertilizer Efficiencies in Irrigated Rice. Agron. J. 1998, 90, 178–185. [Google Scholar] [CrossRef]
- Hatfield, J.L.; Prueger, J.H. Value of Using Different Vegetative Indices to Quantify Agricultural Crop Characteristics at Different Growth Stages Under Varying Management Practices. Remote Sens. 2010, 2, 562. [Google Scholar] [CrossRef] [Green Version]
- Amaral, L.R.; Molin, J.P.; Schepers, J.S. Algorithm for Variable-Rate Nitrogen Application in Sugarcane Based on Active Crop Canopy Sensor. Agron. J. 2015, 107, 1513–1523. [Google Scholar] [CrossRef] [Green Version]
- Gitelson, A.A. Wide Dynamic Range Vegetation Index for Remote Quantification of Biophysical Characteristics of Vegetation. J. Plant Physiol. 2004, 161, 165–173. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thenkabail, P.S.; Smith, R.B.; De Pauw, E. Hyperspectral Vegetation Indices and Their Relationships with Agricultural Crop Characteristics. Remote Sens. Environ. 2000, 71, 158–182. [Google Scholar] [CrossRef]
- Miller, J.J.; Schepers, J.S.; Shapiro, C.A.; Arneson, N.J.; Eskridge, K.M.; Oliveira, M.C.; Giesler, L.J. Characterizing Soybean Vigor and Productivity Using Multiple Crop Canopy Sensor Readings. Field Crop. Res. 2018, 216, 22–31. [Google Scholar] [CrossRef]
- Van Niel, T.G.; McVicar, T.R. Determining Temporal Windows for Crop Discrimination with Remote Sensing: A Case Study in South-Eastern Australia. Comput. Electron. Agric. 2004, 45, 91–108. [Google Scholar] [CrossRef]
- Nguy-Robertson, A.; Gitelson, A.; Peng, Y.; Viña, A.; Arkebauer, T.; Rundquist, D. 2012. Green Leaf Area Index Estimation in Maize and Soybean: Combining Vegetation Indices to Achieve Maximal Sensitivity. Agron. J. 2012, 104, 1336–1347. [Google Scholar] [CrossRef] [Green Version]
- Kanke, Y.; Tubana, B.; Dalen, M.; Harrell, D. Evaluation of Red and Red-Edge Reflectance-Based Vegetation Indices for Rice Biomass and Grain Yield Prediction Models in Paddy Fields. Precis. Agric. 2016, 17, 507–530. [Google Scholar] [CrossRef]
- Cao, Q.; Miao, Y.; Wang, H.; Huang, S.; Cheng, S.; Khosla, R.; Jiang, R. Non-Destructive Estimation of Rice Plant Nitrogen Status with Crop Circle Multispectral Active Canopy Sensor. Field Crop. Res. 2013, 154, 133–144. [Google Scholar] [CrossRef]
- Gnyp, M.L.; Miao, Y.; Yuan, F.; Ustin, S.L.; Yu, K.; Yao, Y.; Huang, S.; Bareth, G. 2014. Hyperspectral Canopy Sensing of Paddy Rice Aboveground Biomass at Different Growth Stages. Field Crop. Res. 2014, 155, 42–55. [Google Scholar] [CrossRef]
- Linquist, B.A.; Sengxua, P. Efficient and Flexible Management of Nitrogen for Rainfed Lowland Rice. Nutr. Cycl. Agroecosys. 2003, 67, 139–146. [Google Scholar] [CrossRef]
- LaHue, G.T.; Chaney, R.L.; Adviento-Borbe, M.A.; Linquist, B.A. Alternate Wetting and Drying in High Yielding Direct-Seeded Rice Systems Accomplishes Multiple Environmental and Agronomic Objectives. Agric. Ecosyst. Environ. 2016, 229, 30–39. [Google Scholar] [CrossRef]
- Perry, H.; Carrijo, D.; Linquist, B. Single Midseason Drainage Events Decrease Global Warming Potential Without Sacrificing Grain Yield in Flooded Rice Systems. Field Crop. Res. 2022, 276, 1–13. [Google Scholar] [CrossRef]
- Kaur, R. Prediction of Grain Yield and Nitrogen Uptake by Basmati Rice through In-Season Proximal Sensing with a Canopy Reflectance Sensor. Precis. Agric. 2021, 23, 733–747. [Google Scholar] [CrossRef]
- Dunn, B.W.; Dunn, T.S.; Beecher, H.G. Nitrogen Timing and Rate Effects on Growth and Grain Yield of Delayed Permanent-Water Rice in South-Eastern Australia. Crop Pasture Sci. 2014, 65, 878–887. [Google Scholar] [CrossRef]
- Dunn, B.W.; Dunn, T.S.; Orchard, B.A. Nitrogen Rate and Timing Effects on Growth and Yield of Drill-Sown Rice. Crop Pasture Sci. 2016, 67, 1149–1157. [Google Scholar] [CrossRef]
- Norman, R.J.; Slaton, N.A.; Roberts, T.L. Soil Fertility. In Rice Production Handbook; Miscellaneous Publication 192, Hardke, J., Eds.; Arkansas Cooperative Extension Service: Little Rock, AR, USA, 2021; pp. 69–101. [Google Scholar]
- Troldahl, D. Rice Growing Guide; New South Wales Government Department of Primary Industries: Orange, NSW, Australia, 2018; pp. 16–19. [Google Scholar]
- Zhou, X.; Zheng, H.B.; Xu, X.Q.; He, J.Y.; Ge, X.K.; Yao, X.; Cheng, T.; Zhu, Y.; Cao, W.X.; Tian, Y.C. Predicting Grain Yield in Rice Using Multi-Temporal Vegetation Indices from UAV-Based Multispectral and Digital Imagery. ISPRS J. Photogramm. 2017, 130, 246–255. [Google Scholar] [CrossRef]


| Site-Year | Soil Series | Taxonomic Classification | Texture (%) | Organic Carbon (%) | Total Nitrogen (%) | pH | ||
|---|---|---|---|---|---|---|---|---|
| Sand | Silt | Clay | ||||||
| Nicolaus-17 | Capay | Fine, smectitic, thermic Typic Haploxererts | 19 | 36 | 45 | 1.51 | 0.12 | 5.5 |
| Williams-17 | Willows | Fine, smectitic, thermic Sodic Endoaquerts | 21 | 39 | 40 | 1.75 | 0.15 | 5.0 |
| Arbuckle-18 | Clear Lake | Fine, smectitic, thermic Xeric Endoaquerts | 30 | 21 | 49 | 1.95 | 0.16 | 6.3 |
| Biggs-18 | Eastbiggs | Fine, mixed, active, thermic Abruptic Durixeralfs | 50 | 30 | 20 | 1.60 | 0.12 | 4.9 |
| Marysville-18 | San Joaquin | Fine, mixed, active, thermic Abruptic Durixeralfs | 39 | 39 | 22 | 1.64 | 0.13 | 4.6 |
| Nicolaus-18 | Capay | Fine, smectitic, thermic Typic Haploxererts | 22 | 36 | 42 | 1.67 | 0.14 | 4.8 |
| Arbuckle-19 | Clear Lake | Fine, smectitic, thermic Xeric Endoaquerts | 8 | 38 | 55 | 1.99 | 0.16 | 6.3 |
| Davis-19 | Sycamore | Fine-silty, mixed, super active, nonacid, thermic Mollic Endoaquepts | 9 | 38 | 53 | 1.98 | 0.18 | 6.3 |
| Marysville-19 | San Joaquin | Fine, mixed, active, thermic Abruptic Durixeralfs | 35 | 41 | 24 | 1.54 | 0.12 | 4.7 |
| RES-19 | Esquon-Neerdobe | Fine, smectitic, thermic Xeric Epiaquerts | 30 | 26 | 44 | 1.38 | 0.11 | 5.3 |
| Vegetation Index | Sensor Type | Year | Sensor | Light Source | Spectral Band | Central Wavelength (nm) | Bandwidth † (nm) | Formula | Reference |
|---|---|---|---|---|---|---|---|---|---|
| NDVI | Proximal | 2017–2019 | GreenSeeker | Active | Red | 670 | 10 | [48] | |
| Near Infrared | 780 | 10 | |||||||
| Aerial | 2017 | SlantRange 3P | Passive | Red | 650 | 40 | |||
| Near Infrared | 850 | 100 | |||||||
| 2018 & 2019 | MicaSense RedEdge-M | Passive | Red | 668 | 10 | ||||
| Near Infrared | 840 | 40 | |||||||
| NDRE | Aerial | 2017 | SlantRange 3P | Passive | Red Edge | 710 | 20 | [49] | |
| Near Infrared | 850 | 100 | |||||||
| 2018 & 2019 | MicaSense Red Edge-M | Passive | Red Edge | 717 | 10 | ||||
| Near Infrared | 840 | 40 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rehman, T.H.; Lundy, M.E.; Linquist, B.A. Comparative Sensitivity of Vegetation Indices Measured via Proximal and Aerial Sensors for Assessing N Status and Predicting Grain Yield in Rice Cropping Systems. Remote Sens. 2022, 14, 2770. https://doi.org/10.3390/rs14122770
Rehman TH, Lundy ME, Linquist BA. Comparative Sensitivity of Vegetation Indices Measured via Proximal and Aerial Sensors for Assessing N Status and Predicting Grain Yield in Rice Cropping Systems. Remote Sensing. 2022; 14(12):2770. https://doi.org/10.3390/rs14122770
Chicago/Turabian StyleRehman, Telha H., Mark E. Lundy, and Bruce A. Linquist. 2022. "Comparative Sensitivity of Vegetation Indices Measured via Proximal and Aerial Sensors for Assessing N Status and Predicting Grain Yield in Rice Cropping Systems" Remote Sensing 14, no. 12: 2770. https://doi.org/10.3390/rs14122770
APA StyleRehman, T. H., Lundy, M. E., & Linquist, B. A. (2022). Comparative Sensitivity of Vegetation Indices Measured via Proximal and Aerial Sensors for Assessing N Status and Predicting Grain Yield in Rice Cropping Systems. Remote Sensing, 14(12), 2770. https://doi.org/10.3390/rs14122770