Drone-Based Characterization of Seagrass Habitats in the Tropical Waters of Zanzibar
Abstract
1. Introduction
2. Materials and Methods
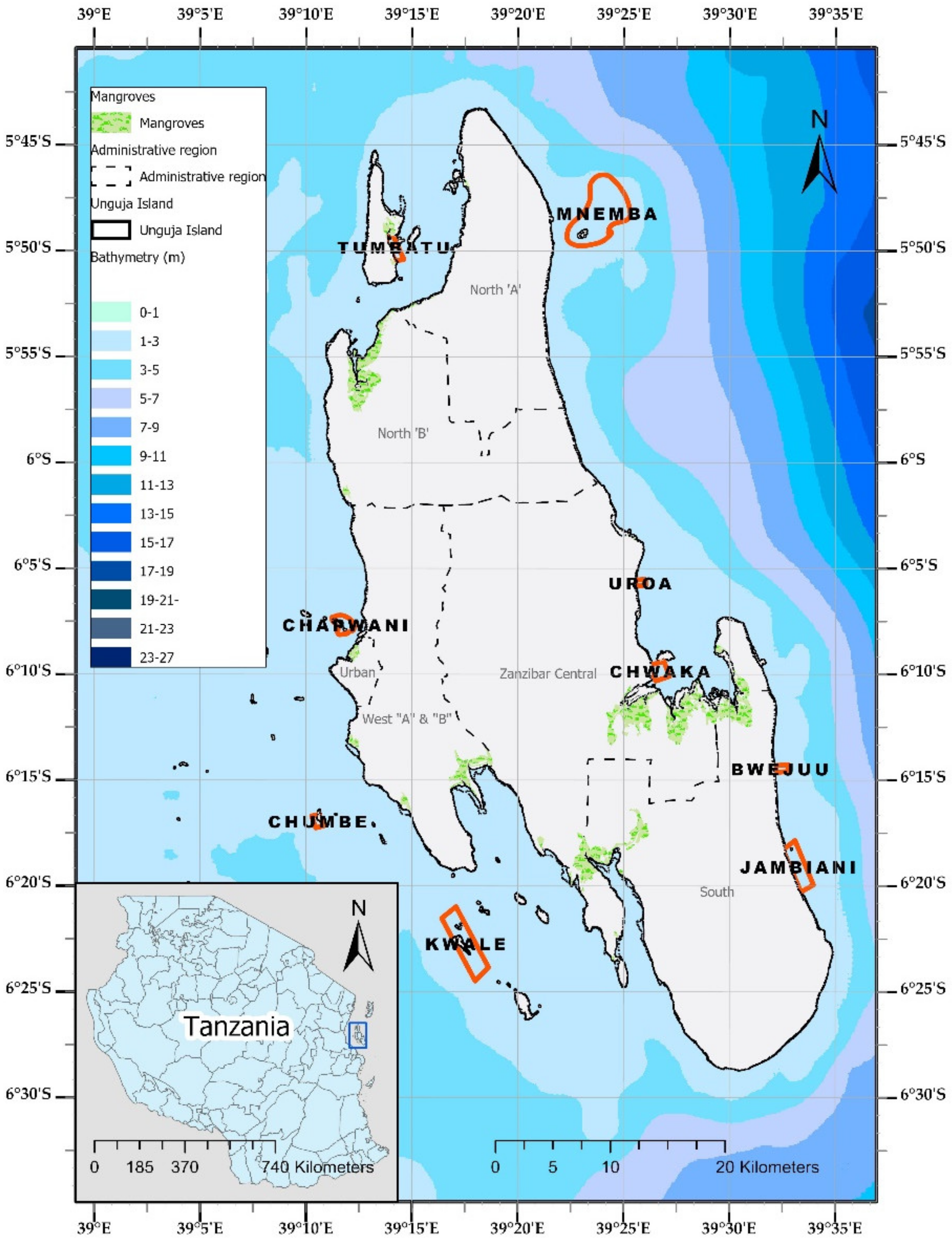
2.1. Study Design and Study Area Characteristics
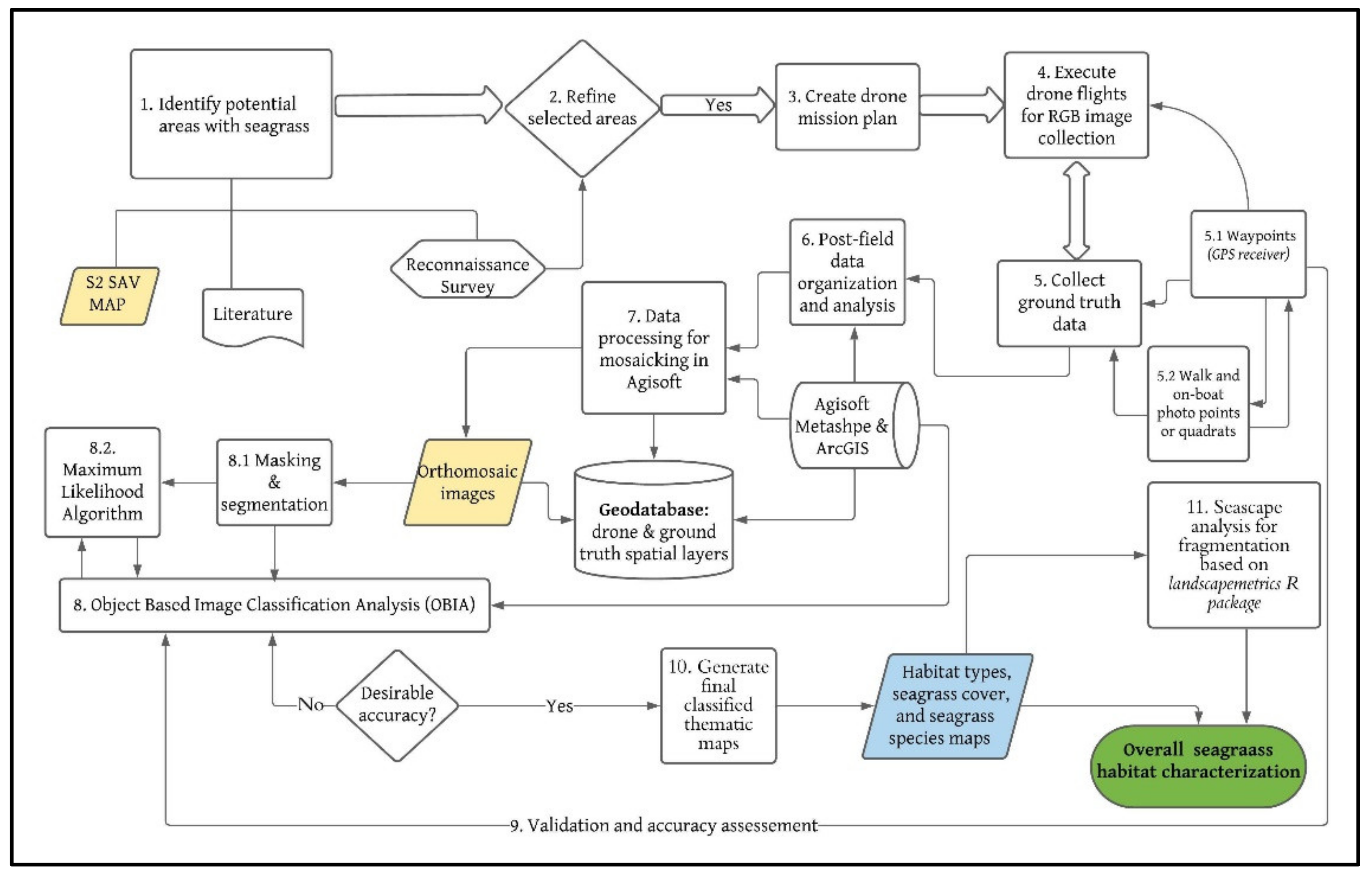
2.2. Data Acquisition Methods
2.2.1. Planning and Execution of Drone Mapping
2.2.2. Drone Image Processing
2.2.3. Collection of Underwater Video Footage and Photos
2.2.4. Depth Dependency
2.3. Analysis of Drone Data
2.3.1. Object-Based Image Analysis
2.3.2. Calibration, Validation, and Accuracy Assessment
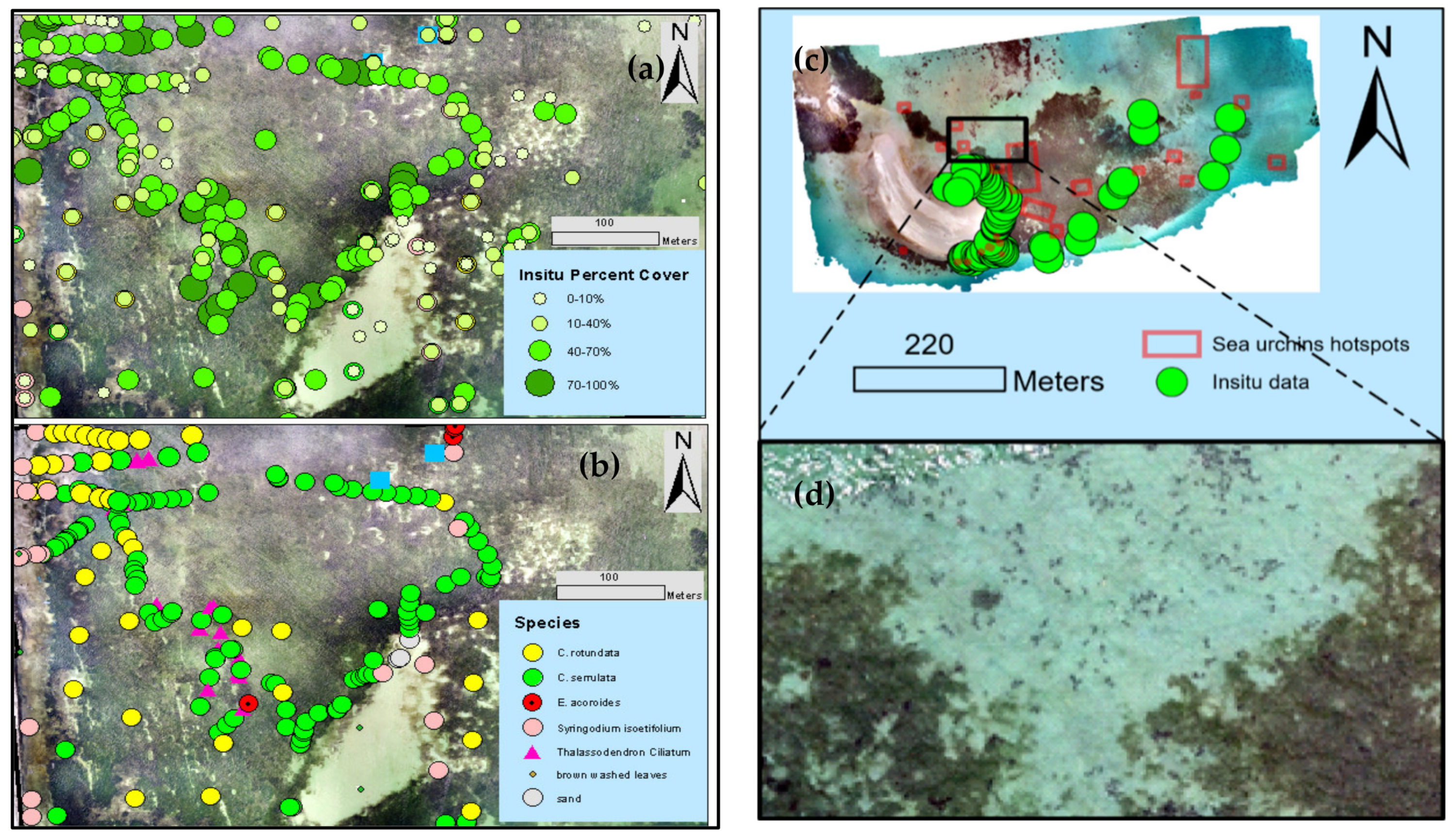
2.3.3. Video Footage and Ground Photo Analysis
2.3.4. Determining Seagrass Total Cover and Species Cover
| Parameter | Measurement Type | Data Type and Method for Data Extraction |
|---|---|---|
| 1.1. Benthic cover class (BC) | Type of cover (seagrass, macroalgae, corals, or bare sediment). | Categorical: The MLA applied a four-class schema; OBIA generated cover habitat maps for these four major classes and in situ data used for calibration and validation. |
| 1.2. Seagrass species | Type and number of species that make the dominant class. | Categorical: The species identification was based on the description made by [85] and on the notes that were taken during the field. Photo points were georectified, and their observations integrated into the spatial dataset. |
| 1.3. Seagrass total cover (TC) | Expressed as an interval scale of 0 to 100% of the seagrass cover, with five classes: Continuous—70 to 100; Dense—40 to 70; Moderate—10 to 40; Low—0 to 10; and Bare. | Continuous: In situ assessment of cover per photo taken. The method is based on the modified approach, as recommended by McMenzie (2003). OBIA applied MLA to generate a seagrass total cover with 5 classes if all were present (TC). |
| 1.4. Seagrass species cover (SC) | Cover distribution by type of species. | Categorical: Seagrass species percent cover classes based on the dominant species. OBIA applied MLA to generate a thematic map of seagrass species cover. |
| 1.5. Sea urchin abundance | Sea urchin frequency and density. | Numerical: By number, e.g., number of sea urchins/photos, and number per ha. |
| 1.6. Dead leaves | Expressed as a continuous scale of 0 to 100 percent of the dead leaves. | Continuous: Visual analysis, the estimate of the count of leaves per photo or point. |
| 1.7. Epiphytic cover | Expressed as 0 to 100% of epiphyte on seagrass leaves. | Continuous: A 0 to 100 visual estimate of the observed epiphytes in the seagrass leaves per photo. |
| 1.8. Bottom substrate | Type of bottom substrate (sand, fine sand, coral sand, mud, or rocky). | Categorical: Based on visual analysis of the camera used and field notes. |
| 1.9. Seagrass patchiness | Analysis at patch, class, and seascape level, e.g., number of patches and mean patch area. | Numerical: The selected seascape metrics measured meadows’ patchiness levels and were used after [49], and computed with R landscapemetrics [85]. |
2.3.5. Analyzing Ecological Conditions Affecting Seagrass Meadow Cover
Epiphyte Cover, Sea Urchin Distribution, and Substrate Conditions
2.3.6. Depth-Cover Seagrass Distribution Analysis
2.3.7. Analysis of Patchiness in Seagrass Meadows
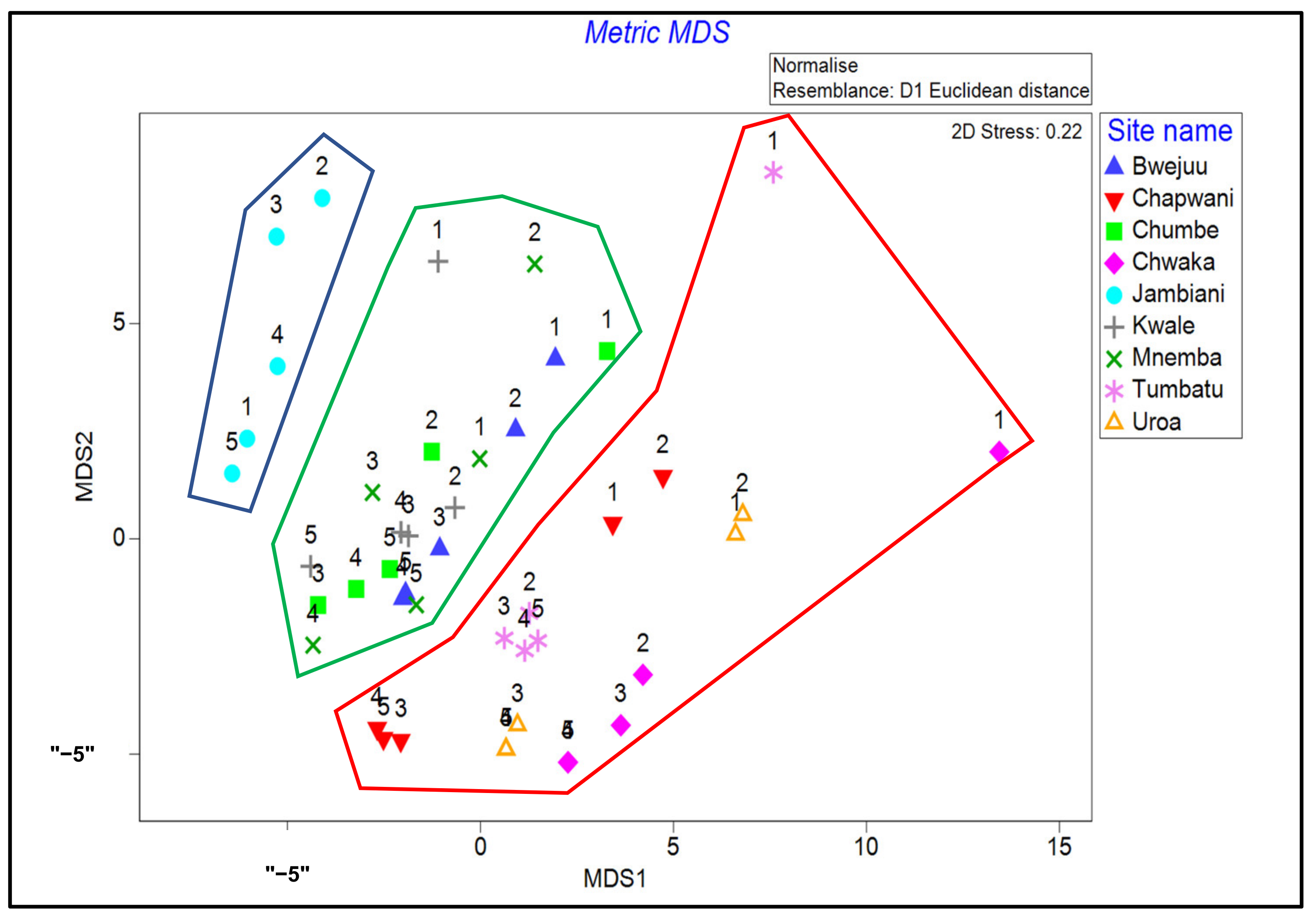
2.3.8. Multivariate Analysis
3. Results
3.1. Field Observatiosn on Habitat Type, Cover, and Species Composition
3.2. Spatial Characterization of Habitats, Percent Cover, and Species Composition
3.2.1. Seagrass Habitat Cover Maps
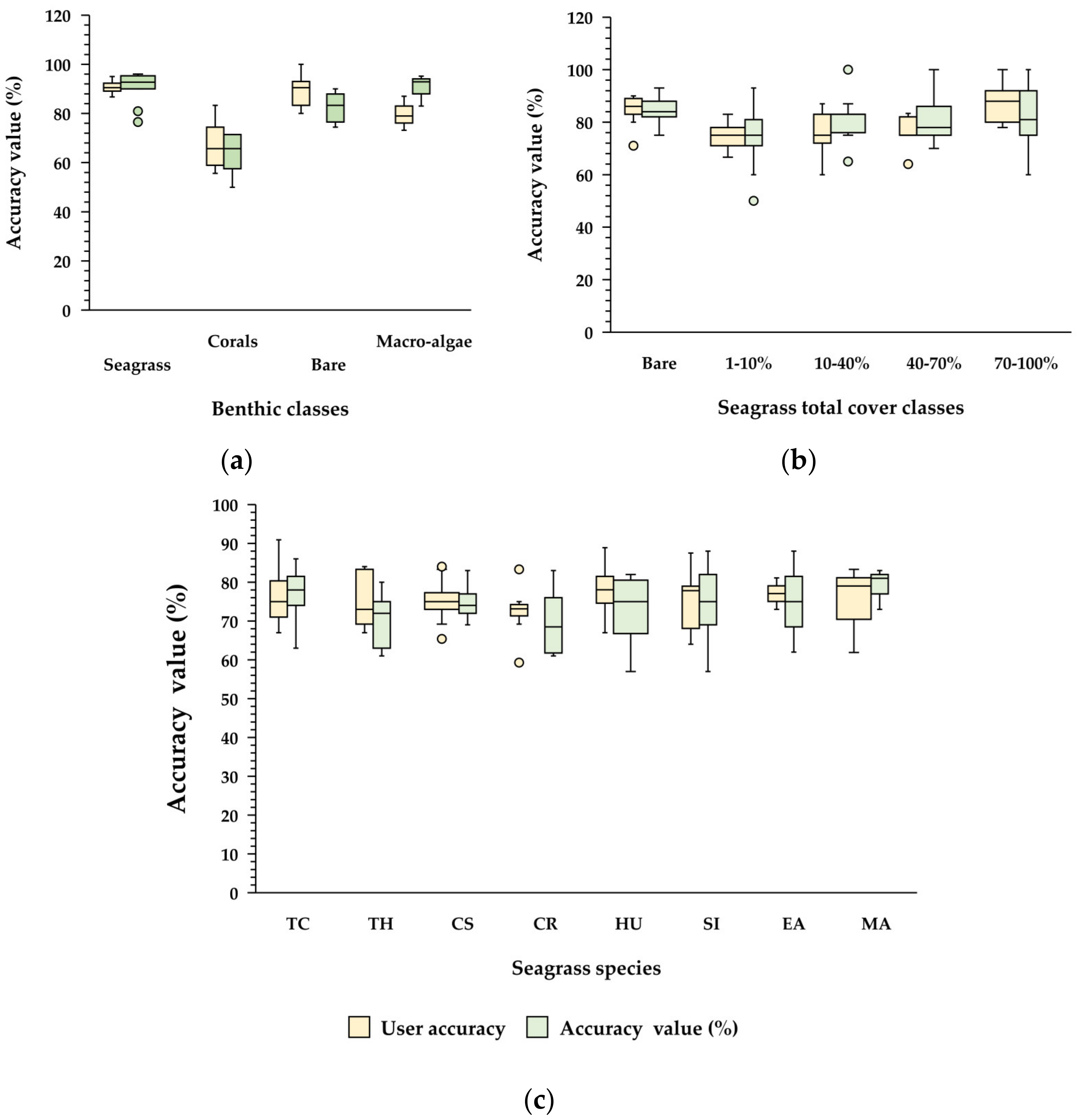
3.2.2. Accuracy of Cover Maps
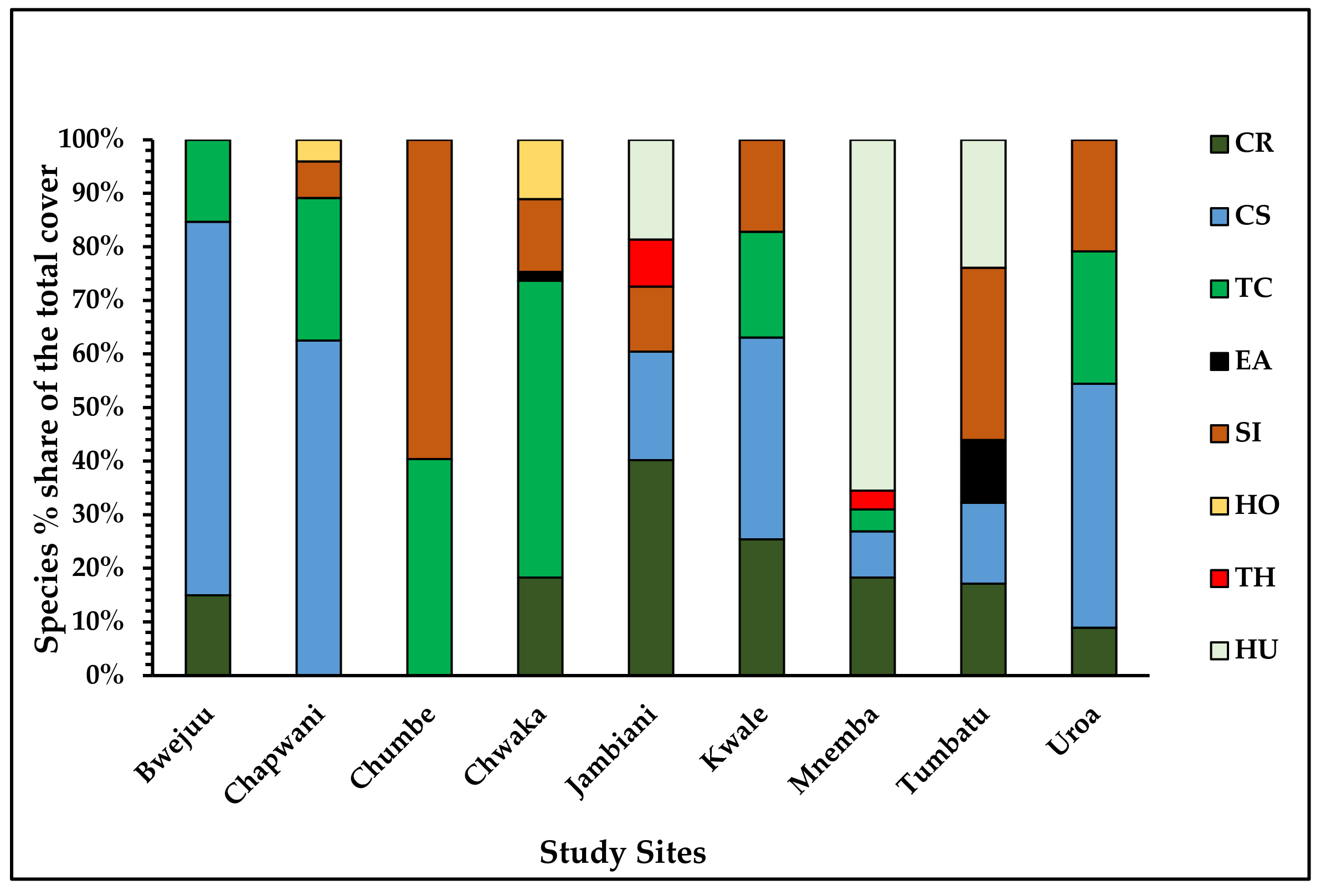
3.2.3. Statistical Analysis of Benthic Cover, Seagrass Total Cover, and Seagrass Species Cover
Benthic Classes, Percent Cover, and Cover of Seagrass Species
3.3. Seagrass Distribution with Depth
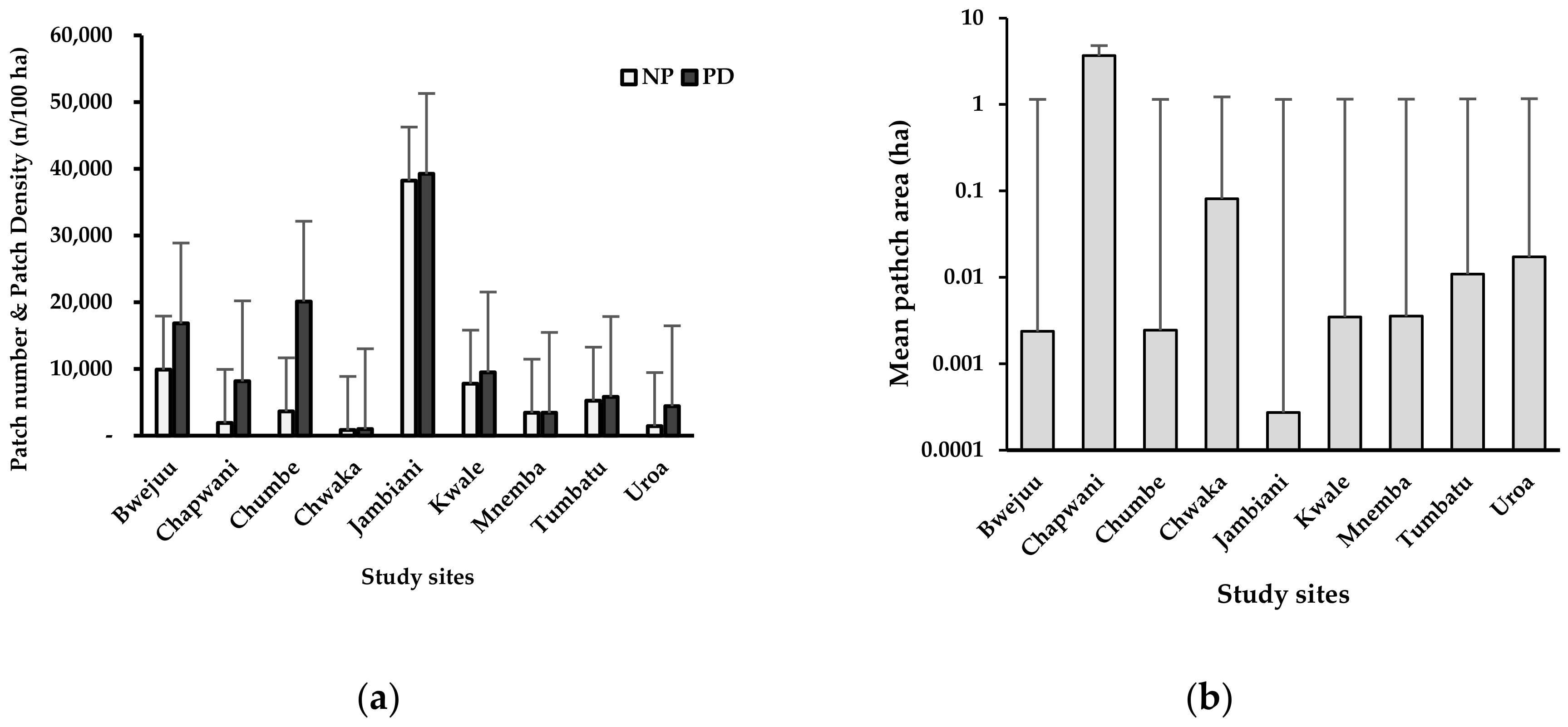
3.4. Patchiness of the Seagrass Seascapes
3.5. Associated Flora and Fauna in Seagrass Meadows
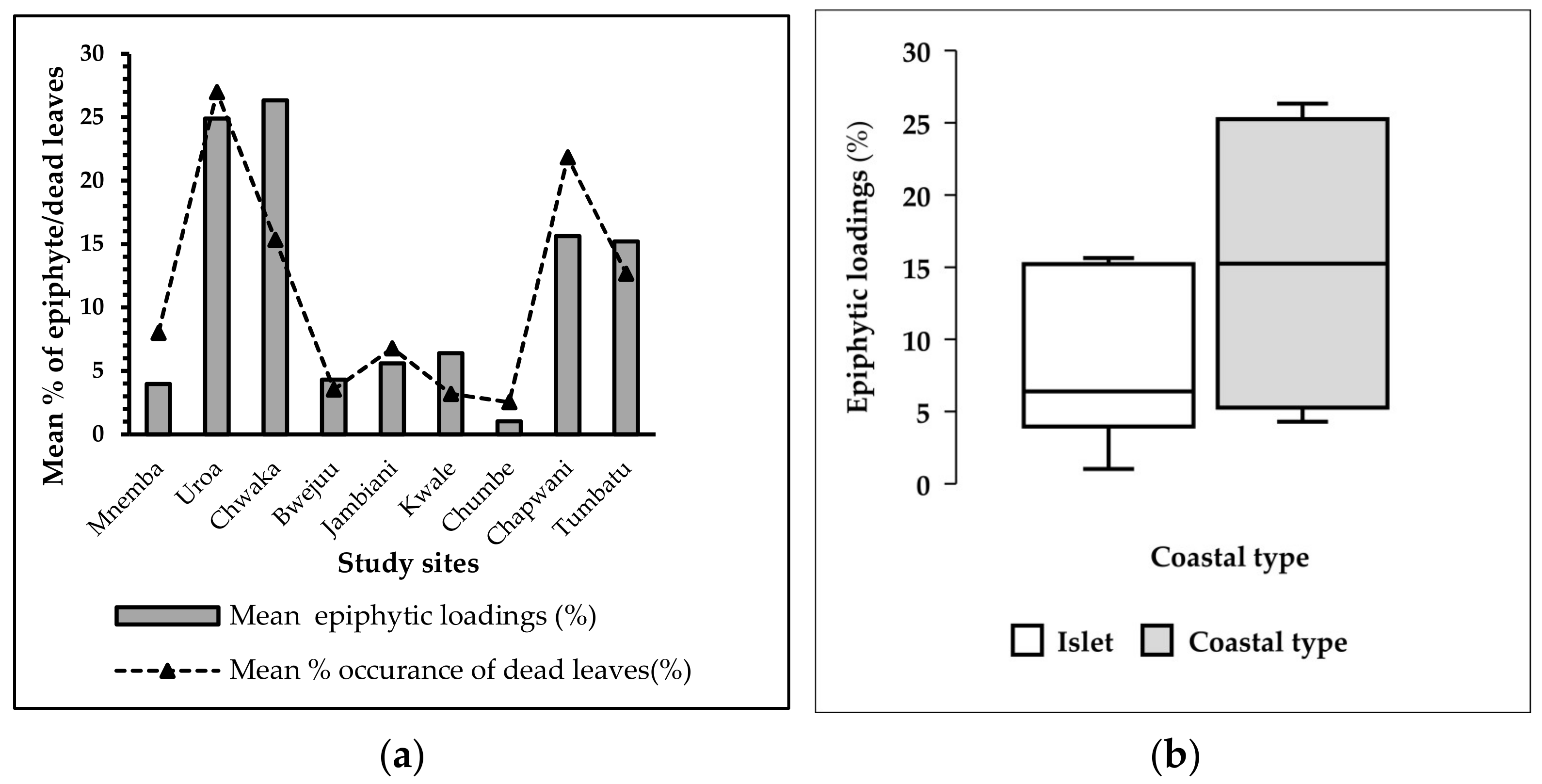
3.5.1. Epiphytes and Dead Leaves
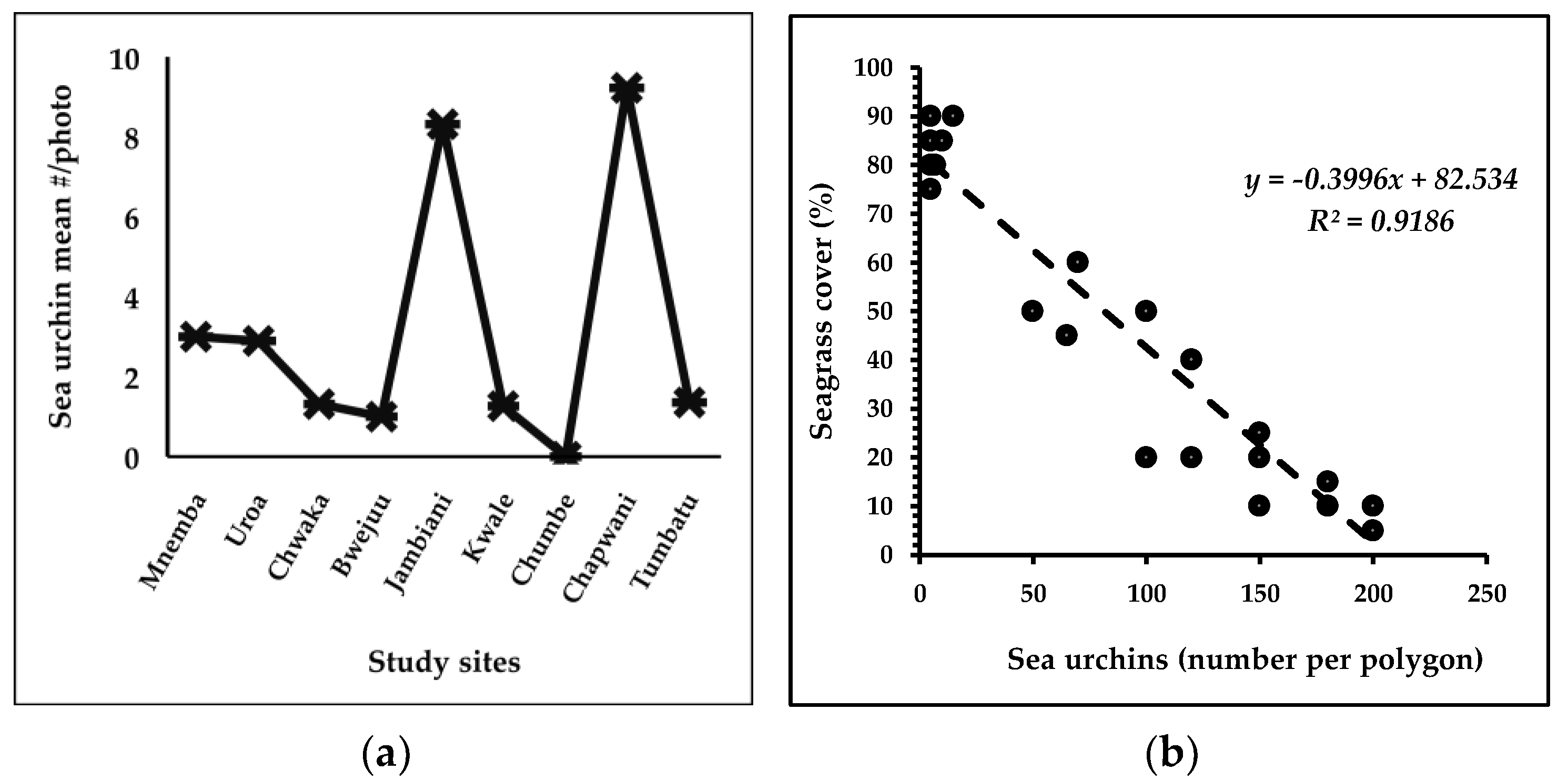
3.5.2. Sea Urchin Interaction with Seagrass Habitats
3.6. Multivariate Analysis
4. Discussion
4.1. Spatial Characterization of Tropical Nearshore Habitats Using Drone Imagery
4.1.1. Cover Maps
Benthic Classes, Seagrass Total Cover, and Species Composition
4.1.2. Accuracy Assessment
4.2. Depth Distribution of Seagrasses
4.3. Patchiness of Seagrass Distributions
4.4. Characterization of Habitats Using In Situ Imagery
4.5. Multivariate Classification of Nearshore Seagrass Habitats
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bandeira, S.; Björk, M. Seagrass research in the eastern Africa region: Emphasis on diversity, ecology and ecophysiology. S. Afr. J. Bot. 2001, 67, 420–425. [Google Scholar] [CrossRef]
- Gullström, M.; de la Torre Castro, M.; Bandeira, S.O.; Björk, M.; Dahlberg, M.; Kautsky, N.; Rönnbäck, P.; Öhman, M.C. Seagrass Ecosystems in the Western Indian Ocean. AMBIO J. Hum. Environ. 2002, 31, 588–596. [Google Scholar] [CrossRef]
- Cullen-Unsworth, L.C.; Nordlund, L.M.; Paddock, J.; Baker, S.; McKenzie, L.J.; Unsworth, R.K. Seagrass meadows globally as a coupled social-ecological system: Implications for human wellbeing. Mar. Pollut. Bull. 2014, 83, 387–397. [Google Scholar] [CrossRef] [PubMed]
- De la Torre-Castro, M.; Di Carlo, G.; Jiddawi, N.S. Seagrass importance for a small-scale fishery in the tropics: The need for seascape management. Mar. Pollut. Bull. 2014, 83, 398–407. [Google Scholar] [CrossRef]
- Lugendo, B.R.; Nagelkerken, I.; van der Velde, G.; Mgaya, Y.D. The importance of mangroves, mud and sand flats, and seagrass beds as feeding areas for juvenile fishes in Chwaka Bay, Zanzibar: Gut content and stable isotope analyses. J. Fish Biol. 2006, 69, 1639–1661. [Google Scholar] [CrossRef]
- Lyimo, T.J.; Mvungi, E.F.; Lugomela, C.; Björk, M. Seagrass Biomass and Productivity in Seaweed and Non-Seaweed Farming Areas in the East Coast of Zanzibar, Tanzania. West. Indian Ocean J. Mar. Sci. 2006, 5, 141–152. [Google Scholar]
- De la Torre-Castro, M.; Rönnbäck, P. Links between humans and seagrasses—an example from tropical East Africa. Ocean Coast. Manag. 2004, 47, 361–387. [Google Scholar] [CrossRef]
- Msuya, F.E.; Namukose, M.; Beltran-Gutierrez, M.; Fabiani, G.; Kunzmann, A. Integrated seaweed–sea cucumber farming in Tanzania. West. Indian Ocean J. Mar. Sci. 2018, 17, 35–50. [Google Scholar]
- Greiner, J.T.; McGlathery, K.J.; Gunnell, J.; McKee, B.A. Seagrass restoration enhances “blue carbon” sequestration in coastal waters. PLoS ONE 2013, 8, e72469. [Google Scholar] [CrossRef]
- Röhr, M.E.; Boström, C.; Canal-Vergés, P.; Holmer, M. Blue carbon stocks in Baltic Sea eelgrass (Zostera marina) meadows. Biogeosciences 2016, 13, 6139–6153. [Google Scholar] [CrossRef]
- Bulthuis, D.A.; Brand, G.W.; Mobley, M.C. Suspended sediments and nutrients in water ebbing from seagrass-covered and denuded tidal mudflats in a southern Australian embayment. Aquat. Bot. 1984, 20, 257–266. [Google Scholar] [CrossRef]
- Fonseca, M.S. Sediment stabilization by Halophila decipiens in comparison to other seagrasses. Estuarine Coast. Shelf Sci. 1989, 29, 501–507. [Google Scholar] [CrossRef]
- Koch, E.W. Hydrodynamics of a shallow Thalassia testudinum bed in Florida, USA. In Seagrass Biology, Proceedings of an International Workshop, Rottnest Island, Australia, 25–29 January 1996; University of Western Australia: Perth, Australia, 1996; pp. 25–29. [Google Scholar]
- James, R.K.; Christianen, M.J.A.; van Katwijk, M.M.; de Smit, J.C.; Bakker, E.S.; Herman, P.M.J.; Bouma, T.J. Seagrass coastal protection services reduced by invasive species expansion and megaherbivore grazing. J. Ecol. 2020, 108, 2025–2037. [Google Scholar] [CrossRef]
- Orth, R.J.; Carruthers, T.J.; Dennison, W.C.; Duarte, C.M.; Fourqurean, J.W.; Heck, K.L.; Hughes, A.R.; Kendrick, G.A.; Kenworthy, W.J.; Olyarnik, S. A global crisis for seagrass ecosystems. Bioscience 2006, 56, 987–996. [Google Scholar] [CrossRef]
- Waycott, M.; Duarte, C.M.; Carruthers, T.J.; Orth, R.J.; Dennison, W.C.; Olyarnik, S.; Calladine, A.; Fourqurean, J.W.; Heck, K.L.; Hughes, A.R. Accelerating loss of seagrasses across the globe threatens coastal ecosystems. Proc. Natl. Acad. Sci. USA 2009, 106, 12377–12381. [Google Scholar] [CrossRef]
- Short, F.T.; Polidoro, B.; Livingstone, S.R.; Carpenter, K.E.; Bandeira, S.; Bujang, J.S.; Calumpong, H.P.; Carruthers, T.J.B.; Coles, R.G.; Dennison, W.C.; et al. Extinction risk assessment of the world’s seagrass species. Biol. Conserv. 2011, 144, 1961–1971. [Google Scholar] [CrossRef]
- Amone-Mabuto, M.; Bandeira, S.; da Silva, A. Long-term changes in seagrass coverage and potential links to climate-related factors: The case of Inhambane Bay, southern Mozambique. West. Indian Ocean J. Mar. Sci. 2017, 16, 13–25. [Google Scholar]
- Short, F.T.; Wyllie-Echeverria, S. Natural and human-induced disturbance of seagrasses. Environ. Conserv. 1996, 23, 17–27. [Google Scholar] [CrossRef]
- Khamis, Z.A.; Kalliola, R.; Käyhkö, N. Spatial modelling of cumulative human pressure in the tropical coastscape of Zanzibar, Tanzania. Afr. J. Mar. Sci. 2019, 41, 337–352. [Google Scholar] [CrossRef]
- Ralph, P.J.; Tomasko, D.; Moore, K.; Seddon, S.; Macinnis, O.C.M. Human impacts on seagrasses: Eutrophication, sedimentation, and contamination. In Seagrasses: Biology, Ecology, and Conservation; Springer: Dordrecht, The Netherlands, 2007; pp. 567–593. [Google Scholar]
- Yaakub, S.M.; McKenzie, L.J.; Erftemeijer, P.L.A.; Bouma, T.; Todd, P.A. Courage under fire: Seagrass persistence adjacent to a highly urbanised city–state. Mar. Pollut. Bull. 2014, 83, 417–424. [Google Scholar] [CrossRef]
- Hallac, D.E.; Sadle, J.; Pearlstine, L.; Herling, F.; Shinde, D. Boating impacts to seagrass in Florida Bay, Everglades National Park, Florida, USA: Links with physical and visitor-use factors and implications for management. Mar. Freshw. Res. 2012, 63, 1117–1128. [Google Scholar] [CrossRef]
- Abadie, A.; Gobert, S.; Bonacorsi, M.; Lejeune, P.; Pergent, G.; Pergent-Martini, C. Marine space ecology and seagrasses. Does patch type matter in Posidonia oceanica seascapes? Ecol. Indic. 2015, 57, 435–446. [Google Scholar] [CrossRef]
- Serrano, O.; Ruhon, R.; Lavery, P.S.; Kendrick, G.A.; Hickey, S.; Masqué, P.; Arias-Ortiz, A.; Steven, A.; Duarte, C.M. Impact of mooring activities on carbon stocks in seagrass meadows. Sci. Rep. 2016, 6, 23193. [Google Scholar] [CrossRef]
- Lyimo, T.J.; Mvungi, E.F.; Mgaya, Y.D. Abundance and diversity of seagrass and macrofauna in the intertidal areas with and without seaweed farming activities in the east coast of Zanzibar. Tanzan. J. Sci. 2008, 34, 41–52. [Google Scholar] [CrossRef][Green Version]
- Stæhr, P.A.; Sheikh, M.; Rashid, R.; Ussi, A.; Suleiman, M.; Kloiber, U.; Dahl, K.; Tairova, Z.; Strand, J.; Kuguru, B. Managing human pressures to restore ecosystem health of zanzibar coastal waters. J. Aquac. Mar. Biol. 2018, 7, 59–70. [Google Scholar] [CrossRef]
- Khamis, Z.A.; Kalliola, R.; Käyhkö, N. Geographical characterization of the Zanzibar coastal zone and its management perspectives. Ocean Coast. Manag. 2017, 149, 116–134. [Google Scholar] [CrossRef]
- Lange, G.-M. Tourism in Zanzibar: Incentives for sustainable management of the coastal environment. Ecosyst. Serv. 2015, 11, 5–11. [Google Scholar] [CrossRef]
- Shah, N.J.; Lindén, O.; Lundin, C.G.; Johnstone, R. Coastal management in Eastern Africa: Status and future. Ambio 1997, 26, 227–234. [Google Scholar]
- Gullström, M.; Lundén, B.; Bodin, M.; Kangwe, J.; Öhman, M.C.; Mtolera, M.S.P.; Björk, M. Assessment of changes in the seagrass-dominated submerged vegetation of tropical Chwaka Bay (Zanzibar) using satellite remote sensing. Estuarine Coast. Shelf Sci. 2006, 67, 399–408. [Google Scholar] [CrossRef]
- Knudby, A.; Nordlund, L. Remote sensing of seagrasses in a patchy multi-species environment. Int. J. Remote Sens. 2011, 32, 2227–2244. [Google Scholar] [CrossRef]
- Phinn, S.; Roelfsema, C.; Dekker, A.; Brando, V.; Anstee, J. Mapping seagrass species, cover and biomass in shallow waters: An assessment of satellite multi-spectral and airborne hyper-spectral imaging systems in Moreton Bay (Australia). Remote Sens. Environ. 2008, 112, 3413–3425. [Google Scholar] [CrossRef]
- Nordlund, L.M.; Unsworth, R.K.F.; Gullström, M.; Cullen-Unsworth, L.C. Global significance of seagrass fishery activity. Fish Fish. 2018, 19, 399–412. [Google Scholar] [CrossRef]
- Hedberg, N.; von Schreeb, K.; Charisiadou, S.; Jiddawi, N.S.; Tedengren, M.; Nordlund, L.M. Habitat preference for seaweed farming—A case study from Zanzibar, Tanzania. Ocean Coast. Manag. 2018, 154, 186–195. [Google Scholar] [CrossRef]
- Poursanidis, D.; Traganos, D.; Teixeira, L.; Shapiro, A.; Muaves, L. Cloud-native seascape mapping of Mozambique’s Quirimbas National Park with Sentinel-2. Remote Sens. Ecol. Conserv. 2021, 7, 275–291. [Google Scholar] [CrossRef]
- Kutser, T.; Hedley, J.; Giardino, C.; Roelfsema, C.; Brando, V.E. Remote sensing of shallow waters—A 50 year retrospective and future directions. Remote Sens. Environ. 2020, 240, 111619. [Google Scholar] [CrossRef]
- Lønborg, C.; Thomasberger, A.; Stæhr, P.A.U.; Stockmarr, A.; Sengupta, S.; Rasmussen, M.L.; Nielsen, L.T.; Hansen, L.B.; Timmermann, K. Submerged aquatic vegetation: Overview of monitoring techniques used for the identification and determination of spatial distribution in European coastal waters. Integr. Environ. Assess. Manag. 2021, n/a. [Google Scholar] [CrossRef]
- Boström, C.; Pittman, S.J.; Simenstad, C.; Kneib, R.T. Seascape ecology of coastal biogenic habitats: Advances, gaps, and challenges. Mar. Ecol. Prog. Ser. 2011, 427, 191–217. [Google Scholar] [CrossRef]
- Nahirnick, N.K.; Reshitnyk, L.; Campbell, M.; Hessing-Lewis, M.; Costa, M.; Yakimishyn, J.; Lee, L.; Horning, N.; Poursanidis, D. Mapping with confidence; delineating seagrass habitats using Unoccupied Aerial Systems (UAS). Remote Sens. Ecol. Conserv. 2018, 5, 121–135. [Google Scholar] [CrossRef]
- Galparsoro, I.; Pınarbaşı, K.; Gissi, E.; Culhane, F.; Gacutan, J.; Kotta, J.; Cabana, D.; Wanke, S.; Aps, R.; Bazzucchi, D.; et al. Operationalisation of ecosystem services in support of ecosystem-based marine spatial planning: Insights into needs and recommendations. Mar. Policy 2021, 131, 104609. [Google Scholar] [CrossRef]
- Yang, W.; Zhang, Z.; Sun, T.; Liu, H.; Shao, D. Marine ecological and environmental health assessment using the pressure-state-response framework at different spatial scales, China. Ecol. Indic. 2021, 121, 106965. [Google Scholar] [CrossRef]
- Roca, G.; Alcoverro, T.; Krause-Jensen, D.; Balsby, T.J.S.; van Katwijk, M.M.; Marbà, N.; Santos, R.; Arthur, R.; Mascaró, O.; Fernández-Torquemada, Y.; et al. Response of seagrass indicators to shifts in environmental stressors: A global review and management synthesis. Ecol. Indic. 2016, 63, 310–323. [Google Scholar] [CrossRef]
- Neto, J.M.; Barroso, D.V.; Barría, P. Seagrass Quality Index (SQI), a Water Framework Directive compliant tool for the assessment of transitional and coastal intertidal areas. Ecol. Indic. 2013, 30, 130–137. [Google Scholar] [CrossRef]
- Marbà, N.; Krause-Jensen, D.; Alcoverro, T.; Birk, S.; Pedersen, A.; Neto, J.M.; Orfanidis, S.; Garmendia, J.M.; Muxika, I.; Borja, A. Diversity of European seagrass indicators: Patterns within and across regions. Hydrobiologia 2013, 704, 265–278. [Google Scholar] [CrossRef]
- Purvaja, R.; Robin, R.; Ganguly, D.; Hariharan, G.; Singh, G.; Raghuraman, R.; Ramesh, R. Seagrass meadows as proxy for assessment of ecosystem health. Ocean Coast. Manag. 2018, 159, 34–45. [Google Scholar] [CrossRef]
- Nelson, W.G. Development of an epiphyte indicator of nutrient enrichment: Threshold values for seagrass epiphyte load. Ecol. Indic. 2017, 74, 343–356. [Google Scholar] [CrossRef]
- Halpern, B.S.; Longo, C.; Hardy, D.; McLeod, K.L.; Samhouri, J.F.; Katona, S.K.; Kleisner, K.; Lester, S.E.; O’Leary, J.; Ranelletti, M.; et al. An index to assess the health and benefits of the global ocean. Nature 2012, 488, 615–620. [Google Scholar] [CrossRef]
- Sleeman, J.C.; Kendrick, G.A.; Boggs, G.S.; Hegge, B.J. Measuring fragmentation of seagrass landscapes: Which indices are most appropriate for detecting change? Mar. Freshw. Res. 2005, 56, 851–864. [Google Scholar] [CrossRef]
- Duffy, J.P.; Pratt, L.; Anderson, K.; Land, P.E.; Shutler, J.D. Spatial assessment of intertidal seagrass meadows using optical imaging systems and a lightweight drone. Estuarine Coast. Shelf Sci. 2018, 200, 169–180. [Google Scholar] [CrossRef]
- Lathrop, R.G., Jr.; Haag, S.M.; Merchant, D.; Kennish, M.J.; Fertig, B. Comparison of remotely-sensed surveys vs. in situ plot-based assessments of sea grass condition in Barnegat Bay-Little Egg Harbor, New Jersey USA. J. Coast. Conserv. 2004, 2014, 299–308. [Google Scholar] [CrossRef]
- Roelfsema, C.M.; Lyons, M.; Kovacs, E.M.; Maxwell, P.; Saunders, M.I.; Samper-Villarreal, J.; Phinn, S.R. Multi-temporal mapping of seagrass cover, species and biomass: A semi-automated object based image analysis approach. Remote Sens. Environ. 2014, 150, 172–187. [Google Scholar] [CrossRef]
- Stæhr, P.A.; Groom, G.B.; Krause-Jensen, D.; Hansen, L.B.; Huber, S.; Jensen, L.Ø.; Rasmussen, M.B.; Upadhyay, S.; Ørberg, S.B. Use of Remote Sensing Technologies for Monitoring Chlorophyll A and Submerged Aquatic Vegetation in Danish Coastal Waters: Part of the Restek Project; Aarhus University, DCE—Danish Centre for Environment and Energy: Roskilde, Denmark, 2019. [Google Scholar]
- Ventura, D.; Bruno, M.; Jona Lasinio, G.; Belluscio, A.; Ardizzone, G. A low-cost drone based application for identifying and mapping of coastal fish nursery grounds. Estuarine Coast. Shelf Sci. 2016, 171, 85–98. [Google Scholar] [CrossRef]
- Harvey, T.; Krause-Jensen, D.; Stæhr, P.A.; Groom, G.B.; Hansen, L.B. Literature Review of Remote Sensing Technologies for Coastal Chlorophyll-A Observations and Vegetation Coverage; Technical Report from DCE—Danish Centre for Environment and Energy No. 112; Aarhus University, DCE—Danish Centre for Environment and Energy: Roskilde, Denmark, 2018; 47p, Available online: http://dce2.au.dk/pub/TR112.pdf (accessed on 15 December 2021).
- Roelfsema, C.M.; Phinn, S.R.; Udy, N.; Maxwell, P. An integrated field and remote sensing approach for mapping Seagrass Cover, Moreton Bay, Australia. J. Spat. Sci. 2009, 54, 45–62. [Google Scholar] [CrossRef]
- Andriolo, U.; Gonçalves, G.; Rangel-Buitrago, N.; Paterni, M.; Bessa, F.; Gonçalves, L.M.S.; Sobral, P.; Bini, M.; Duarte, D.; Fontán-Bouzas, Á.; et al. Drones for litter mapping: An inter-operator concordance test in marking beached items on aerial images. Mar. Pollut. Bull. 2021, 169, 112542. [Google Scholar] [CrossRef]
- Konar, B.; Iken, K. The use of unmanned aerial vehicle imagery in intertidal monitoring. Deep Sea Res. Part II Top. Stud. Oceanogr. 2018, 147, 79–86. [Google Scholar] [CrossRef]
- Monteiro, J.G.; Jiménez, J.L.; Gizzi, F.; Přikryl, P.; Lefcheck, J.S.; Santos, R.S.; Canning-Clode, J. Novel approach to enhance coastal habitat and biotope mapping with drone aerial imagery analysis. Sci. Rep. 2021, 11, 574. [Google Scholar] [CrossRef] [PubMed]
- Knudby, A.; LeDrew, E.; Brenning, A. Predictive mapping of reef fish species richness, diversity and biomass in Zanzibar using IKONOS imagery and machine-learning techniques. Remote Sens. Environ. 2010, 114, 1230–1241. [Google Scholar] [CrossRef]
- Knudby, A.; Nordlund, L.M.; Palmqvist, G.; Wikström, K.; Koliji, A.; Lindborg, R.; Gullström, M. Using multiple Landsat scenes in an ensemble classifier reduces classification error in a stable nearshore environment. Int. J. Appl. Earth Obs. Geoinf. 2014, 28, 90–101. [Google Scholar] [CrossRef]
- Maina, J.; Venus, V.; McClanahan, T.R.; Ateweberhan, M. Modelling susceptibility of coral reefs to environmental stress using remote sensing data and GIS models. Ecol. Model. 2008, 212, 180–199. [Google Scholar] [CrossRef]
- Blakey, T.; Melesse, A.; Hall, M. Supervised Classification of Benthic Reflectance in Shallow Subtropical Waters Using a Generalized Pixel-Based Classifier across a Time Series. Remote Sens. 2015, 7, 5098–5116. [Google Scholar] [CrossRef]
- Duro, D.C.; Franklin, S.E.; Dubé, M.G. A comparison of pixel-based and object-based image analysis with selected machine learning algorithms for the classification of agricultural landscapes using SPOT-5 HRG imagery. Remote Sens. Environ. 2012, 118, 259–272. [Google Scholar] [CrossRef]
- Blaschke, T. Object based image analysis for remote sensing. ISPRS J. Photogramm. Remote Sens. 2010, 65, 2–16. [Google Scholar] [CrossRef]
- Fallati, L.; Saponari, L.; Savini, A.; Marchese, F.; Corselli, C.; Galli, P. Multi-Temporal UAV Data and Object-Based Image Analysis (OBIA) for Estimation of Substrate Changes in a Post-Bleaching Scenario on a Maldivian Reef. Remote Sens. 2020, 12, 2093. [Google Scholar]
- Zhang, C. Applying data fusion techniques for benthic habitat mapping and monitoring in a coral reef ecosystem. ISPRS J. Photogramm. Remote Sens. 2015, 104, 213–223. [Google Scholar] [CrossRef]
- Román, A.; Tovar-Sánchez, A.; Olivé, I.; Navarro, G. Using a UAV-Mounted Multispectral Camera for the Monitoring of Marine Macrophytes. Front. Mar. Sci. 2021, 8, 1225. [Google Scholar] [CrossRef]
- Duarte, C.M.; Kirkman, H. Methods for the measurement of seagrass abundance and depth distribution. In Global Seagrass Research Methods, 1st ed.; Short, F., Coles, R., Eds.; Elsevier: Amsterdam, The Netherlands, 2001. [Google Scholar]
- Fauzan, M.A.; Kumara, I.S.W.; Yogyantoro, R.N.; Suwardana, S.W.; Fadhilah, N.; Nurmalasari, I.; Apriyani, S.; Wicaksono, P. Assessing the Capability of Sentinel-2A Data for Mapping Seagrass Percent Cover in Jerowaru, East Lombok. Indones. J. Geogr. 2017, 49, 195–203. [Google Scholar] [CrossRef]
- Ferwerda, J.G.; de Leeuw, J.; Atzberger, C.; Vekerdy, Z. Satellite-based monitoring of tropical seagrass vegetation: Current techniques and future developments. Hydrobiologia 2007, 591, 59–71. [Google Scholar] [CrossRef]
- Flowers, B.; Huang, K.-T.; Aldana, G.O. Analysis of the Habitat Fragmentation of Ecosystems in Belize Using Landscape Metrics. Sustainability 2020, 12, 3024. [Google Scholar] [CrossRef]
- Wedding, L.M.; Lepczyk, C.A.; Pittman, S.J.; Friedlander, A.M.; Jorgensen, S. Quantifying seascape structure: Extending terrestrial spatial pattern metrics to the marine realm. Mar. Ecol. Prog. Ser. 2011, 427, 219–232. [Google Scholar] [CrossRef]
- DHI GRAS. Satellite-Derived Bathymetry: Mapping and Measuring Water Depths from Space. Available online: https://www.dhi-gras.com/solutions/satellite-derived-bathymetry/ (accessed on 15 December 2021).
- Guzinski, R.; Spondylis, E.; Michalis, M.; Tusa, S.; Brancato, G.; Minno, L.; Hansen, L.B. Exploring the Utility of Bathymetry Maps Derived With Multispectral Satellite Observations in the Field of Underwater Archaeology. Open Archaeol. 2016, 2. [Google Scholar] [CrossRef]
- Klonowski, W.; Fearns, P.; Lynch, M. Retrieving key benthic cover types and bathymetry from hyperspectral imagery. J. Appl. Remote Sens. 2007, 1, 011505. [Google Scholar] [CrossRef]
- Lee, Z.; Carder, K.L.; Chen, R.F.; Peacock, T.G. Properties of the water column and bottom derived from Airborne Visible Infrared Imaging Spectrometer (AVIRIS) data. J. Geophys. Res. Ocean. 2001, 106, 11639–11651. [Google Scholar] [CrossRef]
- Lee, Z.; Carder, K.L.; Mobley, C.D.; Steward, R.G.; Patch, J.S. Hyperspectral remote sensing for shallow waters: 2. Deriving bottom depths and water properties by optimization. Appl. Opt. 1999, 38, 3831–3843. [Google Scholar] [CrossRef]
- Lee, Z.; Carder, K.L.; Mobley, C.D.; Steward, R.G.; Patch, J.S. Hyperspectral remote sensing for shallow waters. I. A semianalytical model. Appl. Opt. 1998, 37, 6329–6338. [Google Scholar] [CrossRef]
- Fethers, J.O. Remote Sensing of Eelgrass using Object Based Image Analysis and Sentinel-2 Imagery. Master’s Thesis, University of Aalborg, Aalborg, Denmark, 2018; p. 95. [Google Scholar]
- Lillesand, T.; Kiefer, R.W.; Chipman, J. Remote Sensing and Image Interpretation; John Wiley & Sons: New Jersey, NJ, USA, 2015; p. 736. [Google Scholar]
- McKenzie, L.; Campbell, S.; Roder, C. Seagrass-Watch: Manual for Mapping & Monitoring Seagrass Resources by Community (Citizen) Volunteers; Information Series Qi01094; Northern Fisheries Centre: Townsville, Australia, 2003. [Google Scholar]
- McKenzie, L.J.; Finkbeiner, M.A.; Kirkman, H. Methods for mapping seagrass distribution. In Global Seagrass Research Methods, 1st ed.; Short, F., Coles, R., Eds.; Elsevier: Amsterdam, The Netherlands, 2001; pp. 101–121. [Google Scholar]
- Richmond, M.D. (Ed.) A Field Guide to the Seashores of Eastern Africa and the Western Indian Ocean Islands, 3rd ed.; Sida/WIOMSA: Dar es Salaam, Tanzania, 2011; p. 464. [Google Scholar]
- Hesselbarth, M.H.K.; Sciaini, M.; With, K.A.; Wiegand, K.; Nowosad, J. landscapemetrics: An open-source R tool to calculate landscape metrics. Ecography 2019, 42, 1648–1657. [Google Scholar] [CrossRef]
- Miller-Myers, R.; Virnstein, R.W. Development and Use of an Epiphyte Photo-Index (EPI) for Assessing Epiphyte Loadings on the Seagrass Halodule wrightii; CRC Press: Boca Raton, FL, USA, 2000. [Google Scholar]
- Schreeb, K.V. Mapping Coastal Biotopes and Resource Use in Zanzibar, Tanzania. Master’s Thesis, Stockholm University, Stockholm, Sweden, 2016. [Google Scholar]
- Bray, J.R.; Curtis, J.T. An ordination of the upland forest communities of southern Wisconsin. Ecol. Monogr. 1957, 27, 326–349. [Google Scholar] [CrossRef]
- Clarke, K.; Gorley, R. PRIMER v7: User Manual/Tutorial; Primer-E Ltd.: Plymouth, UK, 2015. [Google Scholar]
- Eklöf, J.S.; de la Torre-Castro, M.; Gullström, M.; Uku, J.; Muthiga, N.; Lyimo, T.; Bandeira, S.O. Sea urchin overgrazing of seagrasses: A review of current knowledge on causes, consequences, and management. Estuarine Coast. Shelf Sci. 2008, 79, 569–580. [Google Scholar] [CrossRef]
- Aoki, L.R.; McGlathery, K.J.; Wiberg, P.L.; Oreska, M.P.J.; Berger, A.C.; Berg, P.; Orth, R.J. Seagrass Recovery Following Marine Heat Wave Influences Sediment Carbon Stocks. Front. Mar. Sci. 2021, 7, 1170. [Google Scholar] [CrossRef]
- Gullström, M.; Lyimo, T.J.; Eklöf, J.S.; Björk, M.; Semesi, I.S.; de la Torre-Castro, M. Seagrass meadows in Chwaka Bay: Socio-ecological and management aspects. In People, Nature and Research in Chwaka Bay, Zanzibar, Tanzania; De la Torre-Castro, M., Lyimo, T.J., Eds.; Western Indian Ocean Marine Science Association (WIOMSA): Zanzibar, Tanzania, 2012; p. 346. [Google Scholar]
- Dan, A.; Moriguchi, A.; Mitsuhashi, K.; Terawaki, T. Relationship between Zostera marina beds and bottom sediments, and wave action offshore in Naruto, southern Japan. Fish. Eng. 1998, 34, 299–304. [Google Scholar]
- Stæhr, P.A.; Göke, C.; Holbach, A.M.; Krause-Jensen, D.; Timmermann, K.; Upadhyay, S.; Ørberg, S.B. Habitat Model of Eelgrass in Danish Coastal Waters: Development, Validation and Management Perspectives. Front. Mar. Sci. 2019, 6, 175. [Google Scholar] [CrossRef]
- Kendrick, G.A.; Holmes, K.W.; Van Niel, K.P. Multi-Scale Spatial Patterns of Three Seagrass Species with Different Growth Dynamics. Ecography 2008, 31, 191–200. [Google Scholar] [CrossRef]
- Koedsin, W.; Intararuang, W.; Ritchie, R.J.; Huete, A. An Integrated Field and Remote Sensing Method for Mapping Seagrass Species, Cover, and Biomass in Southern Thailand. Remote Sens. 2016, 8, 292. [Google Scholar] [CrossRef]
- Hossain, M.S.; Bujang, J.S.; Zakaria, M.H.; Hashim, M. The application of remote sensing to seagrass ecosystems: An overview and future research prospects. Int. J. Remote Sens. 2014, 36, 61–114. [Google Scholar] [CrossRef]
- Giles, A.B.; Davies, J.E.; Ren, K.; Kelaher, B. A deep learning algorithm to detect and classify sun glint from high-resolution aerial imagery over shallow marine environments. ISPRS J. Photogramm. Remote Sens. 2021, 181, 20–26. [Google Scholar] [CrossRef]
- Duarte, C.M. Seagrass depth limits. Aquat. Bot. 1991, 40, 363–377. [Google Scholar] [CrossRef]
- Aleem, A.A. Distribution and ecology of seagrass communities in the Western Indian Ocean. Deep Sea Res. Part A Oceanogr. Res. Pap. 1984, 31, 919–933. [Google Scholar] [CrossRef]
- Fonseca, M.S.; Bell, S.S. Influence of physical setting on seagrass landscapes near Beaufort, North Carolina, USA. Mar. Ecol. Prog. Ser. 1998, 171, 109–121. [Google Scholar] [CrossRef]
- Wikström, K. Influence of Landscape Configuration and Within-Patch Variation on Fish Abundance, Size and Functional Groups in Tropical Seagrass Beds; Stockholm University: Stockholm, Sweden, 2013. [Google Scholar]
- Hinchey, E.K.; Nicholson, M.C.; Zajac, R.N.; Irlandi, E.A. Preface: Marine and coastal applications in landscape ecology. Landsc. Ecol. 2008, 23, 1–5. [Google Scholar] [CrossRef]
- Li, X.; Mander, Ü. Future options in landscape ecology: Development and research. Prog. Phys. Geogr. Earth Environ. 2009, 33, 31–48. [Google Scholar] [CrossRef]
- Ortiz Cajica, A.K.; Hinojosa-Arango, G.; Garza-Pérez, J.R.; Rioja-Nieto, R. Seascape metrics, spatio-temporal change, and intensity of use for the spatial conservation prioritization of a Caribbean marine protected area. Ocean Coast. Manag. 2020, 194, 105265. [Google Scholar] [CrossRef]
- Vermaat, J.E.; Rollon, R.N.; Lacap, C.D.A.; Billot, C.; Alberto, F.; Nacorda, H.M.E.; Wiegman, F.; Terrados, J. Meadow fragmentation and reproductive output of the SE Asian seagrass Enhalus acoroides. J. Sea Res. 2004, 52, 321–328. [Google Scholar] [CrossRef]
- Björk, M.; Short, F.; McLeod, E.; Beer, S. Managing Seagrasses for Resilience to Climate Change; IUCN International Union for Conservation of Nature: Gland, Switzerland, 2008; Volume 3. [Google Scholar]
- Gullström, M.; Lyimo, L.D.; Dahl, M.; Samuelsson, G.S.; Eggertsen, M.; Anderberg, E.; Rasmusson, L.M.; Linderholm, H.W.; Knudby, A.; Bandeira, S.; et al. Blue Carbon Storage in Tropical Seagrass Meadows Relates to Carbonate Stock Dynamics, Plant–Sediment Processes, and Landscape Context: Insights from the Western Indian Ocean. Ecosystems 2018, 21, 551–566. [Google Scholar] [CrossRef]
- Macreadie, P.I.; Anton, A.; Raven, J.A.; Beaumont, N.; Connolly, R.M.; Friess, D.A.; Kelleway, J.J.; Kennedy, H.; Kuwae, T.; Lavery, P.S.; et al. The future of Blue Carbon science. Nat. Commun. 2019, 10, 3998. [Google Scholar] [CrossRef]
- Cuevas, E.; Uribe-Martínez, A.; Morales-Ojeda, S.M.; Gómez-Ruíz, P.A.; Núñez-Lara, E.; Teutli-Hernández, C.; Herrera-Silveira, J.A. Spatial configuration of seagrass community attributes in a stressed coastal lagoon, southeastern Gulf of Mexico. Reg. Stud. Mar. Sci. 2021, 48, 102049. [Google Scholar] [CrossRef]
- Leliaert, F.; Vanreusel, W.; De Clerck, O.; Coppejans, E. Epiphytes on the seagrasses of Zanzibar Island (Tanzania), floristic and ecological aspects. Belg. J. Bot. 2001, 134, 3–20. [Google Scholar]
- Mamboya, F.; Lugomela, C.; Mvungi, E.; Hamisi, M.; Kamukuru, A.T.; Lyimo, T.J. Seagrass–sea urchin interaction in shallow littoral zones of Dar es Salaam, Tanzania. Aquat. Conserv. Mar. Freshw. Ecosyst. 2009, 19, S19–S26. [Google Scholar] [CrossRef]
- Valentine, J.F.; Heck, K.L. The role of sea urchin grazing in regulating subtropical seagrass meadows: Evidence from field manipulations in the northern Gulf of Mexico. J. Exp. Mar. Biol. Ecol. 1991, 154, 215–230. [Google Scholar] [CrossRef]
- Carnell, P.E.; Ierodiaconou, D.; Atwood, T.B.; Macreadie, P.I. Overgrazing of Seagrass by Sea Urchins Diminishes Blue Carbon Stocks. Ecosystems 2020, 23, 1437–1448. [Google Scholar] [CrossRef]
- Rose, C.D.; Sharp, W.C.; Kenworthy, W.J.; Hunt, J.H.; Lyons, W.G.; Prager, E.J.; Valentine, J.F.; Hall, M.O.; Whitfield, P.E.; Fourqurean, J.W. Overgrazing of a large seagrass bed by the sea urchin Lytechinus variegatus in Outer Florida Bay. Mar. Ecol. Prog. Ser. 1999, 190, 211–222. [Google Scholar] [CrossRef]
- Eklöf, J.S.; Henriksson, R.; Kautsky, N. Effects of tropical open-water seaweed farming on seagrass ecosystem structure and function. Mar. Ecol. Prog. Ser. 2006, 325, 73–84. [Google Scholar] [CrossRef]
- Zanzibar Planning Commission. Human Pressure and Threats on Coastal and Marine Resources, Zanzibar: A Critical Review; Zanzibar Planning Commission: Zanzibar, Tanzania, 2017; p. 135.
- Nchimbi, A.A.; Lyimo, L.D. Socioeconomic Determinants of Mangrove Exploitation and Seagrass Degradation in Zanzibar: Implications for Sustainable Development. J. Mar. Biol. 2019, 2019, 7684924. [Google Scholar] [CrossRef]
- Meehan, A.J.; Williams, R.J.; Watford, F.A. Detecting Trends in Seagrass Abundance Using Aerial Photograph Interpretation: Problems Arising with the Evolution of Mapping Methods. Estuaries 2005, 28, 462–472. [Google Scholar] [CrossRef]
- Unsworth, R.K.F.; van Keulen, M.; Coles, R.G. Seagrass meadows in a globally changing environment. Mar. Pollut. Bull. 2014, 83, 383–386. [Google Scholar] [CrossRef] [PubMed]



| Dataset Type | Unit | Spatial Resolution | Source | Use |
|---|---|---|---|---|
| Sentinel 2 imagery | m | 10 m; island scale. | Sentinel 2 | Site location, SAV detection. |
| Orthomosaic drone imagery | ha | 2 to 4 cm at the site scale; covers nine studied sites. | Aerial drone field survey (2020) | Mapping habitat type; percent cover and analysis of seascape fragmentation. |
| Bathymetry | m | 10 m; covers entire Unguja island waters. | Sentinel 2; DHI GRAS (2018) | Analysis of the depth-cover distribution of seagrasses. |
| Ground truth waypoints (GPS) | m | 3 m; covers nine sites; total number varies with site. | GPS waypoints (2020) | Georectifying of the photo points and videos; geometry correction of orthomosaics. Validation and accuracy assessment. |
| In situ photos and videos | Point | Point (relative); a various number of photo points in the nine sites. | Ground truth field survey (2020) | OBIA classification; analysis of biological parameters, such as species and substrate type. |
| Site | Date of Acquisition | Area (ha) | Drone and Camera Model | Resolution (cm/pixel) | Time of Mapping |
|---|---|---|---|---|---|
| Mnemba | 10 May 2020 | 29 | FC6310 (8.8 mm) | 2.79 | High tide |
| Uroa | 22 August 2020 | 37.5 | FC6310 (8.8 mm) | 2.68 | Low tide |
| Chwaka | 06 January 2020 | 161 | FC6310 (8.8 mm | 2.79 | High tide |
| Bwejuu | 03 January 2020 | 69.6 | L1D-20c (10.26 mm) | 2.96 | Low tide |
| Jambiani | 09 January 2020 | 185 | FC6310 (8.8 mm) | 2.83 | Low tide |
| Kwale | 02 January 2020 | 73.9 | L1D-20c (10.26 mm) | 2.83 | High tide |
| Chumbe | 07 January 2020 | 26.1 | L1D-20c (10.26 mm) | 2.73 | High tide |
| Chapwani | 13 January 2020 | 30.2 | FC6310 (8.8 mm) | 2.78 | Low tide |
| Tumbatu | 24 January 2020 | 110 | FC6310 (8.8 mm) | 4.14 | Low tide |
| Total area | 722.3 | 2.94 (mean) |
| Site | In Situ Points | Substrate Type 1 | Coral Reefs | Seagrass Species | Average Cover (%) |
|---|---|---|---|---|---|
| Bwejuu | 79 | M, S, FS, R | Yes | 5 | 52.1 |
| Chapwani | 105 | S, CS, R | Yes | 6 | 44.2 |
| Chumbe | 37 | S, R, CS | Yes | 5 | 44.8 |
| Chwaka | 72 | M, S, FS, R | No | 9 | 78.6 |
| Jambiani | 290 | S, R, M | No | 6 | 64.4 |
| Kwale | 113 | S, CR, R | Yes | 5 | 55 |
| Mnemba | 35 | S, CS | Yes | 7 | 34 |
| Tumbatu | 270 | M, CS, S, R | No | 6 | 66.6 |
| Uroa | 144 | M, FS, S, R | No | 7 | 71.6 |
| Total | 1145 | 9 (max) | 56.8 (mean) |
| Sites | Classification Type | |||||
|---|---|---|---|---|---|---|
| Benthic Cover (%) | Seagrass Total Cover (%) | Seagrass Species Cover (%) | ||||
| OA | Ka | OA | Ka | OA | Ka | |
| Bwejuu | 87 | 80 | 78 | 78 | 72 | 71 |
| Chapwani | 89 | 79 | 86 | 82 | 78 | 67 |
| Chwaka | 88 | 70 | 79 | 79 | 71 | 70 |
| Chumbe | 81 | 73 | 78 | 72 | 78 | 67 |
| Jambiani | 90 | 90 | 77 | 77 | 78 | 77 |
| Kwale | 84 | 77 | 78 | 78 | 71 | 71 |
| Mnemba | 87 | 79 | 79 | 74 | 77 | 69 |
| Tumbatu | 87 | 80 | 83 | 78 | 86 | 70 |
| Uroa | 94 | 85 | 83 | 78 | 76 | 67 |
| Mean | 87 | 79 | 80 | 77 | 76 | 70 |
| Median | 87 | 79 | 79 | 78 | 77 | 70 |
| SD | 3.4 | 5.6 | 2.9 | 2.7 | 4.4 | 3.0 |
| CV | 3.9 | 7.0 | 3.6 | 3.5 | 5.8 | 4.2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hamad, I.Y.; Staehr, P.A.U.; Rasmussen, M.B.; Sheikh, M. Drone-Based Characterization of Seagrass Habitats in the Tropical Waters of Zanzibar. Remote Sens. 2022, 14, 680. https://doi.org/10.3390/rs14030680
Hamad IY, Staehr PAU, Rasmussen MB, Sheikh M. Drone-Based Characterization of Seagrass Habitats in the Tropical Waters of Zanzibar. Remote Sensing. 2022; 14(3):680. https://doi.org/10.3390/rs14030680
Chicago/Turabian StyleHamad, Idrissa Yussuf, Peter Anton Upadhyay Staehr, Michael Bo Rasmussen, and Mohammed Sheikh. 2022. "Drone-Based Characterization of Seagrass Habitats in the Tropical Waters of Zanzibar" Remote Sensing 14, no. 3: 680. https://doi.org/10.3390/rs14030680
APA StyleHamad, I. Y., Staehr, P. A. U., Rasmussen, M. B., & Sheikh, M. (2022). Drone-Based Characterization of Seagrass Habitats in the Tropical Waters of Zanzibar. Remote Sensing, 14(3), 680. https://doi.org/10.3390/rs14030680







