LiDAR Reveals the Process of Vision-Mediated Predator–Prey Relationships
Abstract
1. Introduction
2. Materials and Methods
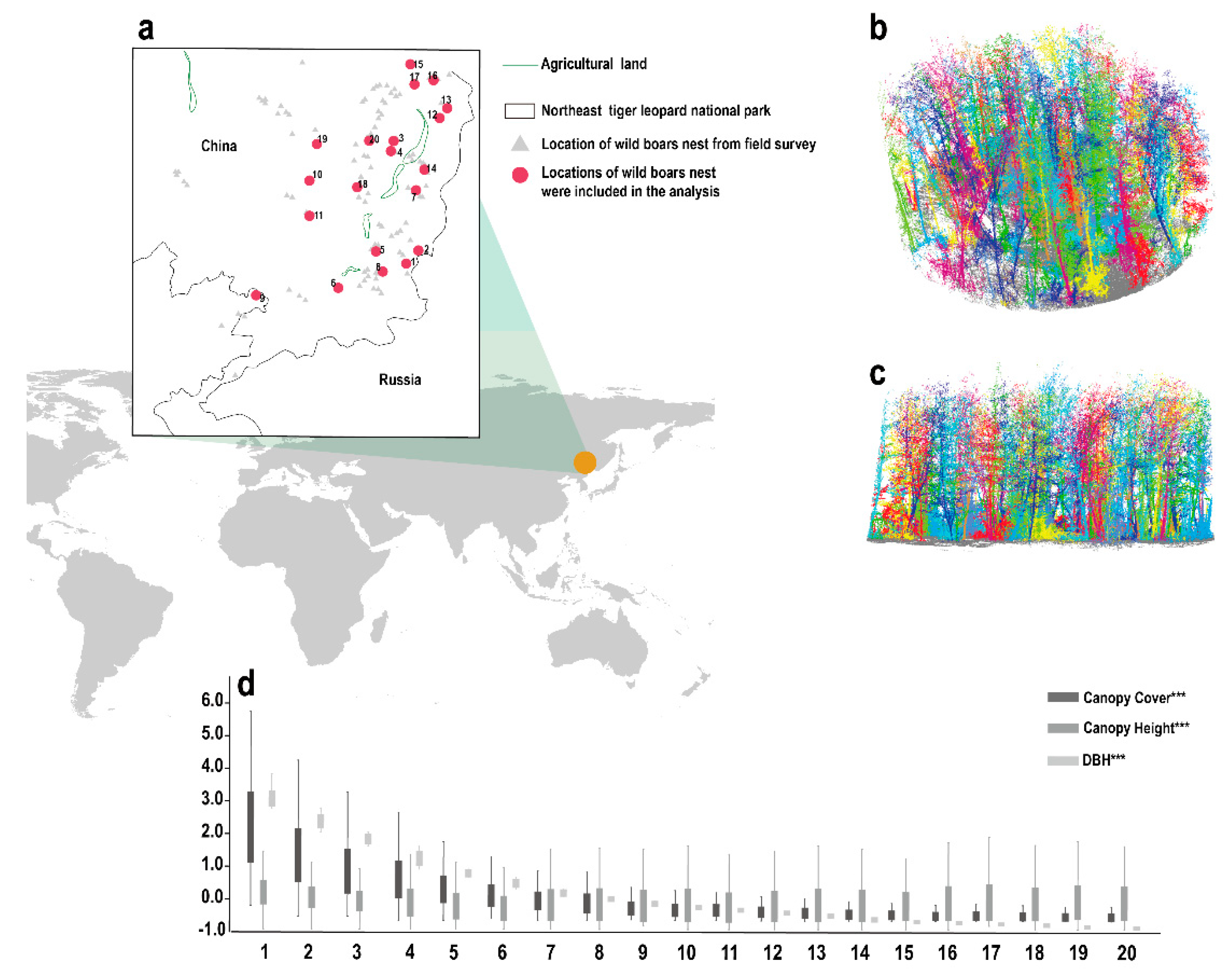
2.1. Study Area
2.2. Data Collection
2.2.1. Location Data of Wild Boar Nests
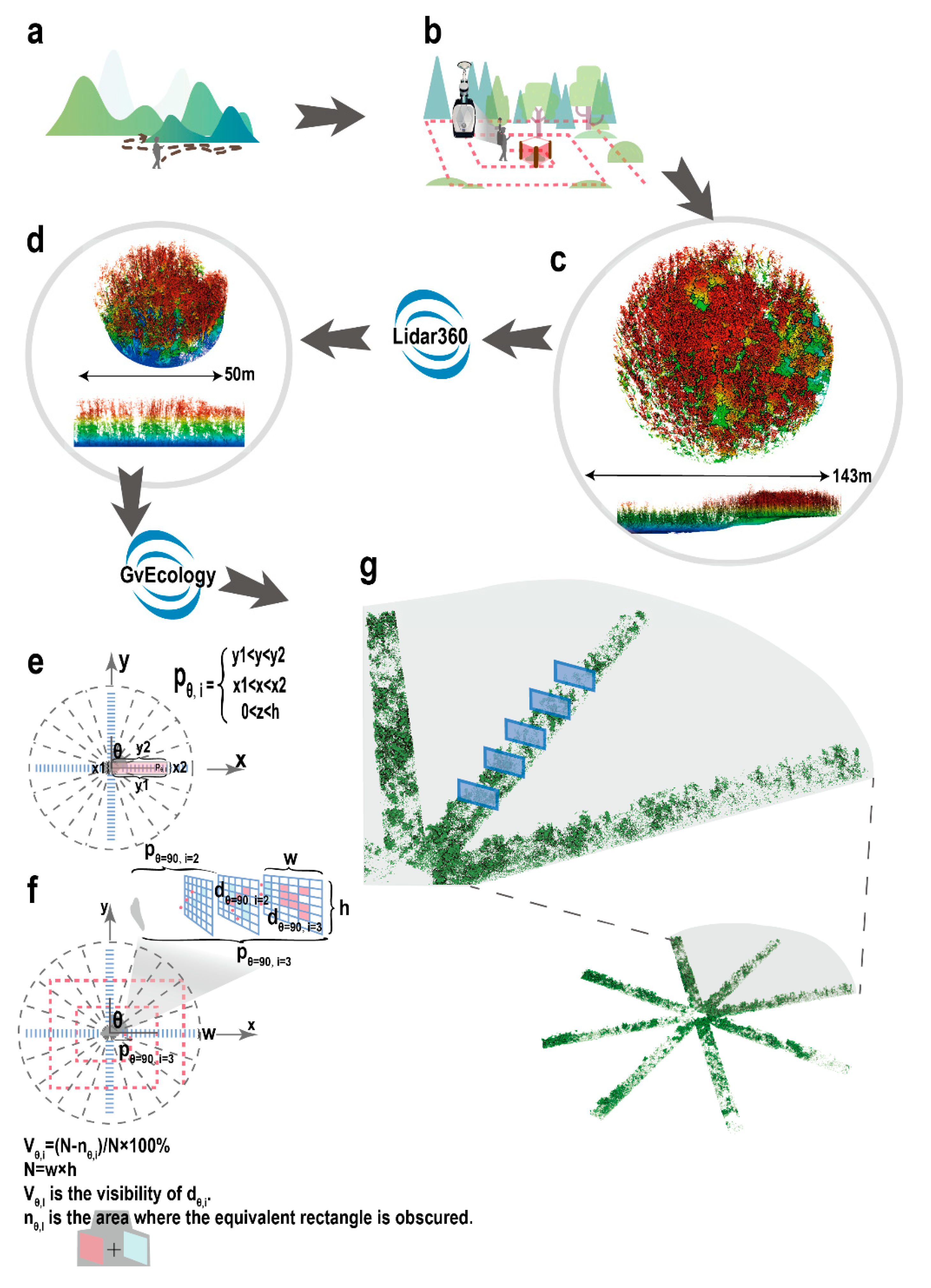
2.2.2. Point Cloud Data of the Environment Surrounding the Wild Boar Nests
2.3. Data Processing
2.3.1. Point Cloud Data Pre-Processing
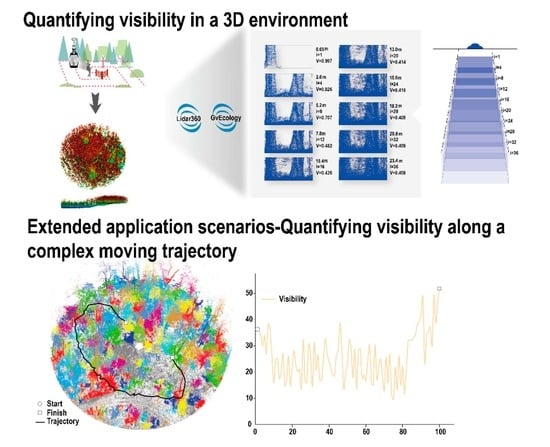
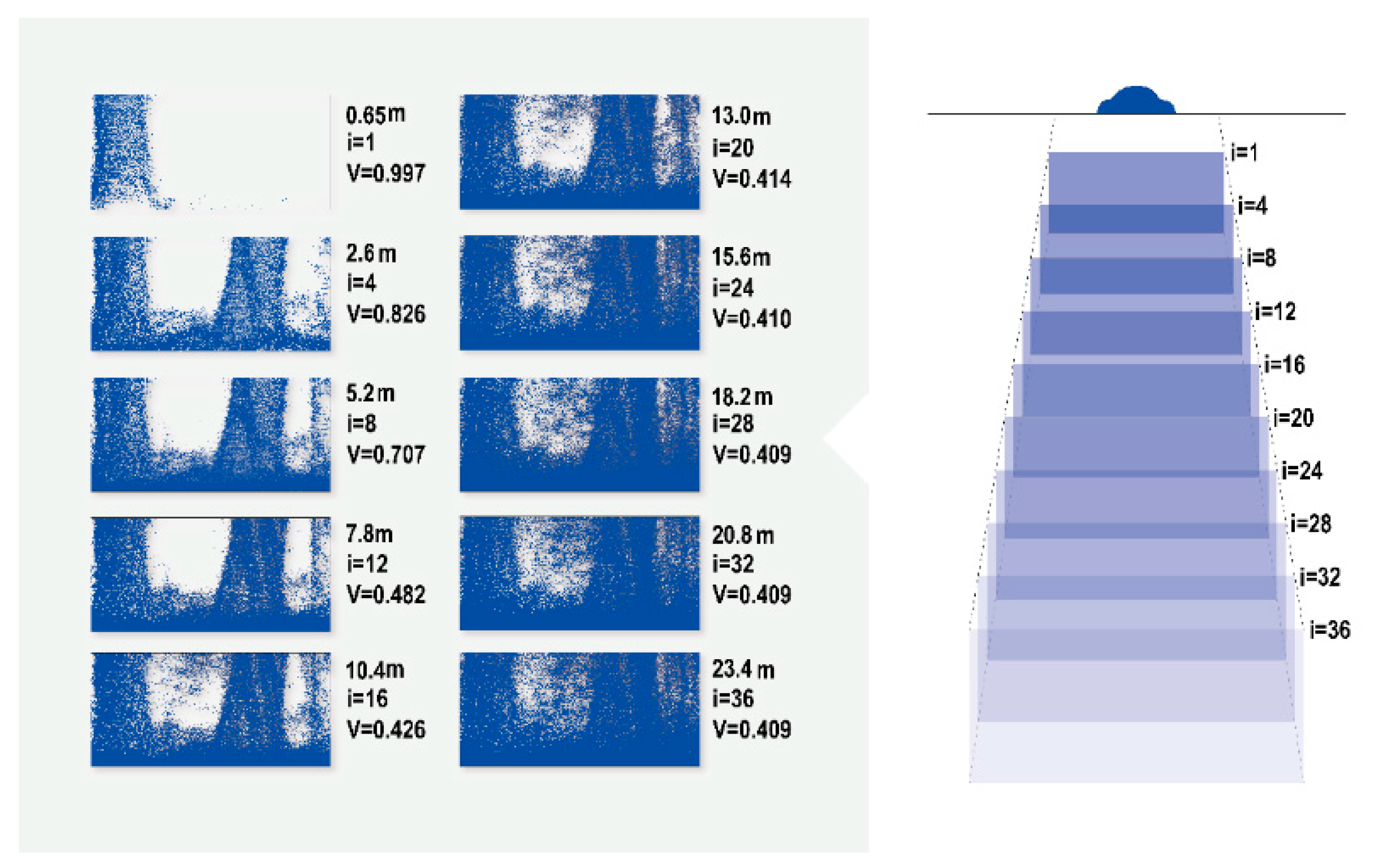
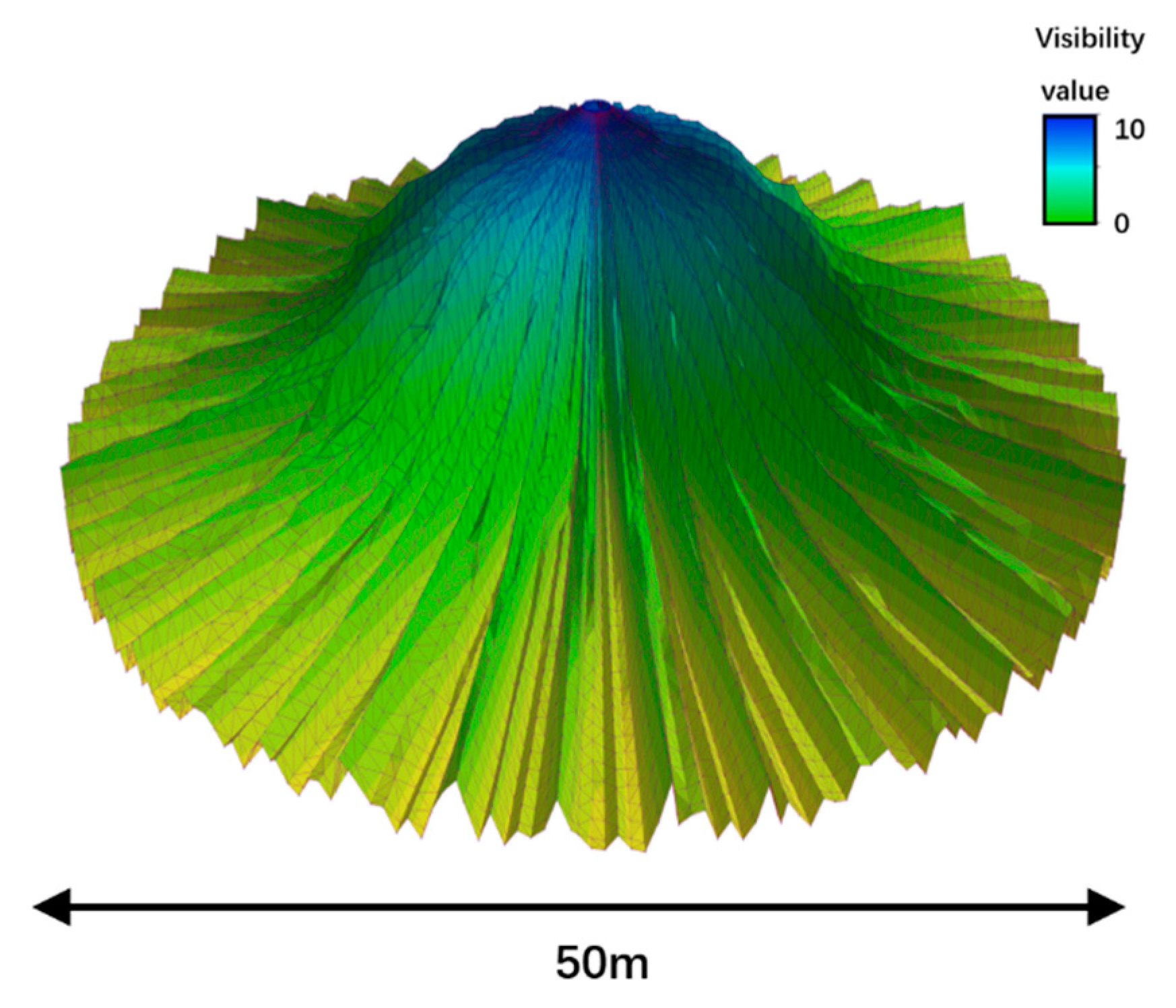
2.3.2. Calculation of the Visibility Index
2.3.3. Simulated Scene Showing the Tiger Near the Nest
2.4. Statistical Analysis
3. Results
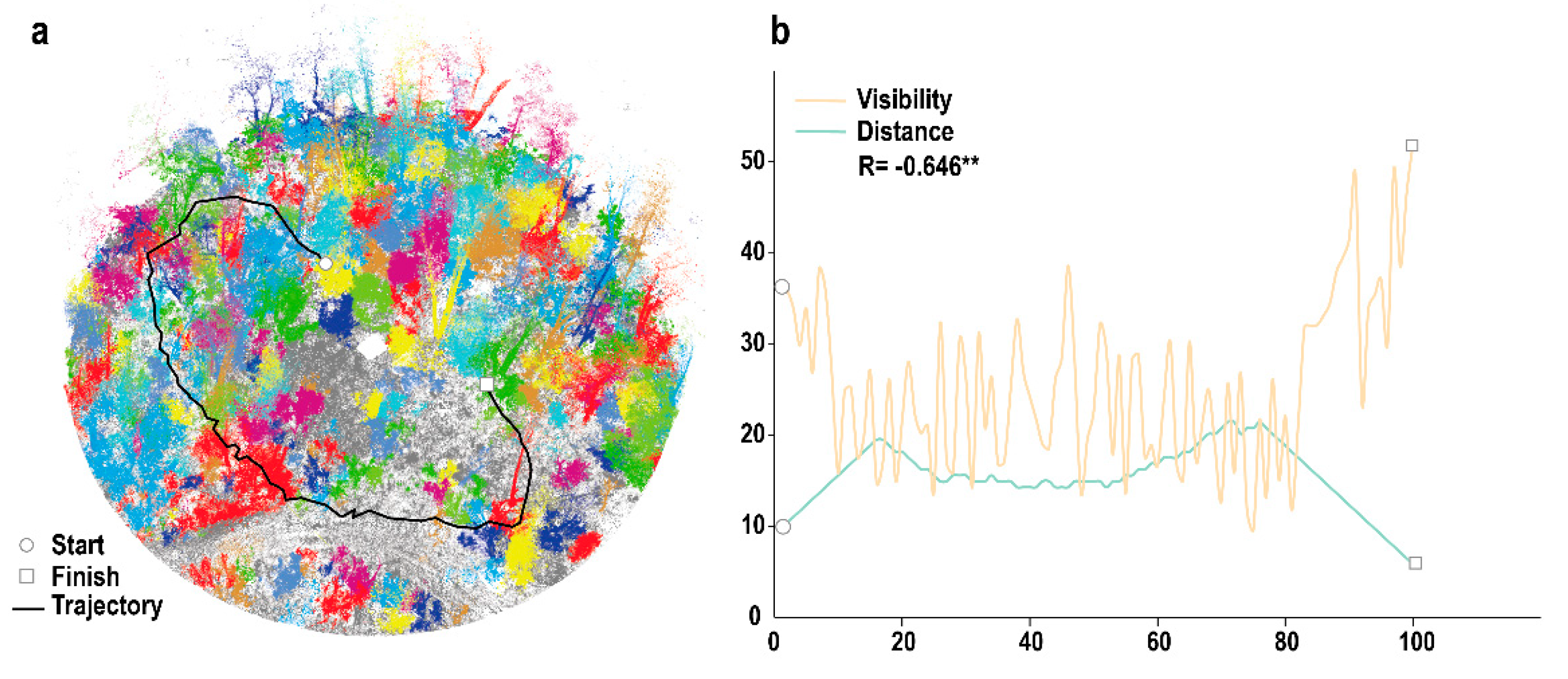
3.1. Visibility Calculation
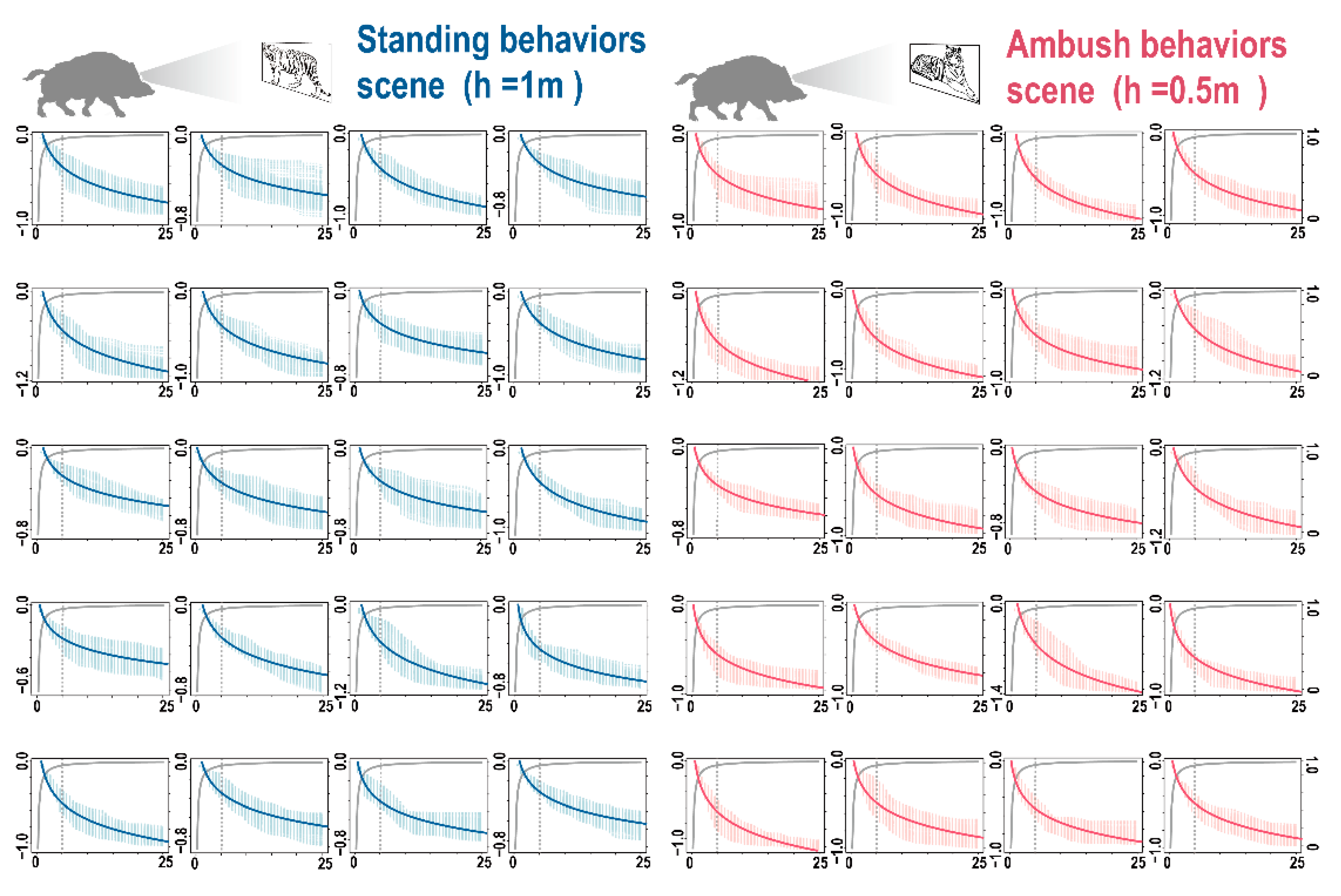
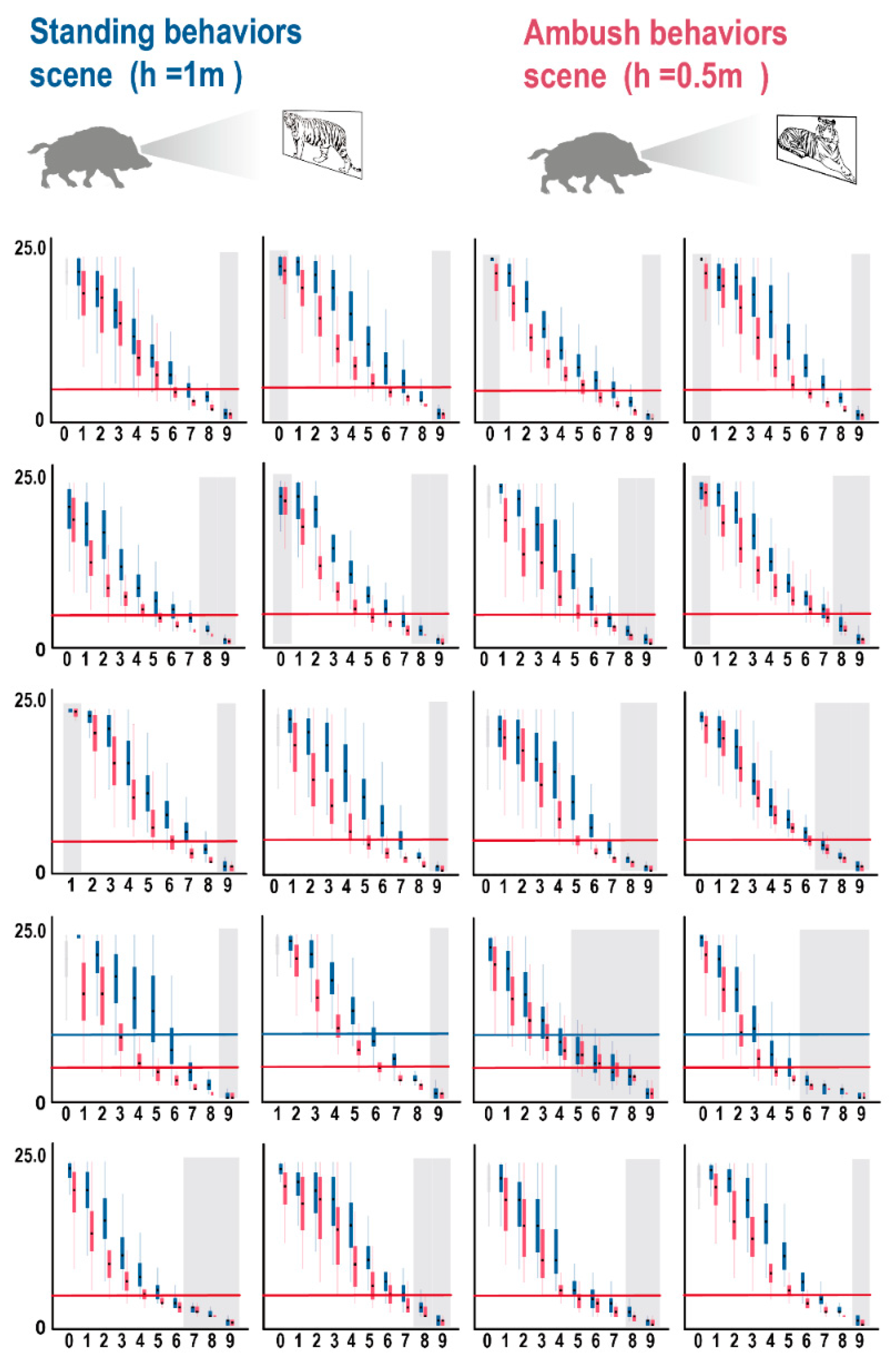
3.2. 3D Forest Structure Mediated Predation Strategy
3.3. 3D Forest Structure and Predation Mediated Anti-Predation Strategy
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Letten, A.D.; Stouffer, D.B. The mechanistic basis for higher-order interactions and non-additivity in competitive communities. Ecol. Lett. 2019, 22, 423–436. [Google Scholar] [CrossRef] [PubMed]
- Gallien, L.; Zimmermann, N.E.; Levine, J.M.; Adler, P.B. The effects of intransitive competition on coexistence. Ecol. Lett. 2017, 20, 791–800. [Google Scholar] [CrossRef]
- Terborgh, J.W. Toward a trophic theory of species diversity. Proc. Natl. Acad. Sci. USA 2015, 112, 11415. [Google Scholar] [CrossRef] [PubMed]
- Chase, J.M.; Abrams, P.A.; Grover, J.P.; Diehl, S.; Chesson, P.; Holt, R.D.; Richards, S.A.; Nisbet, R.M.; Case, T.J. The interaction between predation and competition: A review and synthesis. Ecol. Lett. 2002, 5, 302–315. [Google Scholar] [CrossRef]
- Chesson, P.; Kuang, J.J. The interaction between predation and competition. Nature 2008, 456, 235–238. [Google Scholar] [CrossRef] [PubMed]
- Vázquez, D.P.; Lomáscolo, S.B.; Maldonado, M.B.; Chacoff, N.P.; Dorado, J.; Stevani, E.L.; Vitale, N.L. The strength of plant–pollinator interactions. Ecology 2012, 93, 719–725. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Buckling, A. Antagonistic coevolution limits population persistence of a virus in a thermally deteriorating environment. Ecol. Lett. 2011, 14, 282–288. [Google Scholar] [CrossRef]
- Allesina, S.; Levine, J.M. A competitive network theory of species diversity. Proc. Natl. Acad. Sci. USA 2011, 108, 5638. [Google Scholar] [CrossRef] [PubMed]
- Bairey, E.; Kelsic, E.D.; Kishony, R. High-order species interactions shape ecosystem diversity. Nat. Commun. 2016, 7, 12285. [Google Scholar] [CrossRef]
- Bastolla, U.; Fortuna, M.A.; Pascual-Garcia, A.; Ferrera, A.; Luque, B.; Bascompte, J. The architecture of mutualistic networks minimizes competition and increases biodiversity. Nature 2009, 458, 1018–1020. [Google Scholar] [CrossRef]
- Pringle, R.M.; Kartzinel, T.R.; Palmer, T.M.; Thurman, T.J.; Fox-Dobbs, K.; Xu, C.C.Y.; Hutchinson, M.C.; Coverdale, T.C.; Daskin, J.H.; Evangelista, D.A.; et al. Predator-induced collapse of niche structure and species coexistence. Nature 2019, 570, 58–64. [Google Scholar] [CrossRef]
- Stachowicz, J.J.; Fried, H.; Osman, R.W.; Whitlatch, R.B. Biodiversity, invasion resistance, and marine ecosystem function: Reconciling pattern and process. Ecology 2002, 83, 2575–2590. [Google Scholar] [CrossRef]
- Zhang, H.; Bearup, D.; Nijs, I.; Wang, S.; Barabás, G.; Tao, Y.; Liao, J. Dispersal network heterogeneity promotes species coexistence in hierarchical competitive communities. Ecol. Lett. 2021, 24, 50–59. [Google Scholar] [CrossRef]
- Eklöv, P.; VanKooten, T. Faciliation among piscivorous predators: Effects of prey habitat use. Ecology 2001, 82, 2486–2494. [Google Scholar] [CrossRef]
- Dröge, E.; Creel, S.; Becker, M.S.; M’soka, J. Risky times and risky places interact to affect prey behaviour. Nat. Ecol. Evol. 2017, 1, 1123–1128. [Google Scholar] [CrossRef]
- Stier Adrian, C.; Samhouri Jameal, F.; Novak, M.; Marshall Kristin, N.; Ward Eric, J.; Holt Robert, D.; Levin Phillip, S. Ecosystem context and historical contingency in apex predator recoveries. Sci. Adv. 2016, 2, e1501769. [Google Scholar] [CrossRef]
- Smith, J.G.; Tomoleoni, J.; Staedler, M.; Lyon, S.; Fujii, J.; Tinker, M.T. Behavioral responses across a mosaic of ecosystem states restructure a sea otter–urchin trophic cascade. Proc. Natl. Acad. Sci. USA 2021, 118, e2012493118. [Google Scholar] [CrossRef] [PubMed]
- Molnár, P.K.; Bitz, C.M.; Holland, M.M.; Kay, J.E.; Penk, S.R.; Amstrup, S.C. Fasting season length sets temporal limits for global polar bear persistence. Nat. Clim. Chang. 2020, 10, 732–738. [Google Scholar] [CrossRef]
- Creel, S.; Winnie, J.; Maxwell, B.; Hamlin, K.; Creel, M. Elk alter habitat selection as an antipredator response to wolves. Ecology 2005, 86, 3387–3397. [Google Scholar] [CrossRef]
- Loarie, S.R.; Tambling, C.J.; Asner, G.P. Lion hunting behaviour and vegetation structure in an African savanna. Anim. Behav. 2013, 85, 899–906. [Google Scholar] [CrossRef]
- Swanson, A.; Caro, T.; Davies-Mostert, H.; Mills, M.G.L.; Macdonald, D.W.; Borner, M.; Masenga, E.; Packer, C. Cheetahs and wild dogs show contrasting patterns of suppression by lions. J. Anim. Ecol. 2014, 83, 1418–1427. [Google Scholar] [CrossRef]
- Davies, A.B.; Tambling, C.J.; Marneweck, D.G.; Ranc, N.; Druce, D.J.; Cromsigt, J.P.G.M.; le Roux, E.; Asner, G.P. Spatial heterogeneity facilitates carnivore coexistence. Ecology 2021, 102, e03319. [Google Scholar] [CrossRef] [PubMed]
- Schmitz, O.J. Predators avoiding predation. Proc. Natl. Acad. Sci. USA 2008, 105, 14749. [Google Scholar] [CrossRef] [PubMed]
- Manicom, C.; Schwarzkopf, L.; Alford, R.A.; Schoener, T.W. Self-made shelters protect spiders from predation. Proc. Natl. Acad. Sci. USA 2008, 105, 14903. [Google Scholar] [CrossRef]
- Olsoy, P.J.; Forbey, J.S.; Rachlow, J.L.; Nobler, J.D.; Glenn, N.F.; Shipley, L.A. Fearscapes: Mapping Functional Properties of Cover for Prey with Terrestrial LiDAR. BioScience 2015, 65, 74–80. [Google Scholar] [CrossRef][Green Version]
- Muto, A.; Lal, P.; Ailani, D.; Abe, G.; Itoh, M.; Kawakami, K. Activation of the hypothalamic feeding centre upon visual prey detection. Nat. Commun. 2017, 8, 15029. [Google Scholar] [CrossRef] [PubMed]
- Mugan, U.; MacIver, M.A. Spatial planning with long visual range benefits escape from visual predators in complex naturalistic environments. Nat. Commun. 2020, 11, 3057. [Google Scholar] [CrossRef]
- Aben, J.; Signer, J.; Heiskanen, J.; Pellikka, P.; Travis, J.M.J. What you see is where you go: Visibility influences movement decisions of a forest bird navigating a three-dimensional-structured matrix. Biol. Lett. 2021, 17, 20200478. [Google Scholar] [CrossRef] [PubMed]
- Vierling, K.; Vierling, L.; Gould, W.; Martinuzzi, S.; Clawges, R. LiDAR: Shedding new light on habitat characterization and modeling. Front. Ecol. Environ. 2008, 6, 90–98. [Google Scholar] [CrossRef]
- Davies, A.B.; Asner, G.P. Advances in animal ecology from 3D-LiDAR ecosystem mapping. Trends Ecol. Evol. 2014, 29, 681–691. [Google Scholar] [CrossRef]
- Yang, H.; Dou, H.; Baniya, R.K.; Han, S.; Guan, Y.; Xie, B.; Zhao, G.; Wang, T.; Mou, P.; Feng, L.; et al. Seasonal food habits and prey selection of Amur tigers and Amur leopards in Northeast China. Sci. Rep. 2018, 8, 6930. [Google Scholar] [CrossRef] [PubMed]
- Hailong, D.; Haitao, Y.; James, L.D.S.; Limin, F.; Tianming, W.; Jianping, G. Prey selection of Amur tigers in relation to the spatiotemporal overlap with prey across the Sino–Russian border. Wildl. Biol. 2019, 2019, 1–11. [Google Scholar]
- Luskin, M.S.; Brashares, J.S.; Ickes, K.; Sun, I.F.; Fletcher, C.; Wright, S.J.; Potts, M.D. Cross-boundary subsidy cascades from oil palm degrade distant tropical forests. Nat. Commun. 2017, 8, 2231. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Llario, P. Environmental correlates of nest site selection by wild boar Sus scrofa. Acta Theriol. 2004, 49, 383–392. [Google Scholar] [CrossRef]
- Hayward, M.W.; Jędrzejewski, W.; Jêdrzejewska, B. Prey preferences of the tiger Panthera tigris. J. Zool. 2012, 286, 221–231. [Google Scholar] [CrossRef]
- Van der Zande, D.; Hoet, W.; Jonckheere, I.; van Aardt, J.; Coppin, P. Influence of measurement set-up of ground-based LiDAR for derivation of tree structure. Agric. For. Meteorol. 2006, 141, 147–160. [Google Scholar] [CrossRef]
- Henning, J.; Radtke, P. Ground-based Laser Imaging for Assessing Three-dimensional Forest Canopy Structure. Photogramm. Eng. Remote Sens. 2006, 72, 1349–1358. [Google Scholar] [CrossRef]
- Li, C.; Gan, Y.; Zhang, C.; He, H.; Fang, J.; Wang, L.; Wang, Y.; Liu, J. “Microplastic communities” in different environments: Differences, links, and role of diversity index in source analysis. Water Res. 2021, 188, 116574. [Google Scholar] [CrossRef] [PubMed]
- Yudakov, A.G.; Nikolaev, I.G. Winter ecology of the Amur tiger: Based upon observations in the west-central Sikhote-Alin Mountains 1970–1973, 1996–2010. J. Wildl. Manag. 2014, 1–2. [Google Scholar] [CrossRef]
- Pikunov; Mitchell; Dunishe; Li, B. A Guide to Wildlife Tracks in the Far East; Northeast Forestry University Press: Harbin, China, 2008. [Google Scholar]
- Samia, D.S.M.; Nomura, F.; Blumstein, D.T. Do animals generally flush early and avoid the rush? A meta-analysis. Biol. Lett. 2013, 9, 20130016. [Google Scholar] [CrossRef] [PubMed]
- Hopcraft, J.G.C.; Sinclair, A.R.E.; Packer, C. Planning for success: Serengeti lions seek prey accessibility rather than abundance. J. Anim. Ecol. 2005, 74, 559–566. [Google Scholar] [CrossRef]
- Yudakov, A.G.; Nikolaev, I.G. Winter Ecology of the Amur Tiger; Dalnauka: Vladivostok, Russia, 2012. [Google Scholar]
- Iason, G.R.; Prins, H.H.T. Dangerous Lions and Nonchalant Buffalo. Behaviour 1989, 108, 262–296. [Google Scholar] [CrossRef]
- Lima, S.L. Putting predators back into behavioral predator–prey interactions. Trends Ecol. Evol. 2002, 17, 70–75. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fu, Y.; Xu, G.; Gao, S.; Feng, L.; Guo, Q.; Yang, H. LiDAR Reveals the Process of Vision-Mediated Predator–Prey Relationships. Remote Sens. 2022, 14, 3730. https://doi.org/10.3390/rs14153730
Fu Y, Xu G, Gao S, Feng L, Guo Q, Yang H. LiDAR Reveals the Process of Vision-Mediated Predator–Prey Relationships. Remote Sensing. 2022; 14(15):3730. https://doi.org/10.3390/rs14153730
Chicago/Turabian StyleFu, Yanwen, Guangcai Xu, Shang Gao, Limin Feng, Qinghua Guo, and Haitao Yang. 2022. "LiDAR Reveals the Process of Vision-Mediated Predator–Prey Relationships" Remote Sensing 14, no. 15: 3730. https://doi.org/10.3390/rs14153730
APA StyleFu, Y., Xu, G., Gao, S., Feng, L., Guo, Q., & Yang, H. (2022). LiDAR Reveals the Process of Vision-Mediated Predator–Prey Relationships. Remote Sensing, 14(15), 3730. https://doi.org/10.3390/rs14153730